Abstract
Embedding and immobilisation of living cells and microorganisms is used in a variety of research and commercial applications. Here we report the successful extended immobilisation of coral larvae in a low-gelling temperature agarose. Embryos and larvae of five broadcast-spawning Scleractinian species were immobilised in agarose gel and tested in a series of exploratory survival and settlement assays. The optimal developmental stage for immobilisation was after ciliation at approximately 24 hours post-fertilisation, after which, survival of immobilised larvae of all species was nearly 100%. In long-term assays, 50% of Montipora digitata larvae survived immobilised for 89 days. Furthermore, immobilised larvae of multiple species, that were released from the agarose, generally remained capable of settlement. These results demonstrate that the immobilisation of the early life-history stages of corals is possible for a variety of applications in basic and applied science.
Subject terms: Animal physiology, Embryogenesis, Developmental biology
Introduction
Immobilisation is a well-established biotechnology with the potential to be harnessed for coral-reef research and restoration. Immobilisation is the process of entrapping and restricting the movement of living cells or organisms in a medium, often a hydrogel1. Hydrogels are ideal for immobilisation for several reasons: they retain water without dissolving, solute transport through the gel can be regulated by pore size, they resist degradation, and they have anti-fouling properties2. The immobilisation of a wide variety of organisms has been used for decades in the pharmaceutical, agricultural, food, and biomedical research industries. For example: bacteria immobilised in a hydrogel have been used in anti-fouling agents2; marine diatoms and microalgae have been encapsulated in alginate beads for aquaculture food-delivery systems3; Aiptasia sp. were immobilised in hydrogel for in vitro fertilisation and microinjection4; filamentous fungi were microencapsulated for large-particle flow cytometry5; and nematodes and squid hatchlings were immobilised in a hydrogel for extended microscopy6.
Immobilisation can provide improved control over and survival of cells and living organisms, and when combined with innovations in biomaterials for microencapsulation, can enable delivery systems5. Advances in coral breeding along with the predicted advantages of using sexually produced corals for restoration have stimulated research into methods for the collection and controlled delivery of coral larvae onto degraded reefs7–9. However, larval and post-settlement survival rates remain low after deployment, and current techniques are not well suited for the co-deployment of larvae with any form of enrichment, such as probiotics, nutrients, or endosymbiotic dinoflagellates. Immobilisation and microencapsulation have the potential to be applied to such delivery systems. Therefore, the objectives of this study were to (1) evaluate the effect of immobilisation on coral embryo and larval survival, and (2) test post-embedding settlement success.
Results and Discussion
The immobilisation of coral embryos and larvae was tested across a range of concentrations of low-gelling temperature agarose. Concentrations of 1.0–1.25% provided the best structural integrity of the gel but set at higher temperatures (~31–32 °C), whereas concentrations of 0.65–0.8% resulted in less structural integrity and poorer embedding but set at cooler temperatures (~28–29 °C). Successful embedding rendered larvae completely immobile (i.e. ‘suspended’ in gel and unable to swim). While 31 °C is warmer than normal summer temperatures on the Great Barrier Reef, a short-term exposure to 31 °C had no observable negative impacts, and temperatures cooled to 28 °C within ten minutes of encapsulation. Hardening of coral offspring by exposing them to heat stress has been proposed as a technique to increase thermal tolerance of corals10; controlled larval immobilisation at higher temperatures, therefore, may serve a dual purpose by stress-hardening during the encapsulation process. Yet, whether this transient exposure to heat stress can result in lasting effects must be tested experimentally.
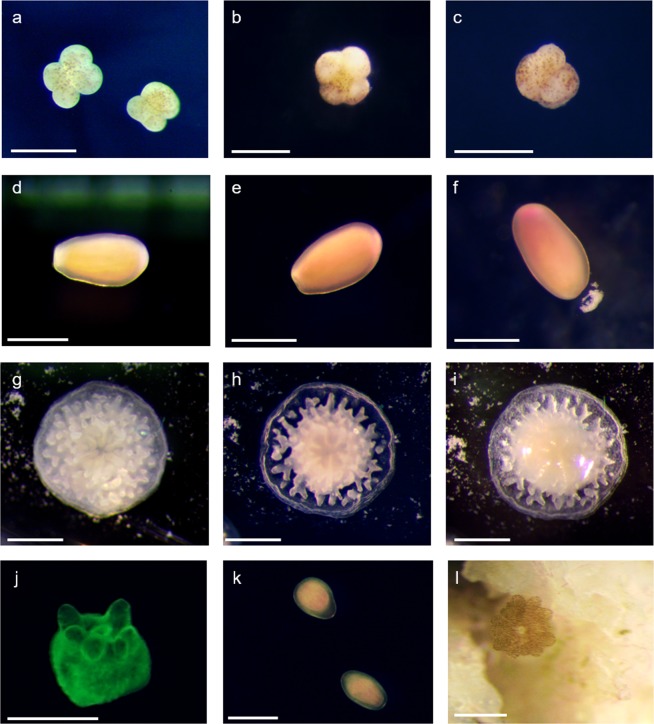
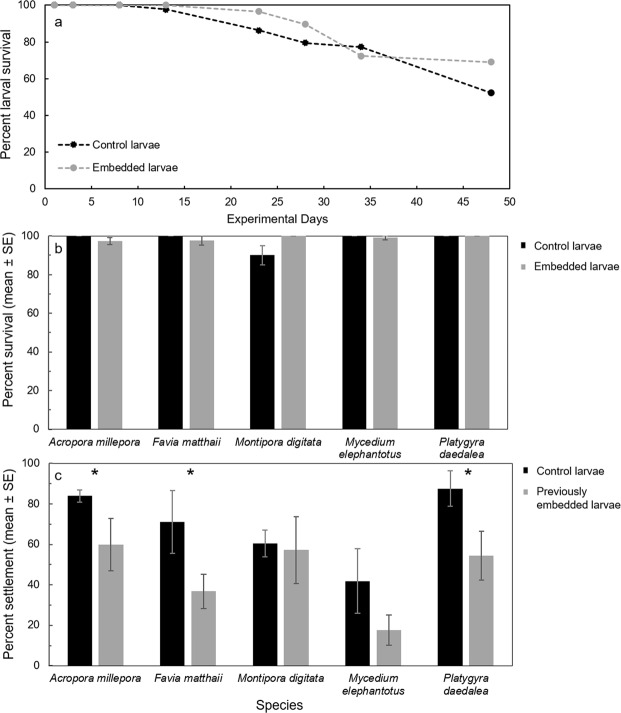
Immobilisation during very early development appeared to halt embryogenesis. Immobilised 4-cell embryos remained in an apparent 4-cell stage, indicating either a cessation of cell divisions, or a continuation of cell divisions in an embryo that was shaped as a 4-cell morula (Fig. 1a–c). After several days, immobilised early embryos began to disintegrate, and survivorship was poor (Fig. 2). Conversely, when early-stage larvae were immobilised after they had developed cilia (Fig. 1d–f), survival rates increased to nearly 100% and were comparable with controls (Figs 2, 3b). In a long-term assay, survival rates remained near 100% for over two weeks before slow attrition began in all treatments, including seawater controls (Fig. 3a). Even after 89 days embedded, ~50% of Montipora digitata larvae in agarose were alive. Immobilised larvae appeared to survive by creating a mucous cocoon, which allowed ciliary action to continue, presumably enabling the exchange of gases and the movement of particulate organic material (Fig. 1f, Supplementary Video). This movement, which allowed the larvae to rotate in place, was observed during encapsulation, as was reported by Jones et al.4 for Aiptasia sp. larvae, and suggests that immobilised ciliated larvae can modify their microenvironments (Supplementary Video). This protective mechanism is like that described for Acropora spp. embryos, which form protective cocoons in response to suspended sediments11. We suggest that the optimal timing of coral encapsulation is, therefore, after embryos are ciliated, but prior to decline in buoyancy, for ease of collection (Fig. 2). These results indicate that immobilisation could serve as a research tool for studies of both coral embryos and larvae; embedded embryos remain alive for several days and larvae for weeks to months, with each organism individually trackable, imageable, and easily manipulated. As such, gel immobilisation offers an alternative to standard ‘fixation’ methods.
Figure 1.
Montipora digitata 4-cell embryos immediately (a), 24 hours after (b), and 7 days after (c) embedding. Acropora millepora larvae immediately (d), 24 hours after (e), and 4 days after (f) embedding. Acropora millepora spat immediately after (g), 4 days after (h), and 7 days after (i) embedding. (j) Montipora digitata metamorphosed in gel matrix and imaged using fluorescence microscopy. (k) Platygyra daedalea in gel matrix immediately after embedding. (l) Montipora digitata settled on a rubble fragment after 89 days immobilised. Scale bars = 0.5 mm.
Figure 2.
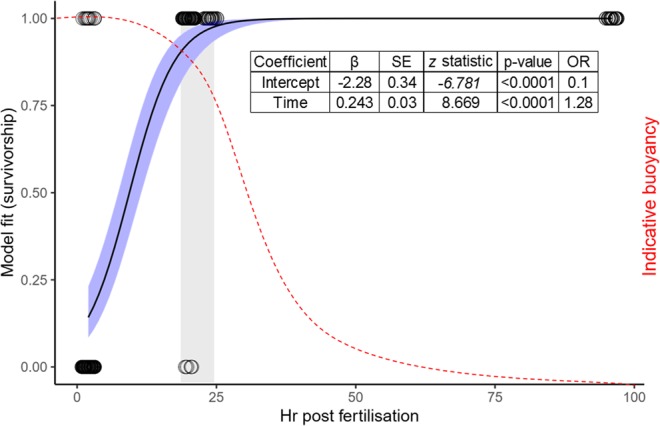
Survival of agarose-embedded Montipora digitata embryos (points are jittered horizontally for clarity) plotted against hours after fertilisation with a logistic model fitted to the data (black curve). Blue shading represents the 95% confidence interval around the model. Red curve indicates the indicative buoyancy of embryos/larvae for many coral species. Grey shading indicates optimal theoretical window for embedding from 19–24 hr corresponding with an estimated 95–99% survival and high larval buoyancy. Model coefficients are presented in the inset table. SE = standard error; OR = odds ratio.
Figure 3.
(a) Long-term survival of agarose-embedded and control Montipora digitata larvae in individual wells of 48-well plates (n = 48 larvae per treatment). (b) Average percent survival of agarose-embedded and control larvae tested in 3– to 6–day assays. (c) Average percent settlement of previously embedded and control larvae tested in 24–48 hr assays. * indicates statistically significant differences (p ≤ 0.05) between treatments for a given species.
M. digitata provides photosynthetic endosymbionts to eggs prior to spawning, whereas the larvae and juveniles of the other species tested take up symbionts from the environment. No differences in embryo or larval survival were observed over 3–6 days among the species tested, suggesting that symbiotic and aposymbiotic larvae respond similarly to immobilisation. We hypothesize that the presence of endosymbionts contributed to the longevity of M. digitata in the long-term experiment, by providing oxygen and other photosynthates to the larvae for nutrition12. While co-embedding with symbiotic dinoflagellates was not tested in this study, there is precedence for the encapsulation of similarly sized, single-celled marine diatoms and microalgae13,14, suggesting that encapsulation of Symbiodiniaceae is possible. Co-encapsulation with a facilitator may enhance survival or performance of coral larvae15,16. The co-encapsulation of yeast with microalgae, for example, increased yeast biomass and lipid productivity by 1.6-fold over mono-encapsulation15. Future coral co-encapsulation techniques could be used to (i) inoculate larvae with stress-tolerant Symbiodiniaceae, (ii) provide probiotics that increase larval and recruit fitness, and (iii) incorporate food for heterotrophic larvae to increase survival and post-settlement growth.
While immobilisation in hydrogel was successful, agarose does not dissolve in seawater. Therefore, to evaluate post-embedding settlement, larvae were manually removed from the gel. Most larvae elongated and swam off within a few seconds of being freed from the gel. The swimming behaviour and morphology of released larvae appeared normal. If any gel remained in contact with a larva after extraction, however, it remained immobile. No significant differences in settlement between previously embedded and control larvae were observed for Montipora digitata or Mycedium elephantotus, although settlement rates on average declined by 35% compared with controls across all species, and were significantly reduced for Acropora millepora, Favia matthaii, and Platygyra daedalea (Fig. 3c). We note, however, that any larvae with remnant gel attached were unable to swim, which contributed to settlement failure. Of those larvae that did settle, morphology appeared normal. While not formally tested, larvae were observed settling after 89 days immobilised (Fig. 1l). The development of a mechanism for the controlled and gel-free release of larvae after embedding would clearly improve settlement.
Immobilised A. millepora spat demonstrated a ‘polyp bail out’-like behaviour (sensu Sammarco17), whereby tissue slowly retracted from the skeleton and amassed around the mouth over a 7-day period (Fig. 2g–i). Spat also appeared to form a protective mucous coating, yet whether they could successfully ‘bail-out’ and return to the larval stage after extraction was not tested.
In conclusion, immobilisation of coral embryos and larvae in agarose gel was tested across a range of developmental stages, for five Indo-Pacific coral species. Survival rates of embedded larvae were high and comparable with controls when immobilisation occurred after ciliation, and larvae were capable of settling post immobilisation. While the technology to embed and encapsulate coral embryos/larvae for restoration applications requires significant advancement and feasibility testing, this study suggests that coral larvae may be excellent candidates. This manipulation, to isolate and maintain planulae, is amenable to use in almost any laboratory. The immobilisation of larvae in discrete, partitionable micro-chambers may also prompt innovative experimental techniques to investigate variability in larval biology and response to the environment.
Materials and Methods
Coral spawning and larval rearing
Gravid corals were collected from Backnumbers Reef (Acropora millepora, Platygyra daedalea, Favia matthaii, Mycedium elephantotus) and Orpheus Island (Montipora digitata) on the central Great Barrier Reef, approximately one week prior to predicted spawning dates in 2017 and 2018, and were transported to the National Sea Simulator at the Australian Institute of Marine Science. The timing of spawning and the numbers of colonies that contributed spawn to mass cultures are reported in Table 1. Gamete bundles were collected, separated, washed, and fertilized as described in Pollock et al.18, and mass cultures were maintained in flow-through aquaria at ambient temperature (26–27 °C), with no aeration during the first 24 hours, and low aeration thereafter.
Table 1.
Summary of spawning corals used to generate mass cultures of coral embryos and larvae tested in immobilisation trials.
| Species | Spawn date | no. nights after full-moon | Spawning commencement time(hh:mm) | Total no. spawning colonies |
|---|---|---|---|---|
| Acropora millepora | 9th Nov 2017 | 5 | 21:15 | 10 |
| 28th Oct 2018 | 3 | 20:40 | 7 | |
| Favia matthaii | 28th Nov 2018 | 5 | 21:20 | 3 |
| Montipora digitata | 4th Apr 2018 | 4 | 19:21 | 9 |
| 6th Apr 2018 | 6 | 19:24 | 18 | |
| Mycedium elephantotus | 28th Nov 2018 | 5 | 21:06 | 4 |
| Platygyra daedalea | 29th Oct 2018 | 4 | 18:45 | 13 |
Immobilisation trials
Immobilisation of embryos and larvae was tested in low-gelling-temperature agarose hydrogel (Sigma-Aldrich A9414) at concentrations of 0.65% to 1.25% weight/volume in 0.2 µm filtered sea water (FSW) at 35.1 ppt (mean ± SD 0.6). A range of agarose concentrations was tested to identify the corresponding gelling temperatures in seawater, and to determine the optimal concentration for immobilisation. Embryos/larvae were either pipetted with 50 µl of FSW directly into liquid agarose and gently mixed using the pipette tip (i.e. ‘agar first’ in Table 2) or pipetted into an empty well plate with 50 µl of FSW after which agarose was pipetted on top of the sample (i.e. ‘embryo first’ in Table 2). We note that the 50 µl FSW added with the embryos/larvae was considered an insignificant volume and thus was not accounted for in the reported concentration of agarose. Immobilisation was trialled in 6-well plates, 48-well plates, and petri dishes. The temperature of the agarose was monitored during the trials and gelling temperatures were recorded.
Table 2.
Summary of conditions and sample sizes for each assay reported.
| Assay | Species | Culture date | Assay type | Embryo/larval age | Conc. agarose (%) | Vol. agarose (ml) | Vol. FSW over agarose (ml) | Embryo/larval embedding technique | No. Reps | No. embryos/larvae per replicate (mean ± SD) | Embedded duration (days) | No. days read post settlement induction |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Embryogenesis | Montipora digitata | 6/04/2018 | 6-well plate | 2 hr | 0.65 | 5.0 | 5.0 | embryo first | 6 | 10 ± 0 | 3 | NA |
| Montipora digitata | 4/04/2108 | Petri dish | 2 hr | 1.00 | 30.0 | 20.0 | embryo first | 1 | 30 | 2 | NA | |
| Montipora digitata | 4/04/2018 | 6-well plate | 20 hr | 0.80 | 3.0 | 7.0 | embryo first | 9 | 10 ± 2 | 4 | NA | |
| Montipora digitata | 4/04/2018 | 6-well plate | 24 hr | 0.80 | 3.0 | 7.0 | embryo first | 6 | 5 ± 0 | 4 | NA | |
| Montipora digitata | 4/04/2018 & 6/04/2018 | 48-well plate | 96 hr & 144 hr | 0.80 | 0.8 | 0.8 | embryo first | 29 | 1 | 3 | NA | |
| Short-term survival | Montipora digitata | 4/04/2018 | 6-well plate | 1 day | 0.65 | 3.0 | 7.0 | embryos first | 6 | 5 ± 0 | 4 | NA |
| Acropora millepora | 28/10/2018 | 6-well plate | 13 days | 0.8 | 7.0 | 0.0 | agar first | 6 | 11 ± 2 | 4 | NA | |
| Favia matthaii | 28/11/2018 | 6-well plate | 36 days | 0.8 | 7.0 | 0.0 | agar first | 6 | 15 ± 1 | 5 | NA | |
| Mycedium elephantotus | 28/11/2018 | 6-well plate | 36 days | 0.8 | 7.0 | 0.0 | agar first | 6 | 17 ± 2 | 6 | NA | |
| Platygyra daedalea | 29/10/2018 | 6-well plate | 12 days | 0.8 | 7.0 | 0.0 | agar first | 6 | 9 ± 4 | 4 | NA | |
| Long-term survival | Montipora digitata | 4/04/2018 & 6/04/2018 | 48-well plate | 96 hr & 144 hr | 0.8 | 0.8 | 0.8 | embryo first | 29 | 1 | cont. | NA |
| Settlement | Montipora digitata | 4/04/2018 | 6-well plate | 17 days | 0.8 | 7.0 | 0.0 | agar first | 6 | 8 ± 1 | 1 | 2 |
| Acropora millepora | 28/10/2018 | 6-well plate | 17 days | 0.8 | 7.0 | 0.0 | agar first | 6 | 10 ± 1 | 4 | 1 | |
| Favia matthaii | 28/11/2018 | 6-well plate | 41 days | 0.8 | 7.0 | 0.0 | agar first | 6 | 11 ± 2 | 5 | 2 | |
| Mycedium elephantotus | 28/11/2018 | 6-well plate | 42 days | 0.8 | 7.0 | 0.0 | agar first | 6 | 15 ± 1 | 6 | 2 | |
| Platygyra daedalea | 29/10/2018 | 6-well plate | 16 days | 0.8 | 7.0 | 0.0 | agar first | 6 | 10 ± 1 | 4 | 1 | |
| Spat | Acropora millepora | 9/11/2017 | Petri dish | 14 days post settlement | 0.7 | 5.0 | 5.0 | spat first | 1 | 5 | 7 | NA |
We note that the volume of agarose (ml) equalled the volume of the filtered seawater (FSW) equivalent in negative controls.
Larval development
To evaluate the optimal developmental stage for immobilisation, Montipora digitata embryos were embedded at the 4-cell stage (2 hr post fertilisation), late gastrula stage (20 hr post fertilisation), early larval stage (24 hr post fertilization) and advanced larval stage (≥96 hr post fertilisation). Survival was modelled as a function of hours post fertilisation using a logistic regression with a binomial distribution and a logit link function using ‘glm’ from the ‘stats’ package in R19.
Larval survival
Larval survival during immobilisation was tested using two sets of assays. Firstly, a short-term (3–6 day) survival assay was completed for all five species. Approximately 10 larvae per well were immobilised in gel in sterile 6-well plates (assay conditions reported in Table 2). Initial numbers of larvae per well were recorded, and survival was assessed after 3–6 days. Larvae were considered ‘alive’ if they (i) were normally pigmented compared with control larvae, (ii) were observed beating their cilia and/or rotating, and (iii) had an intact ectoderm. Secondly, a long-term assay was completed with M. digitata where a single larva (4–6 days old) was immobilised in wells of a 48-well plate (Table 2). In some assays, including long-term survival, FSW was added on top of the gel to prevent desiccation (Table 2). Control wells were prepared with larvae in an equivalent volume of FSW. Well plates were maintained under AquaIllumination® Hydra light emitting diode (LED) aquarium lights (171 photosynthetically active radiation (PAR), 12:12 h diel cycle) in a temperature-controlled experimental room (27 °C), and the status (i.e. live, dead, metamorphosed) of each larva was evaluated over 48 days; a final reading of only embedded larvae was made after 89 days.
Larval settlement
To evaluate the effect of previous immobilisation on larval settlement, larvae were embedded in agarose in sterile 6-well plates (Table 2). Control plates were prepared with larvae of the same age in equivalent volumes of FSW. After a period of immobilisation that ranged from 1 to 6 days depending on the species (see Table 2), larvae in each treatment well were carefully released from the gel using dissection tools under a standard dissection microscope, and gently transferred via pipette into sterile 6-well plates with 10 mL FSW. Control larvae were also pipetted into 10 ml FSW into new sterile plates. All wells were then provided with a fragment of ‘live’ reef rubble (~1 cm2; all fragments in a given assay were from the same parent fragment) to initiate settlement. Percent settlement in each well was assessed after 24 hr and percent settlement of previously immobilised larvae was compared with control larvae using a Welch’s two sample t-test in R (R Core Team 2019). We note that all survival and settlement assays were carried out with only embryos/larvae that were, or had been, fully immobilised.
Spat
Lastly, five A. millepora recruits (<7 days post settlement) attached to clean glass tiles20 were embedded in agarose and observed over seven days. Tiles with living spat were placed in a sterile petri dish. Five millilitres of 0.7% weight/volume agarose in FSW was pipetted into the petri dish at approximately 30 °C. The agarose quickly cooled and gelled, after which five millilitres of FSW was pipetted to cover the layer of gel. The petri dish was maintained in a temperature-controlled experimental room (27 °C), as described above, and assessed for viability each day.
Compliance with ethical standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Corals were collected under permit # G12/35236.1 issued by the Great Barrier Reef Marine Park Authority.
Supplementary information
Acknowledgements
We thank K. Quigley, J. Speaks, T. Whitman, B. Stephenson, M. Nordborg, G. Ricardo, C. Brunner, A. Severati and the SeaSim team for assistance collecting and spawning corals and maintaining cultures. We also thank N. Webster and L. Bay for valuable conceptual and technical contributions. Funding was provided by the Australian Institute of Marine Science Reef Restoration and Adaptation Program.
Author Contributions
All authors conceived of and designed the experiments. C.G. and C.J.R. conducted the experiments. C.R. analysed the data and wrote the manuscript. All authors edited and approved the final draft.
Data Availability
All data are available upon request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-51072-5.
References
- 1.Tsao-Wu GS, Weber CH, Budgeon LR, Cheng KC. Agarose-embedded tissue arrays for histologic and genetic analysis. BioTechniques. 1998;25:614–618. doi: 10.2144/98254st02. [DOI] [PubMed] [Google Scholar]
- 2.Gatenholm P, Holmström C, Maki JS, Kjelleberg S. Toward biological antifouling surface coatings: Marine bacteria immobilized in hydrogel inhibit barnacle larvae. Biofouling. 1995;8:293–301. doi: 10.1080/08927019509378282. [DOI] [Google Scholar]
- 3.Gaudin P, Lebeau T, Robert J-M. Microalgal cell immobilization for the long-term storage of the marine diatom Haslea ostrearia. Journal of Applied Phycology. 2006;18:175–184. doi: 10.1007/s10811-006-9092-0. [DOI] [Google Scholar]
- 4.Jones VAS, Bucher M, Hambleton EA, Guse A. Microinjection to deliver protein, mRNA, and DNA into zygotes of the cnidarian endosymbiosis model Aiptasia sp. Scientific Reports. 2018;8:16437. doi: 10.1038/s41598-018-34773-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delgado-Ramos L, et al. Flow cytometry of microencapsulated colonies for genetics analysis of filamentous Fungi. G3: Genes, Genomes, Genetics. 2014;4:2271–2278. doi: 10.1534/g3.114.014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnett K, Edsinger E, Albrecht DR. Rapid and gentle hydrogel encapsulation of living organisms enables long-term microscopy over multiple hours. Communications Biology. 2018;1:73. doi: 10.1038/s42003-018-0079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heyward A, Smith L, Rees M, Field S. Enhancement of coral recruitment by in situ mass culture of coral larvae. Marine Ecology Progress Series. 2002;230:113–118. doi: 10.3354/meps230113. [DOI] [Google Scholar]
- 8.dela Cruz, D. W. & Harrison, P. L. Enhanced larval supply and recruitment can replenish reef corals on degraded reefs. Scientific Reports7 (2017). [DOI] [PMC free article] [PubMed]
- 9.Doropoulos, C., Elzinga, J., ter Hofstede, R., van Koningsveld, M. & Babcock, R. C. Optimizing industrial-scale coral reef restoration: comparing harvesting wild coral spawn slicks and transplanting gravid adult colonies: Restoration Ecology, 10.1111/rec.12918 (2019).
- 10.van Oppen MJH, Oliver JK, Putnam HM, Gates RD. Building coral reef resilience through assisted evolution. Proceedings of the National Academy of Sciences. 2015;112:2307–2313. doi: 10.1073/pnas.1422301112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricardo GF, Jones RJ, Clode PL, Negri AP. Mucous secretion and cilia beating defend developing coral larvae from suspended sediments. PLoS ONE. 2016;11:e0162743. doi: 10.1371/journal.pone.0162743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muscatine L, Porter JW. Reef corals: mutualistic symbioses adapted to nutrient-poor environments. BioScience. 1977;27:454–460. doi: 10.2307/1297526. [DOI] [Google Scholar]
- 13.Chen Y-C. Immobilization of twelve benthic diatom species for long-term storage and as feed for post-larval abalone Haliotis diversicolor. Aquaculture. 2007;263:97–106. doi: 10.1016/j.aquaculture.2006.12.008. [DOI] [Google Scholar]
- 14.LaJeunesse TC, et al. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Current Biology. 2018;28:2570–2580.e6. doi: 10.1016/j.cub.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Kitcha S, Cheirsilp B. Enhanced lipid production by co-cultivation and co-encapsulation of oleaginous yeast Trichosporonoides spathulata with microalgae in alginate gel beads. Applied Biochemistry and Biotechnology. 2014;173:522–534. doi: 10.1007/s12010-014-0859-5. [DOI] [PubMed] [Google Scholar]
- 16.Perullini M, Orias F, Durrieu C, Jobbágy M, Bilmes SA. Co-encapsulation of Daphnia magna and microalgae in silica matrices, a stepping stone toward a portable microcosm. Biotechnology Reports. 2014;4:147–150. doi: 10.1016/j.btre.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sammarco PW. Polyp bail-out: An escape response to environmental stress and a new means of reproduction in corals. Marine Ecology Progress Series. 1982;10:57–65. doi: 10.3354/meps010057. [DOI] [Google Scholar]
- 18.Pollock FJ, et al. Coral larvae for restoration and research: a large-scale method for rearing Acropora millepora larvae, inducing settlement, and establishing symbiosis. PeerJ. 2017;5:e3732. doi: 10.7717/peerj.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Core Team. R: language and environment for statistical computing. R Foundation for Statistical Computing (2019).
- 20.Heyward AJ, Negri AP. Natural inducers for coral larval metamorphosis. Coral Reefs. 1999;18:273–279. doi: 10.1007/s003380050193. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available upon request.