Abstract
The neonatal capacity for cardiac regeneration in mice is well-studied and has been used to develop many potential strategies for adult cardiac regenerative repair following injury. However, translating these findings from rodents to designing regenerative therapeutics for adult human heart disease remains elusive. Large mammals including pigs, dogs, and sheep are widely used as animal models of humans in preclinical trials of new cardiac drugs and devices. However, very little is known about the fundamental cardiac cell biology and the timing of postnatal cardiac events that influence cardiomyocyte proliferation in these animals. There is emerging evidence that external physiological and environmental cues could be the key to understanding cardiomyocyte proliferative behavior. In this review, we survey available literature on postnatal development in various large-mammal animal models to offer a perspective on the physiological and cellular characteristics that could be regulating cardiomyocyte proliferation. Similarities and differences between developmental milestones, cardiomyocyte maturational events, as well as environmental cues regulating cardiac development, are discussed for various large mammals, with a focus on postnatal cardiac regenerative potential and translatability to the human heart.
Keywords: Postnatal heart development, Cardiomyocyte proliferation, Large mammals, Cardiac regeneration, Human heart disease
I. Introduction
Congenital heart defects are the most lethal of anomalies in the first year of life and cardiovascular disease is the leading cause of death worldwide [1,2]. These lethal conditions are aggravated by the limited ability of cardiac muscle to repair or regenerate in adult mammals. Studies in mice reported several years ago provide evidence that the heart can regenerate after injury in the first few days after birth [3,4]. This finding has led to intensive research efforts directed towards understanding how regeneration in mammalian hearts occurs and what causes loss of regenerative ability. Moreover, there is increasing evidence that inducing developmental or regenerative pathways in adult mouse cardiomyocytes can promote cardiac repair after myocardial infarction [5,6]. However, relatively little is known of cardiac regeneration or repair mechanisms in larger mammals, including humans. Current efforts are directed towards examining postnatal heart development in large animal models as a means to understand human heart development, particularly since the US Food and Drug Administration (FDA) requires large mammal models to translate devices and potential therapies based on studies in mice and zebrafish.
Cardiac maturation events, such as the timing of cell cycle arrest, multinucleation, polyploidy, expression of mature sarcomeric proteins, and induction of cardiomyocyte hypertrophic growth, are likely variable across mammalian species. Available studies support differences in postnatal maturation and regenerative potential in rodents and large animals used for laboratory studies, when compared to the human heart [7]. Further, the impact of physiological differences between the various model animal species, such as gestation time, age at weaning, age at sexual maturity, and growth rate, on postnatal cardiac maturation has also not been taken into account in their use as models of human heart development and regenerative capacity [8]. The conservation of such characteristics across different species has important implications for translating findings from these mammalian model systems to human congenital heart repair or treatment of heart failure.
Before birth, the mammalian heart grows primarily through proliferation of differentiated, mononucleated cardiomyocytes that undergo complete cytokinesis to form new cardiomyocytes [9]. After birth, the ability of differentiated cardiomyocytes to proliferate and generate new muscle is lost, which is a major disadvantage for correction of congenital malformations or cardiac repair after injury [3]. The timing of cardiomyocyte cell cycle arrest and loss of regenerative ability after birth has been extensively studied in rodents, but information for large mammals is lacking [8]. In humans, there is no consensus on cardiomyocyte proliferative capacity after birth [10–12]. One study estimating mitotic and cytokinetic indices in neonates and children suggests that total cardiomyocyte number continues to increase after birth, with about 3-fold increase in cardiomyocyte number measured by 20 years of age [10]. Alternatively, human cardiomyocyte generation rates based on radiolabeling led to lower estimates of newly-formed cardiomyocytes in children and young adults [11,12]. Therefore, the exact proliferative capacity and timing of cardiomyocyte cell cycle arrest in postnatal humans is still not known. A case report published in 2016 described surprising recovery of heart function after neonatal heart injury in a human infant [13]. This has led to much speculation in the field regarding the ability of the postnatal human heart to repair after injury, with implications for the timing of surgical repair of congenital heart defects in infants. However, definitive findings on the proliferative or regenerative abilities of large mammals including pigs, dogs, sheep, and nonhuman primates have not been reported. Here we review published information on postnatal heart maturation and cardiomyocyte cell cycling as a means to assess the potential of the postnatal heart to repair or regenerate in various mammals.
II. Cardiac regeneration in common verterbrate laboratory animal models
Cardiac regeneration was first characterized in salamanders and zebrafish where new cardiac muscle is generated after resection of up to 20% of the adult heart [14,15]. Cell lineage tracing in zebrafish demonstrated that the newly-formed muscle arises from pre-existing cardiomyocytes which dedifferentiate, proliferate, and regenerate [16,17]. Notably, the new heart muscle forms in the absence of a collagen-rich scar, which is in contrast to mammalian adult cardiac injury [15]. Zebrafish heart regeneration is enabled by proliferation of mononucleated cardiomyocytes and signaling from the epicardium [18]. Ploidy of cardiomyocytes affects cardiac regenerative ability with diploid cardiomyocytes capable of proliferation and formation of new muscle after injury [19]. Thus, the innate cardiac regenerative capacity in zebrafish includes cardiomyocyte proliferation, induction of regenerative signals from the epicardium, and lack of fibrosis or scar formation.
Mammalian cardiac regeneration after ventricular resection was first observed in neonatal mice, where new heart muscle was formed by proliferation of existing cardiomyocytes [3]. The regenerative period in mice ends soon after birth, within 3–7 days. During this period, cardiomyocytes become binucleated, undergo cell cycle arrest, and initiate hypertrophic growth [20]. At the same time, circulatory demands increase, oxidative metabolism is induced, cardiac fibroblasts produce collagen-rich extracellular matrix (ECM), and circulating macrophages take up residence in the heart [21]. The ability of hearts to regenerate or repair after injury has been linked to ploidy and mononucleation in a comparative study of mouse strains [22]. ECM stiffness and presence of cardiac fibroblasts also have been implicated in cardiomyocyte maturation and cell cycle arrest [23–25]. Macrophages are also necessary for postnatal heart regeneration, most likely through promoting angiogenesis needed for cardiac vascularization after birth [26]. The exposure of neonatal mice to atmospheric oxygen at birth has been proposed as the means of loss of regenerative capacity through induction of oxidative damage, and hypoxia can prolong the regenerative period after birth or promote repair after injury in adults [27,28]. Moreover, thyroid hormone signaling has been implicated in postnatal cardiomyocyte cell cycle arrest, loss of regenerative ability, and increased metabolic rates in mice [29]. It is currently not known if these mechanisms related to loss of regenerative ability of the postnatal mouse heart extend to larger mammals with much longer gestation times, increased size, and greater cardiac tissue complexity at birth.
III. Cardiac regenerative potential after birth in large animal models
While rodents undergo neonatal cardiomyocyte proliferative arrest and a switch to hypertrophic growth within a few days after birth, how this relates to neonatal proliferative capacity in larger mammals, including humans, is unknown. Further, in order for the regenerative repair induced by various approaches in the injured adult mouse heart to be developed into viable therapies for human heart disease, clinical trials are required in large animal models to show efficacy of the proposed therapeutic strategy. Pigs, sheep, dogs, and non-human primates are the most common animals utilized in cardiac preclinical trials, but cats, cows, and horses also are occasionally used [30]. However, the fundamental cell biology of the heart, particularly cardiomyocyte proliferation and growth mechanisms, is largely uncharacterized in these animals. It is thus important to first know the major events in postnatal heart maturation, as well as the characteristics of the cardiac cellular milieu, when considering which type of large animal to use for preclinical studies of cardiovascular devices and reparative therapies. Recently published initial reports suggest that pigs have a similar neonatal regenerative period as mice, with regenerative potential being lost by 3 days after birth [31,32]. However, it is not known how this neonatal maturation and limited regenerative capacity compares to human postnatal heart repair mechanisms. Ultimately, it is imperative to identify the ideal mammalian model that recapitulates postnatal human cardiac development for translation of therapeutic advances in cardiac repair from animal model systems to humans. Here, we discuss fundamental physiology of various model systems alongside features of postnatal cardiac development, to offer a perspective on the physiological and cellular similarities that could be used as a starting point for translatability from large animal models to human postnatal cardiac development.
A. Impact of physiology and developmental milestones on postnatal heart growth
Species-specific physiological features such as gestation times, intrauterine environment, offspring number per birth, precociality at birth, as well as growth rate post-birth, could all have significant influence on postnatal cardiac development, but are largely underappreciated when selecting large-mammal models to study human cardiac regenerative potential [8]. It may prove important to consider the impact of such developmental milestones when studying postnatal cardiomyocyte proliferative capacity and defining the mammalian heart regenerative period (Table 1).
Table 1.
Comparison of approximate ages of physiological and developmental milestones in mammalian animal models and humans (Citations in text)
| Species | Gestational duration |
Minimum weaning age |
Sexual maturity |
Offspring per pregnancy |
Pregnancy frequency |
Development at birth | Average lifespan |
|---|---|---|---|---|---|---|---|
| Mice | 3 weeks | 3 weeks | 8 weeks | 7– 10 | 3 – 5 per year | Altricial | 2 – 3 years |
| Pigs | 3.7 months | 3 weeks | 5 – 8 months |
7– 10 | 1 – 2 per year | Precocial | 15 – 20 years |
| Dogs | 2 months | 3 weeks | 8 – 18 months |
6 | 2 – 3 per year | Altricial | 10 – 20 years |
| Cats | 2 months | 3 weeks | 5 – 10 months |
6 | 3 – 4 per year | Altricial | 9 – 17 years |
| Sheep | 4.8 months | 2 months | 4 – 8 months |
1–2 | 1 per year | Precocial | 10 – 12 years |
| Cows | 9 months | 7 months | 12 – 18 months |
1 | 1 per year | Precocial | 18 −22 years |
| Humans | 9 months | 1 – 2 years | 12 – 17 years |
1 | 1 per year | Altricial | 70 −85 years |
In mice, gestation lasts for 19 days, following which the neonatal mouse undergoes rapid development post-birth. Weaning occurs in mice at 21 days post-birth (P21) and sexual maturity by 6 – 8 weeks, with a total lifespan of 2 to 3 years in protected environments such as laboratories [33]. In contrast, human gestational duration is 9 months, with sexual maturity reached between 12 to 17 years after birth and an average life span of approximately 85 years [34,35]. Apart from the timing of such physiological milestones, in utero environment could also play a vital role in fetal mammalian heart development as well as the timing of terminal differentiation and cell cycle arrest of cardiomyocytes post-birth. While human pregnancies generally involve the development of one fetus per pregnancy (singletons), in commonly-used mammalian model systems such as rodents, pigs, and dogs, an average of 6–10 offspring (litter) are produced per birth [36–38]. In sheep and cattle, however, singleton births are seen, with a gestational age of 4.7 months and 9 months respectively [34,39,40]. It is unknown how the environmental cues resulting from different numbers of offspring and uterine environment influence cardiac growth and proliferative capacity.
Differences in postnatal growth rates leading to variable demands for cardiac output and cardiac load may also affect cardiac growth patterns of cardiomyocyte hyperplasia and hypertrophy. Farm animals such as domestic pigs, including the large White Yorkshire-Landrace breed, are used for meat production and are hence selected for rapid growth after birth, with over a 100-fold increase in total body weight between birth and one year-of-age [41]. Also, in hoofed mammals, such as pigs, sheep, and cows, newborns are precocial (mobile within the first few hours after birth). In contrast, rodents, canines, felines, and primates are born relatively helpless (altricial) and are entirely dependent on parental care for the first few weeks to years after birth depending on the species, with mobility achieved gradually [42]. These physiological parameters likely affect cardiac development and proliferative capacity, hypertrophic growth (longitudinal vs. concentric growth), and nucleation (mono/binucleation vs. multinucleation) of cardiomyocytes in different species of mammals.
B. Cardiomyocyte characteristics in large animal models
i. Hyperplastic versus hypertrophic growth of the postnatal heart
The transition from hyperplastic to hypertrophic growth coincides with loss of proliferative capacity in murine cardiomyocytes. Beyond two weeks, cardiac growth in rodents is through increasing cell size, with no new myocytes contributing to the increase in overall heart size [43]. Hence, a switch to hypertrophic cardiac growth has been linked to the timing of loss of cardiomyocyte proliferative capacity in the postnatal murine heart. However, the relationship between hyperplastic and hypertrophic growth mechanisms in large mammals is not completely understood. It is also not known whether there could be species-specific variation in cardiac growth mechanisms due to the physiological factors such as gestation time, number of offspring per birth, and maturity at birth.
Some similarities in timing of cardiac hypertrophic switch could be postulated in mammalian animal models on the basis of gestational duration and when comparing animals exhibiting litter births against animals having singleton pregnancies. In swine, which exhibit litter births and a gestation time of 3.7 months, cardiomyocyte length increases by 54% between birth and 2 postnatal weeks, which coincides with a 333% increase in left ventricular weight along with an increase in overall nuclear content [44,45]. This would indicate a combination of both hypertrophic growth and cardiomyocyte proliferation in the neonatal pig hearts. In older pigs greater than 6 months-of-age, however, it was visually seen that cardiomyocytes have increased length, concurrent with extensive multinucleation [46]. In canines, which have a gestation time of 2 months and also exhibit litter births, the right ventricle weighs significantly more than the left ventricle in newborns. However, by postnatal day 3 (P3), the left and right ventricular weights were equalized, and beyond P7, the left ventricle weighed more than the right, consistent with increased circulatory demands of systemic circulation [47]. In another study in canines, left ventricular weight was found to increase linearly after birth without apparent increase in cell size of mononucleated cardiomyocytes for the first 6 weeks after birth [48]. However, at 38 weeks post-birth, canine cardiomyocytes exhibited hypertrophic growth, with cardiomyocyte volume reaching 19 times that of newborn cardiomyocytes. The average diameter of canine cardiomyocytes reaches adult size by 6 – 8 months [49]. Together, this would indicate a brief neonatal window of about a week post-birth in which LV mass increases by proliferation rather than hypertrophy in dogs, with transition towards hypertrophic growth occurring beyond the first postnatal month.
There is, therefore, a delay relative to rodents in the timing of complete switch from hyperplastic to hypertrophic mode of cardiomyocyte growth in larger animals with longer gestational periods, such as dogs and pigs, despite the similarity in timing of weaning (~3 weeks post-birth) and occurrence of litter births among these three species. Further, timing of hypertrophy appears to be similar between canines and swine, though the former exhibits altriciality while the latter is precocial at birth. For both species, definitive studies of cardiomyocyte proliferation and maturation after birth are needed.
In sheep, with a gestation period of nearly 5 months for 1–2 precocial offspring, terminal differentiation and increased cardiomyocyte cross-sectional area occur even before birth, with cell volumes increased in the left ventricular free wall in the last 40 days of gestation [50,51]. Thus, both hyperplasia and hypertrophy contribute to the growth of the perinatal sheep heart. By 4 to 6 weeks after birth, significantly increased cardiomyocyte cell size and a reduction in total myocyte number per area were observed in sheep hearts, indicating transition to hypertrophic cardiomyocyte growth by one month of age [52]. This would suggest that among larger mammals, the timing of the cardiomyocyte hypertrophic switch may be dependent on the number of offspring in utero as well as gestation time, possibly due to differences in environmental cues such as hormones and intrauterine hypoxia that shape cardiac development, discussed in later sections. However, the mode of cardiac hypertrophy, i.e. longitudinal growth vs. concentric growth of muscle fibers, may be related to the precociality of an animal at birth, placing increased cardiac demands in these animals compared to an altricial newborn.
ii. Timing of increased nucleation and ploidy in cardiomyocytes
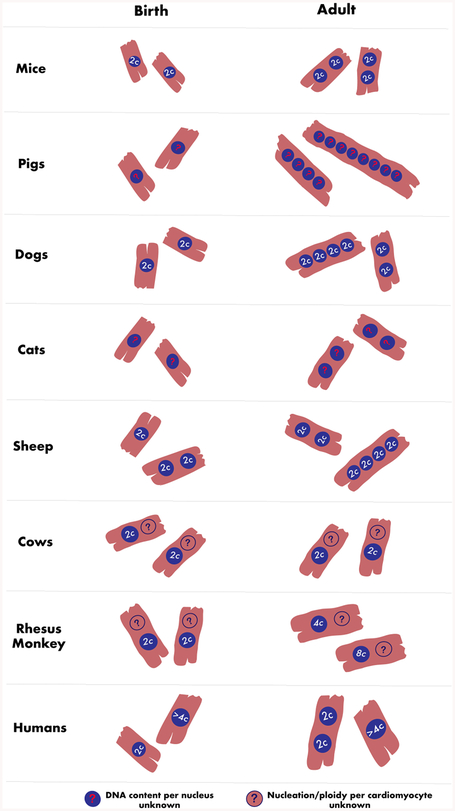
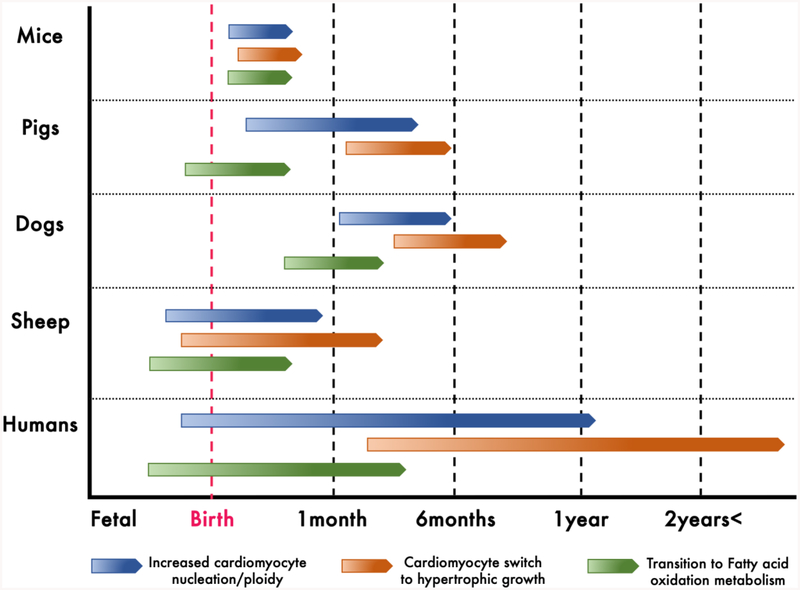
Mononucleated proliferative cardiomyocytes are predominant at birth in rodents [43]. By 2 weeks after birth, greater than 90% of all murine cardiomyocytes are binucleated, with each nucleus having diploid (2C) DNA content, which is maintained in adult animals [43,53,54]. In adult humans, cardiomyocytes are predominantly mononucleated, with lower incidence of bi- and multinucleation, and most cardiomyocytes are polyploid (>4C), with little to no proliferative capacity [10]. Hence, repression of cell cycle activity, along with reduced number of diploid mononucleated cardiomyocytes and/or increased ploidy of cardiomyocyte nuclei could be related to the loss of cardiomyocyte proliferative capacity in older mammals. In most large mammals, very little is known about timing of cardiac cell cycle arrest and loss of proliferative capacity, but there is increasing evidence that the timing and patterns of cardiomyocyte nucleation and ploidy are dynamic and variable across species (Fig. 1, Fig. 2). This not only suggests potential variation in the period of cardiac regenerative capacity, but also points to underlying important differences in basic cardiomyocyte cell biology which must be considered before the use of these animals as a more ‘human-like’ model for cardiac preclinical studies.
Fig. 1.
Schematic comparison of changes in cardiomyocyte nucleation and ploidy between birth and adulthood in mammalian animal models and humans (Citations in text)
Fig. 2.
Schematic of known and estimated timepoints of increased cardiomyocyte nucleation/ploidy, switch to hypertrophic growth and shift to fatty acid oxidation in various mammals (Citations in text)
In altricial animals such as rodents, canines, and felines, similar patterns of cardiomyocyte nucleation and ploidy could be correlated. Like rodents, canines are born with predominantly small, mononucleated cardiomyocytes (96 – 100%) [48]. However, while cardiomyocyte binucleation is nearly complete by 2 weeks in rodents, canines with a longer gestation time of 2 months have a prolonged duration of transitioning from a mononucleated state to a bi-/tetra-nucleated state [7,48,55]. Cardiomyocytes in canines increase significantly in size and number of nuclei beyond 2 weeks after birth. At 2 weeks, 15% of all canine cardiomyocytes are bi- and tetra-nucleated, and by 4 weeks this is increased to 55%. By 2 – 3 months after birth, 60 – 80% of all cardiomyocytes have two or four nuclei. However, each individual nucleus remains predominantly diploid (2C) in dogs. In felines, which have a similar durations of gestation, postnatal maturation, and life span as dogs, cardiomyocytes are predominantly binucleated at 11 weeks post-birth, with no change in the percentage of binucleated cardiomyocytes between 11 and 22 weeks, although a low percentage of small mononucleated cardiomyocytes were seen in adolescent cats [56]. This suggests adult cats primarily possess binucleated cardiomyocytes, and that this transition to a binucleated state likely occurs within the first 3 months after birth. Hence, in these altricial species, cardiomyocytes undergo terminal differentiation by bi-/tetra-nucleation rather than polyploidization of individual nuclei, with timing of nucleation onset later in animals with longer gestation times and life spans.
Adult domestic pig cardiomyocytes exhibit extensive multinucleation going beyond bi- and tetra-nucleation, with up to 8, 16 or even 32 nuclei per cardiomyocyte by a year after birth, though they are born predominantly mononucleated [46,57]. Further, when ploidy in individual cardiomyocyte nuclei was analysed in farm pig hearts, 14–16% of nuclei were tetraploid (4C) and 2–3% octaploid (8C) by 6 months of age [7,55,58]. In contrast, sheep, which are also precocial, exhibit binucleation even before birth [52]. Unlike the other mammalian models wherein cardiomyocytes are primarily mononucleated at birth, binucleation onset occurs in sheep at 115 days (out of 145 days) of fetal development, with over 50% of all cardiomyocytes exhibiting binucleation at birth, which then increases to 83% by P4 [52,59]. After birth, almost all cardiomyocytes in the 1 month-old sheep heart are binucleated, with individual cardiomyocyte nuclei being predominantly diploid [55,59]. It is not known whether older sheep exhibit the high degree of multinucleation seen in adult pigs. In other precocial animals like bovines (ox) and equines (horse), adult cardiomyocytes are predominantly diploid (2C), although the total number of nuclei per cardiomyocyte at birth or later in life was not reported [55]. If bovines and equines are found to undergo binucleation in utero, it could indicate that, despite precociality at birth, time of gestation along with singleton birth (sheep, cows, horses) vs. litter birth (pigs) likely plays a role in determining nucleation pattern and time of onset in these four species.
Among non-human primates, young adult (>4 years) chimpanzee, rhesus, gorilla, and hamadryas baboon all exhibit high percentages of 4C and 8C cardiomyocyte nuclei (30–55% and 10–25% respectively), although interestingly, the 3–4 year-old rhesus monkey still retains 90% of 2C cardiomyocyte nuclei [7,60]. It has not been reported whether non-human primates exhibit multinucleation. However, considering that human adult hearts are mostly mononucleated, with some binucleation though the percentages are under dispute [10,61], it can be hypothesized that primates under 4 years-of-age retain mononucleated, diploid cells which likely possess proliferative potential and the capacity for regenerative repair upon cardiac injury.
iii. Energy metabolism in the postnatal heart
To respond to increased cardiac demand and maintain continual cardiac output, the mammalian heart escalates ATP production and turnover rates after birth [62]. In the normoxic adult mammalian heart, over 95% of the total ATP produced is through oxidative phosphorylation (or fatty acid beta-oxidation) in the mitochondria. However, in the hypoxic embryonic heart, the predominant energy source is glycolysis. In rodents, during the neonatal switch from a hyperplastic to hypertrophic growth in the week after birth, cardiac energy metabolism also transitions from glycolysis to fatty acid oxidation [62–64]. Hence, timing of metabolic switching could influence cardiomyocyte proliferative capacity and hypertrophic growth in large mammals.
In pigs, cardiac glycogen levels are reduced close to parturition, with pig hearts in the perinatal period achieving the capacity to oxidize fatty acids to produce ATP [62]. However, the late gestation pig heart still requires glucose for proper maintenance of contractile function, with fatty acid oxidation alone insufficient for optimal cardiac function, and glycolysis accounting for nearly all of the myocardial oxygen consumption before birth [65,66]. After birth, the neonatal pig heart appears to transition to fatty acid metabolism, with increased triglycerides in the newborn swine heart. By 12 days post-birth, the rate of palmitate oxidation in neonatal swine approaches values comparable to adults [67,68]. In dogs up to 2 weeks post-birth, robust lactate release was still measured in the hearts in vivo, indicating glucose is still an important substrate for the neonatal canine heart [62,69]. Sheep, in contrast, exhibit a reduction in myocardial glycogen levels in utero beyond mid-gestation, although glycolysis remains an important mode of energy production in the late fetal and early neonatal sheep hearts [70]. Thus, with existing data, there does not appear to be a consistent time of metabolic switch from glycolysis to fatty acid oxidation in these large mammals. Cardiac function appears to require both glycolysis and fatty acids in the late fetal to early postnatal ages, with widely variable accompanying oxygen consumption and substrate utilization. Neonatal metabolic substrate switching needs to be examined at greater depth in large mammals to understand its role in physiological heart growth and maturation.
iv. Sarcomeric maturation after birth
The transition from the intrauterine to post-birth environment influences sarcomeric maturation, as the postnatal heart adapts to changes in cardiac flow, output and blood pressure. Cardiac sarcomeric proteins, such as myosin heavy chain slow- and fastisoforms and cardiac troponins undergo maturation from fetal to adult isoforms with downregulation of fetal sarcomeric gene expression [71]. These events are critical for the transition from a fetal-like immature heart with proliferative cardiomyocytes, to the mature adult heart in which cardiomyocytes are post-mitotic.
Generally, the slow skeletal muscle cardiac troponin I isoform ssTnI (TNNI1) is the predominant sarcomeric TnI found in the developing mammalian embryonic heart [71]. With changes in postnatal demands for cardiac load and output, TNNI1 is downregulated rapidly after birth, to be replaced by the mature sarcomeric protein isoform cardiac (c)TnI (TNNI3). This isoform then persists as the predominant adult TnI protein expressed in the cardiac myocytes. In rodents, this switching coincides with postnatal cardiomyocyte cell cycle withdrawal in the first week after birth, and, by 2 weeks of age, only cTnI is actively expressed in rodent ventricles [72]. In sheep, however, this sarcomeric protein isoform switching occurs in late gestation, with adult cTnI already measurable at 90 days of gestation, and there is an approximately 2-fold increase in cTnI expression between 110 days to 142 days of gestation, with downregulation of ssTnI fetal sarcomeric isoform [73]. There are no reported data on the timing of cardiac troponin I isoform switch in other large animal models.
Sarcomeric myosin heavy chain (MYH) and actin contractile proteins also undergo isoform switching between the fetal and adult ages in mammals [71]. Cardiac ventricular myosin heavy chains occur as two isoforms in mammals: α-MHC (MYH6) or β-MHC (MYH7). However, there are fundamental species-specific differences in expression of these isoforms that reflect the contractile properties of their heart chambers. In rodents, fast twitch Myh6 is the predominant myosin heavy chain isoform in the adult ventricular myocardium which beats at 400–600 beats per minute. However, in larger animal models with slower beat rates, including pigs and humans, slow twitch Myh7 is the predominant ventricular myosin heavy chain isoform [71,74].
Titin isoforms, important for myocardial papillary muscle and myofibril formation, are major contributors to ventricular passive tension and overall cardiac compliance [75]. In rats, the fetal myocardium predominantly expresses the compliant N2BA titin isoform, which correlates with lower passive tension in the fetal heart [76,77]. This large isoform of titin is downregulated in the first neonatal week and is replaced by a smaller titin isoform (N2B) which persists throughout adult life, concurrent with increased tension and force production. In neonatal pigs, however, N2BA was present from birth throughout life [77]. The importance of these species-specific differences in titan isoforms is not readily evident. With an emerging understanding of the role played by sarcomeric architecture in regulating cardiomyocyte proliferative capacity, it is thus necessary to consider the various isoforms and time of sarcomeric isoform switching in large animal models, as well as how this contributes to overall cardiac stiffness.
v. External cues regulating postnatal cardiac development
Apart from the intrinsic cell biology of the cardiomyocytes, cues from the external environment, such as ECM remodeling, cardiac tissue compliance and organization, maturation of the immune system, non-cardiomyocyte cardiac cell populations, intrauterine hypoxia during fetal development, postnatal oxidative stress, and thyroid hormone, collectively have a major influence on fetal and postnatal cardiomyocyte growth and maturation.
ECM composition and stiffness, as well as role of specific ECM proteins in guiding cardiomyocyte proliferative capacity, have been implicated in mice for inducing cardiac regenerative repair following injury [78,79]. However, not much is known about cardiac ECM architecture and composition in large mammalian models during development. Similarly, in mice, hypoxia promotes cardiomyocyte proliferation in an oxidative stress signaling-mediated mechanism and has been proposed as a strategy to induce adult cardiac regenerative mechanisms in humans [28]. However, exposure of pregnant ewes to long-term, high-altitude hypoxia has a detrimental effect on fetal cardiac contractility and output [80], possibly due to shift towards fatty acid oxidation occurring prenatally in sheep, in contrast to neonatal switch to fatty acid metabolism as seen in other larger mammals. Additional studies are needed to determine if hypoxia and oxidative stress affect cardiomyocyte proliferation and maturation in preclinical models.
In the postnatal period of mammals, thyroid hormone regulates cardiac contractility, T-tubule maturation and gap junction formation, as well as postnatal cardiac myosin heavy chain isoform switching [81]. In rodents, activation of thyroid hormone (T3) occurs 3 to 5 days before birth, but T3 expression peaks postnatally at P5–6, and also at P12–15, coincident with cardiomyocyte cell cycle withdrawal and switch to hypertrophic growth [81,82]. Likewise, inhibition of T3 signaling can delay cardiomyocyte cell cycle arrest after birth and promote cardiac regeneration in adult mice [29]. However, in sheep T3 is activated during late gestation, and promotes cardiomyocyte differentiation and binucleation along with increased hypertrophic growth and decreased proliferative cell cycling even before birth [83]. There is thus an important hormonal impact on differential timing of cardiomyocyte maturation, proliferative capacity and nucleation in the growing heart. Likewise, hormonal differences between species in utero and postnatally may be important to consider when determining the suitability of a large animal model for clinicial testing of cardiovascular therapies.
vi. Postnatal cardiac regenerative potential in mammalian large animal models
The transient neonatal cardiac regenerative capacity in mice extends for up to a week post-birth, with new myocyte production and limited scar formation seen in P0 to P3 mice hearts upon injury [3]. Adult mice do not normally regenerate after injury, but regenerative repair can be induced genetically through manipulation of cell signaling, miRNAs, developmental transcription factors or chromatin remodeling [84]. In pigs and canines, typically utilized for preclinical trials testing various cardiac regenerative therapeutic strategies, extensive scarring and fibrosis is seen in the adults after cardiac injury [85]. However, recent studies suggest that neonatal swine possess a 2 to 3-day regenerative window after birth [31,32]. In pigs, myocardial infarction (MI) induced 1–2 days after birth was followed by complete healing without fibrosis, as well as recovered myocardial function, a month after injury. However, injury at P7 or P14 resulted in full-thickness myocardial fibrotic scars, alongside ventricular wall thinning and lack of functional recovery [31,32]. Ongoing studies in neonatal pigs in our lab show pig hearts possess mitotic cardiomyocytes and ventricular cell cycle activity up to 2 months post-birth (Velayutham et al., unpublished results). This leads to interesting questions on the role of cardiomyocyte cell cycling and the relative importance of mononucleated cardiomyocytes in cardiac healing after injury. However, there is little available data on the innate or induced capacity of the postnatal heart to regenerate in large mammals.
IV. Cardiac regenerative potential after birth in humans
With evidence of cardiac regenerative potential in neonatal mice and pigs, it is conceivable that human cardiomyocytes also have the capacity for active proliferation for a limited time after birth. Among infants with congenital heart disease, mortality is greatest in the first year [86,87]. Major structural abnormalities of the heart at birth, for instance in Tetralogy of Fallot, require high-risk surgeries early in life [88]. In such cases, neonatal cardiac surgery performed during a period of active cardiomyocyte proliferation could improve patient outcomes by enhancing cardiac repair post-surgery. Hence, it is necessary to determine the duration of neonatal proliferative capacity of the human heart and whether mechanisms controlling the switch in cardiomyocytes from a proliferative to a non-proliferative state are similar for humans, rodents, and larger mammals. Here, we discuss some key findings on human postnatal cardiac development and proliferative capacity in comparison to animal models, that may be informative as to the possible duration of postnatal regenerative potential in the human heart.
i. Hyperplastic versus hypertrophic growth of the human heart
In human adults, cardiomyocyte growth is primarily by hypertrophy [10]. The old dogma was that adult human hearts do not possess proliferating cardiomyocytes [89]. However, in the past decade, evidence of a low rate of cardiomyocyte proliferation in adult humans has been provided by multiple studies, including the study by Bergmann et al., where atmospheric 14C levels were used to assess cardiomyocyte turnover post-birth [11]. Adult human hearts were found to possess a very low level of proliferating cardiomyocytes, with an annual turnover of about 1% in early years, which reduces to 0.3% by 75 years of age. While this indicated cardiomyocyte proliferative capacity in human adults, this low rate of renewal is insufficient for any robust regenerative response in case of injury or disease, and timing of cardiomyocyte cell cycle arrest in humans is still unknown. A study by Mollova et al. suggests that postnatal human hearts grow by both hypertrophy and cardiomyocyte proliferation in the first two decades after birth, with new cardiomyocytes measured in the first two decades [10]. Cardiomyocyte mitosis and cytokinesis were detectable in 0.012% and 0.003% of cardiomyocytes respectively in 1-year-old humans, decreasing with age till cytokinesis is virtually undetectable beyond 20 years of age. However, an opposing study found new cardiomyocyte formation ceases during the early postnatal period, with a steep decline in newly-formed cardiomyocytes by 10 years of age [12]. Other reports indicate cardiomyocyte proliferation in humans occurs primarily in the first few months after birth, with proliferative markers falling dramatically beyond 3 months after birth, in autopsied hearts of human infants [90–93]. In humans, it is therefore accepted that there is a small but detectable level of cardiomyocyte cell cycle activity in the early postnatal period, potentially extending into childhood, which then decreases with age alongside a transition towards hypertrophic mode of cardiac growth. However, the exact timing of the transition from hyperplastic to hypertrophic growth and the mechanisms involved are not known.
ii. Timing of increased nucleation and ploidy in cardiomyocytes
The majority of human cardiomyocytes are mononucleated at birth [94], however there are conflicting reports on the extent of binucleation and polyploidy of human cardiomyocytes in later life [61,94,95]. The number of binucleated cardiomyocytes present in the adult human heart is reported as ranging from 25 to 60%. This variation may be due to polynucleated cardiomyocytes not being detectable by current methods, differences in timepoints of samples that are utilized, and technical difficulties associated with studying human cardiac cells and tissues. It has been noted that with age the human heart retains the same number of mononucleated cardiomyocytes, however those mononucleated cardiomyocytes exhibit increased ploidy via increases in DNA content per nucleus [10,96,97]. Typically, cardiomyocytes at birth are mononucleated with diploid (2C) or tetraploid (4C) nuclei, and adult human cardiomyocytes are mostly mononucleated with polyploid (4C or more) nuclei. However, up to 16C DNA content per nucleus has been observed in the adult human heart [7,60,97,98]. Cardiomyocyte ploidy has also been found to increase in hypertrophied and diseased hearts, with excessive polyploidy (32C) recorded in cases of congenital heart disease, which persists to adulthood [97]. The mechanisms underlying increased nucleation and ploidy and their impact on cardiomyocyte proliferative ability and cardiac health of the human heart still remains to be understood, although studies in zebrafish and mice show polyploidization poses a significant barrier to the regenerative capacity of the heart [19,22].
iii. Energy metabolism in the fetal and postnatal human heart
Compared to rodents, very little is known about cardiac metabolic substrate utilization in the human heart in early postnatal life. In a transcriptomic study of the developing heart, expression of genes for fatty acid oxidation are detected as early as ten weeks in the growing fetus [99]. The expression of these genes increases further with fetal age, supporting the possibility of a metabolic shift towards fatty acid oxidation even before birth in human hearts. Increased ATP generation with increased fatty acid metabolism may be favorable to the maturation and differentiation of cardiomyocytes in the developing human heart. In human embryonic stem cell-derived cardiomyocytes, it has been further shown that a glucose-rich environment favors cardiomyocyte proliferation while inhibiting cardiac maturation [100]. During gestation, glycolytic metabolism likely contributes to cardiac growth by hyperplasia in human hearts. There is a marked reduction in glucose uptake during late gestation and early postnatal life, potentially contributing to the cessation of cardiomyocyte cell cycling activity.
iv. Sarcomeric maturation after birth
Isoform switching of sarcomeric proteins occurs in response to changes in physiological demands such as heart rate and cardiac load during development and after birth. However, the mechanisms of sarcomeric protein isoform transitions during human cardiac development are still poorly understood. In the human heart, there are four isoforms of cardiac troponin T (cTnT1, cTnT2, cTnT3 and cTnT4) expressed differentially during cardiac development [101,102]. cTnT1, cTnT2 and cTnT4 are expressed in the fetal heart, though with cTnT2 having low expression. In late gestation and early postnatal age, cTnT1 decreases with concurrent increase in cTnT3, until ultimately, cTnT3 is the only isoform to be expressed in the normal adult heart. The two TnI isoforms, ssTnI and cTnI are expressed in the human fetal heart, however ssTnI is the predominant isoform during fetal development. After birth, ssTnI decreases with increase in cTnI occurring simultaneously, similar to rodents [103,104]. By 9 months after birth in human hearts, cTnI is the only isoform detectable. This would suggest that human hearts undergo complete cardiomyocyte maturation within the first year after birth.
Myosin heavy and light chains (MHC and MLC) also display specific isoform switching patterns during cardiac development before and after birth. The β-isoform of MHC (MYH7) is predominant in human adult ventricular myocardium, similar to pigs, whereas the α-subunit (MYH6) is predominant in adult atrial tissue [105]. Isoform switching also occurs in cardiac MLCs. Essential MLC-1 has two isoforms, with differential expression during development. In the human, heart muscle expresses the atrial form (ALC-1) more predominantly during fetal stages and early life, than the ventricular form (VLC-1) mostly expressed in adulthood [71]. The developing and adult human heart is very sensitive to external pressure and environmental cues which can influence the activity of contractile filaments. In general, there is a reactivation of fetal contractile protein isoforms in cardiomyopathy and heart failure, but this is not accompanied by reactivation of cardiomyocyte proliferative activity [106,107].
v. External cues regulating cardiac development
The cellular and molecular mechanisms that regulate postnatal cardiomyocyte maturation in human hearts are not well characterized due to difficulty obtaining infant hearts for studies. Thus, the influence of external cues, such as extracellular matrix remodeling, hypoxia, and hormonal fluctuations on cardiomyocyte development in the human heart has been minimally studied. However, from the few studies conducted it is likely such factors may shape how the human heart develops, ultimately influencing the potential for regenerative repair in cardiac disease.
Thyroid hormone is a critical regulator of cardiac development during fetal and postnatal life, thought to play a role in stimulating cardiomyocyte proliferation [108]. Children with congenital heart defects typically have low T3 hormone levels, which can result in impaired cardiovascular function [109]. Therapy with T3 to children with such heart deficiencies has been proposed, to ultimately aid in cardiomyocyte repair and renewal [110]. However, studies in sheep and rodents indicate T3 can also play an inhibitory role in cardiomyocyte proliferation and regeneration [29,81–83]. Oxidative stress due to postnatal increase in oxygen has been implicated as a mechanism for cardiomyocyte cell cycle arrest in mice, with hypoxia investigated as a strategy for cardiac regeneration [27,28]. However, little is known about the role played by hypoxia in human cardiomyocyte proliferative capacity. Maturation of the ECM through remodeling of its composition and compliance is an important process in the transition from neonatal to adult heart in rodents [111], but little is known about ECM reorganization in the early postnatal life in humans. Further studies are required to understand the impact of such environmental cues on cardiomyocyte development from fetal to neonatal stages in humans.
vi. Is there a regenerative period in human hearts after birth?
Clinical cases with neonates suffering myocardial infarction followed by complete functional recovery of the heart have been reported, which suggests a potential neonatal window of cardiac regenerative repair in humans [13,112–114]. However these reports are not definitive evidence, as cardiac tissue is not available from these individuals for rigorous assessment of cardiomyocyte proliferative and reparative mechanisms.
In an interesting case study described by Haubner et al., a newborn suffered severe myocardial infarction with massive cardiac damage, as identified by presence of blood flow blockage in the left anterior descending artery, serum markers for cell death, echo and electrocardiography impairments [13]. Following thrombolytic reperfusion therapy, there was a remarkable recovery within weeks, with long-term cardiac functional recovery observed. Another notable case described by Tsang et al. describes an 11-month girl with severe heart failure receiving a heterotopic cardiac transplantation (placing the donor heart in an ectopic position without removing the native heart) [115]. The patient developed multiple episodes of post-transplant lymphoproliferative disorder with Epstein-Barr virus infection resistant to many therapies. Remarkably, her native heart recovered allowing removal of the donor heart 10.5 years after the initial transplantation. The patient on long-term follow up revealed normal cardiac function, highlighting the ability of the young human heart to recover. These studies demonstrate preliminary evidence for continued cardiomyocyte proliferative activity and potential for regenerative repair in the early postnatal period, leading to longstanding normal cardiac structural and functional recovery.
Surgical correction of anomalous left coronary artery from the pulmonary artery has been shown to have little or no long-term myocardial scarring when performed between 0.2–39 years of age [116]. Time of surgery correlated to outcome, however, has shown mixed results, with no relationship between younger patients and improved myocardial regeneration. However, examples of delayed diagnosis resulting in cardiac damage and ischemic cardiomyopathy, as opposed to cardiac surgery within the first year of age resulting in complete recovery of the heart, are also evident [117]. Thus, there is potential for improved cardiac outcomes if corrective surgery for congenital heart defects is performed in younger individuals. However, whether or not full regeneration of the injured human heart occurs postnatally is yet to be determined. Studies currently describe enhanced cardiac function, even if full regeneration is not achieved. Thus, compensatory mechanisms involving cell types other than cardiomyocyte may play a role in improving overall cardiac health following injury in the neonatal human heart.
V. Conclusions and future perspectives
The field of mammalian cardiac regeneration has primarily utilized the characteristics of rodent cardiomyocytes during cell cycle arrest to estimate capacity for regenerative repair after injury in mammals [118]. However, when other mammalian systems are examined, there is wide variation in the timing and pattern of the cardiomyocyte events associated with loss of regenerative capacity in mice (Fig. 1, Fig. 2). In swine and canines, mature cardiomyocytes exhibit multinucleation, and the switch to hypertrophic growth is not clearly demarcated from cardiomyocyte hyperplasia in the first postnatal month [44,46]. Sheep, in contrast, begin maturational events in late gestation and a significant subset of the cardiomyocytes are already binucleated and hypertrophic at birth [59]. In addition, the timing of sarcomeric protein isoform switching and transition to fatty acid oxidation amongst the large-mammal models are highly variable among species (Fig. 2). It is thus difficult to utilize the hallmarks of postnatal cardiac development as observed in rodents to estimate proliferative capacity of cardiomyocytes in other mammalian systems. There is emerging evidence that the cardiac microenvironment – such as ECM composition and rigidity, maturation of the immune system in the growing neonate, cardiac non-muscle cell activity, and physiological influences such as hypoxia – play important roles in regulating postnatal cardiomyocyte proliferative arrest in mice [24,27,119,120]. These parameters are not yet well-studied in large mammals, and may hold the key to understanding the mechanisms influencing loss of regenerative capacity in large mammals, including humans.
When postnatal cardiac developmental characteristics are appraised across various large mammals, there is great variation in the innate physiology of the myocardium, as outlined in previous sections of this review. Such species-specific differences could prove significant in translatability of findings in large-mammal models to humans, and should be considered when designing preclinical studies for cardiac therapeutics. For instance, pigs are widely utilized as large-mammal animal model in preclinical trials for cardiac regenerative strategies, as necessitated by FDA regulations [30]. However, adult pig multinucleated cardiomyocytes are very different from the polyploid mononucleated cardiomyocytes prevalent in human adult hearts [10,46]. Such fundamental differences in the cell biology and timing of maturational events in the pigs could confound the preclinical testing, particularly in studies for molecular and cellular therapies. Additional information is needed to reach a consensus on which large mammal model is ideal for specific studies, before they can be considered as clinically ‘more relevant’ and reflective of the human heart. Moreover, there may not be a single large-mammal animal model that recapitulates all key developmental and disease mechanisms in the human heart, and multiple large mammal models may need to be utilized for preclinical testing, depending on the type of therapeutics and cardiovascular disease being evaluated. Additional characterization of the basic cellular and molecular mechanisms of postnatal heart development in commonly-used large animal models such as pigs, dogs, and sheep is thus necessary to enhance translatability of research findings from model systems to treatment of human heart disease.
Acknowledgements:
We thank members of the Yutzey lab for valuable input and discussion.
Funding: This study was funded by NIH R01HL135848, R01HL142217, and Cincinnati Children’s Research Foundation.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: The authors have no conflicts of interest to declare.
Ethical Approval: This article does not contain any experiments with human participants or animals performed by any of the authors.
References
- 1.Bui AL, Horwich TB, Fonarow GC (2011) Epidemiology and risk profile of heart failure. Nat Rev Cardiol 8: 30–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A (2013) Temporal trends in survival among infants with critical congenital heart defects. Pediatrics 131: e1502–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA (2011) Transient regenerative potential of the neonatal mouse heart. Science 331: 1078–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haubner BJ, Adamowicz-Brice M, Khadayate S, Tiefenthaler V, Metzler B, Aitman T, Penninger JM (2012) Complete cardiac regeneration in a mouse model of myocardial infarction. Aging (Albany NY) 4: 966–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tzahor E, Poss KD (2017) Cardiac regeneration strategies: Staying young at heart. Science 356: 1035–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Liu S, Heallen T, Martin JF (2018) The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat Rev Cardiol 15: 672–684 [DOI] [PubMed] [Google Scholar]
- 7.Rumyantsev P (1991) Growth and Hyperplasia of Cardiac Muscle Cells, Taylor & Francis, [Google Scholar]
- 8.Vivien CJ, Hudson JE, Porrello ER (2016) Evolution, comparative biology and ontogeny of vertebrate heart regeneration. NPJ Regen Med 1: 16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foglia MJ, Poss KD (2016) Building and re-building the heart by cardiomyocyte proliferation. Development 143: 729–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park SY, Silberstein LE, Dos Remedios CG, Graham D, Colan S, Kuhn B (2013) Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A 110: 1446–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J (2009) Evidence for cardiomyocyte renewal in humans. Science 324: 98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andra M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisen J (2015) Dynamics of Cell Generation and Turnover in the Human Heart. Cell 161: 1566–1575 [DOI] [PubMed] [Google Scholar]
- 13.Haubner BJ, Schneider J, Schweigmann U, Schuetz T, Dichtl W, Velik-Salchner C, Stein JI, Penninger JM (2016) Functional Recovery of a Human Neonatal Heart After Severe Myocardial Infarction. Circ Res 118: 216–221 [DOI] [PubMed] [Google Scholar]
- 14.Oberpriller JO, Oberpriller JC (1974) Response of the adult newt ventricle to injury. J Exp Zool 187: 249–253 [DOI] [PubMed] [Google Scholar]
- 15.Poss KD, Wilson LG, Keating MT (2002) Heart regeneration in zebrafish. Science 298: 2188–2190 [DOI] [PubMed] [Google Scholar]
- 16.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD (2010) Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature 464: 601–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Pater E, Clijsters L, Marques SR, Lin YF, Garavito-Aguilar ZV, Yelon D, Bakkers J (2009) Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development 136: 1633–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao J, Poss KD (2018) The epicardium as a hub for heart regeneration. Nat Rev Cardiol 15: 631–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Rosa JM, Sharpe M, Field D, Soonpaa MH, Field LJ, Burns CE, Burns CG (2018) Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish. Dev Cell 44: 433–446 e437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunthel M, Barnett P, Christoffels VM (2018) Development, Proliferation, and Growth of the Mammalian Heart. Mol Ther 26: 1599–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL (2014) Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci U S A 111: 16029–16034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson M, Barske L, Van Handel B, Rau CD, Gan P, Sharma A, Parikh S, Denholtz M, Huang Y, Yamaguchi Y, Shen H, Allayee H, Crump JG, Force TI, Lien CL, Makita T, Lusis AJ, Kumar SR, Sucov HM (2017) Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nat Genet 49: 1346–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D (2012) In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485: 593–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yahalom-Ronen Y, Rajchman D, Sarig R, Geiger B, Tzahor E (2015) Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. Elife 4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Asfour H, Bursac N (2017) Age-dependent functional crosstalk between cardiac fibroblasts and cardiomyocytes in a 3D engineered cardiac tissue. Acta Biomater 55: 120–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN (2014) Macrophages are required for neonatal heart regeneration. J Clin Invest 124: 1382–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA (2014) The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157: 565–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, Szweda LI, Xing C, Hu Z, Deberardinis RJ, Schiattarella G, Hill JA, Oz O, Lu Z, Zhang CC, Kimura W, Sadek HA (2017) Hypoxia induces heart regeneration in adult mice. Nature 541: 222–227 [DOI] [PubMed] [Google Scholar]
- 29.Hirose K, Payumo AY, Cutie S, Hoang A, Zhang H, Guyot R, Lunn D, Bigley RB, Yu H, Wang J, Smith M, Gillett E, Muroy SE, Schmid T, Wilson E, Field KA, Reeder DM, Maden M, Yartsev MM, Wolfgang MJ, Grutzner F, Scanlan TS, Szweda LI, Buffenstein R, Hu G, Flamant F, Olgin JE, Huang GN (2019) Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science 364: 184–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camacho P, Fan H, Liu Z, He JQ (2016) Large Mammalian Animal Models of Heart Disease. J Cardiovasc Dev Dis 3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye L, D’Agostino G, Loo SJ, Wang CX, Su LP, Tan SH, Tee GZ, Pua CJ, Pena EM, Cheng RB, Chen WC, Abdurrachim D, Lalic J, Tan RS, Lee TH, Zhang J, Cook SA (2018) Early Regenerative Capacity in the Porcine Heart. Circulation 138: 2798–2808 [DOI] [PubMed] [Google Scholar]
- 32.Zhu W, Zhang E, Zhao M, Chong Z, Fan C, Tang Y, Hunter JD, Borovjagin AV, Walcott GP, Chen JY, Qin G, Zhang J (2018) Regenerative Potential of Neonatal Porcine Hearts. Circulation 138: 2809–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagy A, Gertsenstein M, Vintersten K, Behringer R (2003) Manipulating the mouse embryo: a laboratory manual, Firefly Books, [Google Scholar]
- 34.de Magalhaes JP, Costa J (2009) A database of vertebrate longevity records and their relation to other life-history traits. J Evol Biol 22: 1770–1774 [DOI] [PubMed] [Google Scholar]
- 35.Cutler RG (1979) Evolution of human longevity: a critical overview. Mech Ageing Dev 9: 337–354 [DOI] [PubMed] [Google Scholar]
- 36.Finn CA (1963) Reproductive Capacity and Litter Size in Mice: Effect of Age and Environment. J Reprod Fertil 6: 205–214 [DOI] [PubMed] [Google Scholar]
- 37.Chen P, Baas TJ, Mabry JW, Koehler KJ, Dekkers JC (2003) Genetic parameters and trends for litter traits in U.S. Yorkshire, Duroc, Hampshire, and Landrace pigs. J Anim Sci 81: 46–53 [DOI] [PubMed] [Google Scholar]
- 38.Okkens AC, Hekerman TW, de Vogel JW, van Haaften B (1993) Influence of litter size and breed on variation in length of gestation in the dog. Vet Q 15: 160–161 [DOI] [PubMed] [Google Scholar]
- 39.Janssens S, Vandepitte W, Bodin L (2004) Genetic parameters for litter size in sheep: natural versus hormone-induced oestrus. Genet Sel Evol 36: 543–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva del Rio N, Stewart S, Rapnicki P, Chang YM, Fricke PM (2007) An observational analysis of twin births, calf sex ratio, and calf mortality in Holstein dairy cattle. J Dairy Sci 90: 1255–1264 [DOI] [PubMed] [Google Scholar]
- 41.Poore KR, Fowden AL (2004) The effects of birth weight and postnatal growth patterns on fat depth and plasma leptin concentrations in juvenile and adult pigs. J Physiol 558: 295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sibly RM, Brown JH (2009) Mammal reproductive strategies driven by offspring mortality-size relationships. Am Nat 173: E185–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li F, Wang X, Capasso JM, Gerdes AM (1996) Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol 28: 1737–1746 [DOI] [PubMed] [Google Scholar]
- 44.Beinlich CJ, Rissinger CJ, Morgan HE (1995) Mechanisms of rapid growth in the neonatal pig heart. J Mol Cell Cardiol 27: 273–281 [DOI] [PubMed] [Google Scholar]
- 45.Peterson CJ, Whitman V, Watson PA, Schuler HG, Morgan HE (1989) Mechanisms of differential growth of heart ventricles in newborn pigs. Circ Res 64: 360–369 [DOI] [PubMed] [Google Scholar]
- 46.Grabner W, Pfitzer P (1974) Number of nuclei in isolated myocardial cells of pigs. Virchows Arch B Cell Pathol 15: 279–294 [DOI] [PubMed] [Google Scholar]
- 47.Kirk GR, Smith DM, Hutcheson DP, Kirby R (1975) Postnatal growth of the dog heart. J Anat 119: 461–469 [PMC free article] [PubMed] [Google Scholar]
- 48.Bishop SP, Hine P (1975) Carciac muscle cytoplasmic and nuclear development during canine neonatal growth. Recent Adv Stud Cardiac Struct Metab 8: 77–98 [PubMed] [Google Scholar]
- 49.Munnell JF, Getty R (1968) Rate of accumulation of cardiac lipofuscin in the aging canine. J Gerontol 23: 154–158 [DOI] [PubMed] [Google Scholar]
- 50.Thornburg K, Jonker S, O’Tierney P, Chattergoon N, Louey S, Faber J, Giraud G (2011) Regulation of the cardiomyocyte population in the developing heart. Prog Biophys Mol Biol 106: 289–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jonker SS, Louey S, Giraud GD, Thornburg KL, Faber JJ (2015) Timing of cardiomyocyte growth, maturation, and attrition in perinatal sheep. FASEB J 29: 4346–4357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burrell JH, Boyn AM, Kumarasamy V, Hsieh A, Head SI, Lumbers ER (2003) Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. Anat Rec A Discov Mol Cell Evol Biol 274: 952–961 [DOI] [PubMed] [Google Scholar]
- 53.Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ (1996) Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol 271: H2183–2189 [DOI] [PubMed] [Google Scholar]
- 54.Soonpaa MH, Zebrowski DC, Platt C, Rosenzweig A, Engel FB, Field LJ (2015) Cardiomyocyte Cell-Cycle Activity during Preadolescence. Cell 163: 781–782 [DOI] [PubMed] [Google Scholar]
- 55.Adler CP, Friedburg H, Herget GW, Neuburger M, Schwalb H (1996) Variability of cardiomyocyte DNA content, ploidy level and nuclear number in mammalian hearts. Virchows Arch 429: 159–164 [DOI] [PubMed] [Google Scholar]
- 56.Chen X, Wilson RM, Kubo H, Berretta RM, Harris DM, Zhang X, Jaleel N, MacDonnell SM, Bearzi C, Tillmanns J, Trofimova I, Hosoda T, Mosna F, Cribbs L, Leri A, Kajstura J, Anversa P, Houser SR (2007) Adolescent feline heart contains a population of small, proliferative ventricular myocytes with immature physiological properties. Circ Res 100: 536–544 [DOI] [PubMed] [Google Scholar]
- 57.Kim MY, Eiby YA, Lumbers ER, Wright LL, Gibson KJ, Barnett AC, Lingwood BE (2014) Effects of glucocorticoid exposure on growth and structural maturation of the heart of the preterm piglet. PLoS One 9: e93407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfitzer P (1971) [Polyploid nuclei in myocardial cells of the pig]. Virchows Arch B Cell Pathol 9: 180–186 [PubMed] [Google Scholar]
- 59.Bensley JG, De Matteo R, Harding R, Black MJ (2016) Three-dimensional direct measurement of cardiomyocyte volume, nuclearity, and ploidy in thick histological sections. Sci Rep 6: 23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfitzer P (1972) [Polyploid nuclei in myocardial cells of monkeys]. Virchows Arch B Cell Pathol 10: 268–274 [PubMed] [Google Scholar]
- 61.Olivetti G, Cigola E, Maestri R, Corradi D, Lagrasta C, Gambert SR, Anversa P (1996) Aging, cardiac hypertrophy and ischemic cardiomyopathy do not affect the proportion of mononucleated and multinucleated myocytes in the human heart. J Mol Cell Cardiol 28: 1463–1477 [DOI] [PubMed] [Google Scholar]
- 62.Ascuitto RJ, Ross-Ascuitto NT (1996) Substrate metabolism in the developing heart. Semin Perinatol 20: 542–563 [DOI] [PubMed] [Google Scholar]
- 63.Doenst T, Nguyen TD, Abel ED (2013) Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 113: 709–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Makinde AO, Kantor PF, Lopaschuk GD (1998) Maturation of fatty acid and carbohydrate metabolism in the newborn heart. Mol Cell Biochem 188: 49–56 [PubMed] [Google Scholar]
- 65.Werner JC, Whitman V, Fripp RR, Schuler HG, Morgan HE (1981) Carbohydrate metabolism in isolated, working newborn pig heart. Am J Physiol 241: E364–371 [DOI] [PubMed] [Google Scholar]
- 66.Werner JC, Whitman V, Vary TC, Fripp RR, Musselman J, Schuler HG (1983) Fatty acid and glucose utilization in isolated, working newborn pig hearts. Am J Physiol 244: E19–23 [DOI] [PubMed] [Google Scholar]
- 67.Werner JC, Sicard RE, Schuler HG (1989) Palmitate oxidation by isolated working fetal and newborn pig hearts. Am J Physiol 256: E315–321 [DOI] [PubMed] [Google Scholar]
- 68.Ascuitto RJ, Ross-Ascuitto NT, Chen V, Downing SE (1989) Ventricular function and fatty acid metabolism in neonatal piglet heart. Am J Physiol 256: H9–15 [DOI] [PubMed] [Google Scholar]
- 69.Breuer E, Barta E, Pappova E, Zlatos L (1967) Developmental Changes of Myocardial Metabolism .I. Peculiarities of Cardiac Carbohydrate Metabolism in Early Postnatal Period in Dogs . Biologia Neonatorum 11: 367–& [PubMed] [Google Scholar]
- 70.Fisher DJ, Heymann MA, Rudolph AM (1980) Myocardial oxygen and carbohydrate consumption in fetal lambs in utero and in adult sheep. Am J Physiol 238: H399–405 [DOI] [PubMed] [Google Scholar]
- 71.Yin Z, Ren J, Guo W (2015) Sarcomeric protein isoform transitions in cardiac muscle: a journey to heart failure. Biochim Biophys Acta 1852: 47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saggin L, Gorza L, Ausoni S, Schiaffino S (1989) Troponin I switching in the developing heart. J Biol Chem 264: 16299–16302 [PubMed] [Google Scholar]
- 73.Posterino GS, Dunn SL, Botting KJ, Wang W, Gentili S, Morrison JL (2011) Changes in cardiac troponins with gestational age explain changes in cardiac muscle contractility in the sheep fetus. J Appl Physiol (1985) 111: 236–243 [DOI] [PubMed] [Google Scholar]
- 74.Locher MR, Razumova MV, Stelzer JE, Norman HS, Moss RL (2011) Effects of low-level α-myosin heavy chain expression on contractile kinetics in porcine myocardium. Am J Physiol Heart Circ Physiol 300: H869–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Castro-Ferreira R, Fontes-Carvalho R, Falcao-Pires I, Leite-Moreira AF (2011) The role of titin in the modulation of cardiac function and its pathophysiological implications. Arq Bras Cardiol 96: 332–339 [DOI] [PubMed] [Google Scholar]
- 76.Warren CM, Krzesinski PR, Campbell KS, Moss RL, Greaser ML (2004) Titin isoform changes in rat myocardium during development. Mech Dev 121: 1301–1312 [DOI] [PubMed] [Google Scholar]
- 77.Opitz CA, Leake MC, Makarenko I, Benes V, Linke WA (2004) Developmentally regulated switching of titin size alters myofibrillar stiffness in the perinatal heart. Circ Res 94: 967–975 [DOI] [PubMed] [Google Scholar]
- 78.Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J, Riabov Bassat D, Udi Y, Sarig R, Sagi I, Martin JF, Bursac N, Cohen S, Tzahor E (2017) The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547: 179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen WC, Wang Z, Missinato MA, Park DW, Long DW, Liu HJ, Zeng X, Yates NA, Kim K, Wang Y (2016) Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration. Sci Adv 2: e1600844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lewis AM, Mathieu-Costello O, McMillan PJ, Gilbert RD (1999) Effects of long-term, high-altitude hypoxia on the capillarity of the ovine fetal heart. Am J Physiol 277: H756–762 [DOI] [PubMed] [Google Scholar]
- 81.Li M, Iismaa SE, Naqvi N, Nicks A, Husain A, Graham RM (2014) Thyroid hormone action in postnatal heart development. Stem Cell Res 13: 582–591 [DOI] [PubMed] [Google Scholar]
- 82.Fisher DA, Klein AH (1981) Thyroid development and disorders of thyroid function in the newborn. N Engl J Med 304: 702–712 [DOI] [PubMed] [Google Scholar]
- 83.Chattergoon NN, Louey S, Stork P, Giraud GD, Thornburg KL (2012) Mid-gestation ovine cardiomyocytes are vulnerable to mitotic suppression by thyroid hormone. Reprod Sci 19: 642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karra R, Poss KD (2017) Redirecting cardiac growth mechanisms for therapeutic regeneration. J Clin Invest 127: 427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garbern JC, Mummery CL, Lee RT (2013) Model systems for cardiovascular regenerative biology. Cold Spring Harb Perspect Med 3: a014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Best KE, Rankin J (2016) Long-Term Survival of Individuals Born With Congenital Heart Disease: A Systematic Review and Meta-Analysis. J Am Heart Assoc 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Triedman JK, Newburger JW (2016) Trends in Congenital Heart Disease: The Next Decade. Circulation 133: 2716–2733 [DOI] [PubMed] [Google Scholar]
- 88.Egbe AC, Mittnacht AJ, Nguyen K, Joashi U (2014) Risk factors for morbidity in infants undergoing tetralogy of fallot repair. Ann Pediatr Cardiol 7: 13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yutzey KE (2017) Cardiomyocyte Proliferation: Teaching an Old Dogma New Tricks. Circ Res 120: 627–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huttenbach Y, Ostrowski ML, Thaller D, Kim HS (2001) Cell proliferation in the growing human heart: MIB-1 immunostaining in preterm and term infants at autopsy. Cardiovasc Pathol 10: 119–123 [DOI] [PubMed] [Google Scholar]
- 91.Amir G, Ma X, Reddy VM, Hanley FL, Reinhartz O, Ramamoorthy C, Riemer RK (2008) Dynamics of human myocardial progenitor cell populations in the neonatal period. Ann Thorac Surg 86: 1311–1319 [DOI] [PubMed] [Google Scholar]
- 92.Silva TF, Souza GK, Simoes MA, Pabis FC, Noronha L (2012) Immunohistochemical expression of cell differentiation and growth in neonate cardiomyocytes. Arq Bras Cardiol 99: 797–801 [DOI] [PubMed] [Google Scholar]
- 93.Ye L, Qiu L, Zhang H, Chen H, Jiang C, Hong H, Liu J (2016) Cardiomyocytes in Young Infants With Congenital Heart Disease: a Three-Month Window of Proliferation. Sci Rep 6: 23188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmid G, Pfitzer P (1985) Mitoses and binucleated cells in perinatal human hearts. Virchows Arch B Cell Pathol Incl Mol Pathol 48: 59–67 [DOI] [PubMed] [Google Scholar]
- 95.Botting KJ, Wang KC, Padhee M, McMillen IC, Summers-Pearce B, Rattanatray L, Cutri N, Posterino GS, Brooks DA, Morrison JL (2012) Early origins of heart disease: low birth weight and determinants of cardiomyocyte endowment. Clin Exp Pharmacol Physiol 39: 814–823 [DOI] [PubMed] [Google Scholar]
- 96.Eisenstein R, Wied GL (1970) Myocardial DNA and protein in maturing and hypertrophied human hearts. Proc Soc Exp Biol Med 133: 176–179 [DOI] [PubMed] [Google Scholar]
- 97.Brodsky V, Sarkisov DS, Arefyeva AM, Panova NW, Gvasava IG (1994) Polyploidy in cardiac myocytes of normal and hypertrophic human hearts; range of values. Virchows Arch 424: 429–435 [DOI] [PubMed] [Google Scholar]
- 98.Herget GW, Neuburger M, Plagwitz R, Adler CP (1997) DNA content, ploidy level and number of nuclei in the human heart after myocardial infarction. Cardiovasc Res 36: 45–51 [DOI] [PubMed] [Google Scholar]
- 99.Iruretagoyena JI, Davis W, Bird C, Olsen J, Radue R, Teo Broman A, Kendziorski C, Splinter BonDurant S, Golos T, Bird I, Shah D (2014) Metabolic gene profile in early human fetal heart development. Mol Hum Reprod 20: 690–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakano H, Minami I, Braas D, Pappoe H, Wu X, Sagadevan A, Vergnes L, Fu K, Morselli M, Dunham C, Ding X, Stieg AZ, Gimzewski JK, Pellegrini M, Clark PM, Reue K, Lusis AJ, Ribalet B, Kurdistani SK, Christofk H, Nakatsuji N, Nakano A (2017) Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis. Elife 6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anderson PA, Malouf NN, Oakeley AE, Pagani ED, Allen PD (1991) Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circ Res 69: 1226–1233 [DOI] [PubMed] [Google Scholar]
- 102.Swynghedauw B (1986) Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev 66: 710–771 [DOI] [PubMed] [Google Scholar]
- 103.Wilkinson JM, Grand RJ (1978) Comparison of amino acid sequence of troponin I from different striated muscles. Nature 271: 31–35 [DOI] [PubMed] [Google Scholar]
- 104.Sasse S, Brand NJ, Kyprianou P, Dhoot GK, Wade R, Arai M, Periasamy M, Yacoub MH, Barton PJ (1993) Troponin I gene expression during human cardiac development and in end-stage heart failure. Circ Res 72: 932–938 [DOI] [PubMed] [Google Scholar]
- 105.Reiser PJ, Portman MA, Ning XH, Schomisch Moravec C (2001) Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am J Physiol Heart Circ Physiol 280: H1814–1820 [DOI] [PubMed] [Google Scholar]
- 106.Ritter O, Luther HP, Haase H, Baltas LG, Baumann G, Schulte HD, Morano I (1999) Expression of atrial myosin light chains but not alpha-myosin heavy chains is correlated in vivo with increased ventricular function in patients with hypertrophic obstructive cardiomyopathy. J Mol Med (Berl) 77: 677–685 [DOI] [PubMed] [Google Scholar]
- 107.Miyata S, Minobe W, Bristow MR, Leinwand LA (2000) Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res 86: 386–390 [DOI] [PubMed] [Google Scholar]
- 108.Ledda-Columbano GM, Molotzu F, Pibiri M, Cossu C, Perra A, Columbano A (2006) Thyroid hormone induces cyclin D1 nuclear translocation and DNA synthesis in adult rat cardiomyocytes. FASEB J 20: 87–94 [DOI] [PubMed] [Google Scholar]
- 109.Olivieri A, Stazi MA, Mastroiacovo P, Fazzini C, Medda E, Spagnolo A, De Angelis S, Grandolfo ME, Taruscio D, Cordeddu V, Sorcini M, Study Group for Congenital H (2002) A population-based study on the frequency of additional congenital malformations in infants with congenital hypothyroidism: data from the Italian Registry for Congenital Hypothyroidism (1991–1998). J Clin Endocrinol Metab 87: 557–562 [DOI] [PubMed] [Google Scholar]
- 110.Chowdhury D, Ojamaa K, Parnell VA, McMahon C, Sison CP, Klein I (2001) A prospective randomized clinical study of thyroid hormone treatment after operations for complex congenital heart disease. J Thorac Cardiovasc Surg 122: 1023–1025 [DOI] [PubMed] [Google Scholar]
- 111.Lockhart M, Wirrig E, Phelps A, Wessels A (2011) Extracellular matrix and heart development. Birth Defects Res A Clin Mol Teratol 91: 535–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McMahon CJ, Nihill MR, Denfield S (2003) Neoaortic root dilation associated with left coronary artery stenosis following arterial switch procedure. Pediatr Cardiol 24: 43–46 [DOI] [PubMed] [Google Scholar]
- 113.Farooqi KM, Sutton N, Weinstein S, Menegus M, Spindola-Franco H, Pass RH (2012) Neonatal myocardial infarction: case report and review of the literature. Congenit Heart Dis 7: E97–102 [DOI] [PubMed] [Google Scholar]
- 114.Nakagama Y, Inuzuka R, Ichimura K, Hinata M, Takehara H, Takeda N, Kakiuchi S, Shiraga K, Asakai H, Shindo T, Hirata Y, Saitoh M, Oka A (2018) Accelerated Cardiomyocyte Proliferation in the Heart of a Neonate With LEOPARD Syndrome-Associated Fatal Cardiomyopathy. Circ Heart Fail 11: e004660. [DOI] [PubMed] [Google Scholar]
- 115.Tsang V, Yacoub M, Sridharan S, Burch M, Radley-Smith R, Khaghani A, Savoldo B, Amrolia PJ (2009) Late donor cardiectomy after paediatric heterotopic cardiac transplantation. Lancet 374: 387–392 [DOI] [PubMed] [Google Scholar]
- 116.Fratz S, Hager A, Schreiber C, Schwaiger M, Hess J, Stern HC (2011) Long-term myocardial scarring after operation for anomalous left coronary artery from the pulmonary artery. Ann Thorac Surg 92: 1761–1765 [DOI] [PubMed] [Google Scholar]
- 117.Wesselhoeft H, Fawcett JS, Johnson AL (1968) Anomalous origin of the left coronary artery from the pulmonary trunk. Its clinical spectrum, pathology, and pathophysiology, based on a review of 140 cases with seven further cases. Circulation 38: 403–425 [DOI] [PubMed] [Google Scholar]
- 118.Porrello ER, Olson EN (2014) A neonatal blueprint for cardiac regeneration. Stem Cell Res 13: 556–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Godwin JW, Debuque R, Salimova E, Rosenthal NA (2017) Heart regeneration in the salamander relies on macrophage-mediated control of fibroblast activation and the extracellular landscape. NPJ Regen Med 2: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gray GA, Toor IS, Castellan R, Crisan M, Meloni M (2018) Resident cells of the myocardium: more than spectators in cardiac injury, repair and regeneration. Curr Opin Physiol 1: 46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]