Abstract
Background
One of the key factors for the long‐term success of oral implants is the maintenance of healthy tissues around them. Bacterial plaque accumulation induces inflammatory changes in the soft tissues surrounding oral implants and it may lead to their progressive destruction (peri‐implantitis) and ultimately to implant failure. Different treatment strategies for peri‐implantitis have been suggested, however it is unclear which are the most effective.
Objectives
To identify the most effective interventions for treating peri‐implantitis around osseointegrated dental implants.
Search methods
We searched Cochrane Oral Health's Trials Register, CENTRAL, MEDLINE and EMBASE. Handsearching included several dental journals. We checked the bibliographies of the identified randomised controlled trials (RCTs) and relevant review articles for studies outside the handsearched journals. We wrote to authors of all identified RCTs, to more than 55 dental implant manufacturers and an Internet discussion group to find unpublished or ongoing RCTs. No language restrictions were applied. The last electronic search was conducted on 9 June 2011.
Selection criteria
All RCTs comparing agents or interventions for treating peri‐implantitis around dental implants.
Data collection and analysis
Screening of eligible studies, assessment of the methodological quality of the trials and data extraction were conducted in duplicate and independently by two review authors. We contacted the authors for missing information. Results were expressed as random‐effects models using mean differences for continuous outcomes and risk ratios for dichotomous outcomes with 95% confidence intervals (CI). Heterogeneity was to be investigated including both clinical and methodological factors.
Main results
Fifteen eligible trials were identified, but six were excluded. The following interventions were compared in the nine included studies: different non‐surgical interventions (five trials); adjunctive treatments to non‐surgical interventions (one trial); different surgical interventions (two trials); adjunctive treatments to surgical interventions (one trial). Follow‐up ranged from 3 months to 4 years. No study was judged to be at low risk of bias.
Statistically significant differences were observed in two small single trials judged to be at unclear or high risk of bias. After 4 months, adjunctive local antibiotics to manual debridement in patients who lost at least 50% of the bone around implants showed improved mean probing attachment levels (PAL) of 0.61 mm (95% confidence interval (CI) 0.40 to 0.82) and reduced probing pockets depths (PPD) of 0.59 mm (95% CI 0.39 to 0.79). After 4 years, patients with peri‐implant infrabony defects > 3 mm treated with Bio‐Oss and resorbable barriers gained 1.4 mm more PAL (95% CI 0.24 to 2.56) and 1.4 mm PPD (95% CI 0.81 to 1.99) than patients treated with a nanocrystalline hydroxyapatite.
Authors' conclusions
There is no reliable evidence suggesting which could be the most effective interventions for treating peri‐implantitis. This is not to say that currently used interventions are not effective.
A single small trial at unclear risk of bias showed the use of local antibiotics in addition to manual subgingival debridement was associated with a 0.6 mm additional improvement for PAL and PPD over a 4‐month period in patients affected by severe forms of peri‐implantitis. Another small single trial at high risk of bias showed that after 4 years, improved PAL and PPD of about 1.4 mm were obtained when using Bio‐Oss with resorbable barriers compared to a nanocrystalline hydroxyapatite in peri‐implant infrabony defects. There is no evidence from four trials that the more complex and expensive therapies were more beneficial than the control therapies which basically consisted of simple subgingival mechanical debridement. Follow‐up longer than 1 year suggested recurrence of peri‐implantitis in up to 100% of the treated cases for some of the tested interventions. As this can be a chronic disease, re‐treatment may be necessary. Larger well‐designed RCTs with follow‐up longer than 1 year are needed.
Plain language summary
Interventions for replacing missing teeth: treatment of peri‐implantitis
As with natural teeth, dental implants can be lost due to gum disease (peri‐implantitis). This review looked at which are the most effective treatments to arrest peri‐implantitis. Nine studies were included in the review and evaluated eight different treatment modalities. In one small study of short duration (4 months), it was shown that the use of locally applied antibiotics in addition to the deep manual cleaning of the diseased implants decreased the depth of the pockets around the implants by an additional 0.6 mm in patients affected by severe forms of peri‐implantitis. In another small study of 4‐year duration, it was shown that placing an animal‐derived bone substitute with a resorbable barrier decreased the depth of the pockets by an additional 1.4 mm than synthetic bone. The majority of trials testing more complex and expensive therapies did not show any statistically or clinically significant advantages over the deep mechanical cleaning around the affected implants. In conclusion, at present, there is too little evidence to determine which is the most effective way to treat peri‐implantitis. This is not to say that currently used interventions are not effective.
Background
Missing teeth and supporting oral tissues have traditionally been replaced with removable dentures or fixed bridges permitting restoration of masticatory, phonetic function, and aesthetics. In 1977, Brånemark presented his research work carried out over 10 years showing that bone can grow intimately onto the surface of titanium implants (Brånemark 1977). The now well‐accepted concept, termed osseointegration, has undoubtedly been one of the most significant scientific breakthroughs in dentistry over the past 30 years. A multitude of implant designs have been marketed since, and the clinical situations in which osseointegrated implant‐retained prostheses are used have expanded enormously.
One of the key factors for the long‐term success of dental implants is the maintenance of healthy tissues around them. A cause‐effect relationship between bacterial plaque accumulation and the development of inflammatory changes in the soft tissues surrounding dental implants has been shown (Pontoriero 1994). If this reversible condition, called 'peri‐implant mucositis', is left untreated, it may lead to the progressive destruction of the tissues supporting an implant (peri‐implantitis) and ultimately to its failure (Mombelli 1999). In order to maintain healthy tissues around dental implants it is important to institute an effective preventive regimen (supportive therapy) and when a pathological condition of the tissues around implants is diagnosed a therapeutic intervention should be initiated as soon as possible (Esposito 1999). Different treatment strategies for peri‐implantitis (failing implants) have been suggested, however, it is unclear which are the most effective (Esposito 1999). A prospective study on the treatment of peri‐implantitis with surgical exposure and cleaning of the implant surfaces with hydrogen peroxide together with systemic antibiotics of 26 implants in nine patients indicated that 5 years after treatment seven implants failed, four had additional peri‐implant bone loss, nine had unchanged bone levels, whereas six implants showed some bone gain (Leonhardt 2003).
The occurrence of peri‐implantitis is not rare. In a recent single‐cohort study (Roos‐Jansåker 2006), peri‐implantitis, defined as a marginal bone loss of 3 mm or more in combination with bleeding on probing or pus or both, was diagnosed in 16% of patients treated with turned (machined) Brånemark implants 9 to 14 years after loading. The occurrence of peri‐implantitis around implants with roughened surfaces is likely to be even higher, since it was observed in another Cochrane systematic review (Esposito 2007) that statistically significantly more peri‐implantitis occurred at 3 years of loading around implants with roughened surfaces when compared to turned (machined) Brånemark implants.
Marginal bone loss around dental implants may also be caused by occlusal overload as suggested by an experimental animal study (Isidor 1996). However, the present review will focus exclusively on the treatment of peri‐implantitis induced by plaque infection.
In the case of peri‐implantitis, various interventions (often combined) have been suggested including: (a) mechanical debridement; (b) pharmaceutical therapy (subgingival chlorhexidine irrigation, local or systemic antibiotics); and (c) surgical procedures including: open flap debridement aimed at (1) removing bacteria (also using soft lasers), (2) smoothing the implant surface (to decrease surface roughness) and removing unsupported implant threads that protect bacterial plaque, (3) 'decontamination' or 'detoxification' of the implant surface using various chemical agents or laser beam. After the primary goal of surgical intervention (i.e. bacteria‐free implant surface) has been achieved, it may be necessary to correct the anatomical conditions for improving plaque control and for eliminating the favourable environment for anaerobic bacteria (elimination of pathological peri‐implant pockets). This may be achieved either with resective procedures or alternatively with bone regenerative procedures, including guided bone regeneration, autologous or allogenic bone grafts, etc. (Roos‐Jansåker 2003; Schou 2004; Sahrmann 2011).
Objectives
To evaluate different interventions for treating peri‐implantitis around osseointegrated dental implants. Perimplantitis is defined as: plaque‐induced progressive marginal bone loss observed on radiographs with clinical signs of infection of the peri‐implant soft tissues. Trials evaluating the treatment of peri‐implant mucositis (chronic plaque‐induced infection of the marginal peri‐implant soft tissues without appreciable bone loss) are evaluated in another Cochrane review (Grusovin 2010).
Comparisons between interventions will be didactically divided according to a clinical approach into:
non‐surgical versus surgical interventions;
different non‐surgical interventions;
adjunctive treatments to non‐surgical interventions;
different surgical interventions;
adjunctive treatments to surgical interventions.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) evaluating interventions to treat peri‐implantitis, including studies with parallel group and split‐mouth designs.
Types of participants
People who have at least one dental implant affected by peri‐implantitis.
Types of interventions
Any non‐surgical or surgical procedure including the use of local or systemic therapeutic agents as well as any other interventions aimed at the recovery of peri‐implant oral health.
Types of outcome measures
Primary outcomes
Implant failure, defined as implant mobility of previously clinically osseointegrated implants and removal of non‐mobile implants because of progressive marginal bone loss or infection.
Radiographic marginal bone level change on intraoral radiographs taken with a parallel technique.
Complications and side effects.
Recurrence of peri‐implantitis.
Secondary outcomes
Probing 'attachment' level (PAL) change.
Probing pocket depth (PPD) change.
Marginal soft tissue recession (REC) change.
Aesthetics evaluated by patients.
Aesthetics evaluated by dentists.
Cost (treatment time plus material costs).
Search methods for identification of studies
Electronic searches
For the identification of studies included or considered for this review, detailed search strategies were developed for each database searched. These were based on the search strategy developed for MEDLINE (OVID) but revised appropriately for each database. The search strategy used a combination of controlled vocabulary and free text terms and was run with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2009 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] (Higgins 2011). Details of the MEDLINE search are provided in Appendix 1.
Searched databases
Cochrane Oral Health's Trials Register (to 9th June 2011) (Appendix 2).
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 2) (Appendix 3).
MEDLINE via OVID (1950 to 9th June 2011) (Appendix 1).
EMBASE via OVID (1980 to 9th June 2011) (Appendix 4).
The most recent electronic search was undertaken on 9 June 2011.
Language
There were no language restrictions.
Searching other resources
Unpublished studies
We wrote to all the authors of the identified randomised controlled trials (RCTs), we checked the bibliographies of all identified RCTs and relevant review articles, and we used personal contacts in an attempt to identify unpublished or ongoing RCTs. In the first version of this review we also wrote to more than 55 oral implant manufacturers and we requested information on trials through an Internet discussion group (implantology@yahoogroups.com), however we discontinued this due to poor yield.
Handsearching
Details of the journals being handsearched by Cochrane Oral Health's ongoing programme are given on the website: www.ohg.cochrane.org. The following journals have been identified as being potentially important to be handsearched for this review.
British Journal of Oral and Maxillofacial Surgery
Clinical Implant Dentistry and Related Research
Clinical Oral Implants Research
European Journal of Oral Implantology
Implant Dentistry
International Journal of Oral and Maxillofacial Implants
International Journal of Oral and Maxillofacial Surgery
International Journal of Periodontics and Restorative Dentistry
International Journal of Prosthodontics
Journal of Clinical Periodontology
Journal of Dental Research
Journal of Oral Implantology
Journal of Oral and Maxillofacial Surgery
Journal of Periodontology
Journal of Prosthetic Dentistry
Where these have not already been searched as part of the Cochrane Journal Handsearching Programme, the journals were handsearched by one review author up to the month in which the last electronic search was undertaken.
Data collection and analysis
Study selection
The titles and abstracts (when available) of all reports identified through the electronic searches were scanned independently by two review authors. For studies appearing to meet the inclusion criteria, or for which there were insufficient data in the title and abstract to make a clear decision, the full report was obtained. The full reports obtained from all the electronic and other methods of searching were assessed independently by two review authors to establish whether the studies met the inclusion criteria or not. Disagreements were resolved by discussion. Where resolution was not possible, a third review author was consulted. All studies meeting the inclusion criteria then underwent validity assessment and data extraction. Studies rejected at this or subsequent stages were recorded in the table of excluded studies, and reasons for exclusion recorded.
Data extraction
Data were extracted by two review authors independently using specially designed data extraction forms. The data extraction forms were piloted on several papers and modified as required before use. Any disagreement was discussed and a third review author consulted where necessary. All authors were contacted for clarification or missing information. Data were excluded until further clarification was available if agreement could not be reached.
For each trial the following data were recorded.
Year of publication, country of origin and source of study funding.
Details of the participants including demographic characteristics.
Details on the type of intervention.
Details of the outcomes reported, including method of assessment and time intervals.
Risk of bias in included studies
The risk of bias assessment of the included trials was undertaken independently and in duplicate by at least two review authors as part of the data extraction process. In the case that the paper to be assessed had one or more review authors in the authors list, it was independently evaluated only by those review authors not involved in the trial.
This was conducted using the recommended approach for assessing risk of bias in studies included in Cochrane reviews (Higgins 2011). It is a two‐part tool, addressing the six specific domains (namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and 'other issues'). Each domain includes one specific entry in a 'Risk of bias' table. Within each entry, the first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry.
Summarising risk of bias for a study
After taking into account the additional information provided by the authors of the trials, studies were grouped into the following categories. We assumed that the risk of bias was the same for all outcomes and each study was assessed as follows.
| Risk of bias | Interpretation | Within a study | Across studies |
| Low risk of bias. | Plausible bias unlikely to seriously alter the results. | Low risk of bias for all key domains. | Most information is from studies at low risk of bias. |
| Unclear risk of bias. | Plausible bias that raises some doubt about the results. | Unclear risk of bias for one or more key domains. | Most information is from studies at low or unclear risk of bias. |
| High risk of bias. | Plausible bias that seriously weakens confidence in the results. | High risk of bias for one or more key domains. | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results. |
Measure of treatment effect
For dichotomous outcomes, the estimate of effect of an intervention was expressed as risk ratios (RR) together with 95% confidence intervals (CIs). For continuous outcomes, mean differences and standard deviations were used to summarise the data for each group using mean differences and 95% CIs.
Unit of analysis issues
The statistical unit was the patient and not the implant unless the clustering of the implants within the patients had been taken into account in the analysis.
Dealing with missing data
Attempts were made to retrieve missing data from authors of trials. Methods for estimating missing standard deviations in section 7.7.3 of the Cochrane Handbook (Higgins 2011) were to be used. Change data were used and if only cross‐sectional data were available the standard deviation (SD) of the change was to be estimated assuming no within patient correlation, which would give rise to a conservative estimate of the SD for change (Follmann 1992). The techniques described by Follmann (Follmann 1992) were to be used to estimate the standard error of the difference for split‐mouth studies, where the appropriate data were not presented and could not be obtained.
Assessment of heterogeneity
The significance of any discrepancies in the estimates of the treatment effects from the different trials was to be assessed by means of Cochran's test for heterogeneity and heterogeneity would have been considered significant if P < 0.1. The I2 statistic, which describes the percentage total variation across studies that is due to heterogeneity rather than chance, was to be used to quantify heterogeneity with I2 over 50% being considered moderate to high heterogeneity.
Assessment of reporting biases
If there had been sufficient numbers of trials (more than 10) in any meta‐analysis, publication bias would have been assessed according to the recommendations on testing for funnel plot asymmetry (Egger 1997) as described in the Cochrane Handbook (Higgins 2011). If asymmetry was identified we would have examined possible causes.
Data synthesis
Only if there were studies of similar comparisons reporting the same outcome measures a meta‐analysis was done. Risk ratios were to be combined for dichotomous data, and mean differences for continuous data, using random‐effects models provided there were more than three studies in the meta‐analysis. Data from split‐mouth studies were combined with data from parallel group trials with the method outlined by Elbourne (Elbourne 2002), using the generic inverse variance method in Review Manager (RevMan).
Subgroup analysis and investigation of heterogeneity
Clinical heterogeneity was to be assessed by examining the types of participants and interventions for all outcomes in each study. It was decided not to formulate any hypotheses to be investigated for subgroup analyses since no significant meta‐analysis was expected. However, this may be done in future updates of this review.
Sensitivity analyses
It was planned to undertake sensitivity analyses to examine the effect of the study quality assessment on the overall estimates of effect. In addition, the effect of including unpublished literature on the review's findings was also to be examined, but there were insufficient trials to undertake this.
Results
Description of studies
Characteristics of the trial setting and investigators
Of the 15 potentially eligible trials (Tarpey 1996; Bach 2000; Tang 2002; Büchter 2004; Renvert 2004; Karring 2005; Romeo 2005; Schwarz 2005; Schwarz 2006a; Schwarz 2006b; Renvert 2008;Renvert 2009; Renvert 2011; Sahm 2011; Schwarz 2011), six were excluded: one because insufficient data were presented (Tarpey 1996), one because no data were presented (Bach 2000), and the other four because only a minority of the included patients were affected by peri‐implantitis and it was not possible to discriminate those affected by peri‐implantitis from those affected by peri‐implant mucositis (Renvert 2004; Renvert 2008; Renvert 2009; Sahm 2011). The latter four excluded trials are included in another Cochrane review dealing with implant maintenance (Grusovin 2010).
Of the nine included trials, five were conducted in Germany (Büchter 2004; Schwarz 2005; Schwarz 2006a; Schwarz 2006b; Schwarz 2011), one in China (Tang 2002), one in Denmark (Karring 2005), one in Sweden (Renvert 2011), and one in Italy (Romeo 2005).
All included trials had a parallel group study design with one exception (Karring 2005), which was a split‐mouth design.
For seven trials support was received from industry directly involved in the product being tested (Büchter 2004; Karring 2005; Schwarz 2005; Schwarz 2006a; Schwarz 2006b; Renvert 2011; Schwarz 2011). One trial did not receive any support from commercial parties (Romeo 2005). For one trial it was unclear whether support from industry was received (Tang 2002).
All trials were conducted at universities or specialist dental clinics.
All trials included only adult patients.
Characteristics of the interventions
1) Non‐surgical versus surgical interventions: no trials.
2) Different non‐surgical interventions: five trials (Tang 2002; Karring 2005; Schwarz 2005; Schwarz 2006a; Renvert 2011).
Three trials compared different interventions and two trials compared the same interventions, Er:YAG laser versus manual debridement with chlorhexidine subgingival application (Schwarz 2005; Schwarz 2006a):
Local antibiotics versus ultrasonic debridement. Metronidazole gel 25% injected into the pocket at a depth of 3 mm versus ultrasonic debridement with carbon fibre tip inserted 1 to 2 mm into the gingival sulcus at the lowest power for 15 seconds. Both interventions were repeated a second time 1 week after (Tang 2002). The trial included IMZ and Frialit‐2 implants.
Air‐abrasive device versus manual debridement. The test sites were treated with the Vector system (Dürr Dental, Bietigheim‐Bissingen, Germany) with the straight or curved flexible Vector carbon fibre tip combined with aerosol spray of Vector fluid polish with hydroxyapatite particles (grain size approximately 10 µm) whereas the control sites with carbon fibre curettes (Karring 2005). No local anaesthesia was provided and the instrumentation of each implant was carried out for 2 to 3 minutes. The same treatment was repeated after 3 months. Following each treatment patients received oral hygiene instructions. The trial included two pairs of Brånemark, four of ITI and five of Astra implants.
Er:YAG laser versus manual debridement with chlorhexidine subgingival application. Both trials were conducted by the same authors in a similar manner (Schwarz 2005; Schwarz 2006a). Oral hygiene instructions two to four times and supragingival cleaning with rubber caps and polishing paste were given prior to the initiation of the trials. In one group a Er:YAG laser (Key 3, KaVo, Biberach, Germany) emitting a pulsed infrared radiation at a wavelength of 2.94 µm was used. The laser parameters were set at 100 mJ/pulse, 10 Hz, and pulse energy at the tip was approximately 85 mJ/pulse. The laser beam was guided onto the implant surface under water irrigation with a specially designed periodontal handpiece (2061, KaVo, Biberach, Germany) and a cone‐shaped glass fibre tip emitting a radial and axial laser beam. The fibre tip was guided in a circular motion from coronal to apical parallel to the implant surface in contact mode. In the control group manual debridement was performed with plastic curettes followed by pocket irrigation with 0.2% chlorhexidine and subgingival application of 0.2% chlorhexidine gel, followed by 2 weeks of mouthrinses with 0.2% chlorhexidine, twice a day for 2 minutes. For both groups, instrumentation was carried out until the operator felt that the implant surfaces were adequately debrided, on the average 6 minutes per implant. The trials included Brånemark System® (Nobel Biocare, Göteborg, Sweden), Camlog Screw Line® (Camlog, Wimsheim, Germany), Frialit (Dentsply Friadent, Mannheim, Germany), IMZ Twin Plus (Dentsply Friadent, Mannheim, Germany), ITI both SLA and TPS (Straumann, Waldenburg, Switzerland), Spline Twist MTX (Zimmer Dental, Freiburg, Germany), and ZL‐Duraplant Ticer (ZL Microdent, Breckerfeld, Germany) implants.
Er:YAG laser versus air‐abrasive device. Before enrolment in the study, any periodontal lesions at remaining teeth was treated (Renvert 2011). Before the treatments, the supra‐structures were removed. One group was treated with an air‐abrasive device (Perio‐Flow®, EMS, Nyon, Switzerland). The nozzle was placed in the pocket and used for approximately 15 seconds in each position circumferentially around the implant. Careful attempts were made to cover the full circumference of the implant. The Perio‐Flow® device utilizes a 25 mm hydrophobic powder and a flexible tip allowing access to periodontal and implant pockets. The other group was treated with an Er:YAG laser (Key Laser 3 Perio, KaVo, Biberach, Germany) at an energy level of 100 mJ/pulse and 10 Hz (12.7 J/cm2) using a cone‐shaped sapphire tip. The instrument tip was used in a parallel mode using a semicircular motion around the circumferential pocket area of the implant. Routine local anaesthesia was used as needed. The trial included implants with machined (Brånemark System®) or medium rough surfaces (Astra).
3) Adjunctive treatments to non‐surgical interventions: one trial (Büchter 2004).
Adjunctive local antibiotics to manual debridement with chlorhexidine subgingival application. Full mouth debridement and subgingival irrigation with 0.2% chlorhexidine 2 to 18 weeks before baseline examination. Both groups were then subjected to subgingival irrigation with 0.2% chlorhexidine and subgingival debridement with hand plastic instruments and sterilization of the abutments. The test group received 8.5% doxycycline hyclate (Atridox, Block Drug Corporation, Inc, Jersey City, New Jersey, USA) by means of a syringe with a blunt cannula in the peri‐implant sulcus. No placebo was used. The trial included ITI SLA implants.
4) Different surgical interventions: two trials (Schwarz 2006b; Schwarz 2011).
The two trials compared different interventions:
Augmentation with synthetic versus animal‐derived bone substitutes. Patients were previously treated with a single‐course non‐surgical instrumentation with plastic curettes followed by pocket irrigation with 0.2% chlorhexidine and subgingival application of chlorhexidine gel (Schwarz 2006b). After flap elevation and removal of granulation tissues, implants were debrided using plastic curettes and rinsed with saline solution. Sites were filled or with nanocrystalline hydroxyapatite (Ostim, Heraeus Kulzer, Hanau, Germany) injected using a ready‐to‐use paste in a syringe or with a bovine‐derived xenograft (Bio‐Oss, Geistlich, Wolhusen, Switzerland, particle size 0.25 to 1 mm) in combination with a resorbable collagen barrier (Bio‐Gide, Geistlich, Wolhusen, Switzerland) of porcine origin. Authors confirmed that no prophylactic antibiotics were given. The trial included Brånemark System® (Nobel Biocare, Göteborg, Sweden), Camlog Screw Line® (Camlog, Wimsheim, Germany), KSI Bauer Schraube (Bad Nauheim, Germany), ITI both SLA and TPS (Straumann, Waldenburg, Switzerland), Spline Twist MTX and Tapered Screw Vent (Zimmer Dental, Freiburg, Germany), and ZL‐Duraplant Ticer (ZL Microdent, Breckerfeld, Germany) implants.
Surface debridement with laser versus plastic curettes and saline solution before augmentation. Two weeks after a single‐course of non‐surgical instrumentation with plastic curettes followed by pocket irrigation with 0.2% chlorhexidine and subgingival application of chlorhexidine gel (Schwarz 2011), patients were treated with access flap surgery, granulation tissue removal, and implantoplasty at buccally and supracrestally exposed implant surfaces. Implantoplasty was performed in a way to completely plane the threatened areas and smooth the structured implant surface using diamond burs and Arkansas stones under copious irrigation with sterile saline. Particular care was taken to completely remove any titanium deposits/dust from the surrounding tissues. The remaining intra‐bony defects were randomly allocated to two different surface treatments:
Er:YAG laser (Elexxion Delos, Elexxion AG, Radolfzell, Germany) emitting a pulsed infrared radiation at a wavelength of 2.940 nm. The laser beam was guided onto the exposed implant surfaces under water irrigation with a specially designed periodontal handpiece and a lancet‐shaped sapphire tip emitting a lateral laser beam (Experimental Duros Yip, Elexxion AG). Laser parameters were set at 100 mJ/pulse (11.4 J/cm2), 10 Hz, and pulse energy at the tip was approximately 90 mJ/pulse. The fibre tip was guided in a semicircular motion from coronal to apical parallel to the implant surface in the contact mode.
Plastic curettes plus cotton pellets soaked in saline solution followed by a thorough irrigation with saline solution.
In both groups, the intra‐bony component was augmented with BioOss spongiosa granules, particle size 0.25 to 1 mm (Geistlich, Wolhusen, Switzerland). Before its application, the graft material was moistened in sterile saline for 5 minutes. Following grafting, a bioresorbable barrier porcine origin (BioGide, Geistlich) was trimmed and adapted over the entire defect so as to cover 2 to 3 mm of the surrounding alveolar bone. Neither sutures nor pins were used for membrane fixation. Finally, the mucoperiosteal flaps were repositioned coronally and fixed with mattress sutures to ensure a non‐submerged healing procedure.
5) Adjunctive treatments to surgical interventions: one trial (Romeo 2005).
Adjunctive implant surface smoothening to systemic antibiotics plus resective surgery plus two different local antibiotics. Systemic antibiotics (amoxicillin 50 mg/kg/die for 8 days per os) were administered and subgingival debridement with a plastic scaler was performed prior to the initiation of the trial. An apically repositioned flap was performed. Vertical releasing incisions were made, the granulomatous tissue and the inner pocket epithelium were removed with hand curettes, and alveolar bone peaks were removed with bone chisels. A gel of metronidazole (Elyzol 25%, Cabon S.p.A, Milan, Italy) followed by a solution of tetracycline hydrochloride (Ambramicina, Sharper S.p.A, Milan, Italy) was rubbed on the contaminated implant surface for 3 minutes and then washed off with cold sterile physiological saline solution. The exposed titanium plasma‐sprayed (TPS) surface of the implants in the test group were polished with the following sequence of burs assembled on a handpiece rotating at 15,000 rpm: diamond/30 µm particle size egg‐shaped bur and diamond/15 µm particle size egg‐shaped bur (Komet, Gerb. Brasseler GmbH, Lemgo, Germany); Arkansas burs (Dura‐Green and Dura‐White, Shofu Inc, Kyoto, Japan); silicone polishers (Brownie, Greenie, Shofu Inc). The flaps were then apically repositioned. The trial included ITI TPS (Straumann, Waldenburg, Switzerland) dental implants (both full‐body and hollow screws).
Characteristics of outcome measures
Implant failure was evaluated in all trials.
Radiographic marginal bone levels on intraoral radiographs were evaluated in three trials (Karring 2005; Romeo 2005; Renvert 2011), however, the results for one split‐mouth study did not report the within patient standard deviations (Karring 2005). The radiographic data of the other study (Romeo 2005) were not consistent (different patients and patients' numbers were presented in the two reports). Since the authors failed to provide the necessary clarifications, we were unable to use these data.
Complications and side effects were reported in all trials with one exception (Renvert 2011).
Recurrence of peri‐implantitis was evaluated in all trials.
Probing 'attachment' level (PAL) changes were presented or calculated in six trials (Büchter 2004; Karring 2005; Romeo 2005; Schwarz 2005; Schwarz 2006a; Schwarz 2006b), however, we were unable to use the data of two trials (Romeo 2005; Schwarz 2006a). In one study (Romeo 2005) statistical analyses were performed using the implant(s) and not the patient as unit of analysis, one patient was treated in a split‐mouth fashion. In the other study the actual figures were not given (Schwarz 2006a).
Probing pocket depth (PPD) changes were presented or calculated in all trials. We were unable to use data from three trials: the within patient standard deviations for the split‐mouth study were not reported (Karring 2005); statistical analyses were performed using the implant(s) and not the patient as unit of analysis, and one patient was treated in a split‐mouth fashion in one study (Romeo 2005); in the other study the actual figures at patient level were not given (Schwarz 2006a).
Gingival recession (REC) changes were evaluated in five trials (Romeo 2005; Schwarz 2005; Schwarz 2006a; Schwarz 2006b; Schwarz 2011), however, we were unable to use the data of two trials. In one trial (Romeo 2005) the statistical analyses were performed using the implant(s) and not the patient as unit of analysis, and one patient was treated in a split‐mouth fashion. In the other study the figures were not given (Schwarz 2006a).
No study used aesthetics assessed by patients as an outcome measure.
No study used aesthetics assessed by dentists an aesthetic outcome measure (apart for soft tissue recessions).
No study recorded treatment costs as an outcome measure, however, it is possible to estimate them for all trials.
Characteristics at baseline
Inclusion criteria
Stable implants not surrounded by a radiolucent area on radiographs (all trials).
Unspecified peri‐implant bone loss (Romeo 2005; Schwarz 2005; Schwarz 2006a).
Peri‐implant bone loss exceeding 50% of the implant length (Büchter 2004).
Infrabony component > 3 mm (Schwarz 2006b).
Peri‐implant bone loss > 3 mm and PPD > 5 mm with bleeding and/or pus on probing using a standardised force of 0.2 Ncm (about 20 grams) (Renvert 2011).
An infrabony component > 3 mm together with a suprabony component > 1 mm with PPD > 6mm (Schwarz 2011).
Peri‐implant bone loss > 1.5 mm (Karring 2005).
Peri‐implant bone loss < 4 mm (Tang 2002).
PPD > 4 mm bleeding on probing or exudate or both (Romeo 2005; Schwarz 2005; Schwarz 2006a).
PPD > 5 mm bleeding after gentle probing (Karring 2005), however sites with no bleeding on probing (BOP) (four in the Vector group and three in the manually debrided group) at the baseline examination were included.
PPD < 6 mm bleeding on probing using a standardised force of 0.2 Ncm (about 20 grams) (Tang 2002).
PPD > 6 mm (Schwarz 2006b).
Exclusion criteria
Used systemic antibiotics up to 6 months prior to the study (Schwarz 2005; Schwarz 2006a).
Used antibiotics up to 3 months prior to the study (Tang 2002; Büchter 2004; Karring 2005; Renvert 2011).
Used anti‐inflammatory prescription medications up to 3 months prior to the study (Renvert 2011).
Peri‐implantitis treatment during the last 6 months (Schwarz 2006a) or 12 months (Schwarz 2005).
Used mouthrinse with anti‐inflammatory properties up to 1 month prior to the study (Tang 2002).
Subjected to mechanical debridement up to 3 months prior to the study (Karring 2005).
Allergy to the tested antibiotics (Büchter 2004).Test and control implants have to be of the same brand, not next to each other and not differing more than 1 mm in bone loss (Karring 2005).
Absence of keratinised peri‐implant mucosa (Schwarz 2005; Schwarz 2006a; Schwarz 2006b).
Less than 2 mm of keratinised mucosa (Schwarz 2011).
Smokers if not occasional (Schwarz 2005; Schwarz 2006a; Schwarz 2006b).
Hollow cylinder implants (Schwarz 2005; Schwarz 2006a; Schwarz 2006b; Schwarz 2011).
Systemic diseases that could influence the outcome of the therapy, i.e. diabetes, osteoporosis (Schwarz 2005; Schwarz 2006a; Schwarz 2006b; Renvert 2011; Schwarz 2011).
Presence of overhangs or margins (Schwarz 2006b; Schwarz 2011).
Evidence of occlusal overload (Schwarz 2006b; Schwarz 2011).
Presence of acute periodontitis (Schwarz 2006b).
Poor oral hygiene: plaque index > 1 (Schwarz 2006b; Schwarz 2011).
None specified (Romeo 2005).
Heavy smokers (more than 10 cigarettes per day) (Schwarz 2011).
Comparability of control and treatment groups at entry
The two groups were comparable at entry for the outcomes used in the trial for all trials, with one exception (Schwarz 2006a) for which it was unclear whether baseline characteristics were comparable at entry.
Type and frequency of maintenance during the postoperative phase and the follow‐up of the trials
Not reported. Patients were not allowed to use mouthwash or flossing during the entire study period (Tang 2002).
Weekly recall with repeated motivation and instructions in oral hygiene (Büchter 2004).
At baseline, 1, 3, 6 (Schwarz 2005) and 12 months (Schwarz 2006a). Only supragingival cleaning with rubber caps and polishing paste with reinforcement of oral hygiene.
Quarterly with repeated treatment and instructions in oral hygiene (Karring 2005).
Mouthrinse with chlorhexidine 0.2% four times a day for the first 2 weeks (Romeo 2005). Frequency and type of the maintenance regimen not reported.
Mouthrinse with chlorhexidine 0.2% twice a day for the first 2 weeks (Schwarz 2006b; Schwarz 2011). Recalls every second week for the first 2 months and monthly thereafter. Supragingival cleaning with rubber caps and polishing paste with reinforcement of oral hygiene were performed at 1, 3 and 6 months.
Subjects received a sonic toothbrush and individualised oral hygiene instructions (Renvert 2011). They were supplied with new brush heads after 3 months.
Duration of the studies
Three months (Tang 2002).
Four months (Büchter 2004).
Six months (Karring 2005; Schwarz 2005; Renvert 2011; Schwarz 2011).
One year (Schwarz 2006a).
Two years (Romeo 2005).
Four years (Schwarz 2006b).
Sample size
Only two trials reported a sample size calculations (Renvert 2011; Schwarz 2011).
Risk of bias in included studies
The final risk of bias assessment after having incorporated the additional information kindly provided by the authors of the included trials is summarised in Figure 1 and Figure 2. Summarising the risk of bias for each study, three trials were judged to be at an unclear risk of bias (Büchter 2004; Karring 2005; Renvert 2011), whereas six trials were judged to be at high risk of bias (Tang 2002; Romeo 2005; Schwarz 2005; Schwarz 2006a; Schwarz 2006b; Schwarz 2011).
1.
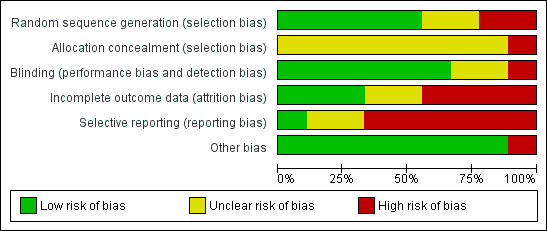
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
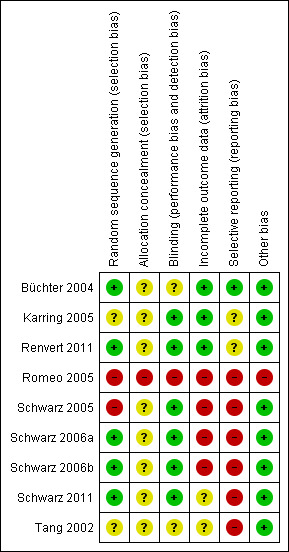
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Random sequence generation (selection bias)
After correspondence with the authors, it was unclear how the random sequence generation was generated for two trials (Tang 2002; Karring 2005), for two trials it was judged not to be adequate (Romeo 2005; Schwarz 2005), and it was considered adequate for five trials (Büchter 2004; Schwarz 2006a; Schwarz 2006b; Renvert 2011; Schwarz 2011).
Allocation concealment (selection bias)
It was unclear whether patients' allocation was concealed for eight trials (Tang 2002; Büchter 2004; Schwarz 2005; Schwarz 2006a; Schwarz 2006b; Karring 2005; Renvert 2011; Schwarz 2011). One trial was not concealed (Romeo 2005).
Blinding of assessors (performance bias and detection bias)
For six trials (Karring 2005; Schwarz 2005; Schwarz 2006a; Schwarz 2006b; Renvert 2011; Schwarz 2011) the outcome assessor was blinded to the delivered interventions. However, in one trial (Karring 2005) the operator recorded the preoperative measurements whereas a blinded outcome assessor recorded the follow‐up measurements. In two trials, it was unclear whether assessors were blinded to the interventions (Büchter 2004; Tang 2002) and in another trial the outcome assessor was not blinded (Romeo 2005).
Incomplete outcome data (attrition bias)
No withdrawals occurred in four trials (Büchter 2004; Karring 2005; Romeo 2005; Renvert 2011). Withdrawals occurred in five trials (Tang 2002; Schwarz 2005; Schwarz 2006a; Schwarz 2006b; Schwarz 2011): three of these trials were judged at high risk of bias (Schwarz 2005; Schwarz 2006a; Schwarz 2006b) and one at unclear risk (Tang 2002). In two trials, one patient from the debridement group (Schwarz 2005) and two patients from the debridement group (Schwarz 2006a) were withdrawn due to persisting pus exudation 4 to 12 weeks after treatment and were treated with the Er:YAG laser. In Schwarz 2006b two patients from the Ostim group and one from the Bio‐Oss group were withdrawn from the study because of severe pus formation 1 and 3 years after initial treatment, respectively. In another trial (Tang 2002), three patients withdrew but reasons for withdrawals were not given. We received no reply to our request for clarifications.
Selective reporting (reporting bias)
Only one trial (Büchter 2004) was judged to be at low risk of bias, two at an unclear risk of bias (Karring 2005; Renvert 2011) whereas all the remaining trials were judged to be at high risk of bias since the data/outcome of some patients were not reported (Romeo 2005; Schwarz 2005; Schwarz 2006a; Schwarz 2006b; Schwarz 2011) or because standard deviations were not reported (Tang 2002; Schwarz 2006a) and therefore we could not use the data in the present review.
Other bias
One trial (Romeo 2005) was judged at high risk of bias because the number and the initials of patients were different in the two published reports. The remaining trials were judged at a low risk of bias.
Effects of interventions
In total 222 patients were originally included in the nine included trials.
1) Non‐surgical versus surgical interventions: no trials.
2) Different non‐surgical interventions: five trials (Tang 2002; Karring 2005; Schwarz 2005; Schwarz 2006a; Renvert 2011).
Local antibiotics versus ultrasonic debridement. One study (Tang 2002) of parallel group design compared metronidazole gel versus ultrasonic debridement. Both interventions were repeated 1 week later. Thirty patients were enrolled and the results are given for 27 patients. Three patients withdrew for unknown reasons, one from the metronidazole group and two from the ultrasonic group. There were no baseline imbalances for plaque, bleeding on probing (BOP), probing pocket depth (PPD) and number of bacteria. After 12 weeks there was no statistically significant difference in PPD changes (Analysis 1.1). No implant failures, complications or recurrence of peri‐implantitis occurred in any of the groups. This trial was judged to be at high risk of bias (Figure 2).
Air‐abrasive device versus manual debridement. One study (Karring 2005) of split‐mouth design compared mechanical debridement with the Vector system versus manual debridement. Both interventions were repeated after 3 months. Eleven patients were enrolled and none was withdrawn. There were no baseline imbalances for plaque, BOP, PPD and bone levels. After 6 months the authors reported no statistically significant difference for marginal bone levels and PPD, however, although the mean values appeared very similar, we were unable to use the data as we did not have the standard deviations. No implant failures, complications or recurrence of peri‐implantitis occurred in any of the groups. This trial was judged to be at an unclear risk of bias (Figure 2).
Er:YAG laser versus manual debridement with chlorhexidine subgingival application. Two studies of identical study design by the same authors evaluated this intervention (Schwarz 2005; Schwarz 2006a).
One study (Schwarz 2005) of parallel group design compared YAG laser versus manual debridement and chlorhexidine irrigation/gel. Twenty patients with 32 implants affected by peri‐implantitis were enrolled (10 patients with 16 implants in each group). One patient with two implants from the chlorhexidine group was withdrawn due to persisting pus formation 8 weeks after treatment. This patient was re‐treated with Er:YAG laser treatment. We therefore substituted the 3‐month for the missing 6‐month data for this patient, so no patients were excluded from the analysis. There were no baseline imbalances for plaque, BOP, PPD, PAL and gingival recession (REC). No implant failures or complications occurred in any of the groups. After 6 months there were no statistically significant differences for changes in PAL, PPD and REC (Analysis 3.3; Analysis 3.4; Analysis 3.5). This trial was judged to be at high risk of bias (Figure 2).
One study (Schwarz 2006a) of parallel group design compared YAG laser versus manual debridement and chlorhexidine irrigation/gel. Twenty patients with 40 implants affected by peri‐implantitis were enrolled (10 patients with 20 implants in each group). Two patients with four implants from the chlorhexidine group were withdrawn due to persisting pus formation between 4 and 12 weeks after treatment. These patients were re‐treated with Er:YAG laser treatment. It was unclear whether there were baseline imbalances for plaque, BOP, PPD, PAL and REC. No implant failures occurred but one patient from the laser group had one perforation of the buccal keratinised mucosa which healed with an increased gingival recession (Analysis 3.1). After 1 year the authors reported there were no statistically significant differences for PAL, PPD and REC, however we were unable to use their data since original figures were not presented in the article and were not provided to us. After the 1‐year follow‐up all patients were re‐treated with laser and augmentation therapies due to deterioration of BOP and PAL. This trial was judged to be at high risk of bias (Figure 2).
1.1. Analysis.
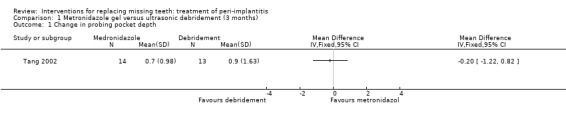
Comparison 1 Metronidazole gel versus ultrasonic debridement (3 months), Outcome 1 Change in probing pocket depth.
3.3. Analysis.

Comparison 3 Er:YAG laser versus manual debridement and chlorhexidine irrigation/gel (6 months), Outcome 3 Change in attachment levels.
3.4. Analysis.
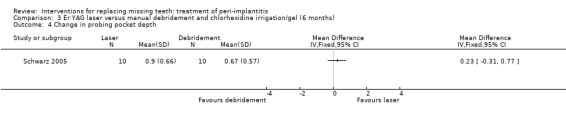
Comparison 3 Er:YAG laser versus manual debridement and chlorhexidine irrigation/gel (6 months), Outcome 4 Change in probing pocket depth.
3.5. Analysis.
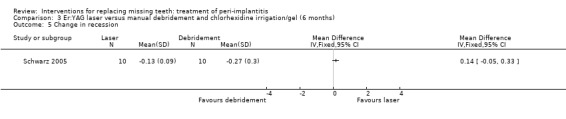
Comparison 3 Er:YAG laser versus manual debridement and chlorhexidine irrigation/gel (6 months), Outcome 5 Change in recession.
3.1. Analysis.

Comparison 3 Er:YAG laser versus manual debridement and chlorhexidine irrigation/gel (6 months), Outcome 1 Complications.
The meta‐analyses of the two trials for recurrence of peri‐implantitis did not indicate a benefit for either intervention (risk ratio (RR) fixed effect 0.25; 95% confidence interval (CI) 0.03 to 2.06; Analysis 3.2) between the two treatment modalities. After 1 year all patients (Schwarz 2006a) were considered to have recurrence of peri‐implantitis.
3.2. Analysis.
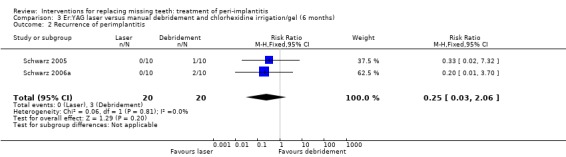
Comparison 3 Er:YAG laser versus manual debridement and chlorhexidine irrigation/gel (6 months), Outcome 2 Recurrence of perimplantitis.
Er:YAG laser versus air‐abrasive device. One study (Renvert 2011) with a parallel group design compared an air‐abrasive device (Perio‐Flow®) with an Er:YAG laser for non‐surgical debridement. Twenty‐one patients with 45 implants were treated with the air‐abrasive and 21 patients with 55 implants with laser. Fourteen implants of the air‐abrasive group and 17 implants of the laser group had pus on probing. There were no baseline imbalances for the groups. After 6 months there were no drop‐outs, implant failures or complications and the study reported no statistically significant differences between the two procedures for PPD, peri‐implant marginal bone levels and peri‐implantitis recurrence (authors reported data: Analysis 7.1; Analysis 7.2; Analysis 7.3). It was reported that 11% of the implants (five implants treated with air‐abrasive and six implants treated with laser) presented pus on probing at 6 months. This trial was judged to be at an unclear risk of bias (Figure 2).
7.1. Analysis.
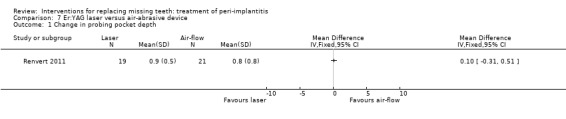
Comparison 7 Er:YAG laser versus air‐abrasive device, Outcome 1 Change in probing pocket depth.
7.2. Analysis.
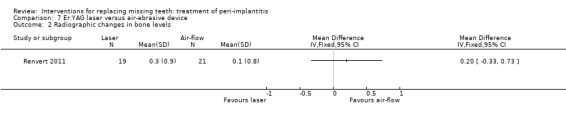
Comparison 7 Er:YAG laser versus air‐abrasive device, Outcome 2 Radiographic changes in bone levels.
7.3. Analysis.
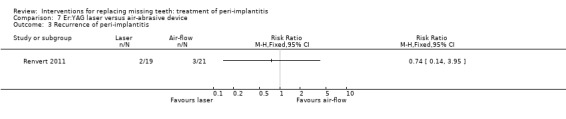
Comparison 7 Er:YAG laser versus air‐abrasive device, Outcome 3 Recurrence of peri‐implantitis.
3) Adjunctive treatments to non‐surgical interventions: one trial (Büchter 2004).
Adjunctive local antibiotics to manual debridement with chlorhexidine subgingival application. One study (Büchter 2004) of parallel group design evaluated doxycycline as adjunct to manual debridement. Twenty‐eight patients were enrolled (14 in each group) and none was withdrawn. There were no baseline imbalances for the groups. After 18 weeks there were statistically significant differences for changes in probing attachment levels (PAL) and PPD, with mean differences of 0.61 mm (95% CI 0.40 to 0.82; Analysis 2.1), and 0.59 mm (95% CI 0.39 to 0.79; Analysis 2.2) in favour of the group treated with adjunctive local antibiotics. No implant failures, complications or recurrence of peri‐implantitis occurred in any of the groups. This trial was judged to be at an unclear risk of bias (Figure 2).
2.1. Analysis.
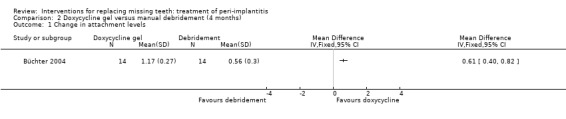
Comparison 2 Doxycycline gel versus manual debridement (4 months), Outcome 1 Change in attachment levels.
2.2. Analysis.
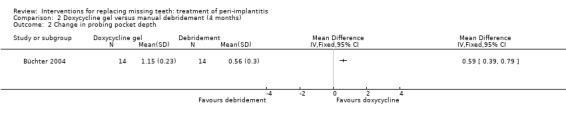
Comparison 2 Doxycycline gel versus manual debridement (4 months), Outcome 2 Change in probing pocket depth.
4) Different surgical interventions: two trials (Schwarz 2006b; Schwarz 2011).
Augmentation with synthetic versus animal‐derived bone substitutes. One study (Schwarz 2006b) of parallel group design compared a nanocrystalline hydroxyapatite (Ostim) with bovine anorganic bone (Bio‐Oss) together with a resorbable barrier to treat peri‐implant infrabony defects deeper than 3 mm. Twenty‐two patients with 22 implants affected by peri‐implantitis were enrolled (11 patients with 11 implants in each group). Two patients from the Ostim group and one from the Bio‐Oss group were withdrawn from the study because of severe pus formation 1 and 3 years after initial treatment, respectively. These patients were re‐treated with Er:YAG laser decontamination and Bio‐Oss with resorbable barriers but the outcome of these interventions was not reported (Analysis 4.1). There were no baseline imbalances for the type of implants, implant position, type of defects, plaque, BOP, PPD, PAL and REC. Results are presented at 6 months, 1, 2 and 4 years after treatment. Four years after treatment, there was a statistically significant difference for PAL (1.4 mm; 95% CI 0.24 to 2.56; Analysis 4.2) and PPD (1.4 mm; 95% CI 0.81 to 1.99; Analysis 4.3) changes in favour of the Bio‐Oss group. There were no statistically significant differences for changes in REC at any of the time intervals (Analysis 4.4). No implant failure or complication occurred in any of the groups. This trial was judged to be at high risk of bias (Figure 2).
Surface debridement with laser versus plastic curettes and saline solution before bone augmentation. One study (Schwarz 2011) of parallel group design compared surface debridement using a Er:YAG laser versus plastic curettes plus cotton pellets soaked in saline solution followed by a thorough irrigation with saline solution, after flap surgery, granulation tissue removal, and implantoplasty at buccally and supracrestally exposed implant surfaces of patients having at least one implant with an infrabony component deeper than 3 mm. Thirty‐two patients with 36 implants affected by peri‐implantitis were randomised to Er:YAG laser (16 patients with 19 implants) versus manual debridement (16 patients with 17 implants). One patient, from each group withdrew, for personal reasons. There were no baseline imbalances for smoking habits, type of implants, type of defects, plaque, BOP, PPD, PAL and REC. All patients experienced flap exposure during healing, though not associated with infective complications and one patient from an unknown group had a slight pigmentation of the peri‐implant soft tissues possibly caused by residual titanium particles (Analysis 6.1). Six‐months after treatment there were neither implant failures nor statistically significant differences for PAL gain (Analysis 6.2), PPD reduction (Analysis 6.3) and soft tissue recessions (Analysis 6.4). This trial was judged to be at high risk of bias (Figure 2).
4.1. Analysis.
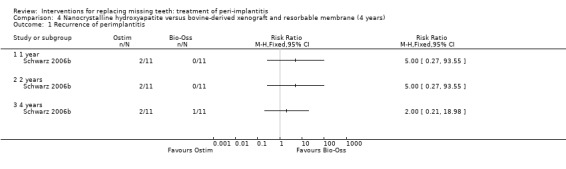
Comparison 4 Nanocrystalline hydroxyapatite versus bovine‐derived xenograft and resorbable membrane (4 years), Outcome 1 Recurrence of perimplantitis.
4.2. Analysis.
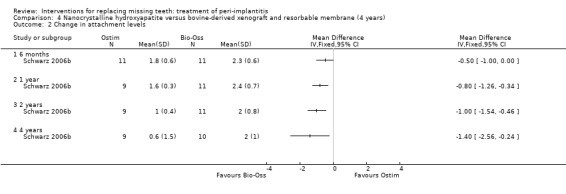
Comparison 4 Nanocrystalline hydroxyapatite versus bovine‐derived xenograft and resorbable membrane (4 years), Outcome 2 Change in attachment levels.
4.3. Analysis.
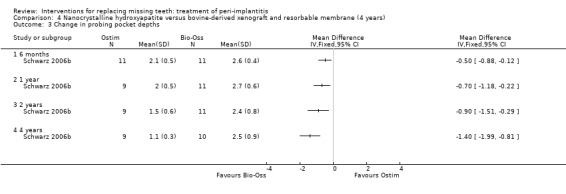
Comparison 4 Nanocrystalline hydroxyapatite versus bovine‐derived xenograft and resorbable membrane (4 years), Outcome 3 Change in probing pocket depths.
4.4. Analysis.
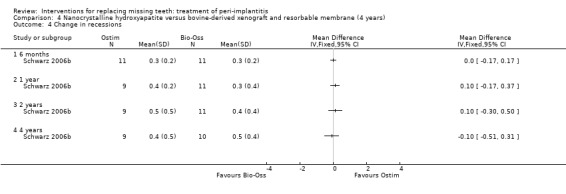
Comparison 4 Nanocrystalline hydroxyapatite versus bovine‐derived xenograft and resorbable membrane (4 years), Outcome 4 Change in recessions.
6.1. Analysis.
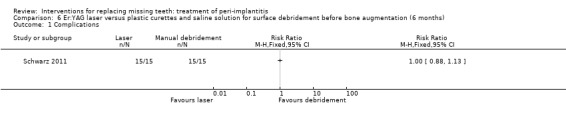
Comparison 6 Er:YAG laser versus plastic curettes and saline solution for surface debridement before bone augmentation (6 months), Outcome 1 Complications.
6.2. Analysis.
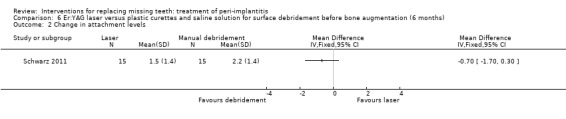
Comparison 6 Er:YAG laser versus plastic curettes and saline solution for surface debridement before bone augmentation (6 months), Outcome 2 Change in attachment levels.
6.3. Analysis.

Comparison 6 Er:YAG laser versus plastic curettes and saline solution for surface debridement before bone augmentation (6 months), Outcome 3 Change in probing pocket depths.
6.4. Analysis.
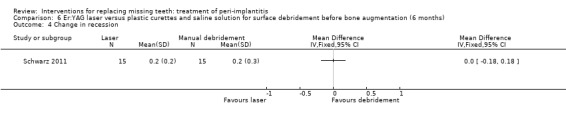
Comparison 6 Er:YAG laser versus plastic curettes and saline solution for surface debridement before bone augmentation (6 months), Outcome 4 Change in recession.
5) Adjunctive treatments to surgical interventions: one trial (Romeo 2005).
Adjunctive implant surface smoothening to systemic antibiotics plus resective surgery plus two different local antibiotics. One study (Romeo 2005) of parallel group design compared implant surface smoothening versus no smoothening after systemic antibiotics, resective surgery and local antibiotics. Ten patients were originally included in the implant surface modification group and eight in the control group, however, a patient was treated in a split‐mouth fashion in both groups and we randomly excluded that patient from one group, therefore there were nine patients (16 implants) in the smoothening group and eight (16 implants) in the no smoothening group. There were no baseline imbalances for plaque, marginal bleeding, PPD, PAL and REC. No withdrawals and no complications occurred in any of the groups. After 2 years, two implants failed in one patient of the no smoothening group, and none in the smoothening group, however, this was not statistically significant (Analysis 5.1). This trial was judged to be at high risk of bias (Figure 2).
5.1. Analysis.
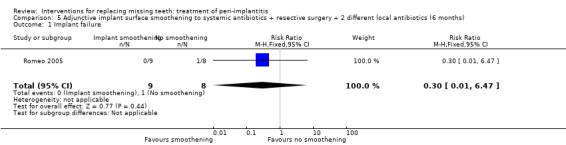
Comparison 5 Adjunctive implant surface smoothening to systemic antibiotics + resective surgery + 2 different local antibiotics (6 months), Outcome 1 Implant failure.
No 'Summary of Findings' table has been constructed as there were various trials investigating different interventions, without any meaningful meta‐analysis.
Discussion
Peri‐implantitis is one of the complications affecting dental implants. Its real incidence is probably underestimated and might be influenced by various factors including the characteristics of the implant surface and design (Esposito 2007). If left untreated is very likely that peri‐implantitis will lead to the failure of the affected implant(s) (Esposito 1999). This topic has grown in importance in the dental implant research agenda possibly because of the increased number of dental implants being placed and their longer follow‐up periods which inevitably means more cases of peri‐implantitis.
Six out of nine included studies were considered to be of high risk of bias. We shall briefly describe some issues which might help readers and investigators to critically evaluate randomised controlled trials (RCTs) in this area. (1) Inclusion criteria. In several trials the initial degree of pathology (in particular the marginal bone loss) around the implants was not accurately described (Romeo 2005; Schwarz 2005). If these parameters are not accurately described it will be difficult to compare trials or to extrapolate the study findings to other populations of interest. This might be particularly important in those situations in which a different degree of pathology may influence the prognosis and the therapy planning. Another four trials (Renvert 2004; Renvert 2008; Renvert 2009; Sahm 2011) had to be excluded since they clearly included an unknown quota of patients affected by peri‐implant mucositis and not by frank peri‐implantitis. The response to treatment and the prognosis of patients affected by peri‐implant mucositis and peri‐implantitis could be different, the former having a better response/prognosis since treated when the disease was at its early stages. (2) Exclusion criteria. Some of the trials adopted too strict exclusion criteria. For instance the exclusion of smokers, of subjects lacking of attached mucosa (this could be quite difficult to evaluate when the peri‐implant marginal tissues are very inflamed, as for many of the subjects in these trials), and patients with systemic diseases that could influence the outcome of the therapy, may limit the extrapolations of the findings to a broader population. (3) Outcome measures. No trial clearly stated that implant failure was the main outcome measure, though information on implant failures was presented or provided by various authors. Among the secondary outcomes it may be that marginal bone level changes provide the most reliable information on the progression of the disease. This was used only in three trials (Karring 2005; Romeo 2005; Renvert 2011), though we were unable to use the data in the way they were presented. No trial evaluated outcome measures such as patient preference or aesthetic outcomes with the exception on data on soft tissue recessions. (4) Interventions. A lot of different interventions or combination of interventions were investigated. Even more therapy variations are currently used in clinical practice. However, if too many treatments are used at the same time, it could be very difficult to discriminate between useful and ineffective ones. In addition, operative procedures become more complicated, treatment time become longer, costs can increase considerably without necessarily providing tangible improvements for the patient. Studies should test those procedures believed to be the most effective and should not use whatever is available to obtain the 'ideal' results, particularly when there is not strong evidence about which could be the most effective interventions. The majority of the trials were sponsored by manufacturers of antibiotics (Büchter 2004), air‐abrasive devices (Karring 2005; Renvert 2011), lasers (Schwarz 2005; Schwarz 2006a; Renvert 2011; Schwarz 2011), and biomaterials (Schwarz 2006b; Schwarz 2011), therefore there might be some commercial 'pressure' to evaluate some interventions and not others. (5) Sample sizes. The number of patients included in the various trials was very small. While it is true that it is not easy to collect large number of patients affected by peri‐implantitis, it should be remembered that existing differences of efficacy among different interventions may not be detected by small sample sizes, therefore conclusions of the majority of these studies are in fact inconclusive. This is however a widespread problem in dentistry. (6) Randomisation and blinding. More efforts should be invested at the protocol design stage to limit bias by using adequate randomisation and allocation concealment procedures as well as blinding at least of the outcome assessors. Trials judged to be at high risk of bias can be less reliable and even misleading in their results, particularly when the proponents have 'a priori very clear ideas' of which are the most reliable interventions. (7) Follow‐up. This question cannot be answered by short‐term follow‐up trials. Peri‐implantitis progresses over the years and years are required to see whether the intervention was effective in saving the implants. However, the majority of the included trials had a follow‐up shorter or up to 6 months. Only two trials (Romeo 2005; Schwarz 2006b), presented data at 2 and 4 years, respectively. (8) Statistical analysis. The patients and not the implants should be the unit of the statistical analyses. Two or more implants in the same mouth are not independent from each other, but are depending on numerous 'patient' factors such as the level of oral hygiene, the type of bacterial flora, the design of the prosthesis, the immunocompetent characteristics and habits (i.e. smoker, etc.) of the subject, etc. The trials should be designed as a split‐mouth or parallel group, or the analysis should take the clustering of the implants within patient into account. One way of doing this would be to use multilevel modelling. (9) Drop outs, withdrawals and failures. There is a clear difference between drop outs, withdrawals and recurrence of peri‐implantitis. Some authors systematically withdrew from the study patients having recurrence of peri‐implantitis (Schwarz 2005; Schwarz 2006a; Schwarz 2006b). These patients were re‐treated and this is obviously correct, but the outcome of the re‐treatment was not presented any longer in the original reports. Recurrence of the diseases means that the treatment was not successful according to certain set parameters, however, if they are considered as drop outs or withdrawals, i.e. excluded from the calculations, results will be biased. When drop outs or withdrawals occur, it is important that the authors clearly describe from which group they occurred and for which reason, since the reason of the drop out/withdrawals may again influence the interpretation of the results.
After these preliminary considerations we can try to evaluate the results of the included trials and their potential impact on clinical practice. Seven out of the nine included studies did not display any statistically significant difference among the tested interventions, but since the sample sizes were small, the absence of the difference could be either true or hidden by an insufficient sample size. So it is difficult to draw definitive conclusions.
In four trials (Tang 2002; Karring 2005; Schwarz 2005; Schwarz 2006a) the control therapy which basically consisted of a simple subgingival mechanical debridement seemed to be sufficient to achieve short‐term results similar to more complex and expensive therapies. In particular two studies with identical design (Schwarz 2005; Schwarz 2006a) compared Er:YAG laser versus manual debridement and chlorhexidine irrigation/gel. Forty patients with 72 implants affected by peri‐implantitis were enrolled (20 patients with 36 implants in each group). Three patients with six implants from the debridement group were withdrawn due to persisting pus formation 4 to 12 weeks after treatment and were treated with the Er:YAG laser. The meta‐analysis for recurrence of peri‐implantitis displayed no statistically significant differences though trends indicated that early recurrence was less likely with laser treatment, however after 1 year all patients were discontinued from the trial (Schwarz 2006a) due to increased bleeding on probing (BOP) values and slight deterioration of mean PAL. All patients were therefore re‐treated with the laser and subjected to bone augmentation procedures. Unfortunately no data were presented, but this may indicate that both plastic curettes and the laser could have been rather ineffective 1 year after treatment despite what appeared to be a good supragingival plaque control. In another trial (Renvert 2011), at baseline about out one third of the patients presented pus on probing. Six months after non‐surgical treatments with Perio‐Flow, an air‐abrasive device, or an Er:YAG laser, 11% of the patients had still pus on probing. These examples emphasise the need for long‐term trials and the need for also publishing negative results. Therefore the actual results of those trials are difficult to interpret and more studies are definitively needed to evaluate which could be the most effective interventions in the long term.
Only two trials showed some statistically significant differences. One investigation (Büchter 2004) showed, after 4 months, that the adjunctive use of a local antibiotic (doxycycline; Atridox) to manual debridement, improved PAL and PPD of about 0.6 mm. The patients included in this trial were affected by a severe form of peri‐implantitis (bone loss exceeding 50% of the implant length at baseline). Even though an overall 0.6 mm of improvement for PAL and PPD is a positive finding, although difficult to be noticed clinically, the follow‐up was limited to 4 months which is definitively too short to provide useful indications of the outcome of the interventions. The other study (Schwarz 2006b), showing a significant difference, compared two bone augmentation techniques at implants with peri‐implant infrabony defects superior to 3 mm: a resorbable nanocrystalline hydroxyapatite (Ostim) and a bovine‐derived xenograft with a resorbable barrier (Bio‐Oss). All augmentation procedures were conducted without prophylactic antibiotic coverage and no complications were reported, however, two patients from the Ostim group and one from the Bio‐Oss group were withdrawn from the study because of severe pus formation 1 and 3 years after initial treatment, respectively. Four years after treatment, both procedures significantly improved PAL and PPD (PAL 0.6 mm and 2.0 mm; PPD 1.1 and 2.5 mm for Ostim and Bio‐Oss, respectively). Unfortunately data of radiographic bone level assessments were not given, therefore it is difficult to fully interpret the results. The combination between a bovine‐derived xenograft and resorbable barrier seemed to provide better results (about 1.4 mm more gain for PAL and in PPD reduction). The different chemical stability characteristics of the bone substitutes could provide a possible explanation for the different clinical outcome. It may be speculated that an almost non‐resorbable anorganic bovine bone could be better suited to fill and remain stable over time in infrabony defects than a synthetic resorbable hydroxyapatite. This study suggests that it is possible to use bone augmentation procedures at infrabony defects induced by peri‐implantitis minimising soft tissue recessions (0.4 to 0.5 mm in both groups) and possibly maintaining an acceptable aesthetics, however, larger trials are needed to confirm these preliminary results. While not a single complication occurred in the previous trial, in a subsequent study by the same authors (Schwarz 2011) on GBR at implants affected by peri‐implantitis, all barriers became exposed though without developing any infection. Such differences are difficult to explain. The same study (Schwarz 2011) failed again to show any benefit of using an Er:YAGlaser over manual debridements with plastic curettes and cleaning with saline solution.
Another interesting study (Roos‐Jansåker 2007a) evaluated a bone substitute (Algipore, Friadent, Malmö, Sweden) with and without a resorbable barrier (Osseoquest, W L Gore & Associates) for treating implants (mostly Brånemark implants with a machined surface) affected by peri‐implantitis. Nineteen patients were included in each group though two patients from the group treated with the barrier died before the 1‐year control. Unfortunately patients were not allocated randomly and the first 19 patients were treated with the adjunctive use of a barrier, whereas the following 19 patients only with the bone substitute. Antibiotic coverage was initiated the day before surgery and continued for 10 days. After 1 year data of 29 implants from the bone substitute plus barrier group and of 36 implants from the bone substitute group were presented. Calculations were conducted at implant level ignoring the patient clustering effect, however, it was reported that there were no statistically significant differences between the different interventions and both therapies achieved a bone gain of 1.5 and 1.4 mm, respectively on intraoral x‐ray. It was also reported that after 2 weeks 44% of the implants treated with the membrane had a barrier exposure versus none of the bone substitute group. While this finding is not surprising, it is in contradiction with another study (Schwarz 2006b) in which no prophylactic antibiotic therapy was administered and not a single complication was reported in the group treated with the resorbable barriers. The same group reported a cases series of 12 patients (Roos‐Jansåker 2007b) who were treated with a bone substitute (Algipore) plus a resorbable membrane (Osseoquest). The implants were then covered by flaps and left healing submerged for 6 months. After 6 months the abutment was reconnected to the suprastructure. After 1 year a mean defect fill of 2.3 mm was obtained. Unfortunately the lack of a proper control group precludes us from understanding whether there could be any advantage when using a submerged healing technique.
We wish to stress that the lack of reliable evidence on which are the most effective interventions for treating peri‐implantitis should not be wrongly interpreted as that the currently used therapies may not be effective. There is some evidence that some treatments may be effective (Mombelli 1992; Haas 2000; Mombelli 2001; Leonhardt 2003; Romeo 2005; Schwarz 2006b), however, at present we do not know which interventions are the most effective, and for the interventions having similar degrees of effectiveness we do not know which one has less side effects, is simpler and cheaper to use.
Authors' conclusions
Implications for practice.
There is no reliable evidence suggesting which could be the most effective interventions for treating peri‐implantitis. This is not to say that currently used interventions are not effective. The use of adjunctive antibiotic therapy (Atridox) to manual debridement was associated with probing attachment level (PAL) and probing pocket depth (PPD) improvements in the range of 0.6 mm after 4 months in patients who had severe forms of peri‐implantitis (i.e. having lost at least 50% of the supporting bone around the implants) in one trial. The use of a bovine‐derived xenograft (Bio‐Oss) with a resorbable barrier (Bio‐Gide) was associated with PAL and PPD improvements of about 1.4 mm after 4 years in infrabony defects deeper than 3 mm when compared to nanocrystalline hydroxyapatite (Ostim) in one trial. In four other trials evaluating local antibiotics, the Vector system and a laser therapy, respectively, no statistically significant differences were observed when compared with subgingival debridement. Follow‐up longer than 1 year suggested recurrence of peri‐implantitis up to 100% of the treated cases for some of the tested interventions which suggests that repeated intervention may be needed. However, sample sizes were very small, therefore these findings have to be considered with great caution.
Implications for research.
More randomised controlled trials powered to detect a difference, using primary outcome measures such as implant failure and with a follow‐up of several years should be conducted. It may be easier to start assessing simpler interventions such as debridement with or without additional antibiotics and placebos using a double‐blind study design and then gradually testing more complex treatments. Clinical trials should evaluate the efficacy of plastic versus metal curettes and ultrasounds tips, implant surface smoothening with elimination of unsupported threads, and adjunctive local or systemic antibiotics. In the presence of deep peri‐implant infrabony defects both resective and regenerative procedures should be evaluated as well as various bone augmentation procedures. Such trials should be reported according to the Consolidated Standards of Reporting Trials (CONSORT) guidelines (www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 10 October 2019 | Review declared as stable | This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future. |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 12 September 2012 | Amended | Minor edit. |
| 5 December 2011 | New citation required but conclusions have not changed | Change in review authors; 2 new included studies; 1 new excluded study; no changes to conclusions. |
| 5 December 2011 | New search has been performed | Searches updated June 2011. |
| 6 May 2010 | New search has been performed | Searches updated January 2010. |
| 6 May 2010 | New citation required but conclusions have not changed | Change in review authors. 2 new excluded studies. |
| 11 June 2008 | Amended | Converted to new review format. |
| 14 April 2008 | New citation required and conclusions have changed | Substantive amendment. This update identified 2 new randomised controlled trials which were included. Conclusions were slightly changed. |
Notes
This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future.
Acknowledgements
We wish to thank Prof Paul Coulthard, Prof Adriano Piattelli, Dr Ioannis Kakisis and Dr Eleni Tzanetea for their contribution to previous versions of the current review; Anne Littlewood (Cochrane Oral Health) and Sylvia Bickley for their assistance with literature searching; Luisa Fernandez Mauleffinch, Philip Riley (Cochrane Oral Health) and Emma Tavender for their help with the preparation of this review; Zongdao Shi (West China School of Stomatology, Sichuan University) for translating the Chinese article; and Andre Büchter, Eva Karring, Stefan Renvert, Eugenio Romeo and Frank Schwarz for kindly providing us with additional information on their trials. We would also like to thank the following referees: Paul Brunton, Lee Hooper, Eva Karring, Ian Needleman, Michele Nieri, Frank Schwarz, and Robin Seymour.
Appendices
Appendix 1. MEDLINE (OVID) search strategy
1. exp Dental Implants/ 2. exp Dental Implantation/ or dental implantation 3. exp Dental Prosthesis, Implant‐Supported/ 4. ((osseointegrated adj implant$) and (dental or oral)) 5. dental implant$ 6. (implant$ adj5 dent$) 7. (((overdenture$ or crown$ or bridge$ or prosthesis or restoration$) adj5 (Dental or oral)) and implant$) 8. "implant supported dental prosthesis" 9. ("blade implant$" and (dental or oral)) 10. ((endosseous adj5 implant$) and (dental or oral)) 11. ((dental or oral) adj5 implant$) 12. OR/1‐11
The above search was run with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2009 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions version 5.0.2 (updated September 2009).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 2. Cochrane Oral Health's Trials Register search strategy
(dental‐implants OR "dental implant*" OR "oral implant*" OR dental‐implantation OR dental‐prosthesis‐implant‐supported OR "implant supported" OR "implant supported prosthesis" OR dental‐implantation‐endosseous‐endodontic OR "endosseous implant*" OR blade‐implantation OR "blade implant*" OR (implant* AND (oral OR dental)) or dental‐implantation‐subperiosteal OR "subperiosteal implant" OR (implant* AND overdenture*) OR ((overdenture* OR crown* OR bridge* OR prosthesis OR prostheses OR restoration*) AND ("dental implant*" OR "Oral implant" OR (zygoma* AND implant*))))
Appendix 3. CENTRAL search strategy
#1 DENTAL IMPLANTS explode all trees (MeSH)
#2 DENTAL IMPLANTATION explode all trees (MeSH)
#3 DENTAL PROSTHESIS IMPLANT‐SUPPORTED single term (MeSH)
#4 ((osseointegrat* near implant*) and (dental* or oral*))
#5 (dental next implant*)
#6 (implant* near dent*)
#7 dental‐implant*
#8 ((overdenture* near dental*) and implant*)
#9 ((overdenture* near oral*) and implant*)
#10 ((crown* near dental*) and implant*)
#11 ((crown* near oral*) and implant*)
#12 ((bridge* near dental*) and implant*)
#13 ((bridge* near oral*) and implant*)
#14 ((prosthesis near dental*) and implant*)
#15 ((prosthesis near oral*) and implant*)
#16 ((prostheses near dental*) and implant*)
#17 ((prostheses near oral*) and implant*)
#18 ((restoration* near dental*) and implant*)
#19 ((restoration* near oral*) and implant*)
#20 (implant next supported next dental next prosthesis)
#21 (blade next implant*)
#22 ((endosseous near implant*) and dental)
#23 ((endosseous near implant*) and oral*)
#24 ((dental* near implant*) or (oral* near implant*))
#25 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24)
Appendix 4. EMBASE search strategy
1. tooth implantation/
2. ((implant‐supported or implant$) adj support$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
3. ((osseointegrated adj implant$) and (dental or oral)).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
4. ((dental implant$ or dental‐implant or implant$) adj (dent$ or oral or tooth)).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
5. (((overdenture$ or crown$ or bridge$ or prosthesis or prostheses or restoration$) adj5 (dental or oral)) and implant$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
6. "implant supported dental prosthesis".mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
7. ("blade implant$" and (dental or oral or tooth or teeth)).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
8. ((endosseous adj5 implant$) and (dental or oral or tooth or teeth)).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
9. ((dental or oral or tooth or teeth) and implant$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
10. or/1‐9
The above search was run with the Cochrane Oral Health Group's search filter for isolating RCTs in EMBASE:
1. random$.ti,ab.
2. factorial$.ti,ab.
3. (crossover$ or cross over$ or cross‐over$).ti,ab.
4. placebo$.ti,ab.
5. (doubl$ adj blind$).ti,ab.
6. (singl$ adj blind$).ti,ab.
7. assign$.ti,ab.
8. allocat$.ti,ab.
9. volunteer$.ti,ab.
10. CROSSOVER PROCEDURE.sh.
11. DOUBLE‐BLIND PROCEDURE.sh.
12. RANDOMIZED CONTROLLED TRIAL.sh.
13. SINGLE BLIND PROCEDURE.sh.
14. or/1‐13
15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/
16. HUMAN/
17. 16 and 15
18. 15 not 17
19. 14 not 18
Data and analyses
Comparison 1. Metronidazole gel versus ultrasonic debridement (3 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in probing pocket depth | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Doxycycline gel versus manual debridement (4 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in attachment levels | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Change in probing pocket depth | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Er:YAG laser versus manual debridement and chlorhexidine irrigation/gel (6 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrence of perimplantitis | 2 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.06] |
| 3 Change in attachment levels | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Change in probing pocket depth | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Change in recession | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Nanocrystalline hydroxyapatite versus bovine‐derived xenograft and resorbable membrane (4 years).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence of perimplantitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 2 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 4 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in attachment levels | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 4 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in probing pocket depths | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 4 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in recessions | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 4 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Adjunctive implant surface smoothening to systemic antibiotics + resective surgery + 2 different local antibiotics (6 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Implant failure | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.01, 6.47] |
Comparison 6. Er:YAG laser versus plastic curettes and saline solution for surface debridement before bone augmentation (6 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Change in attachment levels | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Change in probing pocket depths | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Change in recession | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 7. Er:YAG laser versus air‐abrasive device.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in probing pocket depth | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Radiographic changes in bone levels | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Recurrence of peri‐implantitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Büchter 2004.
| Methods | 18‐week follow‐up, randomised, parallel group study. Unclear whether a blinded outcome assessor was used. No withdrawals. | |
| Participants | Patients having 1 implant affected by peri‐implantitis with bone loss exceeding 50% of the implant length on radiographs. Exclusion criteria were assumption of antibiotics over the last 3 months and allergy to doxycycline. Adults treated at the Department of Cranio‐Maxillofacial Surgery, University of Münster, Germany. 28 patients enrolled (14 in each group) and results given for 28. | |
| Interventions | Full mouth debridement and subgingival irrigation with 0.2% chlorhexidine 2 to 18 weeks before baseline examination. Both groups were then subjected to subgingival irrigation with 0.2% chlorhexidine and subgingival debridement with hand plastic instruments and sterilization of the abutments. The test group received 8.5% doxycycline hyclate (Atridox, Block Drug Corporation, Inc, Jersey City, New Jersey, USA) by means of a syringe with a blunt cannula in the peri‐implant sulcus. The trial included ITI SLA implants. | |
| Outcomes | Implant failure, recurrence of peri‐implantitis, PAL, PPD, BOP and complications/side effects at baseline and 18 weeks. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization being from a computer‐generated table". |
| Allocation concealment (selection bias) | Unclear risk | No information in the article. Authors replied that allocation was done before surgery but did not clarify if it had happened before or after scaling and how allocation was concealed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information in the article. Author's reply: "The measurements at 18 weeks were taken by another surgeon than the one who did the baseline measurements and the treatment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐out. |
| Selective reporting (reporting bias) | Low risk | All data for all outcomes seemed to be reported. |
| Other bias | Low risk | None apparent, though this trial was sponsored by the antibiotic manufacturer. |
Karring 2005.
| Methods | 6‐month follow‐up, randomised, split‐mouth design study. Blinded outcome assessor. No withdrawals. | |
| Participants | Patients having 2 non‐adjacent implants of the same brand affected by peri‐implantitis, showing BOP, with bone loss > 1.5 mm, but with a difference of bone loss < 1 mm among the paired implants. Exclusion criteria were assumption of antibiotics and subjection to debridement over the last 3 months. Adults treated at the Department of Periodontology and Oral Gerontology, University of Aarhus, Denmark. 11 patients enrolled and results given for 11. | |
| Interventions | Test sites were treated with the Vector system (Dürr Dental, Bietigheim‐Bissingen, Germany) with the straight or curved flexible Vector carbon fibre tip combined with aerosol spray of Vector fluid polish with hydroxyapatite particles (grain size approximately 10 µm) whereas the control sites with carbon fibre curettes. No local anaesthesia was provided and the instrumentation of each implant was carried out for 2 to 3 minutes. The same treatment was repeated after 3 months. Following each treatment patients received oral hygiene instructions. The trial included 2 pairs of Brånemark, 4 of ITI and 5 of Astra implants. | |
| Outcomes | Implant failure, recurrence of peri‐implantitis, PPD, bone levels, plaque and BOP and complications/side effects at baseline, 3 and 6 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "One implant was chosen at random (by choosing a sealed envelope out of a bunch of 22 identical envelopes) was treated by Vector system, while the other with submucosal debridement with a carbon fibre curettes". |
| Allocation concealment (selection bias) | Unclear risk | No information presented in the article. The author's reply failed to clarify the issue. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The same investigator (ESK) carried out the treatment of all the implants and made all the recordings at baseline examination, while another investigator who was unaware of the treatment delivered made all the follow‐up treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐out. |
| Selective reporting (reporting bias) | Unclear risk | The standard deviation of the difference between the 2 groups for the changes of the bone levels and PPD were missing, so we were unable to use the data. No reply from the author. |
| Other bias | Low risk | None apparent, though this trial was sponsored by the Vector manufacturer. |
Renvert 2011.
| Methods | 6‐month follow‐up, randomised, parallel group study. Blinded outcome assessor. No withdrawals. | |
| Participants | Patients having at least 1 implant affected by peri‐implantitis, showing bone loss > 3 mm, PPD > 5 mm, BOP and/or pus. Exclusion criteria were poorly controlled diabetes mellitus, use of anti‐inflammatory prescription medications, or antibiotics within the preceding 3 months or during the study, use of medications known to have an effect on gingival growth and subjects requiring prophylactic antibiotics. Adults treated at the at the Specialty Clinic for Periodontology, Kristianstad, Sweden. 42 patients enrolled (21 in each group) and results given for 42. | |
| Interventions | Before enrolment in the study, any periodontal lesions at remaining teeth were treated. Before the treatments, the supra‐structures were removed. One group was treated with an air‐abrasive device (Perio‐Flow®, EMS, Nyon, Switzerland). The nozzle was placed in the pocket and used for approximately 15 seconds in each position circumferentially around the implant. Careful attempts were made to cover the full circumference of the implant. The Perio‐Flow® device utilizes a 25 mm hydrophobic powder and a flexible tip allowing access to periodontal and implant pockets. The other group was treated with an Er:YAG laser (Key Laser 3 Perio, KaVo, Biberach, Germany) at an energy level of 100 mJ/pulse and 10 Hz (12.7 J/cm2) using a cone‐shaped sapphire tip. The instrument tip was used in a parallel mode using a semicircular motion around the circumferential pocket area of the implant. Routine local anaesthesia was used as needed.The trial included implants with machined (Brånemark System®) or medium rough surfaces (Astra). | |
| Outcomes | Implant failure, recurrence of peri‐implantitis, peri‐implant marginal bone level changes, PPD, plaque, BOP, bleeding index at baseline and 6 months. | |
| Notes | Authors provided data for patients outcomes: changes in probing pocket depth and recurrence of peri‐implantitis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomized allocation was performed using a computer software program (SPSS Inc., Chicago, IL, USA)." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A clinician not involved with the study sequenced the study subjects to the therapy allocated." No reply to further request of clarifications. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "When performing their study tasks, the study examiner (M. N.) and the therapist (C. L.) were not jointly present with the study subjects. Study subjects were instructed not to discuss therapy with the study examiner. The study examiner was unaware of study treatment allocations, and performed all clinical measurements." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs. |
| Selective reporting (reporting bias) | Unclear risk | Data data at patient level not presented. |
| Other bias | Low risk | None apparent. Interestingly the trial was sponsored by both biomaterial manufacturers whose products were compared. |
Romeo 2005.
| Methods | 2‐year follow‐up, randomised, parallel group study. Unblinded outcome assessor. No withdrawals. | |
| Participants | Patients having at least 1 implant affected by peri‐implantitis, showing bone loss, BOP and exudate with PD > 4 mm. Exclusion criteria were not specified. Adults treated at the University of Milan, Italy. 17 patients enrolled (9 in the test and 8 in the control group) and results given for 17. | |
| Interventions | Systemic antibiotics (amoxicillin 50 mg/kg/die for 8 days per os) were administered and subgingival debridement with a plastic scaler was performed prior to the initiation of the trial. An apically repositioned flap was performed. Vertical releasing incisions were made, the granulomatous tissue and the inner pocket epithelium were removed with hand curettes, and alveolar bone peaks were removed with bone chisels. A gel of metronidazole (Elyzol 25%, Cabon S.p.A, Milan, Italy) followed by a solution of tetracycline hydrochloride (Ambramicina, Sharper S.p.A, Milan, Italy) was rubbed on the contaminated implant surface for 3 minutes and then washed off with cold sterile physiological saline solution. The exposed TPS surface of the implants in the test group were polished with the following sequence of burs assembled on a handpiece rotating at 15,000 rpm: diamond/30 µm particle size egg‐shaped bur and diamond/15 µm particle size egg‐shaped bur (Komet, Gerb. Brasseler GmbH, Lemgo, Germany); Arkansas burs (Dura‐Green and Dura‐White, Shofu Inc, Kyoto, Japan); silicone polishers (Brownie, Greenie, Shofu Inc). The flaps were then apically repositioned. The trial included ITI TPS (Straumann, Waldenburg, Switzerland) dental implants (both full‐body and hollow screws). | |
| Outcomes | Implant failure, recurrence of peri‐implantitis, PAL, PPD, REC, plaque, bleeding index and complications/side effects at baseline, 6, 12 and 24 months. | |
| Notes | A second report was published in 2007 presenting the 3‐year radiographic results, but the numbers and initials of patients were not consistent with the first report. We enquired for explanations, but we had no reply. We did not use data presented in the second report. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The patients were randomized with a lottery assignment". Author replied that a coin was tossed at diagnosis. |
| Allocation concealment (selection bias) | High risk | No information in the article. We consider that the allocation was not concealed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No information in the article. Author replied that outcome assessor was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patient numbers and initial are different between the 2 publications. Statistical analysis at implant level and not at patient level, 1 patient treated as a split‐mouth patient, though the study appeared to be of parallel group design. |
| Selective reporting (reporting bias) | High risk | Several errors in data reporting. Patients numbers and initials are different between the 2 publications. |
| Other bias | High risk | Patients numbers and initial are different between the 2 publications. |
Schwarz 2005.
| Methods | 6‐month follow‐up, randomised, parallel group study. Blinded outcome assessor. 1 withdrawal in the chlorhexidine group due to persisting pus formation 8 weeks after treatment. Both the affected implants received the Er:YAG laser treatment. | |
| Participants | Patients having at least 1 implant affected by peri‐implantitis, showing bone loss, BOP and exudate with PD > 4 mm. Exclusion criteria were assumption of antibiotics over the last 3 months; treatment for peri‐implantitis over the last 12 months; absence of peri‐implant keratinized mucosa; regular smokers, hollow cylinder implants, and any systemic diseases that could influence the outcome of the therapy. Adults treated in various universities and hospitals in Germany and Switzerland. 20 patients enrolled (10 in each group) and results given for 19. | |
| Interventions | Oral hygiene instructions 2 to 4 times and supragingival cleaning with rubber caps and polishing paste were given prior to the initiation of the trial. Er:YAG laser (Key 3, KaVo, Biberach, Germany) emitting a pulsed infrared radiation at a wavelength of 2.94 µm. The laser parameters were set at 100 mJ/pulse, 10 pps, and pulse energy at the tip was approximately 85 mJ/pulse. The laser beam was guided onto the implant surface under water irrigation with a specially designed periodontal handpiece (2061, KaVo, Biberach, Germany) and a cone‐shaped glass fibre tip emitting a radial and axial laser beam. The fibre tip was guided in a circular motion from coronal to apical parallel to the implant surface in contact mode. In the control group manual debridement was performed with plastic curettes followed by pocket irrigation with 0.2% chlorhexidine and subgingival application of 0.2% chlorhexidine gel, followed by 2 weeks of mouthrinses with 0.2% chlorhexidine, twice a day for 2 minutes. For both groups, instrumentation was carried out until the operator felt that the implant surfaces were adequately debrided, on the average 6 minutes per implant. The trial included implants with TPS and SLA surfaces. | |
| Outcomes | Implant failure, recurrence of peri‐implantitis, PAL, PPD, REC, plaque, BOP and complications/side effects at baseline, 3 and 6 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Before treatment the patients were randomly assigned to the treatment groups. Randomization was performed by coin toss". |
| Allocation concealment (selection bias) | Unclear risk | No information in the article. Author's reply failed to clarify the issue. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All measurements were made at six aspects per implants by one blinded and previously calibrated investigator. All treatments were performed by the same experienced surgeon". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 patient (2 implants) belonging to the mechanical debridement group was excluded due to severe pus formation. Those implants were successfully treated with laser but no measurements were provided. |
| Selective reporting (reporting bias) | High risk | Missing data for the patient excluded by the authors. |
| Other bias | Low risk | None apparent, though this trial was sponsored by the laser manufacturer. |
Schwarz 2006a.
| Methods | 1‐year follow‐up, randomised, parallel group study. Blinded outcome assessor. 2 withdrawals in the chlorhexidine group due to persisting pus formation 4 to 12 weeks after treatment. The affected implants received the Er:YAG laser treatment. | |
| Participants | Patients having at least 1 implant affected by peri‐implantitis, showing bone loss, BOP and exudate with PD > 4 mm. Exclusion criteria were assumption of antibiotics over the last 6 months; treatment for peri‐implantitis over the last 12 months; absence of peri‐implant keratinized mucosa; regular smokers, hollow cylinder implants, and any systemic diseases that could influence the outcome of the therapy. Adults treated in various universities and hospitals in Germany and Switzerland. 20 patients enrolled (10 in each group) and results given for 18. | |
| Interventions | Oral hygiene instructions 2 to 4 times and supragingival cleaning with rubber caps and polishing paste were given prior to the initiation of the trial. Er:YAG laser (Key 3, KaVo, Biberach, Germany) emitting a pulsed infrared radiation at a wavelength of 2.94 µm. The laser parameters were set at 100 mJ/pulse, 10 pps, and pulse energy at the tip was approximately 85 mJ/pulse. The laser beam was guided onto the implant surface under water irrigation with a specially designed periodontal handpiece (2061, KaVo, Biberach, Germany) and a cone‐shaped glass fibre tip emitting a radial and axial laser beam. The fibre tip was guided in a circular motion from coronal to apical parallel to the implant surface in contact mode. In the control group manual debridement was performed with plastic curettes followed by pocket irrigation with 0.2% chlorhexidine and subgingival application of 0.2% chlorhexidine gel, followed by 2 weeks of mouthrinses with 0.2% chlorhexidine, twice a day for 2 minutes. For both groups, instrumentation was carried out until the operator felt that the implant surfaces were adequately debrided, on the average 6 minutes per implant. The trial included implants with TPS and SLA surfaces. | |
| Outcomes | Implant failure, recurrence of peri‐implantitis, PAL, PPD, REC, plaque, BOP and complications/side effects at baseline, 3, 6 and 12 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly assigned to the following test and the control groups according to a computer‐generated protocol (RandList, DatInf GmbH, Tubingen, Germany)". |
| Allocation concealment (selection bias) | Unclear risk | No information in the article. Author's reply failed to clarify the issue. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No information in the article. The author replied that the assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between 4 and 12 weeks following treatment 2 patients (4 implants) of the mechanical debridement group were excluded due to severe pus formation. They were treated with laser as in the other group. No data were presented regarding the outcomes of the their re‐treatments. |
| Selective reporting (reporting bias) | High risk | Pre and postoperative radiographs at baseline and 12 months were taken and evaluated but there was no description of how they were assessed nor data apart from an approximate subjective quantification. Missing data for the patients excluded by the authors. |
| Other bias | Low risk | None apparent, though this trial was sponsored by the laser manufacturer. |
Schwarz 2006b.
| Methods | 4‐year follow‐up, randomised, parallel group study. Blinded outcome assessor. 3 patients were withdrawn: 2 1 year after treatment from the nanocrystalline hydroxyapatite group and 1 3 years after treatment from the anorganic bovine bone group because of recurrence of peri‐implantitis. | |
| Participants | Patients having at least 1 implant affected by peri‐implantitis with an infrabony component > 3 mm and PD > 6 mm. Exclusion criteria were absence of peri‐implant keratinised mucosa, regular smokers, hollow cylinder implants, evidence of overload and any systemic diseases that could influence the outcome of the therapy. Adults treated in Heinrich Heine University, Düsseldorf, Germany. 22 patients enrolled (11 in each group) and results given for 19. | |
| Interventions | Patients were previously treated with a single‐course non‐surgical instrumentation with plastic curettes followed by pocket irrigation with 0.2% chlorhexidine and subgingival application of chlorhexidine gel. After flap elevation and removal of granulation tissues, implants were debrided using plastic curettes and rinsed with saline solution. Sites were filled or with nanocrystalline hydroxyapatite (Ostim, Heraeus Kulzer, Hanau, Germany) injected using a ready‐to‐use paste in a syringe or with a bovine‐derived xenograft (Bio‐Oss, Geistlich, Wolhusen, Switzerland, particle size 0.25 to 1 mm) in combination with a resorbable collagen barrier (Bio‐Gide, Geistlich) of porcine origin. The trial included Brånemark System® (Nobel Biocare, Göteborg, Sweden), Camlog Screw Line® (Camlog, Wimsheim, Germany), KSI Bauer Schraube (Bad Nauheim, Germany), ITI both SLA and TPS (Straumann, Waldenburg, Switzerland), Spline Twist MTX and Tapered Screw Vent (Zimmer Dental, Freiburg, Germany), and ZL‐Duraplant Ticer (ZL Microdent, Breckerfeld, Germany) implants. | |
| Outcomes | Implant failure, recurrence of peri‐implantitis, PAL, PPD, REC, plaque, BOP and complications/side effects at baseline and 6, 12, 18, 24, 36 and 48 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The defects were randomly assigned before surgery to the following test and control groups according to a computer‐generated protocol (RandList, DatInf, GmbH, Germany)". |
| Allocation concealment (selection bias) | Unclear risk | No information in the article. Author's reply failed to clarify the issue. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All measurements were made at 6 aspects per implants by one blinded and previously calibrated investigator (K. B.). All treatments were performed by the same experienced surgeon (F. S.)". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 patients from the Ostim group and 1 from the Bio‐Oss group were withdrawn from the study because of severe pus formation 1 and 3 years after initial treatment, respectively. They were treated with Er:YAG laser decontamination and augmented Bio‐Oss and Bio‐Gide barriers as in the control group. No data were presented regarding the outcomes of the their re‐treatments. |
| Selective reporting (reporting bias) | High risk | Pre and postoperative radiographs at 6 and 24 months were taken and evaluated but there was no description of how they were assessed nor data apart from an approximate subjective quantification. Missing data for the patient excluded by the authors. |
| Other bias | Low risk | None apparent. Interestingly the trial was sponsored by both biomaterial manufacturers whose products were compared. |
Schwarz 2011.
| Methods | 6‐month follow‐up, randomised, parallel group study. Blinded outcome assessor. 2 patients, 1 from each group, refused to continue participation in the trial because of personal reasons. | |
| Participants | Patients having at least 1 implant affected by peri‐implantitis with an infrabony component > 3 mm together with a suprabony component > 1 mm with PPD > 6 mm. Exclusion criteria were less than 2 mm of peri‐implant keratinised mucosa, heavy smokers (more than 10 cigarettes/day), hollow cylinder implants, evidence of overload, presence of overhangings or margins, poor oral hygiene and any systemic diseases that could influence the outcome of the therapy. Adults treated in Heinrich Heine University, Düsseldorf, Germany. 32 patients enrolled (16 in each group) and results given for 30. | |
| Interventions | Patients were treated with access flap surgery, granulation tissue removal, and implantoplasty at buccally and supracrestally exposed implant surfaces. The remaining intra‐bony defects were randomly allocated to 2 different surface treatments: Er:YAG laser (Elexxion Delos, Elexxion AG, Radolfzell, Germany) emitting a pulsed infrared radiation at a wavelength of 2.940 nm versus plastic curettes plus cotton pellets soaked in saline followed by a thorough irrigation with saline solution. | |
| Outcomes | Implant failure, peri‐implant marginal bone level changes, PAL, PPD, REC, plaque, BOP and complications/side effects at baseline and 6 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "According to a computer‐generated protocol (RandList DatInf GmbH, Tubingen, Germany), all defect sites were randomly assigned to the following methods of surface decontamination..." |
| Allocation concealment (selection bias) | Unclear risk | No information provided in the article. No reply from the author. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All measurements were made at six aspects per implant: mesiovestibular (mv), midvestibular (v), distovestibular (dv), mesiooral (mo), midoral (o), and distooral (do) by one blinded and previously calibrated investigator. Pre and postoperative non‐standardized radiographs at 6 months were taken with the long cone paralleling technique and evaluated descriptively by the same blinded investigator (N.S.)." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two drop‐outs, one from each group and reason given. |
| Selective reporting (reporting bias) | High risk | Radiographic marginal bone level changes data considered by the trial authors as a primary outcome were not provided. |
| Other bias | Low risk | None apparent, though this trial was sponsored by the laser and the GBR biomaterials manufacturers. |
Tang 2002.
| Methods | 12‐week follow‐up, randomised, parallel group study. Unclear whether outcome assessors were blinded. 3 withdrawals at 12 weeks for unknown reasons, 1 from the metronidazole group and 2 from the ultrasonic debridement group. | |
| Participants | Patients in good general health having 1 stable implant affected by peri‐implantitis (BOP; PPD equal to or < 6 mm; bone loss < 4 mm). Exclusion criteria were assumption of antibiotics over the last 3 months and antimicrobial mouthwashes over the last month. Adults treated at the Centre for Dental Implantation, Peking University, China. 30 patients enrolled (possibly 15 in each group) and results given for 27. | |
| Interventions | Metronidazole gel 25% injected into the pocket at a depth of 3 mm versus ultrasonic debridement with carbon fibre tip inserted 1 to 2 mm into the gingival sulcus at the lowest power for 15 seconds around IMZ or Frialit‐2 implants. Both interventions were repeated a second time 1 week after. | |
| Outcomes | Implant failure, recurrence of peri‐implantitis, PPD, BOP, plaque index, BANA test for bacterial quantification (Periochek, Sunstar, Japan) and side effects at baseline, 1, 2, 6 and 12 weeks. 12‐week data used. We could not calculate change data as the standard deviations for these were not given. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information in the text. No reply from the author. |
| Allocation concealment (selection bias) | Unclear risk | No information in the text. No reply from the author. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information in the text. No reply from the author. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information on withdrawals. |
| Selective reporting (reporting bias) | High risk | We could not calculate change data as the standard deviations for these were not given. No reply from the author. |
| Other bias | Low risk | None apparent. |
BOP = bleeding on probing PAL = probing attachment level PPD = probing pocket depth REC = gingival recession
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bach 2000 | No data presented. Written to authors but no reply. |
| Renvert 2004 | The majority of the included patients were affected by peri‐implant mucositis and not by peri‐implantitis as specified by the author in his reply to our request for information. |
| Renvert 2008 | The majority of the included patients were affected by peri‐implant mucositis and not by peri‐implantitis as specified by the author in his reply to our request for information. |
| Renvert 2009 | An unknown quota of the included patients were affected by peri‐implant mucositis and not by peri‐implantitis (mean bone loss at study initiation of 1.5 mm + 1.2). |
| Sahm 2011 | Patients with peri‐implant bone loss < 30% of the implant length and pockets > 4 mm were included in the trial. According to these inclusion criteria, a patient with 0.1 mm of bone loss would have been included even thought they were not actually effected by peri‐implantitis but by peri‐implant mucositis. Written to the authors for asking more details about their inclusion criteria but no reply to our letter. |
| Tarpey 1996 | Insufficient information presented. Written to authors but no reply. |
Differences between protocol and review
A new outcome measure was added: recurrence of peri‐implantitis.
Contributions of authors
Conceiving, designing and co‐ordinating the review (Marco Esposito (ME)). Developing search strategy and undertaking searches (ME). Screening search results and retrieved papers against inclusion criteria (ME, Maria Gabriella Grusovin (MG)). Appraising quality (ME, MG). Extracting data from papers (Helen Worthington (HW), ME, MG). Writing to authors for additional information (ME, HW). Data management for the review and entering data into RevMan (HW, ME, MG). Analysis and interpretation of data (ME, HW, MG). Writing the review (ME). Providing general advice on the review (MG). Performing previous work that was the foundation of current study (ME, HW).
Sources of support
Internal sources
Division of Dentistry, The University of Manchester, UK.
-
MAHSC, UK.
Cochrane Oral Health is supported by the Manchester Academic Health Sciences Centre (MAHSC) and the NIHR Manchester Biomedical Research Centre
External sources
-
British Orthodontic Society (BOS), UK.
The BOS have provided funding for Cochrane Oral Health Global Alliance (see www.ohg.cochrane.org).
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed are those of the authors and not necessarily those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Declarations of interest
From April 2011 Marco Esposito became a full‐time free‐lance consultant in dentistry specialising in implantology. Therefore he receives funding for conducting clinical trials and presenting the results of trials/Cochrane reviews at international dental meetings. This funding comes from universities, companies (in alphabetical order: Apollonia e Fama Implant, Biomax, Biomet 3i, Bioteck, Bone System, Branemark Integration, CMS Dental, Dentsply‐Friadent, Geistlich Pharma, Geass, Keystone Dental, MegaGen Implant, Mozo‐Grau, Nano Bridging molecules, Nobel Biocare, Ricerfarma, Saint Jude Medical, Southern Implants, Supercharched production, Techoss Dental Thommen Medical, Tutogen Medical, Zimmer Dental, Z‐Systems), scientific societies, publishing companies, and private dentists. This list of companies was provided by Marco on Friday 4th November 2011 and the funders will change all the time.
This is to certify that: a) Marco does not own stock in companies that produce products included in the reviews; b) he does not have patents on any of the products included in the reviews; c) his salary will not be affected by company sales of any of the products included in the reviews.
Marco’s authorship has been authorised by The Cochrane Collaboration Funding Arbiter (reference 071111/057: Oral Health Group).
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Büchter 2004 {published and unpublished data}
- Büchter A, Meyer U, Kruse‐Lösler B, Joos U, Kleinheinz J. Sustained release of doxycycline for the treatment of peri‐implantitis: randomised controlled trial. British Journal of Oral and Maxillofacial Surgery 2004;42(5):439‐44. [DOI] [PubMed] [Google Scholar]
Karring 2005 {published and unpublished data}
- Karring ES, Stavropoulos A, Ellegaard B, Karring T. Treatment of peri‐implantitis by the vector system. Clinical Oral Implants Research 2005;16(3):288‐93. [DOI] [PubMed] [Google Scholar]
Renvert 2011 {published data only}
- Renvert S, Lindahl C, Roos Jansaker AM, Persson GR. Treatment of peri‐implantitis using an Er:YAG laser or an air‐abrasive device: a randomized clinical trial. Journal of Clinical Periodontology 2011;38(1):65‐73. [DOI] [PubMed] [Google Scholar]
- Rutger Persson G, Roos Jansaker AM, Lindahl C, Renvert S. Microbiologic Results After Non‐Surgical Erbium‐Doped:Yttrium, Aluminum, and Garnet Laser or Air‐Abrasive Treatment of Peri‐Implantitis: A Randomized Clinical Trial. Journal of Periodontology 2011;82(9):1267‐78. [DOI] [PubMed] [Google Scholar]
Romeo 2005 {published data only}
- Romeo E, Ghisolfi M, Murgolo N, Chiapasco M, Lops D, Vogel G. Therapy of peri‐implantitis with resective surgery. A 3‐year clinical trial on rough screw‐shaped oral implants. Part I: clinical outcome. Clinical Oral Implants Research 2005;16(1):9‐18. [DOI] [PubMed] [Google Scholar]
- Romeo E, Lops D, Chiapasco M, Ghisolfi M, Vogel G. Therapy of peri‐implantitis with resective surgery. A 3‐year clinical trial on rough screw‐shaped oral implants. Part II: radiographic outcome. Clinical Oral Implants Research 2007;18(2):179‐87. [DOI] [PubMed] [Google Scholar]
Schwarz 2005 {published data only}
- Schwarz F, Sculean A, Rothamel D, Schwenzer K, Georg T, Becker J. Clinical evaluation of an Er:YAG laser for nonsurgical treatment of peri‐implantitis: a pilot study. Clinical Oral Implants Research 2005;16(1):44‐52. [DOI] [PubMed] [Google Scholar]
Schwarz 2006a {published and unpublished data}
- Schwarz F, Bieling K, Bonsmann M, Latz T, Becker J. Nonsurgical treatment of moderate and advanced periimplantitis lesions: a controlled clinical study. Clinical Oral Investigations 2006;10(4):279‐88. [DOI] [PubMed] [Google Scholar]
Schwarz 2006b {published and unpublished data}
- Schwarz F, Bieling K, Latz T, Nuersy E, Becker J. Healing of intrabony peri‐implantitis defects following application of a nanocrystalline hydroxyapatite (Ostim) or a bovine‐derived xenograft (Bio‐Oss) in combination with a collagen membrane (Bio‐Gide). A case series. Journal of Clinical Periodontology 2006;33(7):491‐9. [DOI] [PubMed] [Google Scholar]
- Schwarz F, Sahm N, Bieling K, Becker J. Surgical regenerative treatment of peri‐implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane: a four‐year clinical follow‐up report. Journal of Clinical Periodontology 2009;36(9):807‐14. [DOI] [PubMed] [Google Scholar]
- Schwarz F, Sculean A, Bieling K, Ferrari D, Rothamel D, Becker J. Two‐year clinical results following treatment of peri‐implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane. Journal of Clinical Periodontology 2008;35(1):80‐7. [DOI] [PubMed] [Google Scholar]
Schwarz 2011 {published data only}
- Schwarz F, Sahm N, Iglhaut G, Becker J. Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of peri‐implantitis: a randomized controlled clinical study. Journal of Clinical Periodontology 2011;38(3):276‐84. [DOI] [PubMed] [Google Scholar]
Tang 2002 {published data only}
- Tang Z, Cao C, Sha Y, Lin Y, Wang X. Effects of non‐surgical treatment modalities on peri‐implantitis. Chinese Journal of Stomatology 2002;37(3):173‐5. [PubMed] [Google Scholar]
References to studies excluded from this review
Bach 2000 {published data only}
- Bach G, Neckel C, Mall C, Krekeler G. Conventional versus laser‐assisted therapy of periimplantitis: a five‐year comparative study. Implant Dentistry 2000;9(3):247‐51. [DOI] [PubMed] [Google Scholar]
Renvert 2004 {published data only}
- Renvert S, Lessem J, Dahlén G, Lindahl C, Svensson M. Topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement of incipient peri‐implant infections: a randomized clinical trial. Journal of Clinical Periodontology 2006;33(5):362‐9. [DOI] [PubMed] [Google Scholar]
- Renvert S, Lessem J, Lindahl C, Svensson M. Treatment of incipient peri‐implant infections using topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement. Journal of the International Academy of Periodontology 2004;6(Suppl 4):154‐9. [PubMed] [Google Scholar]
Renvert 2008 {published data only}
- Renvert S, Lessem J, Dahlen G, Renvert H, Lindahl C. Mechanical and repeated antimicrobial therapy using a local drug delivery system in the treatment of peri‐implantitis: a randomized clinical trial. Journal of Periodontology 2008;79(5):836‐44. [DOI] [PubMed] [Google Scholar]
Renvert 2009 {published data only}
- Persson GR, Samuelsson E, Lindahl C, Renvert S. Mechanical non‐surgical treatment of peri‐implantitis: a single‐blinded randomized longitudinal clinical study. II. Microbiological results. Journal of Clinical Periodontology 2010;37(6):563‐73. [DOI] [PubMed] [Google Scholar]
- Renvert S, Samuelsson E, Lindahl C, Persson GR. Mechanical non‐surgical treatment of peri‐implantitis: a double‐blind randomized longitudinal clinical study. I: clinical results. Journal of Clinical Periodontololgy 2009;36(7):604‐9. [DOI] [PubMed] [Google Scholar]
Sahm 2011 {published data only}
- Sahm N, Becker J, Santel T, Schwarz F. Non‐surgical treatment of peri‐implantitis using an air‐abrasive device or mechanical debridement and local application of chlorhexidine: a prospective, randomized, controlled clinical study. Journal of Clinical Periodontology 2011;38(9):872‐8. [DOI] [PubMed] [Google Scholar]
Tarpey 1996 {published data only}
- Tarpey MV, Lynch E, Beighton D. Effect of Dentomycin on clinical and microbiological parameters of peri‐implantitis (Abs No 1311). Journal of Dental Research 1996;75(Special Issue):181. [Google Scholar]
Additional references
Brånemark 1977
- Brånemark PI, Hansson BO, Adell R, Breine U, Lindstrom J, Hallen O, et al. Osseointegrated Implants in the Treatment of the Edentulous Jaw. Experience from a 10‐year Period. Stockholm: Almqvist & Wiksell Int, 1977. [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Esposito 1999
- Esposito M, Hirsch J‐M, Lekholm U, Thomsen P. Differential diagnosis and treatment strategies for biologic complications and failing oral implants: a review of the literature. International Journal of Oral and Maxillofacial Implants 1999;14(4):473‐90. [PubMed] [Google Scholar]
Esposito 2007
- Esposito M, Murray‐Curtis L, Grusovin MG, Coulthard P, Worthington HV. Interventions for replacing missing teeth: different types of dental implants. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003815.pub3] [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [DOI] [PubMed] [Google Scholar]
Grusovin 2010
- Grusovin MG, Coulthard P, George P, Worthington HV, Esposito M. Interventions for replacing missing teeth: maintaining and recovering soft tissue health around dental implants. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD003069.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haas 2000
- Haas R, Baron M, Dörtbudak O, Watzek G. Lethal photosensitization, autogenous bone, and e‐PTFE membrane for the treatment of peri‐implantitis: preliminary results. The International Journal of Oral and Maxillofacial Implants 2000;15(3):374‐82. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Isidor 1996
- Isidor F. Loss of osseointegration caused by occlusal load of oral implants. A clinical and radiographic study in monkeys. Clinical Oral Implants Research 1996;7(2):143‐52. [DOI] [PubMed] [Google Scholar]
Leonhardt 2003
- Leonhardt A, Dahlen G, Renvert S. Five‐year clinical, microbiological, and radiological outcome following treatment of peri‐implantitis in man. Journal of Periodontology 2003;74(10):1415‐22. [DOI] [PubMed] [Google Scholar]
Mombelli 1992
- Mombelli A, Lang NP. Antimicrobial treatment of peri‐implant infections. Clinical Oral Implants Research 1992;3(4):162‐8. [DOI] [PubMed] [Google Scholar]
Mombelli 1999
- Mombelli A. Prevention and therapy of peri‐implant infections. Proceedings of the 3rd European Workshop on Periodontology. Implant Dentistry. Berlin, Germany: Quintessence Publishing Co., Inc, 1999:281‐303.
Mombelli 2001
- Mombelli A, Feloutzis A, Bragger U, Lang NP. Treatment of peri‐implantitis by local delivery of tetracycline. Clinical, microbiological and radiological results. Clinical Oral Implants Research 2001;12(4):287‐94. [DOI] [PubMed] [Google Scholar]
Pontoriero 1994
- Pontoriero R, Tonelli MP, Carnevale G, Mombelli A, Nyman SR, Lang NP. Experimentally induced peri‐implant mucositis. A clinical study in humans. Clinical Oral Implants Research 1994;5(4):254‐9. [DOI] [PubMed] [Google Scholar]
Roos‐Jansåker 2003
- Roos‐Jansåker AM, Renvert S, Egelberg J. Treatment of peri‐implant infections: a literature review. Journal of Clinical Periodontology 2003;30(6):467‐85. [DOI] [PubMed] [Google Scholar]
Roos‐Jansåker 2006
- Roos‐Jansåker AM, Lindahl C, Renvert H, Renvert S. Nine‐ to fourteen‐year follow‐up of implant treatment. Part II: presence of peri‐implant lesions. Journal of Clinical Periodontology 2006;33(4):290‐5. [DOI] [PubMed] [Google Scholar]
Roos‐Jansåker 2007a
- Roos‐Jansåker AM, Renvert H, Lindahl C, Renvert S. Surgical treatment of peri‐implantitis using a bone substitute with or without a resorbable membrane: a prospective cohort study. Journal of Clinical Periodontology 2007;34(7):625‐32. [DOI] [PubMed] [Google Scholar]
Roos‐Jansåker 2007b
- Roos‐Jansåker AM, Renvert H, Lindahl C, Renvert S. Submerged healing following surgical treatment of peri‐implantitis: a case series. Journal Clinical Periodontology 2007;34(8):723‐37. [DOI] [PubMed] [Google Scholar]
Sahrmann 2011
Schou 2004
- Schou S, Berglundh T, Lang NP. Surgical treatment of peri‐implantitis. International Journal of Oral and Maxillofacial Implants 2004;19(7):140‐9. [PubMed] [Google Scholar]
References to other published versions of this review
Esposito 2002
- Esposito M, Worthington HV, Coulthard P, Jokstad A. Interventions for replacing missing teeth: maintaining and re‐establishing healthy tissues around dental implants. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003069] [DOI] [PubMed] [Google Scholar]
Esposito 2003
- Esposito M, Worthington HV, Coulthard P, Thomsen P. Maintaining and re‐establishing health around osseointegrated oral implants: a Cochrane systematic review comparing the efficacy of various treatments. Periodontology 2000 2003;33:204‐12. [DOI] [PubMed] [Google Scholar]
Esposito 2004
- Esposito M, Worthington HV, Coulthard P. Interventions for replacing missing teeth: treatment of perimplantitis. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004970] [DOI] [PubMed] [Google Scholar]
Esposito 2006
- Esposito M, Grusovin MG, Coulthard P, Worthington HV. Interventions for replacing missing teeth: treatment of perimplantitis. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004970.pub2] [DOI] [PubMed] [Google Scholar]
Esposito 2008a
- Esposito M, Grusovin MG, Kakisis I, Coulthard P, Worthington HV. Interventions for replacing missing teeth: treatment of perimplantitis. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD004970.pub3] [DOI] [PubMed] [Google Scholar]
Esposito 2008b
- Esposito M, Grusovin MG, Coulthard P, Worthington HV. The efficacy of interventions to treat perimplantitis. A Cochrane systematic review of randomized controlled clinical trials. European Journal of Oral Implantology 2008;1:111‐25. [PubMed] [Google Scholar]
Esposito 2010
- Esposito M, Grusovin MG, Tzanetea E, Piattelli A, Worthington HV. Interventions for replacing missing teeth: treatment of perimplantitis. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD004970.pub4] [DOI] [PubMed] [Google Scholar]


