Abstract
Tenosynovial giant cell tumors (TGCT), are rare colony stimulating factor-1(CSF-1)-driven proliferative disorders affecting joints. Diffuse-type TGCT often causes significant morbidity due to local recurrences necessitating multiple surgeries. Imatinib mesylate (IM) blocks the CSF-1 receptor. This study investigated the long term effects of IM in TGCT. We conducted an international multi-institutional retrospective study to assess the activity of IM: data was collected anonymously from individual patients with locally advanced, recurrent or metastatic TGCT. Sixty-two patients from 12 institutions across Europe, Australia and the United States were identified. Four patients with metastatic TGCT progressed rapidly on IM and were excluded for further analyses. Seventeen of 58 evaluable patients achieved complete response (CR) or partial response (PR). One- and five-year progression-free survival rates were 71% and 48%, respectively. Thirty-eight (66%) patients discontinued IM after a median of 7 (range 1–80) months. Reported adverse events in 45 (78%) patients were among other edema (48%) and fatigue (50%), mostly grade 1–2 (89%). Five patients experienced grade 3–4 toxicities. This study confirms, with additional follow-up, the efficacy of IM in TGCT. In responding cases we confirmed prolonged IM activity on TGCT symptoms even after discontinuation, but with high rates of treatment interruption and additional treatments.
Subject terms: Outcomes research, Targeted therapies
Introduction
Tenosynovial giant-cell tumor (TGCT), historically known as pigmented villonodular synovitis (PVNS), is a rare, at times locally aggressive neoplasm affecting the joints or tendon sheaths in young adults. It is most common around large joints such as the knees, ankles and hips1,2. Known subtypes are localized and diffuse TGCT. The localized subtype comprises a single nodule and has a favorable course while the diffuse subtype involves the synovial lining as well as surrounding structures and is associated with a significant risk of recurrence (>50% depending on follow up times), despite being a benign neoplasm2–4. Metastatic forms have been described, but seem to occur very rarely5,6.
Surgical resection is the primary treatment for both subtypes. However, diffuse TGCT is difficult to remove completely and often requires a total synovectomy, or at times a joint replacement, or rarely even amputation1,2,7. In patients with extensive and/or recurrent TGCT, other available treatment modalities include radiation synovectomy8, external beam radiation therapy9, and cryosurgery10. Their therapeutic value has only been assessed in retrospective, in most cases single center series and their long term side effects and complications are poorly described11.
Recurrent TGCT is rarely lethal, but frequently becomes a debilitating chronic illness with substantial morbidity to the joints and quality of life impairment, caused by the disease itself and the multiple treatments2,12.
In TGCT, a neoplastic clone constitutes a subpopulation (2–16%)13 of cells that overexpress colony-stimulating factor-1 (CSF-1). A t(1;2) translocation that links the CSF1 gene on chromosome 1p13 to the COL6A3 gene on chromosome 2q35 has been described and is believed to be responsible for the overproduction of CSF1 by neoplastic cells13,14. Inhibition of CSF1/CSF-1 receptor (CSF-1R) signaling has shown efficacy in the treatment of locally advanced and recurrent diffuse TGCT15–17.
Imatinib mesylate (IM) inhibits the CSF-1R kinase among other kinases17. It has been shown that inhibition of CSF-1R by imatinib is competitive with ATP, with a Ki value of 120 nmol/L18. We have previously reported on the efficacy of IM in TGCT. In the present study we provide long term follow-up on these initial patients and data on 33 additional consecutive patients.
Methods
This retrospective study was conducted at 12 referral centers across Europe (9 institutions), the United States of America (2 institutions), and Australia (1 institution). The file of all patients with locally advanced, recurrent or metastatic TGCT, treated with IM were reviewed. Patient information was extracted from individual patients’ files at each institution by the local investigators and was provided in an anonymous form for final analyses. Histopathologic examination was performed at center of origin by pathologists with extensive experience in mesenchymal tumors. Response was measured using version 1.0 of Response Evaluation Criteria in Solid Tumors (RECIST). Data were described using percentages for qualitative variables and medians with ranges for continuous variables.
Patients were not treated following a fixed regimen. The study protocol and retrospective analysis was approved by the Ethics Committee in Lyon (Committee for the Protection of Individuals, Sud-Est IV, Lyon, France – L10-153 dated 9 December 2010) and was carried out in accordance with the applicable rules concerning the review of research ethics committees. Patients provided written informed consent to treatment with ‘off-label’ medication, for research review and analysis of medical records. Treatment decision was left to the treating physician. The study was conducted in accordance with ethical requirements that differed per country. National investigators dealt with it according to standard practice. All 12 centers at which the study was carried out approved access to the data.
Survival was plotted using the Kaplan-Meier method. Progression-free survival (PFS) was calculated from the date IM was started to the date of disease progression or death. The time to treatment failure (TTF) was calculated from the date IM was started to the date it was stopped because of toxicity, disease progression, or death, whichever occurred first. For patients with a surgical resection or other additional therapy after treatment with IM, PFS and TTF were censored at the time of surgery. Disease specific survival was calculated from the date IM was started to the date of death due to TGCT. Symptomatic response was defined as improvement of pain and/or joint function in patients who had symptoms at baseline. All statistical analyses were performed using R version 3.4.0 (R Foundation, Vienna, Austria) with packages ‘ggplot2’, ‘rms’, and ‘survival’.
Results
Patients
A total of 62 patients with histopathologically proven TGCT treated with imatinib were identified, their main characteristics are described in Table 1. Briefly, median age at diagnosis was 39 (interquartile range (IQR) 31–53) years and 45 (IQR 36–56) years at start of treatment with IM, the majority of patients were female (N = 39, 63%), and the knee (N = 35, 56%) was the most commonly affected joint (Table 1). At start of IM treatment, three (5%) patients had biopsy proven metastatic disease, 15 (24%) locally advanced disease and 44 (71%) locally recurrent disease. Among patients with prior operations for TGCT (n = 47), the median number of prior operations was 2 (range 1–9), and the time since the last operation was 23 (range 1–192) months. Median follow up of all patients was 52 (IQR 18–83) months.
Table 1.
Descriptive of diffuse-type TGCT patients receiving imatinib mesylate treatment.
| Patients N (%) | |
|---|---|
| Total | 62 (100) |
| Median age at diagnosis (IQR), yrs. | 39 (31–53) |
| Median time from diagnosis to start IM (IQR), yrs. | 3.5 (1–8) |
| Sex | |
| Male | 23 (37) |
| Female | 39 (63) |
| Tumor location | |
| Knee | 35 (56) |
| Ankle | 11 (18) |
| Hip | 6 (10) |
| Foot | 4 (6) |
| Shoulder | 1 (2) |
| Elbow | 1 (2) |
| Head and Neck | 2 (3) |
| Wrist | 2 (3) |
| Surgery before start IM | |
| None | 15 (24) |
| 1–2 | 24 (39) |
| 3–4 | 13 (21) |
| >4 | 10 (16) |
| Median N of surgeries (range) | 2 (1–9) |
| Median time since last surgery (range), mo. | 23 (1–192) |
| Disease status | |
| Locally advanced | 20 (32) |
| Recurrence after surgery | 39 (63)* |
| Metastatic disease | 3 (5) |
Abbreviations: TGCT = Tenosynovial Giant Cell tumor, IM = imatinib mesylate, N = Number of patients, mo = months, yrs = years. *One of the locally recurrent patients progressed to metastatic disease.
Treatment efficacy
Sixty-one patients received 400 mg and one patient received 600 mg IM daily, all as a single dose/day. The 3 patients with metastatic disease at treatment start progressed rapidly on IM and were excluded from further analysis. One other patient with metastatic disease after multiple surgical treatments and IM, was excluded for further analyses too, leaving 58 patients for the rest of the analysis.
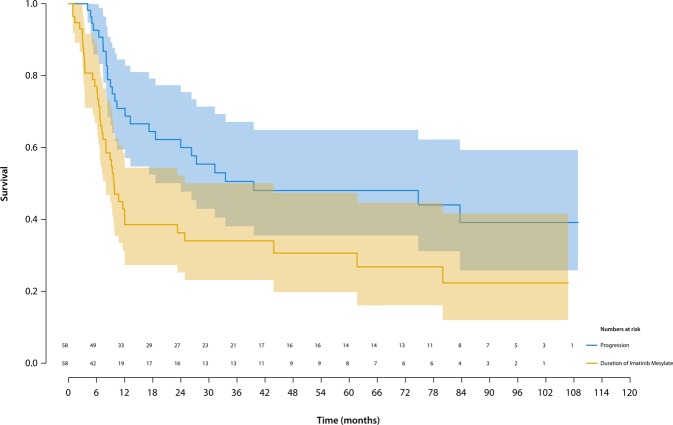
Median duration of IM treatment was 9 (IQR 5–27) months. At last follow-up, the majority of patients (n = 38; 66%) had discontinued treatment. Seventy-seven percent (95% CI 67–89), 41% (95% CI 29–57) and 36% (95% CI 25–52) of patients were still on IM after 6-, 12- and 24-months, respectively (Fig. 1). The treatment failure-rate was 18% (95% CI 6–29) after 12 months.
Figure 1.
Kaplan–Meier survival curves showing the duration of imatinib mesylate treatment (yellow line) and progression free survival (PFS; blue line) in patients with locally advanced or recurrent diffuse-type TGCT. PFS was calculated from the date imatinib mesylate was started to the date of disease progression or death. The shaded areas are 95% confidence intervals (CI). Over half of the patients discontinued IM within a year. The overall PFS after 5 years was ~50%.
Response could not be assessed in 3 patients, two of which were lost to follow-up and one who discontinued early due to febrile neutropenia, leaving 55 patients with locally advanced or locally recurrent TGCT assessable for response. Seventeen patients (31%; 95% CI 19–43) had a RECIST-defined response, including 2 (3%) patients with a complete response. The median time to best response was 6 (range 1–23) months.
Forty of 51 patients (78%) reported symptom improvement (Table 2), including 14 of 15 patients with radiological response (CR or PR). Among patients with radiological stable disease (SD), 22 of 30 patients (73%), for whom data was available, had symptom improvement.
Table 2.
Summary of imatinib mesylate efficacy in patients with locally advanced or recurrent diffuse-type TGCT.
| Parameter | Patients N (%) |
|---|---|
| RECIST best response* | |
| Complete remission | 2 (4) |
| Partial response | 15 (27) |
| Stable disease | 36 (65) |
| Progressive disease | 2 (4) |
| Overall response rate | 17 (31) |
| Rate of disease control | 53 (96) |
| Symptomatic response | 40 (78)** |
| Median IM treatment duration (IQR), mo. | 9.3 (5–26) |
| Median PFS (IQR), mo. | 18 (8–55) |
Abbreviations: TGCT = Tenosynovial Giant Cell tumour, IM = imatinib mesylate, N = Number of patients, mo = months, yrs = years, IQR = inter quartile range.
Overall response rate includes complete remission and partial response; Rate of disease control includes complete remission, partial response and stable disease; Symptomatic response was indicated as present or not (40/51 = 78%). Metastatic patients (n = 4) were excluded.
*N = 3 RECIST best response not available; **N = 9 symptomatic response not available.
The 1-, 2- and 5-years overall PFS, metastatic patients (N = 4) excluded, was 71% (95% CI 60–85), 60% (95% CI 48–75) and 48% (95% CI 36–65) respectively(Fig. 1).
Follow-up
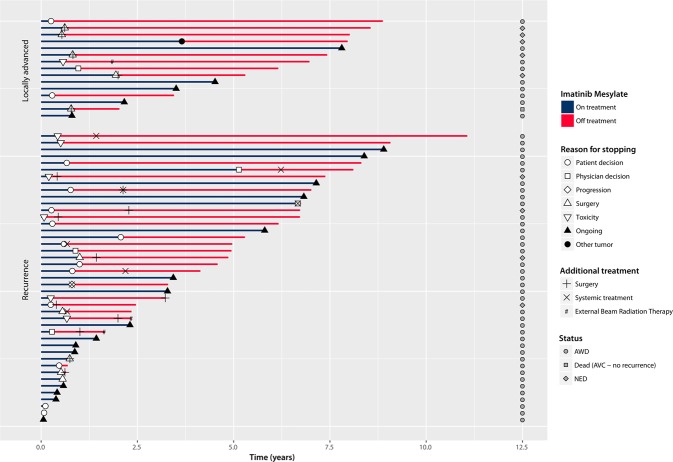
Overall 38/58 patients (66%), metastatic patients (N = 4) excluded, eventually discontinued IM after a median of 7.0 (range 1–80 months). The most common reason for treatment discontinuation was patient decision to stop (n = 14, which possibly reflect low grade chronic toxicity), followed by planned surgery (n = 10), toxicity (n = 7), physician’s decision (n = 5) and progression (n = 1). One patient discontinued IM because of the diagnosis of another tumor requiring therapy. Among the 27 patients who discontinued treatment for reasons other than surgery or progression, progression (either radiological progression or requirement for another line of therapy – i.e. surgery, other medical therapy or radiotherapy) eventually occurred 17 patients after a median of 12 (range 4–84) months, while 10 patients never progressed (nor required additional therapy) after a median follow-up to 78 (range 1–109) months, suggesting that IM was able to provide prolonged symptomatic relief at least in a proportion of patients. Detailed information on each patient is presented in Fig. 2.
Figure 2.
Response and follow up of imatinib mesylate in patients with locally advanced or recurrent diffuse-type TGCT. NED = No evidence of disease, AWD = Alive with disease.
Safety
Forty-five of 58 patients (78%), metastatic patients (N = 4) excluded, reported at least one adverse event with IM. The most common adverse events were edema (N = 28, 48%), fatigue (N = 29, 50%), nausea (N = 21, 34%) and skin rash/dermatitis (N = 7, 12%), mostly grade 1–2 (89%). Additional grade 1–2 complaints were diarrhea, reflux, auditory hallucinations, conjunctivitis, sexual impairment, asthenia, alopecia, cramps and dyspnea. Five (11%) patients had grade 3–4 toxicities, including neutropenia, acute hepatitis, facial edema, skin toxicity and fatigue (Table 3).
Table 3.
Main toxicities associated with imatinib mesylate and reasons for discontinuation, metastatic patients excluded.
| Patients N (%) | ||
|---|---|---|
| Variable | All grades | Grade 3–4 |
| Edema/fluid retention | 28 (48) | 1 (2) |
| Fatigue | 29 (50) | 1 (2) |
| Nausea | 20 (34) | |
| Skin rash/dermatitis | 7 (12) | 2 (3) |
| Other* | 15 (26) | 3 (5) |
| Treatment status | ||
| Continued on IM | 20 (34) | |
| Stopped IM | 38 (66) | |
| Reason for stopping | ||
| Progression | 1 (2) | |
| Toxicity | 7 (12) | |
| Surgery | 10 (17) | |
| Patient choice | 14 (24) | |
| Physician decision | 5 (9) | |
| Other tumor | 1 (2) | |
IM = imatinib mesylate, N = Number of patients. Forty-five (78%) patients reported at least one adverse event with IM. *Other grade 1–2 complaints were diarrhea, reflux, auditory hallucinations, conjunctivitis, sexual impairment, asthenia, alopecia, cramps and dyspnea. Five (11%) patients had grade 3–4 toxicities, including neutropenia, acute hepatitis, auditory hallucinations.
Discussion
To our knowledge, this retrospective study provides the largest case series, with long follow-up, of patients with locally advanced, recurrent or metastatic diffuse-type TGCT treated with IM. We confirmed that IM has activity in TGCT with an overall response rate of 31% in patients with locally advanced/recurrent TGCT. Interestingly all patients with metastatic TGCT progressed on IM, suggesting that metastatic TGCT is either a different disease or loses its dependency on the CSF1/CSF1R axis during malignant transformation. The main issue, is the drop-off rate, with more than half of the patients discontinuing therapy within a year of therapy (59%; 95% CI 29–57), in most cases for unclear reasons (patients decision, physician’s decision) suggesting an unfavorable efficacy/toxicity balance. Eleven percent of patients reported grade 3–4 toxicities, which is consistent with the rates reported with IM for adjuvant gastrointestinal stromal tumors (GIST) or chronic myeloid leukemia (CML)19–22.
To date, surgical resection remains the treatment of choice for diffuse-type TGCT, but is associated with high recurrence rates and multiple additional surgeries11. It is challenging to balance between increased morbidity of multiples or invasive surgeries12,23, alternative therapeutic options, and daily symptoms of the tumor. A more aggressive resection or other multimodality treatments, such as external beam radiation therapy, radiosynovectomy and cryosurgery, may adversely affect joint function, quality of life and development of osteoarthrosis, which, given the young age group, are relevant factors2,24. This would justify a less invasive approach, using systemic therapy, provided those are associated with tumor shrinkage and, most importantly, symptomatic improvements25.
In the present study, age, localization and gender distribution were consistent with the literature10,24,26. The extent of disease in our patient group is emphasized by a disease specific survival of 90% including four metastatic patients and 49% of patients had three or more surgeries before start IM. Similar to previous case-series, we calculated a 1- and 5-years PFS of 71% and 48%, metastatic patients excluded, respectively10,24,26. Because of heterogeneity of patients and a variety of treatments, it is debatable to compare these numbers.
The overall response rate appears higher compared to nilotinib 6% (95% CI unknown), a different tyrosine kinase inhibitor, with similar potency against CSF1R27. Our overall response rate 31% (95% CI 19–43, metastatic patients (N = 4) excluded) was consistent with our previous report on the short term results of IM 19% (95% CI 4–34) with similar disease control rate (96% versus 93%)17. In the present study, 38 (66%) patients discontinued IM; 14 (37%) without subsequent treatment, of which ten patients had stable disease at final follow up. Of the 38 patients who discontinued IM, 21 patients (55%) discontinued IM for toxicity or non-specific medical reasons. 13 (62%) out of these 21 patients eventually progressed. Both stable and progressive patients can be a result of discontinuing IM treatment or the natural course of disease.
Newer, more specific inhibitors of CSF1R, currently only available in trial-setting such as emactuzumab (RG7155)28, pexidartinib (PLX3397)15,29,30, and cabiralizumab31 (FPA008, Five-Prime), have shown promising clinical activity on similar groups of diffuse TGCT patients in prospective clinical studies with more formal criteria and timelines for response assessment than this retrospective series. Emactuzumab (N = 29)16 had an overall response rate of 86% (two patients with a complete response) and a rate of disease control of 96%, including a significant functional and symptomatic improvement (median follow up 12 months). Pexidartinib showed (N = 23)15 an overall response rate of 52% (all patients had a partial response) and a rate of disease control of 83%. At ASCO 2018 results of a pexidartinib placebo controlled, phase 3 study showed a significant improved overall response rate (39.3% vs 0%) and PROMIS physical function (4.06 vs 0.89), after a median 6 months follow up29. The preliminary results with cabiralizumab (N = 22) are consistent, with radiographic response and improvement in pain and function in five out of 11 patients31. However, long term efficacy data have not yet been reported with these newer agents.
Virtually all patients treated with IM for either CML or GISTs, experience32 at least one mild or moderate adverse effect (grade 1–2). Toxicities of IM are determined by the disease stage and the doses used, advanced disease and higher doses result in more frequent and severe toxicities. Most side effects occur early in the course of treatment and tend to decrease in frequency and intensity in time32. We consider a 10–15% rate of grade 3–4 toxicities in a generally benign but locally aggressive disease, such as diffuse TGCT, too high. Only 22% of patients did not experience any side effects.
Although target anti-cancer therapies are described as ‘well tolerated’, the perception of tolerability may vary in the context of a, most often, benign condition. Understanding, monitoring and managing the side effects will be important to optimize systemic therapy for patients with TGCT.
Discontinuation of treatment due to toxicities was seen for IM (this series), emactuzumab15 and pexidartinib16 in 12%, 20% and 9% patients, respectively. TGCT patients might be less willing to cope with adverse event-related and study-related procedures. Here, we report prolonged clinical benefit and symptomatic relief, even after discontinuation of treatment. A similarly persistent effect was observed with monoclonal antibodies and more specific CSF1R tyrosine kinase inhibitors25. This suggest that intermittent treatment administration may be an option to improve long term tolerability.
The place of systemic treatment in a benign, locally aggressive disease, such as TGCT, and how to optimally deliver this treatment, remains unclear. More specifically, the role of CSF1R inhibitors in the peri-operative setting still needs to be explored: the number of patients who underwent operation after IM in our series is too low to draw any conclusions. Despite limitations related to its retrospective nature, this study adds to the knowledge of targeting the CSF-1/CSF-1R pathway in patients with TGCT. An optimal treatment strategy should be developed for the patient group that benefits most from systemic therapy. The combination of a short period of treatment and the durable effect after discontinuation, should be pursued. It is challenging to maintain compliance for years, especially with, even “minor”, toxicities, in the context of a non-life-threatening disease.
So far, less or more specific tyrosine kinase inhibitors have been tested in selected patients who had inoperable, progressive, or recurrent disease15–17,30,31,33. These selected individuals represent a small group of severely affected patients that are a part of a much larger, often less complicated group of patients. The patients who would benefit most from systemic therapy should be identified on the basis of molecular tumour features sensitive to that specific treatment34. In addition, recently an objective MR imaging-based TGCT severity classification has been developed35. The proposed severity classification may be helpful to identify the more aggressive TGCT subtypes eligible for systemic therapy or trials for novel agents.
A limitation of all, including this, clinical TGCT studies is the lack of a control group and the absence of specific and validated patient-reported outcome measures to document treatment-induced symptomatic, functional and economic (back to work) improvement16. Quality of life and functional forms should be implemented. These measures are critical endpoints in demonstrating clinical relevance and impact of treatments for benign diseases in which death is not a relevant outcome variable36. Clinical benefit necessitates objective measures to correlate with tumor reduction.
Conclusion
Identification of a biologic aggressive subgroup of diffuse TGCT, at risk of increased surgical morbidity or recurrent disease, should aid to decide which patients benefit most of systemic treatments. With the advent of more potent CSF-1R inhibitors, such as emactuzumab, pexidartinib and cabiralizumab, the role of IM in extensive TGCT might weaken, but may be balanced by the favorable safety profile of IM. Availability of these new compounds, both in terms of registration and reimbursement, will ultimately define the prescribed drug in daily practice37.
Acknowledgements
Parts of this study have been published in the dissertation of one of the authors (FGMV): “Tenosynovial Giant Cell Tunours: the good, the bad and the ugly”. The authors received no financial support for the research, authorship, and publication of this article.
Author Contributions
The study concept and design were developed by F.G.M.V., M.J.L.M., H.G. and P.A.C. F.G.M.V., M.J.L.M., R.G.M., A.W., E.B., J.D., A.I., B.S., W.G., J.Y.B., M.B., L.E., S.S., O.M., M.A.J.S., G.H. and P.A.C. carried out the data acquisition. The quality control of data and algorithms’ were carried out by F.G.M.V., W.G., J.Y.B., M.A.J.S., G.H. and P.A.C. F.G.M.V. and G.H. did the data analysis, interpretations and statistical analysis. The manuscript preparation and editing was done by F.G.M.V., M.J.L.M., W.G., J.Y.B., M.A.J.S., G.H. and P.A.C. All authors reviewed and approved the final manuscript.
Competing Interests
RGM., AW., JYB., OM., PAC. received personal fees or honorarium for research support outside the submitted work as mentioned in the next paragraph and ‘international committee of medical journal editors form for disclosure of potential conflicts of interest’. All other authors received no financial support for the research and/or authorship of this article. R.G.M. was in the advisory board of Novartis and received consulting fees at some point during the treatment of these patients. AW received consulting fees from Eli Lilly and Five Prime Therapeutics, also he reported research support from Plexxikon, Daiichi-Sankyo, Five Prime Therapeutics, Karyopharm, and Eli Lilly to the institution (Dana Farber Cancer Institute). JYB received grants and honoraria from Roche and Novartis (CentreLéon Bérard). BS was in advisory boards of Plexicon and Lily within the last 2 years. SS reported research grants from Novartis and Daiichi-Sankyo to the institution (Istituto Nazionale Tumori). OM received personal fees from Astra-Zeneca, Amgen, Bayer, BMS, Eli-Lilly, Novartis, Pfizer, Roche, and Servier outside the submitted work. MAJS reported research grants from Daiichi Sankyo and Implantcast to the institution (Leiden University Medical Center). WTAG reported a research grant from Novartis to the institution (Radboud University Medical Center). HG reported a research grant from Novartis and Daiichi to the institution (Leiden University Medical Center). PAC reports grants and personal fees from Novartis, Roche, Amgen; non-financial support from Plexxikon; grants, personal fees and non-financial support from Astra Zeneca during the conduct of the study; grants from Bayer, BMS, MSD, Toray, Taiho, BluePrint and from Eli Lilly outside the submitted work. The other authors (FGMV, MJLM, GH, EB, JD, AI, MB and LE) have declared no conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
F. G. M. Verspoor and and M. J. L. Mastboom contributed equally.
References
- 1.Ottaviani S, Ayral X, Dougados M, Gossec L. Pigmented Villonodular Synovitis: A Retrospective Single-Center Study of 122 Cases and Review of the Literature. Seminars in Arthritis and Rheumatism. 2011;40(6):539–546. doi: 10.1016/j.semarthrit.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Verspoor FGM, et al. Long-term follow-up results of primary and recurrent pigmented villonodular synovitis. Rheumatology (Oxford) 2014;53:2063–2070. doi: 10.1093/rheumatology/keu230. [DOI] [PubMed] [Google Scholar]
- 3.Ravi, V., Wang, W. L. & Lewis, V. O. Treatment of tenosynovial giant cell tumor and pigmented villonodular synovitis. Curr Opin Oncol (2011). [DOI] [PubMed]
- 4.de saint Aubain Somerhausen, N. & van de Rijn, M. Tenosynovial giant cell tumor, localized type/diffuse type. In: WHO classification of tumors of soft tissue and bone (Eds Fletcher, C. D., Bridge, J. A., Hogendoorn, P. C. & Mertens, F.). Lyon: IARC Press, 100–103 (2013).
- 5.Yoon HJ, Cho YA, Lee JI, Hong SP, Hong SD. Malignant pigmented villonodular synovitis of the temporomandibular joint with lung metastasis: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:e30–36. doi: 10.1016/j.tripleo.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Righi A, et al. Metastasizing tenosynovial giant cell tumour, diffuse type/pigmented villonodular synovitis. Clin Sarcoma Res. 2015;5:15. doi: 10.1186/s13569-015-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mastboom MJL, Verspoor FGM, Gelderblom H, van de Sande MAJ. Limb Amputation after Multiple Treatments of Tenosynovial Giant Cell Tumour: Series of 4 Dutch Cases. Case Rep Orthop. 2017;2017:7402570. doi: 10.1155/2017/7402570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shabat S, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology. 2002;41(10):1113–1118. doi: 10.1093/rheumatology/41.10.1113. [DOI] [PubMed] [Google Scholar]
- 9.Heyd, R., Seegenschmiedt, M. H. & Micke, O. The Role of External Beam Radiation Therapy in the Adjuvant Treatment of Pigmented Villonodular Synovitis. Z Orthop Unfall (2011). [DOI] [PubMed]
- 10.Verspoor FG, Scholte A, van der Geest IC, Hannink G, Schreuder HW. Cryosurgery as Additional Treatment in Tenosynovial Giant Cell Tumors. Sarcoma. 2016;2016:3072135. doi: 10.1155/2016/3072135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verspoor FG, et al. Pigmented villonodular synovitis: current concepts about diagnosis and management. Future Oncol. 2013;9:1515–1531. doi: 10.2217/fon.13.124. [DOI] [PubMed] [Google Scholar]
- 12.van der Heijden L, Mastboom MJ, Dijkstra PD, van de Sande MA. Functional outcome and quality of life after the surgical treatment for diffuse-type giant-cell tumour around the knee: a retrospective analysis of 30 patients. Bone Joint J. 2014;96-B:1111–1118. doi: 10.1302/0301-620X.96B8.33608. [DOI] [PubMed] [Google Scholar]
- 13.West RB, et al. A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(3):690–695. doi: 10.1073/pnas.0507321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cupp JS, et al. Translocation and expression of CSF1 in pigmented villonodular synovitis, tenosynovial giant cell tumor, rheumatoid arthritis and other reactive synovitides. American Journal of Surgical Pathology. 2007;31(6):970–976. doi: 10.1097/PAS.0b013e31802b86f8. [DOI] [PubMed] [Google Scholar]
- 15.Tap WD, et al. Structure-Guided Blockade of CSF1R Kinase in Tenosynovial Giant-Cell Tumor. N Engl J Med. 2015;373:428–437. doi: 10.1056/NEJMoa1411366. [DOI] [PubMed] [Google Scholar]
- 16.Cassier PA, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015;16:949–956. doi: 10.1016/S1470-2045(15)00132-1. [DOI] [PubMed] [Google Scholar]
- 17.Cassier PA, et al. Efficacy of imatinib mesylate for the treatment of locally advanced and/or metastatic tenosynovial giant cell tumor/pigmented villonodular synovitis. Cancer. 2012;118:1649–1655. doi: 10.1002/cncr.26409. [DOI] [PubMed] [Google Scholar]
- 18.Guo J, et al. Inhibition of phosphorylation of the colony-stimulating factor-1 receptor (c-Fms) tyrosine kinase in transfected cells by ABT-869 and other tyrosine kinase inhibitors. Mol Cancer Ther. 2006;5:1007–1013. doi: 10.1158/1535-7163.MCT-05-0359. [DOI] [PubMed] [Google Scholar]
- 19.Casali PG, et al. Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated With Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol. 2015;33:4276–4283. doi: 10.1200/JCO.2015.62.4304. [DOI] [PubMed] [Google Scholar]
- 20.Essat M, Cooper K. Imatinib as adjuvant therapy for gastrointestinal stromal tumors: a systematic review. Int J Cancer. 2011;128:2202–2214. doi: 10.1002/ijc.25827. [DOI] [PubMed] [Google Scholar]
- 21.Joensuu H, Trent JC, Reichardt P. Practical management of tyrosine kinase inhibitor-associated side effects in GIST. Cancer Treat Rev. 2011;37:75–88. doi: 10.1016/j.ctrv.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Kalmanti L, et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia. 2015;29:1123–1132. doi: 10.1038/leu.2015.36. [DOI] [PubMed] [Google Scholar]
- 23.Stephan, S. R., Shallop, B., Lackman, R., Kim, T. W. & Mulcahey, M. K. Pigmented Villonodular Synovitis: A Comprehensive Review and Proposed Treatment Algorithm. JBJS Rev4 (2016). [DOI] [PubMed]
- 24.Palmerini E, et al. Tenosynovial giant cell tumour/pigmented villonodular synovitis: outcome of 294 patients before the era of kinase inhibitors. Eur J Cancer. 2015;51:210–217. doi: 10.1016/j.ejca.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Brahmi M, Vinceneux A, Cassier PA. Current Systemic Treatment Options for Tenosynovial Giant Cell Tumor/Pigmented Villonodular Synovitis: Targeting the CSF1/CSF1R Axis. Curr Treat Options Oncol. 2016;17:10. doi: 10.1007/s11864-015-0385-x. [DOI] [PubMed] [Google Scholar]
- 26.Mastboom, M. J. L. et al. Higher incidence rates than previously known in tenosynovial giant cell tumors. Acta Orthop, 1–7 (2017). [DOI] [PMC free article] [PubMed]
- 27.Gelderblom, H. et al. An open-label international multicentric phase II study of nilotinib in progressive pigmented villo-nodular synovitis (PVNS) not amenable to a conservative surgical treatment. Proc Am Soc Clin Oncol31 (suppl) (2013).
- 28.Ries CH, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Tap William D., Gelderblom Hans, Stacchiotti Silvia, Palmerini Emanuela, Ferrari Stefano, Desai Jayesh, Bauer Sebastian, Blay Jean-Yves, Alcindor Thierry, Ganjoo Kristen N., Martin Broto Javier, Ryan Christopher W., Shuster Dale Edward, Zhang Ling, Wang Qiang, Hsu Henry, Lin Paul S., Tong Sandra, Wagner Andrew J. Final results of ENLIVEN: A global, double-blind, randomized, placebo-controlled, phase 3 study of pexidartinib in advanced tenosynovial giant cell tumor (TGCT) Journal of Clinical Oncology. 2018;36(15_suppl):11502–11502. doi: 10.1200/JCO.2018.36.15_suppl.11502. [DOI] [Google Scholar]
- 30.Giustini N, Bernthal NM, Bukata SV, Singh AS. Tenosynovial giant cell tumor: case report of a patient effectively treated with pexidartinib (PLX3397) and review of the literature. Clin Sarcoma Res. 2018;8:14. doi: 10.1186/s13569-018-0101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sankhala, K. K. et al. A phase I/II dose escalation and expansion study of cabiralizumab (cabira; FPA-008), an anti-CSF1R antibody, in tenosynovial giant cell tumor (TGCT, diffuse pigmented villonodular synovitis D-PVNS). American Society of Clinical Oncology Conference: 2017 Annual Meeting ASCO. United States. 35 (15 Supplement 1) (2017).
- 32.Thanopoulou E, Judson I. The safety profile of imatinib in CML and GIST: long-term considerations. Arch Toxicol. 2012;86:1–12. doi: 10.1007/s00204-011-0729-7. [DOI] [PubMed] [Google Scholar]
- 33.Gelderblom, H. et al. Nilotinib in locally advanced pigmented villonodular synovitis: a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol (2018). [DOI] [PubMed]
- 34.Verspoor, F. G. M. & Hannink, G. Systemic treatment of tenosynovial giant cell tumours in context. Lancet (2019). [DOI] [PubMed]
- 35.Mastboom MJL, et al. Severity classification of Tenosynovial Giant Cell Tumours on MR imaging. Surg Oncol. 2018;27:544–550. doi: 10.1016/j.suronc.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Gelhorn HL, et al. Patient-reported Symptoms of Tenosynovial Giant Cell Tumors. Clin Ther. 2016;38:778–793. doi: 10.1016/j.clinthera.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verspoor, F. G. M. Tenosynovial Giant Cell Tunours: the good, the bad and the ugly PhD thesis, Radboud University Medical Center, https://repository.ubn.ru.nl/bitstream/handle/2066/196847/196847.pdf (2018).