Abstract
Hepatic stellate cells (HSCs), as the most important stromal cells in the liver microenvironment, play crucial roles in hepatic fibrosis, hepatocellular carcinoma, liver regeneration and fetal liver development after transdifferentiating into myofibroblasts (MFs). Transforming growth factor β1 (TGF-β1), as an important polyergic cytokine, is involved in HSCs activation process. However, the specific mechanisms of HSCs transdifferentiation process are not clearly demonstrated. Here we added exogenous recombinant TGF-β1 protein and transforming growth factor β receptor 1 (TGF-βR1) inhibitor SB431542 into mouse HSCs to detect the detailed impact of TGF-β1 signaling on HSCs activation. TGF-β1 signaling significantly increased phosphorylated (P)-Smad2/3 level and promoted Smad2/3 translocation from the cytoplasm to the nucleus, which also caused transdifferentiation of HSCs into MFs. Importantly, TGF-β1 signaling also resulted in high expression of Notch pathway markers Notch1, Jagged1, Hes1 in HSCs. In contrast, expression of those above markers in mouse HSCs were obviously decreased after hampering TGF-β1 signaling via TGF-βR1 inhibitor SB431542. To further examine the effect of Notch pathway on HSCs activation process, TGF-β1-stimulated HSCs and control HSCs were treated with or without LY450139, a specific inhibitor of Notch pathway. LY450139 evidently decreased the expression of Notch1 and MFs marker α-smooth muscle actin (α-SMA) expression in HSCs. These above results may provide a novel insight that TGF-β1 signaling controls HSCs activation process through regulating the expression of Notch pathway markers.
Keywords: Hepatic stellate cells, Transdifferentiation, TGF-β1, Notch pathway, Myofibroblasts
Introduction
Hepatic stellate cells (HSCs) reside in the Disse space and account for 5–10% of total hepatocytes in the liver microenvironment. HSCs have the function of vitamin A lipids storage and directly interact with hepatic parenchymal cells and sinusoid endothelial cells to obtain the stable hemodynamics in some chronic liver diseases (Ekihiro and Schwabe 2015; Chunyue et al. 2013). HSCs have a quiescent phenotype in the healthy liver, and they undergo a process of activation to a proliferative and contractile myofibroblastic phenotype after liver injury. Once activated, HSCs transdifferentiate into myofibroblasts (MFs) and secrete more extracellular matrix (ECM) as well as cytokines to participate in complex pathophysiological processes (Scott 2008; Lorenzini et al. 2010). Activated HSCs play significant roles in acute or chronic liver injuries of various causes, including hepatic fibrosis, hepatocellular carcinoma, liver regeneration and fetal liver development (Scott 2011; Roong and Duncan 2010; Nagahara et al. 2015). Thus, it is of great significance to clearly identify HSCs activation process.
Transdifferentiation from HSCs into MFs is a considerably complicated process, during which a series of growth factors and cytokines excreted by Kuffer cells and sinus endothelial cells are involved, including the pluripotent signaling molecular TGF-β1 (Li et al. 2013; Liu et al. 2018). After binding to the receptor, TGF-β1 signaling promotes activation and further transformation of HSCs into MFs that are characterized by excess production of ECM and high level expression of α-smooth muscle actin (α-SMA) (Xiaobao et al. 2013). In animal models, TGF-β1 signaling induces epithelial–mesenchymal transition (EMT) in embryonic development and osteolytic bone metastasis in cancer cells via mediating Notch pathway (Jiri et al. 2004; Bai et al. 2008). Notch signaling is an evolutionarily conserved pathway that profoundly impacts tissue fibrosis and fetal development by regulating cell survival, proliferation, and fate decision in a context-dependent manner (Gao et al. 2017; Krebs et al. 2000). Moreover, in a recent study, it has also been shown that TGF-β1 signaling controls intrahepatic bile duct development via the Jagged1-Notch-Sox9 signaling axis (Wang et al. 2018). Although Zhang and Chen et al. have revealed that Notch pathway affects α-SMA expression and there may exist a link connecting TGF-β1 signaling with Notch pathway in the process of HSCs activation and liver fibrogenesis (2017, 2018), the precise interaction between TGF-β1 signaling and Notch pathway in the activation and further transdifferentiation of HSCs into MFs has yet to be determined. Herein, in this paper, we hypothesize that TGF-β1 signaling may activate HSCs through regulating Notch pathway markers expression in HSCs.
Materials and methods
Cell lines and culture conditions
Mouse HSCs-T25 cell lines were provided by Bei Chuanglian Biotech BNCC Cell Repository (Bei Chuanglian Biotech, Beijing, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS; Gibco), and 1% penicillin–streptomycin (Gibco). Mouse HSCs were cultured in serum-free medium for 24 h, and then replaced with DMEM (Gibco) containing 15% KnockOut Serum Replacement (KSR; Invitrogen, Carlsbad, CA, USA) and 1% penicillin–streptomycin (Gibco). Meanwhile, mouse HSCs were treated with exogenous muse recombinant TGF-β1 protein (10 ng/mL; R&D Systems, Minneapolis, MN, USA) and TGF-βR1 inhibitor SB431542 (l μmol/L; Sigma–Aldrich, St. Louis, MO, USA) for 48 h and divided into three groups: activation group (HSCs + TGF-β1), control group (HSCs) and inhibition group (HSCs + SB431542). The activation group and control group HSCs were treated with specific Notch pathway inhibitor LY450139 (10 μM; Abmole Bioscience Inc, USA) at the same time for 48 h to determine the effect of Notch pathway on HSCs activation process.
Immunofluorescence staining
Mouse HSCs (3 × 103 per well) were seeded into 96-well plates (Beyotime Institute of Biotechnology, Jiangsu, China). 24 h later, the cells were treated with exogenous 10 ng/mL TGF-β1 and l μmol/L TGF-βR1 inhibitor SB 431542 for 48 h. Then, differently-treated HSCs were rinsed with phosphate-buffered saline (PBS; Beyotime) three times and fixed with 4% paraformaldehyde (PFA; Beyotime) for 20 min at room temperature. 1% Triton X-100 (Beyotime) was used for 10 min to penetrate cell membranes at 4 °C. After rinsing with PBS three times, 50% goat serum (prepared with PBS; Beyotime) was used for 2 h to block non-specific binding sites at 37 °C. Then, the cells were treated with primary antibodies specific to rabbit polyclonal antibody against α-SMA (1:200; Abcam, Cambridge, MA, USA), rabbit monoclonal anti-mouse antibody Smad2/3 (1:500; Cell Signaling Technology, Danvers, MA, USA), rabbit polyclonal antibodies against Jagged1 (1:100; Santa Cruz Biotechnology, CA, USA) overnight at 4 °C. Next day, the cells were incubated with Cyanine (CY3)-conjugated or RTC-conjugated secondary antibody (1:200; Proteintech, Rosemont, IL, USA) for 1 h in the dark. 4′,6-Diamidino-2-phenylindole (DAPI, Beyotime) was used to stain nuclei before capturing images. The images were acquired using a fluorescence microscope (Leica Microsystems, Wetzlar, Germany). Multiple pictures of several areas were taken for further analysis. These experiments were performed in triplicate.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from differently-treated HSCs using Trizol reagent (TaKaRa Biotechnology, Shiga, Japan), which was used as qRT-PCR substrates and first-strand cDNA was synthesized using a Prime Script RT reagent kit (TaKaRa). QRT-PCR was conducted using SYBR Green (SYBR Premix Ex Ta qII, TaKaRa) for fluorescent quantification on the iQ5 gradient real-time PCR detection system (CFX96, Bio-Rad, Hercules, CA, USA). Cycling condition was as follows: 30 s at 95 °C, followed by 40 cycles of 5 s at 95 °C and 30 s at 60 °C. The occurrence of primer dimers and secondary products was inspected using melting curve analysis. Our data indicated that the amplification of primers was specific. The qRT-PCR results were quantified using the threshold cycle (Ct) method, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used to normalize relative RNA inputs. Relative gene expression was calculated by the 2−ΔΔCq method (Livak and Schmittgen 2001), and the results were reported as arbitrary units relative to control conditions. All primers were synthesized by TaKaRa Biotechnology and were listed in Table 1.
Table 1.
QRT-PCR primers
| Gene name | Sense primer sequence (5′→3′) | Anti-sense primer sequence (5′→3′) |
|---|---|---|
| TGF-β1 | GAGGCGGTGCTCGCTTTGTA | CGTTGTTGCGGTCCACCATTA |
| TGF-βR1 | TTATGAGAGAATGCTGGTATGCC | CCTTCCTGTTGGCTGAGTTGT |
| α-SMA | GGCATCCACGAAACCACCTA | CGTCGTATTCCTGTTTGCTGAT |
| Smad2 | AGCTCAAGGCAATCGAAAACTG | TGGAATGGAGTGGGTGTAGTCA |
| Smad3 | CGTGGAACTTACAAGGCGACA | TGGGAGACTGGACGAAAATAGC |
| Notch1 | GGCCACCTGGGCCGGAGCTTC | GCGATCTGGGACTGCATGCTG |
| Jagged1 | AGTGGCTTGGGTCTGTTGCTT | ACCGTGTTGGCTGAGTTGT |
| Hes1 | AAGAGGCGAAGGGCAAGAATAA | GGTGCTTCACAGTCATTTCCAGA |
| GAPDH | GTGCTGAGTATGTCGTGGAGTCT | AGTCTTCTGGGTGGCAGTGA |
Western blotting
Total protein was extracted from differently-treated HSCs using radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime). Bicinchoninic acid (BCA) protein assay kit (Beyotime) was used to measure protein concentrations, 40 μg of which was suitable in this study. Protein extracts from differently-treated HSCs were separated with 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a polyvinylidene difluoride membrane (PVDM; Merck-Millipore, Darmstadt, Germany). After blocking non-specific binding sites with 5% non-fat dry milk (prepared with PBS; Beyotime), membranes were incubated with primary antibodies specific to mouse monoclonal anti-mouse antibody against TGF-β1 (1:1000; Abcam); rabbit polyclonal antibodies against TGF-βR1 (1:500; Santa Cruz Biotech); rabbit polyclonal antibody against α-SMA (1:1000; Abcam); rabbit monoclonal anti-mouse antibody Smad2/3 (1:1600; Cell Signaling Tech); rabbit monoclonal anti-mouse antibody p-Smad2/3 (1:1600; Cell Signaling Tech); rabbit polyclonal antibodies against Notch1 (1:1000; Abcam); rabbit polyclonal antibodies against Jagged1 (1:500; Santa Cruz Biotech); rabbit polyclonal antibodies against Hes1 (1: 500; Santa Cruz Biotech) and rabbit monoclonal anti-mouse antibody GAPDH (1:1000; Cell Signaling Tech) at 4 °C overnight. All primary antibodies were prepared with PBS. After washing with tris-buffered saline containing 0.1% Tween 20 (TBST), membranes were incubated with peroxidase-conjugated secondary antibodies (1:5000; Proteintech) for 1 h at 37 °C. Immunobands were visualized using enhanced chemiluminescence kit (Beyotime) according to the manufacturer’s instructions. GAPDH was used as a loading control. The immunoblots were quantified using FuSion software (Vilber, France). Relative protein expression was measured by the ratio of target protein gray value to GAPDH, which was performed in triplicate.
Statistical analysis
All data are expressed as mean ± SEM from three independent experiments. Statistical comparisons of parameters between groups were made by Student’s t test. Repeated measures ANOVA was used for comparison between groups. p < 0.05 was considered statistically significant. Data analysis was conducted using SPSS 20.0 (SPSS, Stanford, CA, USA) and statistical graphs was drawn using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA).
Results
TGF-β1 signaling induces significant alterations in cell morphology and translocation of Smad2/3
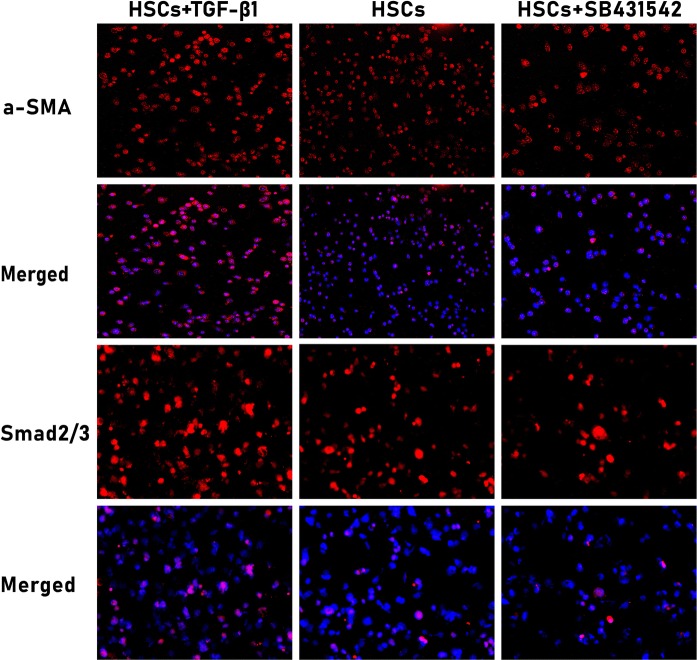
The impact of exogenous recombinant TGF-β1 protein and TGF-βR1 inhibitor on HSCs is time- and dose-dependent, and they make the maximum effect at the concentration of 10 ng/mL and l μmol/L for 48 h respectively (Zhang et al. 2017; Zhu et al. 2014). One of important morphological features of HSCs in response to exogenous stimulation is high level expression of activation marker α-SMA (Li et al. 2015). Thus, we selected the above concentration and time point, at which effects of exogenous TGF-β1 and TGF-βR1 inhibitor on HSCs are the most significant. After this treatment, we confirmed morphological alterations through measuring α-SMA and Smad2/3 expression by immunofluorescence staining (Fig. 1). In line with our expectation, α-SMA showed evidently positive expression in the cytoplasm of TGF-β1-stimulated HSCs, but MFs marker α-SMA was weakly expressed in the cytoplasm of the control and inhibition group HSCs. The results also showed that TGF-β1 signaling promoted the translocation of Smad2/3 from the cytoplasm to the nucleus in HSCs, while blocking TGF-β1 signaling by selective TGF-βR1 inhibitor SB431542 dramatically decreased Smad2/3 translocation. In the control HSCs, there was only few Smad2/3 expression in the HSCs nucleus, further confirming that TGF-β1 is the most significant molecule in HSCs activation process, and that there indeed occur significant morphological changes in HSCs after activation.
Fig. 1.
Morphological changes and translocation of Smad2/3 in differently-treated HSCs. Fluorescence images were taken after treating mouse HSCs with exogenous TGF-β1 and TGF-βR1 inhibitor for 48 h (red for α-SMA and Smad2/3). Images were captured under a fluorescence microscope (α-SMA 200×; Smad2/3 400×). TGF-β1-stimulated HSCs highly expressed MFs marker α-SMA in the cytoplasm, and TGF-β1 signaling also promoted translocation of Smad2/3 from the cytoplasm to the nucleus. In contrast, HSCs weakly expressed α-SMA and Smad2/3 in the cytoplasm and only few Smad2/3 protein was seen in the nucleus in the control and inhibition group, n = 6/group
Transdifferentiation of HSCs via TGF-β1 signaling
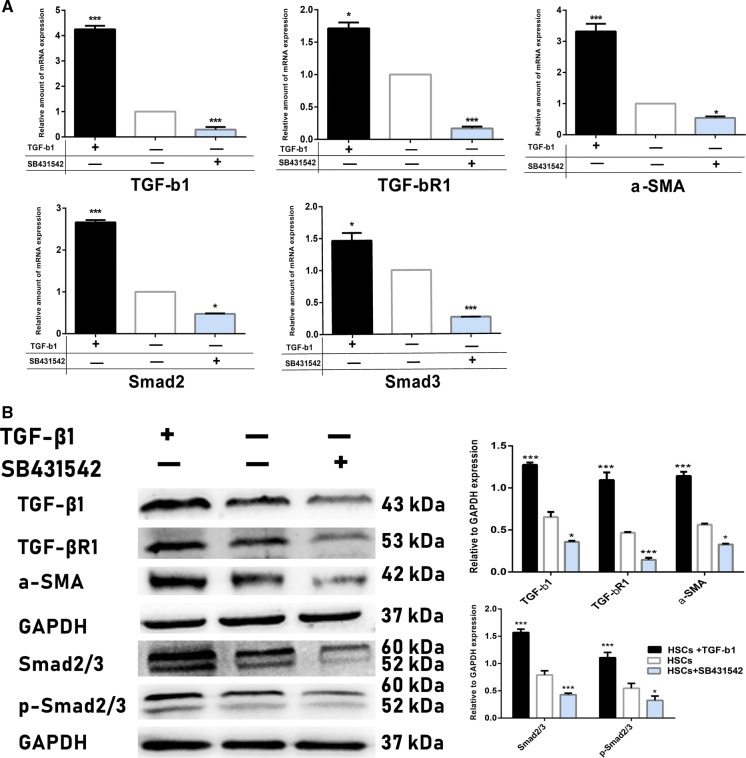
It has been shown that TGF-β1 controls HSCs transdifferentiation process through the canonical Smad signaling pathway involving TGF-βR1-mediated phosphorylation of Smad2/3 (Yun-Lian et al. 2009). Thus, we measured TGF-β1, TGF-βR1, α-SMA, Smad2, Smad3 mRNA expression by qRT-PCR (Fig. 2a) and TGF-β1, TGF-βR1, α-SMA, total Smad2/3, phosphorylated (p)-Smad2/3 protein expression by western blotting (Fig. 2b) methods in differently-treated HSCs. In our results, it was shown that after treating with exogenous TGF-β1, expression of target markers TGF-β1 and TGF-βR1 were significantly increased, as well as MFs marker α-SMA expression in HSCs. Moreover, in western blot analysis study, expression of total Smad2/3 and p-Smad2/3 in the activation group was increased 2.2- and 1.9-fold compared with the control group respectively. However, the expression of above target genes and proteins expression was significantly decreased after interrupting TGF-β1 signaling via specific TGF-βR1 inhibitor. Taken together, these above results further confirmed that TGF-β1 signaling up-regulated the expression of Smad2/3 and promoted its phosphorylation in the transformation process of MFs from HSCs.
Fig. 2.
Transdifferentiation of HSCs into MFs through TGF-β1 signaling. a After treating mouse HSCs with exogenous TGF-β1 and TGF-βR1 inhibitor for 48 h, mRNA expression of target transcription factors was assessed by qRT-PCR. It showed that TGF-β1, TGF-βR1, α-SMA, Smad2, Smad3 mRNA expression in TGF-β1-affected HSCs were significantly increased compared with those in the control group; however, expression of these above transcription factors was significantly decreased in the selective TGF-βR1 inhibitor SB 431542-treated HSCs. b Target proteins expression in differently-treated HSCs was measured by western blotting analysis. The data detected that TGF-β1-activated HSCs expressed high level of TGF-β1, TGF-βR1, α-SMA, Smad2/3 and p-Smad2/3 compared with control HSCs; nevertheless, HSCs obviously decreased those above proteins expression level after being treated with TGF-βR1 inhibitor. ***p < 0.01 and *p < 0.05 compared with the control group respectively; Scale bars = mean ± SEM, n = 3/group
TGF-β1 signaling promotes the expression of Notch pathway markers to activate HSCs
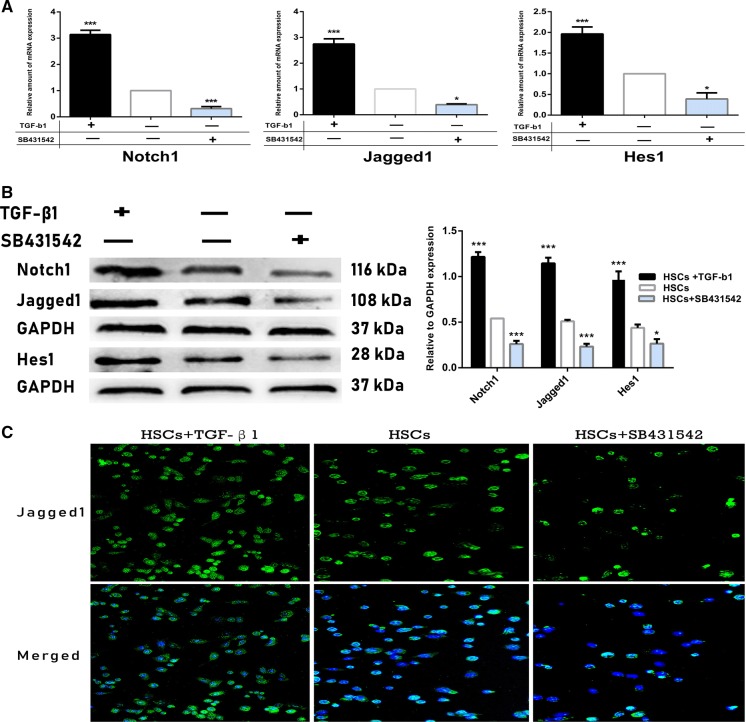
It has been shown in previous studies that most α-SMA+ MFs also highly express Notch pathway ligand Jagged1 (Xiaobao et al. 2013; Kalinichenko et al. 2010). Thus, we analyzed Jagged1 expression in HSCs by immunofluorescence (Fig. 3c). Interestingly, it was found that Jagged1 expression increased significantly in the cytoplasm of exogenous TGF-β1-stimulated HSCs, where α-SMA expression was also highly positive. In contrast, HSCs only expressed low levels of Jagged1 in the cytoplasm of control group and inhibition group, suggesting that there may exist a link between TGF-β1 signaling and Notch pathway in HSCs activation process and further transformation into MFs. To further confirm this hypothesis, we measured Notch pathway-related molecules and target genes Notch1, Jagged1 and Hes1 (Kopan and Ilagan 2009). QRT-PCR (Fig. 3a) analysis showed that in the TGF-β1-activatd HSCs, mRNA expression of Notch1, Jagged1 and Hes1 were increased 3.4-, 2.7- and 2.0-fold compared to the control HSCs. By contrast, expression of these above markers was downregulated in SB431542-treated HSC. Western blotting study (Fig. 3b) also demonstrated that Notch1, Jagged1 and Hes1 proteins expression was all obviously upregulated in the activation group compared to the control group (1.22 ± 0.12 vs 0.54 ± 0.24; 1.15 ± 0.11 vs 0.51 ± 0.13; 0.98 ± 0.25 vs 0.44 ± 0.20; p < 0.01), but their expression was very low in the inhibition group (0.26 ± 0.15 vs 0.54 ± 0.24; 0.23 ± 0.18 vs 0.51 ± 0.13; 0.98 ± 0.25 vs 0.21 ± 0.12; p < 0.05), which was consistent with the previous findings that Notch pathway-related markers are downstream target of TGF-β1 signaling (Wang et al. 2018). The above results further confirm that TGF-β1 signaling regulates Notch pathway-related target molecules expression in HSCs, which is associated with HSCs activation and further transformation into MFs.
Fig. 3.
Regulation of Notch pathway-related markers expression in HSCs by TGF-β1 signaling. a mRNA expression of Notch pathway-related target genes Notch1, Jagged1 and Hes1 were tested by qRT-PCR. Notch1, Jagged1, and Hes1 mRNA expression was significantly upregulated in HSCs after stimulating with exogenous TGF-β1; however, expression of these markers was at the lowest level in SB431542-treated HSCs. b Protein expression of Notch1, Jagged1, Hes1 was assessed with western blotting. Expression of Notch pathway-related proteins was all significantly upregulated in the TGF-β1-activated HSCs compared with control HSCs, but their expression was obviously downregulated after inhibiting TGF-β1 signaling in HSCs. ***p < 0.01 and *p < 0.05 compared with the control group respectively; Scale bars = mean ± SEM, n = 3/group. c Images were captured under a fluorescence microscope (200×). HSCs expressed high levels of Jagged1 in the cytoplasm of activation group; however, Jagged1 was seldom expressed in the control and TGF-βR1-inhibited HSCs, n = 6/group
Notch pathway inhibitor blocks transformation of MFs from HSCs
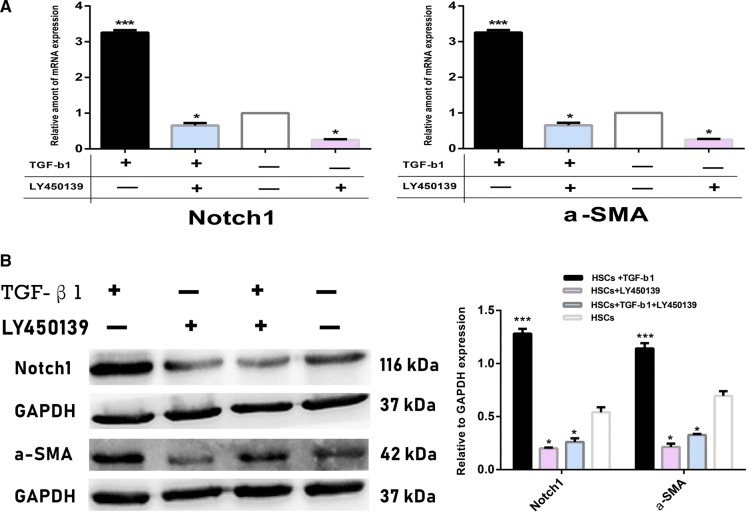
To examine the precise effect of Notch pathway on the activation process of HSCs, we used γ-secretase inhibitor LY450139. QRT-PCR (Fig. 4a) and Western blotting analysis (Fig. 4b) showed that expression of Notch pathway-related marker Notch1 was decreased > fivefold in HSCs after treating HSCs with this inhibitor, and thus, we can use LY450139 as the specific inhibition of Notch pathway with complete confirmation. As expected, cells treated with LY450139 and exogenous TGF-β1 at the same time expressed low levels of MFs marker α-SMA, whose expression was also significantly downregulated in the control HSCs after solely inhibiting Notch pathway. Importantly, there were no statistical differences in α-SMA expression between TGF-β1-stimualted HSCs and control HSCs after interrupting Notch pathway with LY450319, further suggesting that Notch pathway may serve as a downstream target in the transdifferentiation process of HSCs into MFs by TGF-β1 signaling.
Fig. 4.
Inhibition of HSCs transformation into MFs by Notch pathway inhibitor LY450139. a mRNA expression of Notch pathway marker Notch1 and α-SMA were examined by qRT-PCR. mRNA expression of Notch1 and α-SMA was evidently decreased in HSCs after treating with LY450139 for 48 h compared to those in TGF-β1-stimualted and control HSCs. b Protein expression of Notch1 and α-SMA was tested by western blotting. Expression of Notch1 and α-SMA proteins was also distinctly downregulated after inhibiting Notch pathway in HSCs. ***p < 0.01 and *p < 0.05 compared with the control group respectively; Scale bars = mean ± SEM, n = 3/group
Discussion
It is widely recognized that activated HSCs are the most relevant cell type for the development of liver fibrosis, cirrhosis, portal hypertension, and most of these pathological processes can be reversed to recover spontaneously (Scott 2011; Razao et al. 2003; Miyazaki et al. 2009). However, little information exists on the specific mechanisms of HSCs activation process. These above results provide a novel mechanistic role of TGF-β1 signaling in HSCs activation process. Our findings from this study persuasively confirm that Notch pathway-related markers expression in HSCs was indispensable for MFs transformation from HSCs, which was controlled by TGF-β1 signaling. Relevant studies have reported that specific roles of TGF-β1 signaling in HSCs activation process is Smads-dependent and HSCs may occur morphological alterations in response to exogenous TGF-β1 stimulation (Li et al. 2015; Yun-Lian et al. 2009; Shi and Massagué 2003). Indeed, exogenous TGF-β1 significantly increased the level of target molecules TGF-βR1, α-SMA, Smad2/3, p-Smad2/3 expression, which also promoted translocation of Smad2/3 from the cytoplasm to the nucleus. In contrast, blockage of TGF-β1 signaling through selective TGF-βR1 inhibitor SB431542 brought about massive loss of MFs marker expression and was sufficient to downregulate Smads expression. Taken together, the above results suggested that TGF-β1 signaling is definitely possible to determine the initiation of HSCs activation and further transdifferentiation into MFs through controlling their expression of α-SMA and Smads in various liver diseases.
It has been revealed in accumulating studies that a variety of pathways are involved in liver fibrosis, including TGF-β1/Smads, Notch and Hedgehog pathways (Zhang et al. 2017; Xie et al. 2013). Additionally, Dana and Nakano et al. have reported that α-SMA+ MFs expressed high levels of Notch pathway ligand Jagged1 in animal models of CCL4-induced liver cirrhosis. In return, α-SMA expression in HSCs was also regulated by Jagged1 (2010, 2017). Since TGF-β1 signaling controls α-SMA expression in HSCs, we wondered whether expression of Notch pathway markers in HSCs was also affected. As expected, activation of mouse HSCs via exogenous TGF-β1 dramatically increased Notch pathway-related molecule Notch1 expression, accompanied by highly expressed Notch ligand Jagged1 and target gene Hes1, which also resulted in high level expression of MFs marker α-SMA. By contrast, blocking TGF-β1 signaling in HSCs inhibited the expression of Notch pathway-related markers. Importantly, α-SMA expression was obviously downregulated in TGF-β1-stimulated HSCs and control HSCs after preventing Notch pathway with LY450139. These above results provided further evidence for our hypothesis that transdifferentiation of HSCs into MFs is caused by the regulatory roles of TGF-β1 signaling on Notch pathway-related markers expression. HSCs activation and further transformations into MFs is an essential mechanism for promoting chronic end-stage liver diseases, and TGF-β1 signaling is an important molecular in HSCs activation process (Nagahara et al. 2015; Razao et al. 2003; Brenner 2009). TGF-β1 signaling inhibitors have been demonstrated to reduce liver fibrosis progression in rodents, but TGF-β1 signaling is a prototypical cytokine that regulates a plethora of cellular pathways, and non-specific inhibition of TGF-β1 signaling is likely to produce multiple unpredictable adverse effects (Iimuro and Brenner 2008). Moreover, Therapies against TGF-β1/Smads pathway in HSCs only inhibit the initiating process of various chronic liver disease, thereby limiting their further application in clinical settings (Connolly et al. 2010). Therefore, understanding the specific mechanistic roles of TGF-β1 signaling in the mediation of pathophysiological activity of HSCs may ultimately contribute to identifying new therapeutic targets of drugs for various end-stage liver diseases.
Conclusion
To sum up, it is demonstrated in this study that TGF-β1 signaling regulates the expression of Notch pathway-related markers in HSCs, which is closely related to transdifferentiation of HSCs into MFs. However, further ex vivo and animal studies are required to determine precise relationship between TGF-β1 signaling and Notch pathway in regulating HSCs biological activities.
Acknowledgements
The authors would like to acknowledge the technical support from State Key Laboratory on Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia, Xinjiang Medical University. This study was supported by the Postgraduate Scientific Research Innovation Project of Xinjiang Medical University (Grant No. CXCY2018025), Xinjiang Uyghur Autonomous Region Key Laboratory Open Research Program (Grant No. 2017D04004) and Xinjiang Uyghur Autonomous Region Key Project (Grant No. 201430123).
Authors’ contributions
YA: Conception and design; provision of study material; collection and assembly of data; Manuscript writing and revision. MY: Collection and assembly of data; manuscript writing and revision. WL: Data Collection. TT: Provision of study material; collection and assembly of data. AS: Provision of study material; Collection and assembly of data. AM and G: Data collection. AA: Provision of study material. HW: Provision of study material; collection and assembly of data. AT: Conception and design; administrative support. YSS: Conception and design; administrative support. WH: Conception and design; administrative support; Final approval of manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yasen Aimaiti and Maimaitinijiati Yusufukadier contributed equally to this work
Contributor Information
Yasen Aimaiti, Email: 1205739931@qq.com.
Maimaitinijiati Yusufukadier, Email: 1512237458@qq.com.
Wending Li, Email: 813227097@qq.com.
Tusun Tuerhongjiang, Email: 1307673050@qq.com.
Apar Shadike, Email: 410439129@qq.com.
Aisan Meiheriayi, Email: 1748649942@qq.com.
Gulisitan, Email: 2801832846@qq.com.
Aini Abudusalamu, Email: 1307673050@qq.com.
Hui Wang, Email: 125305639@qq.com.
Aji Tuerganaili, Email: tuergan78@sina.com.
Yingmei Shao, Email: Syingmei3000@163.com.
Hao Wen, Email: Dr.wenhao@163.com.
References
- Bai S, Kopan R, Hilton WM, Ong C, Long F, Ross F, et al. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem. 2008;283:6509–6518. doi: 10.1074/jbc.M707000200. [DOI] [PubMed] [Google Scholar]
- Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc. 2009;120:361–368. [PMC free article] [PubMed] [Google Scholar]
- Chen E, Cen Y, Lu D, Luo W, Jiang H. IL-22 inactivates hepatic stellate cells via downregulation of the TGF-β1/Notch signaling pathway. Mol Med Rep. 2018;17:5449–5453. doi: 10.3892/mmr.2018.8516. [DOI] [PubMed] [Google Scholar]
- Chunyue Y, Evason KJ, Kinji A, Stainier DYR. Hepatic stellate cells in liver development, regeneration and cancer. J Clin Invest. 2013;123:1902–1910. doi: 10.1172/JCI66369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly MK, Bedrosian AS, Ashim M, Henning JR, Junaid I, Valery V, et al. In hepatic fibrosis, liver sinusoidal endothelial cells acquire enhanced immunogenicity. J Immunol. 2010;185:2200–2208. doi: 10.4049/jimmunol.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana GP, Thomas DS, Seh-hoon O, Susan VS, Houda D, Bryon EP. Hepatic stellate cells’ involvement in progenitor-mediated liver regeneration. Lab Invest J Tech Methods Pathol. 2010;90:1199–1208. doi: 10.1038/labinvest.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekihiro S, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066–1079. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Chen R, Yu C, Yuan W, Kang S, Zhang Y, et al. Emodin suppresses TGF-β1-induced epithelial–mesenchymal transition in alveolar epithelial cells through Notch signaling pathway. Toxicol Appl Pharmacol. 2017;318:1–7. doi: 10.1016/j.taap.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Iimuro Y, Brenner DA. Matrix metalloproteinase gene delivery for liver fibrosis. Pharm Res. 2008;25:249–258. doi: 10.1007/s11095-007-9311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiri Z, Lukas C, Noem SN, Ttinger EPB. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23:1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichenko VV, Dibyendu B, Yan Z, Gusarova GA, Wooram K, Brian S, et al. Foxf1 ± mice exhibit defective stellate cell activation and abnormal liver regeneration following CCl4 injury. Hepatology. 2010;37:107–117. doi: 10.1053/jhep.2003.50005. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MXG. The canonical notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Develop. 2000;14:1343. [PMC free article] [PubMed] [Google Scholar]
- Li R, Dai G, Zhao M, Zhang Y, Hui L, Zhang X, et al. Preventative effect of Astragalus flavescens on hepatic fibrosis in rats and its mechanism of action. Exp Ther Med. 2013;6:904–908. doi: 10.3892/etm.2013.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Ju D, Zhang DW, Li H, Kong LM, Guo Y, et al. Activation of TGF-Î21-CD147 positive feedback loop in hepatic stellate cells promotes liver fibrosis. Sci Rep. 2015;5:16552. doi: 10.1038/srep16552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang J, Xing W, Peng Y, Huang Y, Fan X. Role of DDAH/ADMA pathway in TGF-β1-mediated activation of hepatic stellate cells. Mol Med Rep. 2018;17:2549. doi: 10.3892/mmr.2017.8107. [DOI] [PubMed] [Google Scholar]
- Livak, Schmittgen Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCq method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lorenzini S, Bird TL, Bellamy C, Samuel K, Aucott R, Clayton E, et al. Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut. 2010;59:645–654. doi: 10.1136/gut.2009.182345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Bouscarel B, Ikegami T, Honda A, Matsuzaki Y. The protective effect of taurine against hepatic damage in a model of liver disease and hepatic stellate cells. Adv Exp Med Biol. 2009;643:293–303. doi: 10.1007/978-0-387-75681-3_30. [DOI] [PubMed] [Google Scholar]
- Nagahara T, Shiraha H, Sawahara H, Uchida D, Takeuchi Y, Iwamuro M, et al. Hepatic stellate cells promote upregulation of epithelial cell adhesion molecule and epithelial–mesenchymal transition in hepatic cancer cells. Oncol Rep. 2015;34:1169–1177. doi: 10.3892/or.2015.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Nakao S, Sumiyoshi H, Mikami K, Tanno Y, Sueoka M, et al. Identification of a novel alpha-fetoprotein-expressing cell population induced by the Jagged1/Notch2 signal in murine fibrotic liver. Hepatol Commun. 2017;1:215–229. doi: 10.1002/hep4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razao I, Xiaoying Z, Nathan T, Harry MS, Stephen K, Christopher B, et al. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. Faseb J Off Publ Federat Am Soc Exp Biol. 2003;17:47. doi: 10.1096/fj.02-0494fje. [DOI] [PubMed] [Google Scholar]
- Roong Z, Duncan SA. Embryonic development of the liver. Hepatology. 2010;41:956–967. doi: 10.1002/hep.20691. [DOI] [PubMed] [Google Scholar]
- Scott L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195–206. doi: 10.1016/j.bpg.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- Wang W, Feng Y, Aimaiti Y, Jin X, Mao X, Li D. TGFβ signaling controls intrahepatic bile duct development may through regulating the Jagged1-Notch-Sox9 signaling axis. J Cell Physiol. 2018;233:5780. doi: 10.1002/jcp.26304. [DOI] [PubMed] [Google Scholar]
- Xiaobao F, Qiannan Z, Shuang L, Yifei L, Houqiang S, Huiping J, et al. Attenuation of CCl4-induced hepatic fibrosis in mice by vaccinating against TGF-β1. PLoS ONE. 2013;8:e82190. doi: 10.1371/journal.pone.0082190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Karaca G, Swiderska M, Michelotti GA, Krüger L, Choi SS, et al. Notch signaling regulates hepatic stellate cell fate by cross-talking with hedgehog signaling. Gastroenterology. 2013;144:S995. doi: 10.1016/S0016-5085(13)63693-8. [DOI] [Google Scholar]
- Yun-Lian L, Chia-Yu L, Chin-Wen C, Yi-Tsau H. Study on antifibrotic effects of curcumin in rat hepatic stellate cells. Phytother Res. 2009;23:927–932. doi: 10.1002/ptr.2764. [DOI] [PubMed] [Google Scholar]
- Zhang K, Han X, Zhang Z, Zheng L, Hu Z, Yao Q, et al. The liver-enriched lnc-LFAR1 promotes liver fibrosis by activating TGFβ and Notch pathways. Nat Commun. 2017;8:144. doi: 10.1038/s41467-017-00204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, He X, Duan Y, Chen J, Wang J, Sun X, et al. Expression of microRNA-454 in TGF-β1-stimulated hepatic stellate cells and in mouse livers infected with Schistosoma japonicum. Parasit Vectors. 2014;7:148. doi: 10.1186/1756-3305-7-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.