Abstract
Necrotic enteritis (NE) caused by Clostridium perfringens infection has reemerged as a prevalent poultry disease worldwide due to reduced usage of prophylactic antibiotics under consumer preferences and regulatory pressures. The lack of alternative antimicrobial strategies to control this disease is mainly due to limited insight into the relationship between NE pathogenesis, microbiome, and host responses. Here we showed that the microbial metabolic byproduct of secondary bile acid deoxycholic acid (DCA), at as low as 50 µM, inhibited 82.8% of C. perfringens growth in Tryptic Soy Broth (P < 0.05). Sequential Eimeria maxima and C. perfringens challenges significantly induced NE, severe intestinal inflammation, and body weight (BW) loss in broiler chickens. These negative effects were diminished (P < 0.05) by 1.5 g/kg DCA diet. At the cellular level, DCA alleviated NE-associated ileal epithelial death and significantly reduced lamina propria cell apoptosis. Interestingly, DCA reduced C. perfringens invasion into ileum (P < 0.05) without altering the bacterial ileal luminal colonization. Molecular analysis showed that DCA significantly reduced inflammatory mediators of Infγ, Litaf, Il1β, and Mmp9 mRNA accumulation in ileal tissue. Mechanism studies revealed that C. perfringens induced (P < 0.05) elevated expression of inflammatory mediators of Infγ, Litaf, and Ptgs2 (Cyclooxygenases-2 (COX-2) gene) in chicken splenocytes. Inhibiting the COX signaling by aspirin significantly attenuated INFγ-induced inflammatory response in the splenocytes. Consistent with the in vitro assay, chickens fed 0.12 g/kg aspirin diet protected the birds against NE-induced BW loss, ileal inflammation, and intestinal cell apoptosis. In conclusion, microbial metabolic product DCA prevents NE-induced BW loss and ileal inflammation through attenuating inflammatory response. These novel findings of microbiome protecting birds against NE provide new options on developing next generation antimicrobial alternatives against NE.
Subject terms: Animal physiology, Infection
Introduction
Antimicrobial resistance is one of the emerging challenges requiring immediate and sustainable counter-actions from agriculture to healthcare1. Increasing antimicrobial resistance has caused the emergence of multiple drug-resistant microbes or “superbugs”. Recently, a “superbug” of an Escherichia coli strain resistant to the last resort antibiotic, Colistin, was reported in USA2. Overuse of antimicrobial agents in medical and agricultural practice is contributing to exacerbating the episodes of emerging antimicrobial resistant microbes1. Withdrawing antimicrobials in chicken production, however, has caused new problems for the chicken industry by reducing production efficiency and increasing diseases, such as Eimeria maxima- and Clostridium perfringens-induced necrotic enteritis (NE)3. NE is a multi-factorial disease and has a significant economic impact to the chicken industry with annual loss at billions of dollars4. Despite no clinical NE outbreak worldwide, subclinical NE (cholangiohepatitis) incidences were doubled from 2013 to 2014 in South-Eastern Norway and were associated with reduced in-feed antimicrobial usage5. Clinical signs of acute NE include watery to bloody (dark) diarrhea, severe depression, decreased appetite, closed eyes, and ruffled feathers. Dissection of dead or severely ill birds shows that the intestine is often distended with gas, very friable, and contains a foul-smelling brown fluid, with clearly visible necrotic lesions6. At the cellular level, NE birds display intestinal inflammation with diffuse and coagulative necrosis of villi and crypts, infiltration of immune cells into lamina propria, and crypt abscesses7,8. Although progress has been made toward understanding risk factors influencing the outcome of NE such as C. perfringens virulence, coccidiosis, and feed9, few effective non-antimicrobial strategies are available.
The human and animal intestine harbors up to trillions of microbes and this intestinal microbiota regulates various host functions such as the intestinal barrier, nutrition and immune homeostasis10–12. The enteric microbiota regulates granulocytosis and neonatal response to Escherichia coli K1 and Klebsiella pneumoniae sepsis13, suggesting the key role of the microbiota in protecting the host against systemic infection. At the gut level, fecal transplantation was reported decades ago to prevent Salmonella infantis chicken infection14. More recently, microbiota transplantation has shown tremendous success against recurrent human Clostridium difficile infection15 and Clostridium scindens metabolizing secondary bile acids have been shown to inhibit C. difficile infection16. Bile acids synthesized in the liver are released in the intestine and metabolized by gut microbiota into final forms of secondary bile acids17. Secondary bile acid of deoxycholic acid (DCA) is associated with a variety of human chronic diseases, such as obesity, diabetes, and colorectal cancer18,19. Recently, mouse anaerobes and their metabolic product DCA has been found to prevent and treat Campylobacter jejuni-induced intestinal inflammation in germ-free mice through attenuating host inflammatory signaling pathways20. However, whether bile acids play any role in NE pathogenesis is unknown.
NE is frequently concurrent with the episode of coccidiosis21. Coccidiosis is associated with strong immune response and intestinal inflammation in chickens22,23. Coccidia-induced intestinal inflammation may render a favorable environment for C. perfringens overgrowth, virulence expression, and invasion21. C. perfringens produces various toxins24 and induces hemolysis, epithelial barrier dysfunction, tissue necrosis and severe inflammation in non-chicken models25,26. Among inflammatory signaling pathways, cyclooxygenases (COX)-catalyzed prostanoids regulate various activities including cell proliferation, apoptosis and migration27, gastrointestinal secretion28, body temperature29, inflammation30, and pain sensation31. COX-1 and COX-3, translated from alternative splicing gene of Ptgs1, are constitutively expressed in intestinal cells and they maintain intestinal epithelial integrity. Inducible COX-2 (Ptgs2) activity is associated with various inflammatory diseases including inflammatory bowel disease32 and radiation-induced small bowel injury33. COX-2 increases gut barrier permeability and bacterial translocation across the intestinal barrier34,35. Paradoxically, COX-2 enhances inflammation resolution through prostaglandin D236. Although non-selective COX inhibitor, aspirin, is used to prevent various chronic diseases, it inflicts intestinal inflammation to the healthy intestine37. However, the role of COX signaling on NE remains elusive.
Currently, limited knowledge is available on the relationship among the NE pathogenesis, the microbiome, and the host inflammatory response. Because DCA attenuates C. jejuni-induced intestinal inflammation20 and prevents in vitro growth of C. difficile16, a same genus member of C. perfringens, we hypothesized that the microbiota metabolic product DCA attenuated NE through inhibiting intestinal inflammation. We found that DCA decreased NE-induced intestinal inflammation, C. perfringens invasion, intestinal cell death, and body weight loss. Blocking the inflammatory COX signaling pathways by aspirin reduced NE-induced intestinal inflammation. These novel findings of microbiome DCA and COX inhibitor against NE offer new strategies to prevent and treat C. perfringens-induced diseases.
Materials and Methods
Bile acid inhibition of C. perfringens assay
Similar to bile acid inhibition assay on Campylobacter jejuni growth38, 103 CFU C. perfringens was inoculated into 10 ml Tryptic Soy Broth (TSB) supplemented with 0.5% sodium thioglycollate, in the presence of taurocholic acid (TCA, 0.2 mM, final concentration), cholic acid (CA, 0.2 mM), or DCA (0, 0.01, 0.05, 0.1, or 0.2 mM), or 0, 0.2, or 1 mM of lithocholic acid (LCA) or ursodeoxycholic acid (UDCA). The different treatments of bacterial broth were cultured at 42 °C overnight (16–18 hours) under anaerobic conditions. The bacterial growth in tubes was observed for inhibition (clear broth) or no inhibition (cloudy broth). The bacterial growth was quantified by OD600 nm using a spectrophotometer (Nanodrop, Thermo Fisher).
Chicken experiment
Animal experiments performed were in accordance with the Animal Research: Reporting of In Vivo Experiments (https://www.nc3rs.org.uk/arrive-guidelines). The experiments were approved by the Care and Use Committee of the University of Arkansas. Cohorts of thirteen zero-day-old broiler chicks per group were obtained from Cobb-Vantress Hatchery (Siloam Springs, AR). Chicks were neck-tagged and randomly allocated to floor pens with new pine shavings as litter in an environmentally controlled isolated room suitable for up to biosafety level (BSL) III animal experiments. The birds were provided with their respective diet and water ad libitum, and temperature was maintained at 34 °C for the first 5 days of age and was then gradually reduced until a temperature of 23 °C was achieved at day 26 days of age. The birds were fed a corn-soybean meal-based starter diet during 0–10 days of age and a grower diet during 11–26 days of age. The basal diet was formulated as described before39. Treatment diets were supplemented with 1.5 g/kg CA or DCA (all from Alfa Aesar). No antibiotics, coccidiostats or enzymes were added to the feed. E. maxima kindly provided by Dr. John Barta was M6 strain, which was a single oocyst-derived isolate at Ontario Veterinary College in 1973. The E. maxima was propagated in chickens as describe before40. The recovered oocysts were sporulated as previously described41. C. perfringens kindly donated from USDA-ARS, College Park, TX42,43 was confirmed alpha-toxin positive using a multiplex PCR assay42,44. An aliquot of frozen C. perfringens was grown in TSB plus sodium thioglycollate overnight for the NE challenge study, and was serially diluted and plated on Tryptic Soy Agar plus sodium thioglycolate for enumerating CFU. In previous experiments, birds infected with this aliquot of C. perfringens alone didn’t show any signs of NE and had comparable body weight gain to noninfected birds (data not showed). In current experiments, birds were infected with 20,000 sporulated E. maxima oocytes/bird at 18 days of age and 109 CFU C. perfringens/bird at 23 and 24 days of age. Chicken body weight and feed intake were individually measured at 0, 18, 23, and 26 days of age. Bird health status was monitored daily after the pathogen infection. Four birds with average BW of the treatment group were sacrificed at 23 and 26 days of age. Because gross necrotic lesion was often observed in upper ileum in this NE model, the ileal tissue and digesta samples from all sacrificed birds were collected for RNA and DNA analysis. The ileal tissue of ~8 cm length was also Swiss-rolled for H&E staining and histopathology analysis. Images were acquired using a Nikon TS2 fluorescent microscope. Ileal inflammation was scored blindly using the H&E Swiss-roll slides (4 slides (birds)/treatment). Briefly, each Swiss-roll slide was divided into 4 areas and was scored. Total histopathological scores were then calculated by adding the four area scores. The following score scales used were based on previous ileitis45,46 and colitis scoring systems47: score 0: no inflammation, villi and crypt intact; score 1: small number infiltration cells in laminar propria of villi and crypts or villi minimally shortened; score 2: more extensive infiltration cells in laminar propria of villi and crypts, villi shortened >1/4 and edema, or crypt hyperplasia; score 3: pronounced infiltration cells in laminar propria of villi, crypts, submucosa, and muscularis, villi shortened >1/2 and edema, or crypt hyperplasia and regeneration; and score 4: necrosis, villus diffuse, ulcers, crypt abscesses, or transmural inflammation (may extend to serosa).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Cell apoptosis in intestinal tissue was visualized using TUNEL assay similar to described before48. Briefly, ileal tissue slides were deparaffinized with xylene bath for 3 times and then rehydrated with 100%, 95%, and 70% ethanol. The tissue was then incubated with TUNEL solution (5 µM Fluorescein-12-dUTP (Enzo Life Sciences), 10 µM dATP, 1 mM pH 7.6 Tris-HCl, 0.1 mM EDTA, 1U TdT enzyme (Promega)) at 37 °C for 90 min. The slides were counter-stained with DAPI for nucleus visualization. The fluorescent green apoptosis cells were evaluated and imaged using a Nikon TS2 fluorescent microscopy. The green dots in representative 3 areas per slide were counted as apoptosis cells and blue dots of nuclei were counted as total cells using ImageJ49 particle analysis and its plugin of Nikon ND2 reader. The results were showed as apoptosis cells per 1,000 total intestinal cells.
Real time PCR of mRNA gene expression and C. perfringens quantification
Total RNA from ileal tissue or splenocytes was extracted using TRIzol as described before20,50. cDNA was prepared using M-MLV (NE Biolab). mRNA levels of proinflammatory genes were determined using SYBR Green PCR Master mix (Bio-Rad) on a Bio-Rad 384-well Real-Time PCR System and normalized to Gapdh. To quantify C. perfringens intestinal luminal colonization or tissue invasion, ileal digesta or tissue were weighed, bead-beaten, and extracted for DNA using phenol-chloroform method as described before20. C. perfringens in the digesta and tissue was quantified using specific C. perfringens 16S rDNA primers by the qPCR. The PCR reactions were performed according to the manufacturer’s recommendation. The following gene primers were used:
Cp16S_forward: 5′-CAACTTGGGTGCTGCATTCC-3′; Cp16S_reverse: 5′-GCCTCAGCGTCAGTTACAG-3′; Mmp9_forward: 5′-CCAAGATGTGCTCACCAAGA-3′; Mmp9_reverse: 5′-CCAATGCCCAACTTCTCAAT-3′; Litaf_forward: 5′-AGATGGGAAGGGAATGAACC; Litaf_reverse: 5′-GACGTGTCACGATCATCTGG-3′; Il1β_forward: 5′-GCATCAAGGGCTACAAGCTC-3′; Il1β_reverse: 5′-CAGGCGGTAGAAGATGAAGC-3′; Infγ_forward: 5′-AGCCGCACATCAAACACATA-3′; Infγ_reverse: 5′-TCCTTTTGAAACTCGGAGGA-3′; Ptgs2_forward: 5′-ACCAGCATTTCAACCTTTGC-3′; Ptgs2_reverse: 5′-CCAGGTTGCTGCTCTACTCC-3′; Gapdh_forward: 5′-GACGTGCAGCAGGAACACTA-3′; Gapdh_reverse: 5′-CTTGGACTTTGCCAGAGAGG-3′. Gene expression of fold change was calculated using ΔΔCt method51 and Gapdh as internal control.
Fluorescence in situ hybridization (FISH)
C. perfringens at ileal tissue was visualized using FISH assay similarly to previously described47. Briefly, tissue slides were deparaffinized, hybridized with the FISH probe, washed, stained with DAPI, and imaged using a Nikon TS2 fluorescent microscope system. The FISH probe sequence of Cp85aa18: 5′-/Cy3/TGGTTGAATGATGATGCC-3′52 was used to probe the presence of C. perfringens similar to a previous report50. Briefly, deparaffinized, formalin-fixed 5 μm thick sections were incubated for 15 min in lysozyme (300,000 Units/ml lysozyme; Sigma-Aldrich) buffer (25 mM Tris pH 7.5, 10 mM EDTA, 585 mM sucrose, and 0.3 mg/ml sodium taurocholate) at room temperature and hybridized overnight at 46 °C in hybridization chambers with the oligonucleotide probe (final concentration of 5 ng/μl in a solution of 30 percent formamide, 0.9 M sodium chloride, 20 mM Tris pH 7.5, and 0.01% sodium dodecyl sulfate). Tissue sections were washed for 20 min at 48 °C in washing buffer (0.9 M NaCl, 20 mM Tris pH 7.2, 0.1% SDS, 20% Formamide, and 10% Dextran Sulfate) and once in distilled water for 10 seconds. The slides were stained with DAPI for 2 min and dried at room temperature, mounted with 50% glycerol. C. perfringens in intestinal tissues were visualized using a Nikon TS2 fluorescent microscopy and representative images of each treatment were presented in the Results section.
C. perfringens-induced inflammatory response using primary splenocytes
Splenocytes were isolated similarly to described previously53. Briefly, chicken spleen was resected, homogenized into splenocytes using frosted glass slides, and pooled together in RPMI 1640 medium supplemented with 2% fetal bovine serum, 2 mM L-glutamine, 50 µM 2-mercaptoethanol. After lysed the red blood cells, the collected cells were plated at 2 × 106 cells/well in 6-well plates. The cells were pre-treated with 1.2 mM aspirin for 45 min. Cells were then challenged with murine INFγ (1 μg/ml, Pepro Tech), chicken INFγ (1 μg/ml, IBI Scientific), or C. perfringens (multiplicity of infection 100). The cells were lysed in TRIzol (Invitrogen) for RNA isolation after 2 or 4 hours of cytokines or C. perfringens treatment, respectively.
Dietary aspirin against NE
Birds fed diet supplemented with 0 and 0.12 g/kg aspirin were raised, infected, and sampled as abovementioned. Ileal histopathology images and scores were collected. To evaluate cell death, TUNEL assay was performed in the ileal slides of the birds and the apoptotic cells were quantified. The impact of aspirin on body weight gain was also measured.
Statistical analysis
For in vitro assay of bile acids against C. perfringens growth, data were first analyzed by One-way ANOVA for significant difference and then Bonferroni’s multiple comparison test using Prism 5.0 software. For other data, differences between treatments were analyzed pairwise using the nonparametric Mann–Whitney U test performed using Prism 5.0 software. The specific pairwise comparisons were showed in Results section and Figures. Sample (individual birds or cell wells) numbers of body weight, histology, and other assays were listed in respective subsections of Methods section. Values are shown as mean of samples in the treatment ± standard error of the mean as indicated. Experiments were considered statistically significant if P values were < 0.05.
Ethics approval and consent to participate
All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Arkansas at Fayetteville.
Results
DCA prevents C. perfringens in vitro growth
Based on previous findings and current state of knowledge, we reasoned that DCA would prevent C. perfringens growth. To test this hypothesis, in vitro inhibition experiments were conducted, in which C. perfringens was inoculated in TSB with sodium thioglycollate under anaerobic condition. The TSB was also supplemented with various concentrations of bile acids, including conjugated primary bile acid TCA, primary bile acid CA, and secondary bile acid DCA. The results showed that DCA inhibited C. perfringens growth at 0.01 (−33.8%) and 0.05 mM (−82.8%, clear broth), respectively, compared to control, while TCA (−16.4%) and CA (−8.2%) didn’t prevent the bacterial growth (cloudy broth) even at 0.2 mM (Fig. 1A). We then examined whether other secondary bile acids were also bacteriostatic in TSB. Interestingly, C. perfringens growth was inhibited by lithocholic acid (LCA; −22.6 and −23.8%) and ursodeoxycholic acid (UDCA; −10.0 and −25.3%) at 0.2 and 1 mM, respectively (Fig. 1B) and it was far less effective compared to DCA.
Figure 1.
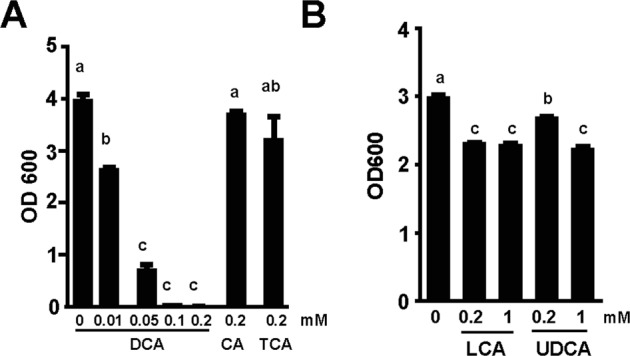
DCA inhibits C. perfringens in vitro growth. C. perfringens (103 CFU) were inoculated into TSB supplemented with various concentrations of conjugated primary bile acid TCA and CA, secondary bile acids DCA, LCA, and UDCA. (A) OD600 reading of broth supplemented with DCA inhibiting C. perfringens growth. (B) OD600 reading of other secondary bile acids inhibiting C. perfringens growth at less efficiency compared to DCA. All graphs depict mean ± SEM. Different letters of a, b and c mean P < 0.05. Results are representative of 3 independent experiments.
DCA prevents NE-induced intestinal inflammation in chicken ileum
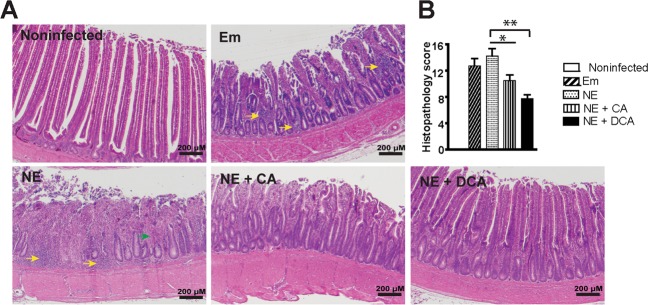
To further address whether DCA reduced coccidia E. maxima- and C. perfringens-induced NE in birds, broiler chicks were fed CA or DCA diet. Because coccidiosis and NE induce severe intestinal inflammation7,8, the impact of DCA on chicken NE was investigated. Upper ileum tissue was collected as Swiss-roll, processed with H&E staining, and performed histopathology analysis. Notably, E. maxima (Em) infection induced severe intestinal inflammation (ileitis) as seen by immune cell infiltration (yellow arrows) into lamina propria, crypt hyperplasia, and mild villus height shortening compared to uninfected birds (Fig. 2A). NE birds displayed worse ileitis as seen by necrosis and fusion of villi and crypt (Green arrow), massive immune cell infiltration, and severe villus shortening. In contrast, DCA diet dramatically attenuated NE-induced ileitis and histopathology score (Fig. 2B), while CA diet also reduced NE-induced ileitis and histopathology score.
Figure 2.
DCA attenuates NE-induced intestinal inflammation. Cohorts of thirteen broiler chicks were fed basal, 1.5 g/kg CA or DCA diets. The birds were infected with E. maxima at 18 days of age and C. perfringens at 23 and 24 days of age. Four birds per group were sacrificed and sampled at 26 days of age. (A) H&E staining showing representative intestinal histology images. (B) Quantification of histological intestinal damage score. Scale bar is 200 μm. All graphs depict mean ± SEM. *P < 0.05; **P < 0.01. NE + CA, NE birds fed CA diet; NE + DCA, NE birds fed DCA diet. Yellow arrows: immune cell infiltration; green arrow: fusion of villi and crypts. Results are representative of 3 independent experiments.
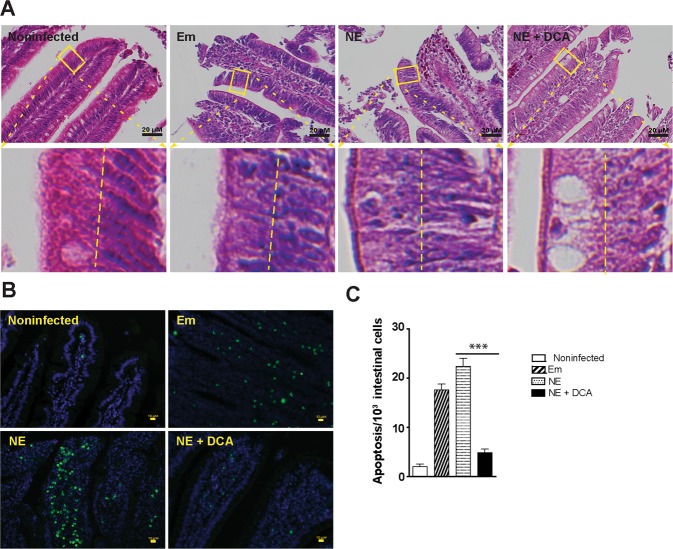
DCA attenuates NE-induced intestinal cell necrosis and apoptosis
Because inflammation often induces cell death54, it was then sought to examine whether cell death was relevant in DCA attenuating NE-induced ileitis. Since it was difficult to find reliable chicken antibodies to detect apoptosis or necrosis in chicken histology slides, we first resorted to classical histological analysis under high magnification. Consistently, the epithelial nuclei (dark blue) in heathy control bird villi were distributed close to the basal membrane (at the right side of the yellow dash line, Fig. 3A lower left panel). In contrast, the nuclei in inflamed villi epithelial cells of Em and NE birds were scattered from basal to the apical membranes, indicating epithelial cell death in villi of those birds. As a contrast, the DCA diet prevented epithelial cell nucleus translocation to apical side, suggesting cell death reduction. To further characterize the villus cell death, TUNEL assay was used, which detects later stage of cell apoptosis. Consistent with histopathology results, coccidiosis and NE induced scattered (Em birds) or concentrated (NE birds) apoptosis cells (green dots) in villus lamina propria, while cellular apoptosis was attenuated in the DCA treatment birds (Fig. 3B). To quantify the apoptosis, ImageJ was used to count apoptosis cells (TUNEL, green) and total cells (DAPI, blue). Consistent with histopathology results, DCA reduced NE-induced cell apoptosis (Fig. 3C).
Figure 3.
DCA attenuates NE-induced intestinal cell death and apoptosis. Cohorts of broiler chicks were fed different diets, infected, and sampled as in Fig. 2. (A) Representative intestinal cell death (deviated nuclei) using H&E staining. (B) Representative villus cell apoptosis (green) using TUNEL assay. (C) Quantified apoptotic cells using ImageJ. Scale bars are 20 μm (A) and 10 μm (B). NE + DCA, NE birds fed DCA diet. All graphs depict mean ± SEM. ***P < 0.001. Results are representative of 3 independent experiments.
DCA reduces C. perfringens invasion, NE-induced inflammatory response, and productivity loss
Given DCA inhibited C. perfringens growth in vitro, it was logic to reason that DCA might also reduce C. perfringens intestinal overgrowth in NE birds. To examine this possibility, C. perfringens colonization level was measured in the intestinal lumen using real-time PCR of C. perfringens 16S rDNA. Surprisingly, ileal luminal C. perfringens colonization in DCA birds was not significantly different from NE control birds (Fig. 4A), while bird histopathology was distinct between the two groups of birds. We then reasoned that the pathogen invasion into tissue was the main driving factors of NE pathogenesis, but not the pathogen luminal colonization level. To quantify the bacterial invasion, total DNA in ileal tissue was isolated and C. perfringens was measured using real time PCR. DCA attenuated more than 95% of C. perfringens invasion into ileal tissue (Fig. 4B). To have a better overview of the bacterial tissue invasion distribution, we used a fluorescence in situ hybridization (FISH) technique. We found that while C. perfringens was present deeply in the inflamed villus and crypt lamina propria of NE control birds, the bacterium was barely detectable in the ileal tissue of DCA birds (Fig. 4C). Because DCA reduced C. perfringens invasion and intestinal inflammation, the impact of DCA on various proinflammatory mediators was evaluated in ileal tissue using Real-Time PCR. C. perfringens induced inflammatory Infγ, Litaf (Tnfα), and Mmp9 mRNA accumulation in chicken ileal tissue, an effect attenuated by 51, 82, and 93%, respectively, in DCA fed chickens (Fig. 4D).
Figure 4.
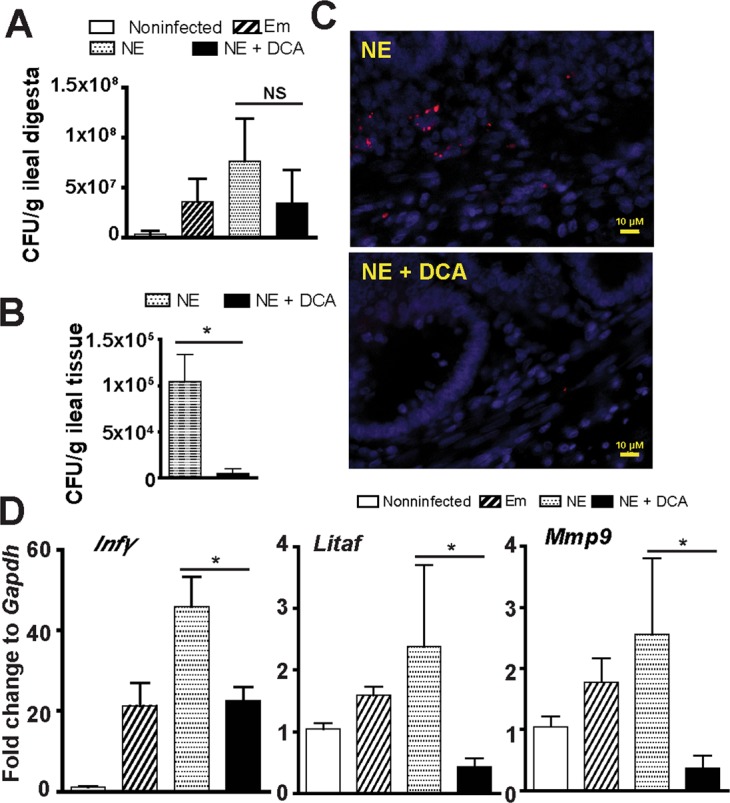
DCA reduces C. perfringens invasion and inflammatory response. Chickens were fed different diets, infected, and sampled as in Fig. 2. (A) Luminal C. perfringens colonization level quantified by 16 S rDNA real-time PCR. (B) C. perfringens invasion into intestinal tissue quantified by 16 S RNA real-time PCR. (C) Presence of C. perfringens (red dots) in ileal sections of NE and NE + DCA birds, detected using fluorescence in situ hybridization (FISH) assay. (D) Ileal Infγ, Litaf (Tnfα), and Mmp9 mRNA qPCR fold change relative to uninfected birds and normalized to Gapdh. Scale bar is 10 μm. NE + DCA, NE birds fed DCA diet. All graphs depict mean ± SEM. NS, not significant; *P < 0.05. Results are representative of 3 independent experiments.
Body weight (BW) is one of important productivity parameters for meat chickens and reflects collective response during NE. The productivity results showed that DCA (solid black bar) but not CA (vertical-line bar) diet promoted bird daily BW gain during 0–18 days of age compared to birds fed control diets (open, tilted line, and dotted bars, Fig. 5). BW gain was reduced in birds infected with E. maxima (Em) (tilted line and dotted bars) during 18–23 days of age (coccidiosis phase) compared to noninfected birds (open bar). Subsequent C. perfringens infection reduced NE control birds (dotted bar) BW gain during 23–26 days of age (NE phase) compared to Em birds (tilted line bar). Consistent with histopathology analysis, DCA prevented productivity loss at coccidiosis and NE phases compared to the NE control birds. Interestingly, the primary bile acid CA diet attenuated body weight loss at NE phase but failed at coccidiosis phase compared to the NE control birds.
Figure 5.
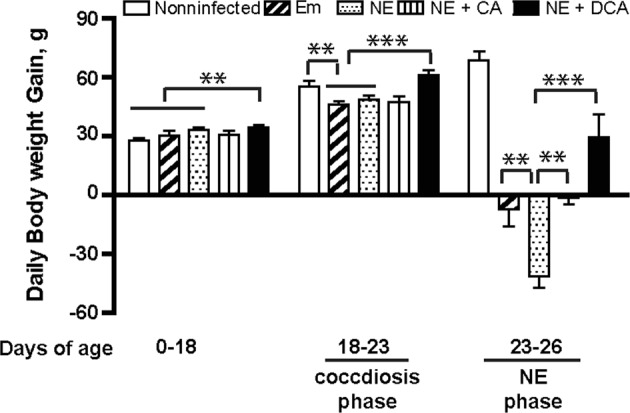
DCA attenuates NE-induced productivity loss. Cohorts of thirteen broiler chicks were fed different diets and infected as in Fig. 2. Bird body weight gain was measured at 18 (13 birds/group), 23 (13 birds/group), and 26 (8–9 birds/group) days of age. Showed was daily periodic body weight gain. NE + DCA, NE birds fed DCA diet. All graphs depict mean ± SEM. **P < 0.01; ***P < 0.001. Results are representative of 3 independent experiments.
COX inhibitor aspirin alleviates C. perfringens-induced inflammatory response in splenocytes
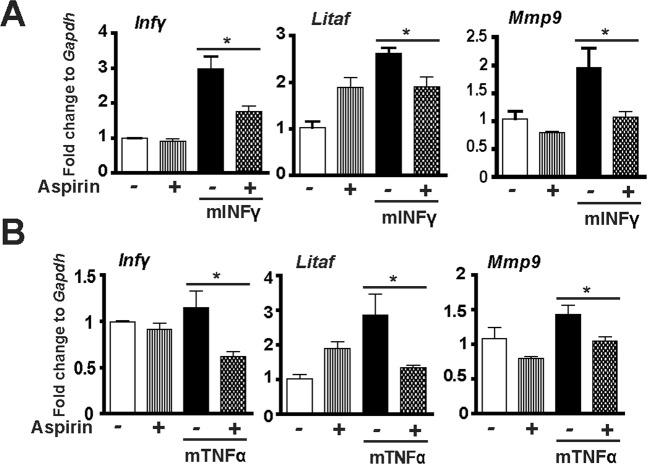
Inflammatory events shape intestinal diseases and targeting the inflammatory response attenuates disease progress such as in Inflammatory Bowel Disease55 and campylobacteriosis53. To dissect how the host inflammatory response is involved in NE-induced ileitis, a primary chicken splenocyte cell culture system was then used similarly to previous report47. After isolation from chickens, the splenocytes were infected with C. perfringens (MOI 100) for 4 hours. The results showed that C. perfringens increased inflammatory mediators of Infγ, Litaf (Tnfα), Mmp9 and Ptgs2 (protein COX-2) mRNA accumulation by 1.54, 1.69, 1.72 and 8.65 folds, respectively, compared to uninfected splenocytes (Fig. 6A). Because COX-2 is an important mediator in the inflammatory response32, COX inhibitor aspirin was then used in C. perfringens-infected chicken splenocytes. Interestingly, aspirin failed to reduce C. perfringens-induced inflammatory gene expression (data not shown). We then reasoned that COX signaling acted on C. perfringens-induced inflammatory cytokines. Inflammatory cytokine of recombinant chicken INFγ (ch-INFγ) was then used to challenge splenocytes in the presence of aspirin. Aspirin reduced ch-INFγ-induced inflammatory gene expression of Infγ, Litaf (Tnfα), and Mmp9 by 44, 45, and 65%, respectively (Fig. 6B). Because no chicken TNFα was available, murine INFγ (mINFγ) and mTNFα were used. Consistently, aspirin also reduced murine INFγ (mINFγ)-induced inflammatory gene expression of Infγ, Litaf, and Mmp9 by 41, 27, and 45%, respectively (Fig. 7A). Similarly, aspirin reduced mTNFα-induced inflammatory gene expression of Infγ, Litaf, and Mmp9 by 49, 53, and 27%, respectively (Fig. 7B). These data indicate that C. perfringens induces inflammatory cytokines and COX-2 while blocking COX signaling by aspirin reduces inflammatory cytokine-induced responses, suggesting that aspirin poses protection potential against NE detrimental inflammatory response.
Figure 6.
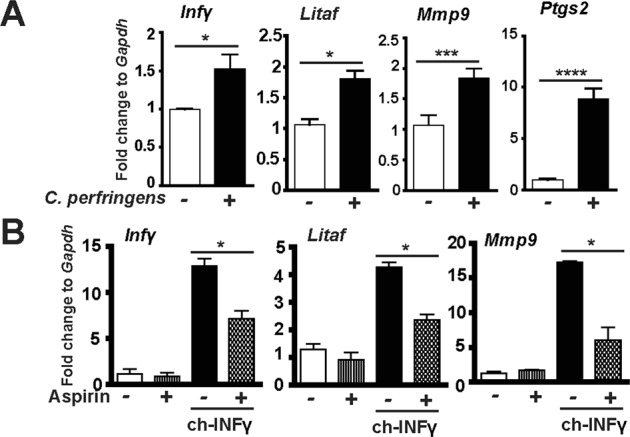
COX inhibitor aspirin alleviates C. perfringens-induced inflammatory response in chicken splenocytes. Splenocytes isolated from broiler chickens were infected with C. perfringens (MOI 100) for 4 hr or stimulated with chicken INFγ (1 μg/ml) for 2 hours in the presence of 1.2 mM aspirin. RNA was extracted, reverse-transcribed, and quantified using a Bio-Rad 384 PCR platform. (A) Infγ, Litaf, Mmp9, and Ptgs2 mRNA fold change normalized to Gapdh. (B) Gene expression fold change in the presence of chicken INFγ (ch-INFγ) and aspirin. All graphs depict mean ± SEM. *P < 0.05; ***P < 0.001. Results are representative of 2 independent experiments.
Figure 7.
COX inhibitor aspirin alleviates murine cytokine-induced inflammatory response in chicken splenocytes. Splenocytes isolated from broiler chickens were stimulated with murine mINFγ (1 μg/ml) or mTNFα (5 ng/ml) for 2 hr in the presence of 1.2 mM aspirin. RNA was extracted, reverse-transcribed, and quantified using a Bio-Rad 384 PCR platform. (A) Infγ, Litaf, and Mmp9 mRNA fold change normalized to Gapdh in the presence of mINFγ. (B) Infγ, Litaf, and Mmp9 mRNA fold change normalized to Gapdh in the presence of mTNFα. All graphs depict mean ± SEM. *P < 0.05. Results are representative of 2 independent experiments.
Aspirin attenuates NE-induced ileitis, intestinal cell apoptosis, and productivity loss
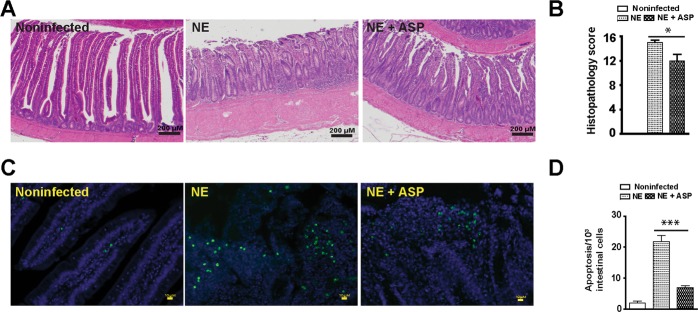
To functionally assess the protective effect of aspirin against NE-induced ileitis, broiler chickens fed with aspirin diet (ASP) were infected with E. maxima and C. perfringens as describe before. Ileal tissues were collected and histopathology analysis was performed to assess NE. Consistently, NE birds had severe ileal necrosis with immune cell infiltration and villus shortening (Fig. 8A). Notably, ASP attenuated NE-induced intestinal inflammation and histopathological score (Fig. 8A,B). At cellular level, ASP reduced NE-induced immune cell apoptosis in villus lamina propria (Fig. 8C,D). On growth performance, ASP birds grew slower compared to control diet birds during 0–18 days of age (Fig. 9). This is because aspirin inhibits all COX isoforms and COX-1 and -3 are important for intestinal homeostasis and growth. Notably, ASP attenuated NE-induced BW loss by 60% during NE phase of 23–26 days of age, while no difference between ASP and NE birds during coccidiosis phase of 18–23 days of age.
Figure 8.
Aspirin attenuates NE-induced intestinal inflammation and apoptosis. Cohorts of thirteen chickens were fed basal and 0.12 g/kg aspirin diet. The birds were infected as in Fig. 2 and four birds per group were sampled at 26 days of age. (A) H&E staining showing representative intestinal histology images. (B) Quantification of histological intestinal damage score. (C) Representative cell apoptosis (green) using TUNEL assay. (D) Quantification of apoptotic cells. Scale bars are 200 μm in panel A and 10 μm in panel C. NE + ASP, NE birds fed aspirin diet. All graphs depict mean ± SEM. *P < 0.05; ***P < 0.001. Results are representative of 2 independent experiments.
Figure 9.
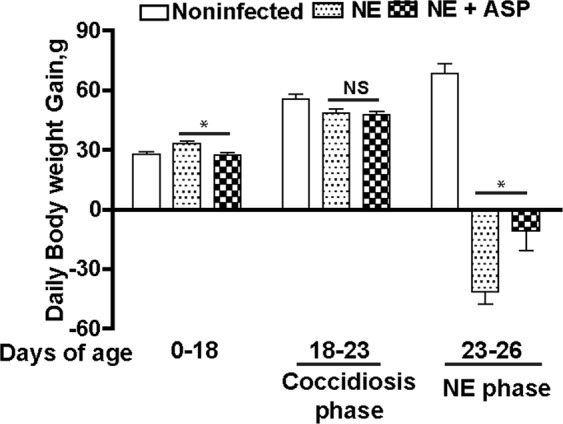
Aspirin attenuates NE-induced productivity loss. Cohorts of 13 broiler chicks were fed different diets and infected as in Fig. 6. Bird body weight gain was measured at 18 (13 birds/group), 23 (13 birds/group), and 26 (8 birds/group) days of age. Showed was daily periodic body weight gain. NE + ASP, NE birds fed aspirin diet. All graphs depict mean ± SEM. NS, not significant; *P < 0.01; Results are representative of 2 independent experiments.
Discussion
Although NE has reemerged as a prevalent chicken disease worldwide in the antimicrobial free era5, the lack of comprehensive molecular mechanism insight into NE severely hinders the development of antimicrobial alternatives to control this disease56. Many virulence factors of Eimeria and C. perfringens are identified but few findings are effective to control NE in chicken production9,57, suggesting that important players/factors in NE pathogenesis were overlooked, such as microbiome and host response. We reported here that microbiota metabolic product DCA attenuated chicken NE by reducing chicken inflammatory COX signaling pathways. These new findings offer approaches for exploring novel antimicrobial alternatives to control C. perfringens-induced diseases.
It is a relatively new concept to manipulate microbiota and its metabolic products against infectious diseases. Fecal transplantation was used in chickens decades ago to prevent S. infantis infection14. Microbiome plays an important role in susceptibility to human C. difficile infection58. Anaerobe C. scindens-transformed secondary bile acids prevent C. difficile germination and growth16. LCA and DCA but not primary bile acid CA inhibit C. difficile vegetable growth and toxin production16,59. However, whether secondary bile acids prevent or treat C. difficile infection in human or animal models is still unknown. It has been recently found that orally gavaging DCA attenuated C. jejuni-induced intestinal inflammation in germ-free mice20. Based on the knowledge, we then reasoned that DCA might prevent E. maxima- and C. perfringens-induced chicken NE. Indeed, dietary DCA prevented NE and its associated productivity loss. The reduction of ileitis was coupled with reduced C. perfringens intestinal tissue invasion and intestinal inflammation and cell death. Intriguingly, DCA failed to reduce C. perfringens ileal luminal colonization, suggesting that the mechanism of DCA action is independent of intestinal luminal colonization exclusion and is possibly through modulating other factors such as inflammation.
At the cellular level, the intestinal tract of NE-inflicted birds displays severe small intestinal inflammation, showing massive immune cells infiltration into lamina propria, villus breakdown, and crypt hyperplasia60,61. Inflammation often induces cell death54. Normal intestinal epithelial cells have polarity62 and their nuclei are located toward the basal membrane63, while stressed dying (apoptosis or necrosis) cells lose polarity64 and their nuclei disperse from basal to apical membranes65. Intestinal inflammation is also critical to clear invaded microbes and to resolve inflammation, while overzealous inflammation causes more bacterial invasion and further collateral damage and inflammation66. Infectious bacteria often hijack the inflammatory pathways to gain survival and invasion advantage. For example, Salmonella Typhimurium induces extensive inflammation in mouse intestine and thrives on the inflammation67. Interestingly, S. Typhimurium infection causes immunosuppressive effect in neonatal chickens because of lymphocyte depletion68. Unlike S. Typhimurium infection in neonatal birds, coccidia infection induces strong immune response and intestinal inflammation in chickens22,23. Furthermore, NE birds suffered more intestinal inflammation compared to Em birds in current study. Consistent with the “over-inflammation” model, NE birds with severe intestinal inflammation showed extensive immune cell infiltration, increased inflammatory cytokine gene expression, and epithelial cell hyperplasia and death (dispersed nuclei and apoptosis). Conversely, DCA attenuated the intestinal inflammation and inflammatory cytokines and improved the growth performance and reduced the NE pathology. Consistent with the reasoning of anti-inflammation reducing NE, blocking inflammatory COX-2 signaling pathway by aspirin alleviated intestinal inflammation, villus apoptosis and NE-induced BW loss. These findings indicate that DCA attenuates NE through decreasing inflammatory signaling pathways.
Different strains of C. perfringens produce a variety of toxins including alpha (CPA), beta (CPB), epsilon (ETX), iota (ITX), enterotoxin (CPE), necrotic enteritis B-like toxin (NetB), and others69. Among them, researchers have reported that NetB but not CPA induced NE in chickens70. However, NE doesn’t have strong association with NetB positive C. perfringens in US71. In our NE model, no difference of NetB gene expression in ileal digesta between healthy control and NE birds (data not shown) suggests limited role of the toxin. Generally, C. perfringens toxins induce cell death of apoptosis, necrosis, necroptosis, and pyroptosis in the presence of extracellular calcium influx69. Higher dietary calcium increases chicken mortality and reduces growth performance in coccidiosis-induced NE model72, suggesting the important role of toxin-induced and calcium-mediated cell death in NE. The reduction of intestinal cell death by DCA and aspirin suggests possible toxin involvement in our NE model. Future work on C. perfringens toxins, DCA, and inflammatory response will be conducted.
In contrast to the accumulating evidence of bile acids against infectious enteritis in recent studies16,20, the microbial-derived metabolites have long been implicated in chronic diseases18. Plasm bile acids is correlated with increased body mass index of obese patients73. Plasma chenodeoxycholic acid, CA and DCA concentrations are higher in type 2 diabetes patients compared to healthy subjects74. Bile acid promoting fatty acid digestion and absorption may play important role in the diseases. Furthermore, DCA and LCA in the colonic contents are increased in humans consuming a high fat diet75. The increase of the two bile acids in feces is associated with elevated incidence of colorectal cancer76. DCA damages genomic DNA by oxidation77 and deficiency in base excision repair of oxidative DNA damage is linked to increased risk of intestinal tumors in mice78. Future work is needed to investigate how DCA induces the chronic diseases but attenuates the infectious enteritis.
Altogether, these data reveal that the microbial metabolic product secondary bile acid DCA attenuates NE, through reducing NE-induced host inflammatory response. These findings highlight the importance of elucidating the molecular relationship between infectious pathogen, microbiome, and host response. These discoveries could be applied to control NE and other intestinal diseases targeting microbiome and host inflammatory response.
Acknowledgements
The authors would like to thank the support from staff at Poultry Health Laboratory and Feed Mill in Department of Poultry Science at University of Arkansas, Fayetteville. We also thank P. L. Matsler on helping our histology slides. This research was supported grants of Arkansas Biosciences Institute, USDA National Institute of Food and Agriculture (NIFA) Hatch project 1012366, USDA NIFA Hatch/Multi State project 1018699, USDA NIFA project 2018-06686 to X. Sun. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
H.W. and X.S. designed the experiments and wrote the manuscript with input from co-authors. H.W. and X.S. performed animal and cell experiments and most analysis with the participation from co-authors. All authors read and approved the final manuscript.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Neu HC. The crisis in antibiotic resistance. Science. 1992;257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 2.McGann P, et al. Escherichia coli Harboring mcr-1 and blaCTX-M on a Novel IncF Plasmid: First report of mcr-1 in the USA. Antimicrob Agents Chemother. 2016 doi: 10.1128/AAC.01103-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casewell M, Friis C, Marco E, McMullin P, Phillips I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. The Journal of antimicrobial chemotherapy. 2003;52:159–161. doi: 10.1093/jac/dkg313. [DOI] [PubMed] [Google Scholar]
- 4.Wade B, Keyburn A. The true cost of necrotic enteritis. Poultry World. 2015;31:16–17. [Google Scholar]
- 5.Kaldhusdal M, Benestad SL, Lovland A. Epidemiologic aspects of necrotic enteritis in broiler chickens - disease occurrence and production performance. Avian pathology: journal of the W.V.P.A. 2016;45:271–274. doi: 10.1080/03079457.2016.1163521. [DOI] [PubMed] [Google Scholar]
- 6.Timbermont L, Haesebrouck F, Ducatelle R, Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- 7.Olkowski AA, Wojnarowicz C, Chirino-Trejo M, Drew MD. Responses of broiler chickens orally challenged with Clostridium perfringens isolated from field cases of necrotic enteritis. Research in veterinary science. 2006;81:99–108. doi: 10.1016/j.rvsc.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Olkowski AA, Wojnarowicz C, Chirino-Trejo M, Laarveld B, Sawicki G. Sub-clinical necrotic enteritis in broiler chickens: novel etiological consideration based on ultra-structural and molecular changes in the intestinal tissue. Research in veterinary science. 2008;85:543–553. doi: 10.1016/j.rvsc.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Prescott JF, Parreira VR, Mehdizadeh Gohari I, Lepp D, Gong J. The pathogenesis of necrotic enteritis in chickens: what we know and what we need to know: a review. Avian pathology: journal of the W.V.P.A. 2016;45:288–294. doi: 10.1080/03079457.2016.1139688. [DOI] [PubMed] [Google Scholar]
- 10.Caricilli AM, Castoldi A, Camara NO. Intestinal barrier: A gentlemen’s agreement between microbiota and immunity. World J Gastrointest Pathophysiol. 2014;5:18–32. doi: 10.4291/wjgp.v5.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramanian S, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014 doi: 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deshmukh HS, et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med. 2014 doi: 10.1038/nm.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rantala M, Nurmi E. Prevention of the growth of Salmonella infantis in chicks by the flora of the alimentary tract of chickens. British poultry science. 1973;14:627–630. doi: 10.1080/00071667308416073. [DOI] [PubMed] [Google Scholar]
- 15.Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol. 2010;8:471–473. doi: 10.1016/j.cgh.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Buffie CG, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517:205–208. doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. Journal of lipid research. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Ma H, Patti ME. Bile acids, obesity, and the metabolic syndrome. Best practice & research. Clinical gastroenterology. 2014;28:573–583. doi: 10.1016/j.bpg.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernstein C, et al. Carcinogenicity of deoxycholate, a secondary bile acid. Archives of toxicology. 2011;85:863–871. doi: 10.1007/s00204-011-0648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun X, et al. Microbiota-Derived Metabolic Factors Reduce Campylobacteriosis in Mice. Gastroenterology. 2018;154:1751–1763 e1752. doi: 10.1053/j.gastro.2018.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams RB. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian pathology: journal of the W.V.P.A. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- 22.Rose ME, Long PL, Bradley JW. Immune responses to infections with coccidia in chickens: gut hypersensitivity. Parasitology. 1975;71:357–368. doi: 10.1017/S0031182000047132. [DOI] [PubMed] [Google Scholar]
- 23.Dalloul RA, Lillehoj HS. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev Vaccines. 2006;5:143–163. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- 24.Paiva D, McElroy A. Necrotic enteritis: Applications for the poultry industry. The Journal of Applied Poultry Research. 2014;23:557–566. doi: 10.3382/japr.2013-00925. [DOI] [Google Scholar]
- 25.Uzal FA, et al. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol. 2014;9:361–377. doi: 10.2217/fmb.13.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedman John, Shrestha Archana, McClane Bruce. Clostridium perfringens Enterotoxin: Action, Genetics, and Translational Applications. Toxins. 2016;8(3):73. doi: 10.3390/toxins8030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biondi C, et al. Prostaglandin E2 inhibits proliferation and migration of HTR-8/SVneo cells, a human trophoblast-derived cell line. Placenta. 2006;27:592–601. doi: 10.1016/j.placenta.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Dey I, Lejeune M, Chadee K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br J Pharmacol. 2006;149:611–623. doi: 10.1038/sj.bjp.0706923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazarus M. The differential role of prostaglandin E2 receptors EP3 and EP4 in regulation of fever. Mol Nutr Food Res. 2006;50:451–455. doi: 10.1002/mnfr.200500207. [DOI] [PubMed] [Google Scholar]
- 30.Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol. 2004;36:1187–1205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Lee Y, Rodriguez C, Dionne RA. The role of COX-2 in acute pain and the use of selective COX-2 inhibitors for acute pain relief. Curr Pharm Des. 2005;11:1737–1755. doi: 10.2174/1381612053764896. [DOI] [PubMed] [Google Scholar]
- 32.Wallace JL. Prostaglandin biology in inflammatory bowel disease. Gastroenterol Clin North Am. 2001;30:971–980. doi: 10.1016/S0889-8553(05)70223-5. [DOI] [PubMed] [Google Scholar]
- 33.Tessner TG, Muhale F, Riehl TE, Anant S, Stenson WF. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. J Clin Invest. 2004;114:1676–1685. doi: 10.1172/JCI22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin-Venegas R, Roig-Perez S, Ferrer R, Moreno JJ. Arachidonic acid cascade and epithelial barrier function during Caco-2 cell differentiation. Journal of lipid research. 2006;47:1416–1423. doi: 10.1194/jlr.M500564-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Zamuner SR, Warrier N, Buret AG, MacNaughton WK, Wallace JL. Cyclooxygenase 2 mediates post-inflammatory colonic secretory and barrier dysfunction. Gut. 2003;52:1714–1720. doi: 10.1136/gut.52.12.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatazawa R, Ohno R, Tanigami M, Tanaka A, Takeuchi K. Roles of endogenous prostaglandins and cyclooxygenase isozymes in healing of indomethacin-induced small intestinal lesions in rats. J Pharmacol Exp Ther. 2006;318:691–699. doi: 10.1124/jpet.106.103994. [DOI] [PubMed] [Google Scholar]
- 37.Pavlidis P, Bjarnason I. Aspirin Induced Adverse Effects on the Small and Large Intestine. Curr Pharm Des. 2015;21:5089–5093. doi: 10.2174/1381612821666150915110058. [DOI] [PubMed] [Google Scholar]
- 38.Alrubaye B, et al. Microbial metabolite deoxycholic acid shapes microbiota against Campylobacter jejuni chicken colonization. PloS one. 2019;14:e0214705. doi: 10.1371/journal.pone.0214705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galarza-Seeber R, et al. Leaky Gut and Mycotoxins: Aflatoxin B1 Does Not Increase Gut Permeability in Broiler Chickens. Frontiers in veterinary science. 2016;3:10. doi: 10.3389/fvets.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basak SC, Lee S, Barta JR, Fernando MA. Differential display analysis of gene expression in two immunologically distinct strains of Eimeria maxima. Parasitol Res. 2006;99:28–36. doi: 10.1007/s00436-005-0087-6. [DOI] [PubMed] [Google Scholar]
- 41.Long PL, Millard BJ, Joyner LP, Norton CC. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet Lat. 1976;6:201–217. [PubMed] [Google Scholar]
- 42.Shivaramaiah S, et al. The role of an early Salmonella Typhimurium infection as a predisposing factor for necrotic enteritis in a laboratory challenge model. Avian diseases. 2011;55:319–323. doi: 10.1637/9604-112910-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 43.McReynolds JL, et al. Evaluation of immunosuppressants and dietary mechanisms in an experimental disease model for necrotic enteritis. Poult Sci. 2004;83:1948–1952. doi: 10.1093/ps/83.12.1948. [DOI] [PubMed] [Google Scholar]
- 44.Heikinheimo A, Korkeala H. Multiplex PCR assay for toxinotyping Clostridium perfringens isolates obtained from Finnish broiler chickens. Letters in applied microbiology. 2005;40:407–411. doi: 10.1111/j.1472-765X.2005.01702.x. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka M, Riddell RH. The pathological diagnosis and differential diagnosis of Crohn’s disease. Hepato-gastroenterology. 1990;37:18–31. [PubMed] [Google Scholar]
- 46.Odze R. Diagnostic problems and advances in inflammatory bowel disease. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2003;16:347–358. doi: 10.1097/01.MP.0000064746.82024.D1. [DOI] [PubMed] [Google Scholar]
- 47.Sun X, Threadgill D, Jobin C. Campylobacter jejuni induces colitis through activation of mammalian target of rapamycin signaling. Gastroenterology. 2012;142:86–95 e85. doi: 10.1053/j.gastro.2011.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joo YE, et al. Tomato lycopene extract prevents lipopolysaccharide-induced NF-kappaB signaling but worsens dextran sulfate sodium-induced colitis in NF-kappaBEGFP mice. PloS one. 2009;4:e4562. doi: 10.1371/journal.pone.0004562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rueden CT, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18:529. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun X, Jobin C. Nucleotide-binding oligomerization domain-containing protein 2 controls host response to Campylobacter jejuni in Il10−/− mice. J Infect Dis. 2014;210:1145–1154. doi: 10.1093/infdis/jiu148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan JS, Reed A, Chen F, Stewart CN., Jr. Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sangild PT, et al. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology. 2006;130:1776–1792. doi: 10.1053/j.gastro.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 53.Sun X, Liu B, Sartor RB, Jobin C. Phosphatidylinositol 3-kinase-gamma signaling promotes Campylobacter jejuni-induced colitis through neutrophil recruitment in mice. J Immunol. 2013;190:357–365. doi: 10.4049/jimmunol.1201825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Y, Jiang G, Zhang P, Fan J. Programmed cell death and its role in inflammation. Military Medical Research. 2015;2:12. doi: 10.1186/s40779-015-0039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nielsen OH, Rask-Madsen J. Mediators of inflammation in chronic inflammatory bowel disease. Scandinavian journal of gastroenterology. Supplement. 1996;216:149–159. doi: 10.3109/00365529609094569. [DOI] [PubMed] [Google Scholar]
- 56.Caly DL, D’Inca R, Auclair E, Drider D. Alternatives to Antibiotics to Prevent Necrotic Enteritis in Broiler Chickens: A Microbiologist’s Perspective. Frontiers in microbiology. 2015;6:1336. doi: 10.3389/fmicb.2015.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chapman HD, et al. A selective review of advances in coccidiosis research. Advances in parasitology. 2013;83:93–171. doi: 10.1016/B978-0-12-407705-8.00002-1. [DOI] [PubMed] [Google Scholar]
- 58.Abt MC, McKenney PT, Pamer EG. Clostridium difficile colitis: pathogenesis and host defence. Nature reviews. Microbiology. 2016;14:609–620. doi: 10.1038/nrmicro.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol. 2008;190:2505–2512. doi: 10.1128/JB.01765-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Long JR, Pettit JR, Barnum DA. Necrotic enteritis in broiler chickens. II. Pathology and proposed pathogenesis. Canadian journal of comparative medicine: Revue canadienne de medecine comparee. 1974;38:467–474. [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Sheikhly F, Truscott RB. The pathology of necrotic enteritis of chickens following infusion of broth cultures of Clostridium perfringens into the duodenum. Avian diseases. 1977;21:230–240. doi: 10.2307/1589343. [DOI] [PubMed] [Google Scholar]
- 62.Royer C, Lu X. Epithelial cell polarity: a major gatekeeper against cancer? Cell death and differentiation. 2011;18:1470–1477. doi: 10.1038/cdd.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xin, Z. S. 188–270 (Springer, 1998).
- 64.Delgado ME, Grabinger T, Brunner T. Cell death at the intestinal epithelial front line. The FEBS journal. 2016;283:2701–2719. doi: 10.1111/febs.13575. [DOI] [PubMed] [Google Scholar]
- 65.Grant TD, Specian RD. Epithelial cell dynamics in rabbit cecum and proximal colon P1. The Anatomical record. 2001;264:427–437. doi: 10.1002/ar.1161. [DOI] [PubMed] [Google Scholar]
- 66.Sherman MA, Kalman D. Initiation and resolution of mucosal inflammation. Immunologic Research. 2004;29:241. doi: 10.1385/ir:29:1-3:241. [DOI] [PubMed] [Google Scholar]
- 67.Winter SE, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hassan JO, Curtiss R., 3rd Virulent Salmonella typhimurium-induced lymphocyte depletion and immunosuppression in chickens. Infect Immun. 1994;62:2027–2036. doi: 10.1128/iai.62.5.2027-2036.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Navarro Mauricio, McClane Bruce, Uzal Francisco. Mechanisms of Action and Cell Death Associated with Clostridium perfringens Toxins. Toxins. 2018;10(5):212. doi: 10.3390/toxins10050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keyburn AL, et al. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008;4:e26. doi: 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin TG, Smyth JA. Prevalence of netB among some clinical isolates of Clostridium perfringens from animals in the United States. Vet Microbiol. 2009;136:202–205. doi: 10.1016/j.vetmic.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 72.Paiva DM, Walk CL, McElroy AP. Influence of dietary calcium level, calcium source, and phytase on bird performance and mineral digestibility during a natural necrotic enteritis episode. Poult Sci. 2013;92:3125–3133. doi: 10.3382/ps.2013-03298. [DOI] [PubMed] [Google Scholar]
- 73.Prinz P, et al. Plasma bile acids show a positive correlation with body mass index and are negatively associated with cognitive restraint of eating in obese patients. Frontiers in neuroscience. 2015;9:199. doi: 10.3389/fnins.2015.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cariou B, et al. Fasting plasma chenodeoxycholic acid and cholic acid concentrations are inversely correlated with insulin sensitivity in adults. Nutrition & metabolism. 2011;8:48. doi: 10.1186/1743-7075-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reddy BS, et al. Effect of high-fat, high-beef diet and of mode of cooking of beef in the diet on fecal bacterial enzymes and fecal bile acids and neutral sterols. The Journal of nutrition. 1980;110:1880–1887. doi: 10.1093/jn/110.9.1880. [DOI] [PubMed] [Google Scholar]
- 76.Hill MJ. Bile flow and colon cancer. Mutation research. 1990;238:313–320. doi: 10.1016/0165-1110(90)90023-5. [DOI] [PubMed] [Google Scholar]
- 77.Rosignoli P, et al. Genotoxic effect of bile acids on human normal and tumour colon cells and protection by dietary antioxidants and butyrate. European journal of nutrition. 2008;47:301–309. doi: 10.1007/s00394-008-0725-8. [DOI] [PubMed] [Google Scholar]
- 78.Sakamoto K, et al. MUTYH-null mice are susceptible to spontaneous and oxidative stress induced intestinal tumorigenesis. Cancer research. 2007;67:6599–6604. doi: 10.1158/0008-5472.CAN-06-4802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.