Abstract
The present study investigated the role of colchicine in the treatment of RSV infection. Treatment of BEAS-2B cells following RSV infection with colchicine caused a significant decrease in the number of viral plaques. In RSV-infected BEAS-2B cells’ treatment with colchicine leads to a significant up-regulation of both IFN-β1 and RIG-I genes. The levels of interleukin, NO, and MDA were suppressed in BEAS-2B cells infected with RSV by colchicine. The phosphorylation of Stat3, COX-2, and p38 was also suppressed significantly by colchicine. The phosphorylation of IkBα was promoted in RSV-infected BEAS-2B cells’ oncolchicine treatment. In neonatal rats, replication of RSV was inhibited significantly by colchicine treatment which was evident by suppression of RSV-L gene expression. A significant decrease in the level of IL-6 and TNF-α was caused in neonatal rat BALF by colchicine treatment. The production of MDA, NO and MPO in the neonatal rat BALF was suppressed markedly by colchicine treatment. Treatment of the neonatal rats infected by RSV with colchicine suppressed the release of IκBα and COX-2 in the pulmonary epithelial cells. Colchicine treatment of the neonatal rats promoted the expression of IFN-α and IFN-β1. In summary, the current study showed that colchicine inhibited RSV infection in neonatal rats through regulation of anti-oxidative factor production. The expression of IFN-β1 and RIG-I genes was also up-regulated in the RSV-infected alveolar epithelial cells by treatment with colchicine. Therefore, colchicine may be developed as the therapeutic agent for the treatment of RSV infection.
Keywords: Bronchiolitis, Pneumonia, Anti-oxidant, Inflammation, Microtubule
Introduction
Human respiratory syncytial virus (RSV), a member of Paramyxoviridae family, is the causal organism responsible for inducing pneumonia and bronchiolitis. Infection by RSV causes bronchiolitis in more than 50% cases and pneumonia in around 20% cases because of non-availability of the effective vaccines against it (Shay et al. 1999). In infants, infection with RSV leads to the asthma and has very high rate of morbidity throughout the globe (Monick et al. 2001). Infant deaths by the RSV infection sensitised the clinicians and researchers to research for new molecules which can effective inhibit the infection. RSV infection has been found to cause epithelial cell damage and inflammation of the airways leading to the severe breathing problems. Inflammation of airways stimulates the production of cytokines and increased mucous release in children and immune-compromised patients (Rudd et al. 2005).There is huge recruitment of inflammatory cells in the perivascular spaces of lungs in the RSV-infected people (Rudd et al. 2005). Prevention of inflammation during RSV infection has vital therapeutic importance for the treatment of various RSV-induced diseases (Rudd et al. 2005).
In traditional system of medicine, colchicine has been used for the treatment of acute gout as well as in prevention of gout (Nuki 2008). Mechanistic study has shown that colchicine plays anti-inflammatory role by inhibition of neutrophil migration and chemotaxis (Hastie 1991). The motility of various motile cells is inhibited by colchicine through depolymerisation of the microtubule (Hastie 1991). Colchicine has also shown satisfactory results for the treatment of Behçet’s syndrome and Mediterranean fever (Slobodnick et al. 2015). Some of the heart diseases such as pericarditis, acute cardiovascular syndrome, and postsurgical atrial fibrillation are also treated by the use of colchicine (Slobodnick et al. 2015). Moreover, colchicine suppresses synthesis of collagen thereby showing anti-fibrotic activity in the animal models of fibrosis (Rodríguez et al. 1998; Ledwozyw 1994). Treatment of the animal models with colchicine exhibits preventive effect on the renal injury induced during various disorders (Disel et al. 2004; McClurkin et al. 1990; Li et al. 2009; Guan et al. 2013). Taking into consideration the promising anti-inflammatory property and good bioavailability of colchicine the present study was designed to investigate its role in the inhibition of RSV infection. The study showed that colchicine treatment inhibited RSV infection both in vitro in BEAS-2B cells as well in vivo in the neonatal rats by suppression of inflammatory cytokines, oxidative markers and activation of IFN-α and IFN-β1 expression.
Materials and methods
Cell line and culture
The BEAS-2B cell line was supplied by the American Type Culture Collection (ATCC; Rockville, MD, USA). The culture of cells was carried out in DMEM which contained fetal calf serum (10%; v/v) and antibiotics [penicillin (100 U/ml) and streptomycin (100 mg/ml)]. The DMEM medium also contained l-glutamine (2 mM). The culture of cells was performed in an incubator under humidified 5% carbon dioxide atmosphere at 37 °C.
Cell treatment
HEp-2 cells were used to grow the RSV-A2 plaque-purified viral strains in accordance with the reported procedure (Guan et al. 2013). The transgenic strain of RSV employed in titration assay was also grown in HEp-2 cells. BEAS-2B cells at 2 × 105 cells per well density were grown for 36 h before actual experiments in 96 well plates. RSV at five multiplicity of infection was added to the plates using immunoplaque assay according to the known protocol (Wang et al. 2006). Following RSV infection, BEAS-2B cells were treated with 50, 100, 200, 250, 300, 350, and 400 µM concentrations of colchicine for 24 h. The cell viability measurement was performed using MTS–PMS assay. There was no change in BEAS-2B cell viability on treatment with different concentrations of colchicine.
Animal experiments
Neonatal rats
The female Sprague–Dawley rats ten in number were obtained from the Guangdong Medical Laboratory Animal Center, Guandong, China. The rats were acclimatised to the environment in laboratory over 1 week at 24 ± 1 °C temperature, 55–60% humidity and exposed to 12 h light/dark cycles. The rats were housed in individual cages and provided free access to food and water. The animals were monitored carefully from postnatal day 0 and the pups were grown under standard laboratory conditions. The pups (around 25 mg in body weight) 50 in number were assigned to 5 groups of 10 each; RSV, control, and three treatment groups. Using isoflurane, the neonatal rats in RSV and treatment groups on postnatal day 7 were infected with 2 × 105 PFU of RSV through intranasal route in 30 μl of PBS. The plaque assay according to the manual was used for the confirmation of establishment of RSV neonatal rat model. The neonatal rats in three treatment groups were administered 10, 50, and 100 mg/kg doses of colchicine intra-gastrically at 2 h of RSV infection. Approval for the present study was obtained from Animal Care and Ethical Committee, Huazhong Agriculture University (Wuhan, China). The study was performed in accordance with the guidelines for Use of Laboratory Animals issued by the National Institute of Health (Wang et al. 2013).
Histopathology and Immunohistochemistry
Five neonatal rats were sacrificed on postnatal day 12 using isoflurane anaesthesia. The lungs of the rats were excised and using cannula 3 × 0.5 ml ice-cold PBS aliquots were instilled through the tracheal tube to collect the BALF. The pulmonary tissues were then subjected to formalin (10%) and subsequently embedded in paraffin. The paraffin-embedded tissues were put on glass slides and cut into thin 2 mm sections. The sections after de-paraffintion in boiling xylene were blocked on treatment with 5% bovine serum albumin. Incubation of the tissues was performed with primary antibodies against IκBα and COX–2 (Cell Signaling Technology, Inc.) according to manufacturer instructions. Hematoxylin and eosin (H and E)-stained tissues were subjected to immunochemistry assay in accordance with the standard protocols. The signals were monitored using commercially available detection system known as PowerVision UltraVision Quanto (Thermo Scientific) in accordance with the supplier instructions. The light microscope (CKX31; Olympus, Tokyo, Japan) was used for the examination of the tissue sections by three pathologists blindly.
BALF analysis
Inflammation of the pulmonary airways was determined using hemocytometer (XB.K.25; Shanghai Quijing Bohao Biotech Co., Ltd., Shanghai, China) to measure the counts of cells in BALF. The calculation of neutrophils and lymphocytes present in the BALF was performed using fluorescence-activated cell sorting (FACS). In this, neutrophils were distinguished from the lymphocytes on the basis of presence of surface markers, GR-1 and CD3 (Belperio et al. 2002).
Reverse transcription–quantitative polymerase chain reaction (RT–qPCR)
Viral replication and pulmonary inflammation in neonatal rats was determined using RT-PCR assay. From BEAS-2B cells and pulmonary tissues of neonatal rats, total RNA was isolated using commercially available extraction kit (BioTeke Corp., Beijing, China). After quantification, 1 μg RNA samples were subjected to reverse transcription into cDNA using PCR (Invitrogen, Carlsbad, CA, USA). The real-time PCR was carried out using FAM-labeled TaqMan probes and TaqMan Universal Master Mix (Applied Biosystems) using Chromo4 (Bio-Rad Laboratories, Cambridge, MA, USA). The sequence of processes used for PCR consisted of: incubation at 48 °C for 2-min, denaturation at 48 °C for 12 min followed by 48 cycles at 93 °C for 15 s and then at 58 °C for 1 min. The internal loading control used was β-actin and the products of PCR were analysed on 2% agarose gel using ethidium bromide. The visualization of products was performed by a UV illuminator. The sequence of primers used was as follows:
| Gene | Forward | Reverse |
|---|---|---|
| β-actin | TGACGTGGACATCCGCAAAG | CTGGAAGGTGGACAGCGAGG |
| RSV-L | GAACTCAGTGTAGGTAGAATGTTTGCA | TTCAGCTATCATTTTCTCTGCCAAT |
| RIG-I | GCCATTACACTGTGCTTGGAGA | CCAGTTGCAATATCCTCCACCA |
| IFN-β1 | TGCTCTGGCACAACAGGTAG | CAGGAGAGCAATTTGGAGGA |
Western blot analysis
Colchicine-treated BEAS-2B cells were collected, PBS washed, and then treated with RIPA lysis buffer containing phenylmethylsulfonyl fluoride (1%). The lysate obtained was subjected to centrifugation at 4 °C for 20 min at 12,000 ×g to eliminate any insoluble cell debris. The protein concentration present in the lysate was measured by employing a bicinchoninic acid assay using commercially available BCA kit (Pierce Chemical Co., Rockford, IL, USA). The protein samples (20 μg) were resolved on 10% sodium dodecyl sulfate–polyacrylamide gel by the electrophoresis (SDS-PAGE). The proteins were then transferred to the nitrocellulose membrane (Millipore Corp., Billerica, MA, USA) which were blocked on treatment with non-fat milk powder (5%; w/v) and Tween-20 (0.05%). Incubation of the membranes with primary antibodies was performed at 4 °C for overnight followed by membrane washing and then incubation for 2 h with HRP-conjugated secondary antibodies at room temperature. The protein bands were visualized using enhanced chemiluminescence system.
Statistical analysis
The presented data are mean of three independently performed experiments. The values are presented as the mean ± SD. The data were compared between various groups statistically using one-way ANOVA and Student’s t test. Differences were considered significant statistically at P < 0.05.
Results
Inhibition of RSV replication by colchicine
Treatment of BEAS-2B cells following RSV infection with colchicine caused a significant decrease in the number of viral plaques formed in dose-based manner (Fig. 1). BEAS-2B cells after RSV infection were treated with 50, 100, 200, 250, 300, 350, and 400 nM concentrations of colchicine for 24 h. The decrease in number of viral plaques formed was significant from 100 nM concentration of colchicine and the effect was maximum at 350 nM. These findings suggest inhibitory effect of colchicine on replication of RSV virus.
Fig. 1.
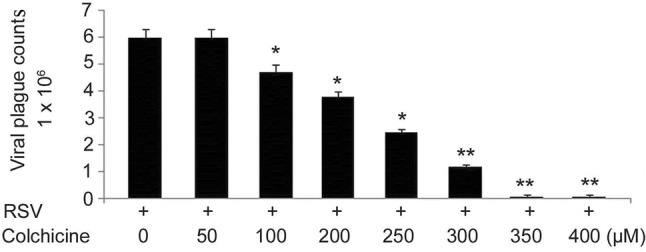
Effect of different colchicine concentrations on RSV replication. After RSV infection, BEAS-2B cells were treated with 50, 100, 200, 250, 300, 350, and 400 µM concentrations of colchicine for 24 h. Immunostaining was used for counting viral plagues to determine replication. *P < 0.05 and **P < 0.01 vs. RSV-infected cells
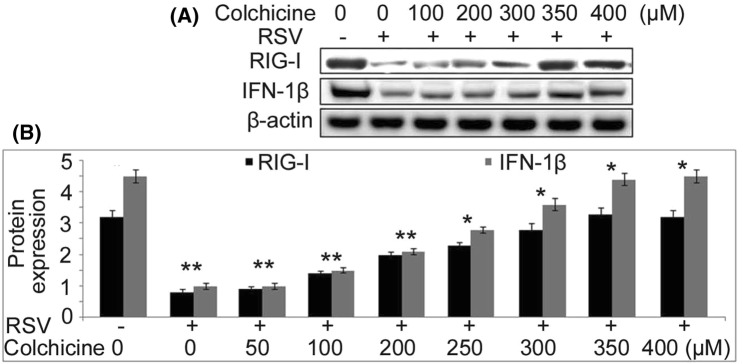
Up-regulation of IFN-β1 and RIG-I gene expression by colchicine in BEAS-2B cells
The effect of colchicine on expression of IFN-β1 and RIG-I genes in BEAS-2B cells following RSV infection was studied using RT-PCR assay and western blotting (Fig. 2). In RSV-infected BEAS-2B cells treatment with colchicine for 24 h lead to a significant up-regulation of both IFN-β1 and RIG-I genes. Colchicine treatment up-regulated IFN-β1 and RIG-I gene expression in RSV-infected BEAS-2B cells in concentration-based manner. No significant increase in IFN-β1 and RIG-I gene expression by colchicine was observed at 50 nM concentration. The increase was significant from 100 nM and the effect was maximum at 350 nM concentration at 24 h.
Fig. 2.
Up-regulation of IFN-β1 and RIG-I genes by colchicine in BEAS-2B cells infected with RSV. The RSV-infected or non-infected BEAS-2B cells were treated with different concentrations of colchicine for 24 h. a Western blot and b RT-PCR was used for analysis of IFN-β1 and RIG-I expression, respectively. *P < 0.05 and **P < 0.01 vs. non-infected cells
Colchicine reduces inflammatory cytokine secretion in BEAS-2B cells following RSV infection
Effect of colchicine on inflammation of lung airways in BEAS-2B cells following RSV infection was assessed by analysis of interleukin (IL)-6, interleukin-8, and MDA levels (Fig. 3). Treatment of BEAS-2B cells following RSV infection with colchicine suppressed the levels of interleukin, NO, and MDA. The levels of IL-6-, IL-8, and MDA were also suppressed in concentration-dependent manner by colchicine in BEAS-2B cells following RSV infection.
Fig. 3.
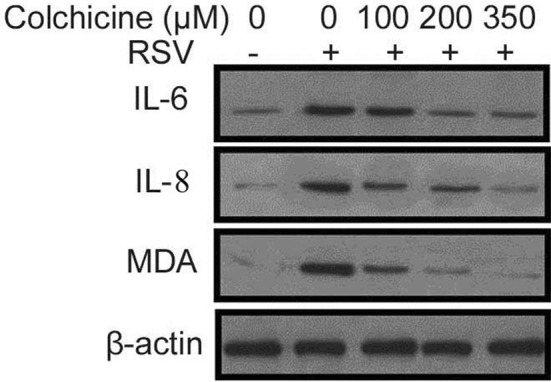
Effect of different colchicine concentrations on secretion of inflammatory cytokines. The RSV-infected or non-infected BEAS-2B cells were treated with 100, 300 and 350 nM concentrations of colchicine for 24 h. Western blotting was used for analysis of inflammatory cytokine release
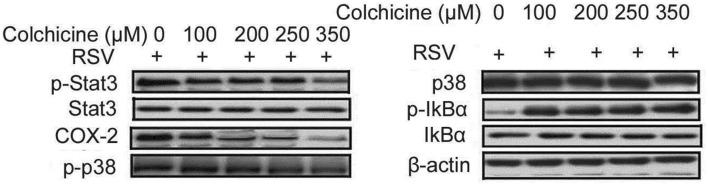
Colchicine reduces oxidative stress molecule secretion in BEAS-2B cells following RSV infection
The expression of COX-2 was reduced by colchicine treatment in BEAS-2B cells (Fig. 4). The phosphorylation of Stat3, COX-2, and p38 was significantly suppressed by colchicine treatment in BEAS-2B cells following RSV infection. On the other hand, IkBα phosphorylation was promoted by treatment of RSV-infected BEAS-2B cells with colchicine. The suppression of oxidative stress molecules by colchicine treatment was concentration dependent with maximum inhibition at 350 nM. The phosphorylation of ERK and JNK was not changed to any significant level by colchicine treatment in BEAS-2B cells following RSV infection.
Fig. 4.
Effect of different colchicine concentrations on secretion of oxidative stress molecules. The RSV-infected or non-infected BEAS-2B cells were treated with 100, 200, 250, and 350 nM concentrations of colchicine for 24 h. Western blotting was used for analysis of oxidative stress molecules
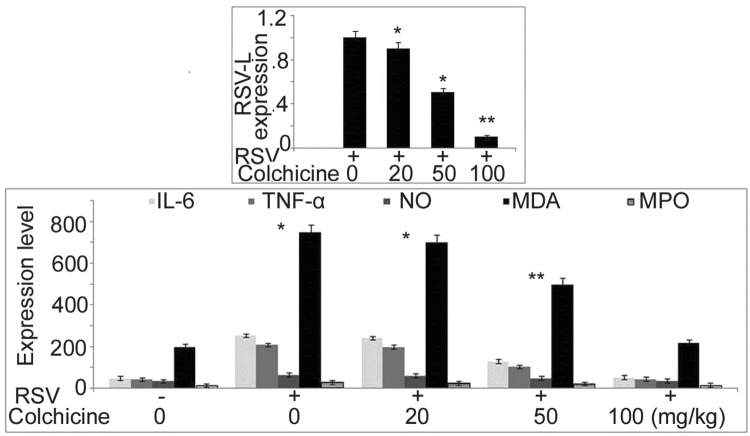
RSV replication inhibition in neonatal rats by colchicine
In neonatal rats, replication of RSV was inhibited significantly by colchicine treatment which was evident by suppression of RSV-L gene expression (Fig. 5). A significant decrease in the level of IL-6 and TNF-α was caused in the neonatal rat BALF by colchicine treatment in dose-based manner with maximum inhibition at 100 mg/kg dose. The production of MDA, NO, and MPO in the neonatal rat BALF was suppressed markedly by colchicine treatment. Although the reduction of MDA, NO, and MPO was significant at all the three doses by the effect was maximum at 100 mg/kg dose.
Fig. 5.
Effect of colchicine on RSV-L expression and oxidative stress molecules in neonatal rat BALF. The neonatal rats after 1 h of RSV infection were treated with 20, 50 and 100 mg/kg doses of colchicine. At 48 h post infection the neonatal rats were sacrificed to determine RSV-L expression and oxidative stress molecules by RT-PCR. *P < 0.05 and **P < 0.02 vs. untreated group
Effect of colchicine on pro-inflammatory molecules
Treatment of the neonatal rats infected by RSV with colchicine suppressed the release of COX-2 and promoted IκBα level in the pulmonary epithelial cells (Fig. 6). The suppression of COX-2 and promotion of IκBα by colchicine in the neonatal rat pulmonary cells were dose dependent. Colchicine treatment of the neonatal rats promoted the expression of IFN-α and IFN-β1. These findings proved that colchicine treatment inhibited RSV infection in neonatal rats by targeting IFN-α and IFN-β1 pathways.
Fig. 6.
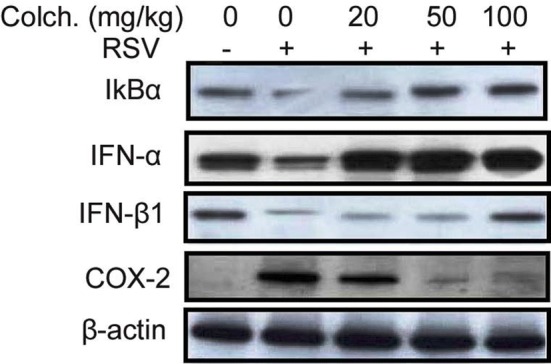
Colchicine treatment suppresses pro-inflammatory and promotes IFN-α and IFN-β1 expression in neonatal rats. In pulmonary tissues IκBα, COX-2, IFN-α, and IFN-β1 levels were analysed using western blot
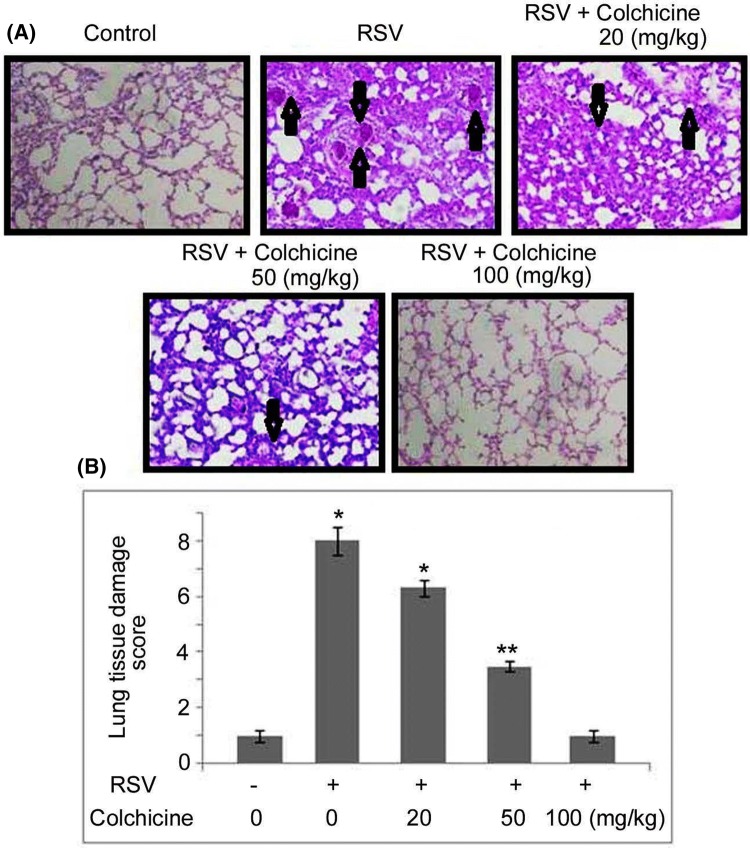
Effect of colchicine on pulmonary histology of the neonatal rats
The H and E staining of pulmonary tissues of the RSV-infected neonatal rats showed alleviated scores for inflammation in the airway and parenchyma tissues (Fig. 7). The pulmonary airway and parenchyma tissue inflammation scoring was markedly lower in the RSV-infected neonatal rats on treatment with colchicine. The accumulation of peribronchial, perivascular, and alveolar infiltrates was also markedly inhibited in the RSV-infected neonatal rats by colchicine treatment.
Fig. 7.
Effect of colchicine on lung histological parameters in RSV-infected neonatal rats. The neonatal rats after 1 h of RSV infection were treated with 20, 50, and 100 mg/kg doses of colchicine. a At 48 h post infection, the neonatal rats were sacrificed to determine airway inflammation severity and histologic parameters using H and E staining. b Average pulmonary tissue damage scores were determined in each experimental group. *P < 0.05 and **P < 0.02 vs. untreated group
Discussion
The present study investigated the effect of colchicine on RSV infection in vitro in BEAS-2B cells and in vivo in the neonatal rats. The study showed that colchicine treatment inhibited RSV infection both in vitro as well in vivo in the neonatal rats by suppression of inflammatory cytokines, oxidative markers, and activation of IFN-α and IFN-β1 expression.
RSV infection to human beings leads to the development of diseases to varied extent ranging from simple coldness to serious problem like bronchiolitis and pneumonia (Hall et al. 1976). RSV on entering the host body damages the cells by decomposing the lipids and proteins and then uses its machinery to replicate own genetic material. Death of the cells in RSV-infected patients is believed to be mainly due to the activation of oxidative pathway (Collins and Graham 2008). RSV infection causes shifting of equilibrium between secretion of ROS and production of anti-oxidants which leads to oxidative damage in the cells (Collins and Graham 2008). The pathogenesis of RSV infection is associated with the increased production of cytokines in lung alveolar cells (Zeng et al. 2011). The oxidative stress induced by RSV activates transcription molecule, nuclear factor-κB, and mitogen-activated protein kinase (Jie et al. 1984). In the present study, colchicine treatment inhibited RSV replication in dose-based manner in vitro in BEAS-2B cells and in vivo in the neonatal rats. The RSV replication inhibition by colchicine treatment was evident by a significant reduction in the number of viral plaques formed in treatment neonatal group in comparison with the untreated rats. The colchicine treatment of RSV-infected neonatal rats led to a marked reduction of pulmonary airway and parenchyma tissue inflammation scoring. Treatment with colchicine suppressed accumulation of peribronchial, perivascular, and alveolar infiltrates in the neonatal rats.
Up-regulation of the IFN-β1 and RIG-I genes has been found to be associated with the inhibition of replication of the RVS (Icardi et al. 2012; Feng et al. 2016). In the present study, colchicine treatment caused a significant up-regulation of IFN-β1 and RIG-I genes in dose-based manner in alveolar epithelial cells infected with RSV. Thus, colchicine exhibits inhibitory effect on replication of RSV partly by up-regulation of IFN-β1 and RIG-I genes.
Oxidative stress plays a vital role in the development of bronchiolitis induced by RSV infection. The alveolar epithelial cells and endothelial cells secrete oxidative stress molecules which target several cellular components such as membrane lipids, proteins, and the chromatin material resulting in tissue damage (Hosakote et al. 2012). In alveolar epithelial cells, enhanced secretion of ROS activates the expression of transcription factor associated, IRF, and chemokine genes (Jamaluddin et al. 2009). This is believed to be linked with the suppression of antioxidant enzyme level and their activity (Jamaluddin et al. 2009). In the present study, secretion of oxidative molecules such as nitric oxide, MDA, and MPO was markedly higher in the alveolar epithelial cells and pulmonary tissues of neonatal rats infected with RSV. Colchicine treatment significantly reduced the levels of nitric oxide, MDA and MPO in both in vitro as well as in neonatal rats. These findings suggested that colchicine treatment down-regulated RSV replication by preventing oxidative damage to the host tissues and debarring the virus to use the host machinery.
Conclusion
In conclusion, the current study showed that colchicines inhibited RSV infection in neonatal rats through regulation of anti-oxidative factor production. The expression of IFN-β1 and RIG-I genes was also up-regulated in the RSV-infected alveolar epithelial cells by treatment with colchicine. Therefore, colchicine may be developed as the therapeutic agent for the treatment of RSV infection.
Acknowledgements
This study was supported by Projects in Yunnan Science and Technology Plan (No: 2017FB147).
References
- Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM. Critical role for CXCR1 and CXCR1 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest. 2002;110:1703–1716. doi: 10.1172/JCI0215849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82:2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disel U, Paydas S, Dogan A, Gulfiliz G, Yavuz S. Effect of colchicine on cyclosporine nephrotoxicity, reduction of TGF–beta overexpression, apoptosis, and oxidative damage: an experimental animal study. Transpl Proc. 2004;36:1372–1376. doi: 10.1016/j.transproceed.2004.05.078. [DOI] [PubMed] [Google Scholar]
- Feng Q, Su Z, Song S, Χu H, Zhang B, Yi L, Tian M, Wang H. Histone deacetylase inhibitors suppress RSV infection and alleviate virus-induced airway inflammation. Int J Mol Med. 2016;38:812–822. doi: 10.3892/ijmm.2016.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan T, Gao B, Chen G, Chen X, Janssen M, Uttarwar L, Ingram AJ, Krepinsky JC. Colchicine attenuates renal injury in a model of hypertensive chronic kidney disease. Am J Physiol Renal Physiol. 2013;305:F1466–F1476. doi: 10.1152/ajprenal.00057.2013. [DOI] [PubMed] [Google Scholar]
- Hall CB, Geiman JM, Biggar R, Kotok DI, Hogan PM, Douglas GR., Jr Respiratory syncytial virus infections within families. N Engl J Med. 1976;294:414–419. doi: 10.1056/NEJM197602192940803. [DOI] [PubMed] [Google Scholar]
- Hastie SB. Interactions of colchicine with tubulin. Pharmacol Ther. 1991;51:377–401. doi: 10.1016/0163-7258(91)90067-V. [DOI] [PubMed] [Google Scholar]
- Hosakote YM, Komaravelli N, Mautemps N, Liu T, Garofalo RP, Casola A. Antioxidant mimetics modulate oxidative stress and cellular signaling in airway epithelial cells infected with respiratory syncytial virus. Am J Physiol Lung Cell Mol Physiol. 2012;303:L991–L1000. doi: 10.1152/ajplung.00192.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icardi L, Lievens S, Mori R, Piessevaux J, De Cauwer L, De Bosscher K, Tavernier J. Opposed regulation of type I IFN-induced STAT3 and ISGF3 transcriptional activities by histone deacetylases (HDACS) 1 and 2. FASEB J. 2012;26:240–249. doi: 10.1096/fj.11-191122. [DOI] [PubMed] [Google Scholar]
- Jamaluddin M, Tian B, Boldogh I, Garofalo RP, Brasier AR. Respiratory syncytial virus infection induces a reactive oxygen species-MSK1-phospho-Ser-276 RelA pathway required for cytokine expression. J Virol. 2009;83:10605–10615. doi: 10.1128/JVI.01090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie YH, Cammisuli S, Baggiolini M. Immunomodulatory effects of Panax Ginseng C.A. Meyer in the mouse. Agents Actions. 1984;15:386–391. doi: 10.1007/BF01972376. [DOI] [PubMed] [Google Scholar]
- Ledwozyw A. The effect of colchicine and vinblastine on bleomycin–induced lung fibrosis in rats. Acta Physiol Hung. 1994;82:383–389. [PubMed] [Google Scholar]
- Li JJ, Lee SH, Kim DK, Jin R, Jung DS, Kwak SJ, Kim SH, Han SH, Lee JE, Moon SJ, et al. Colchicine attenuates inflammatory cell infiltration and extracellular matrix accumulation in diabetic nephropathy. Am J Physiol Renal Physiol. 2009;297:F200–F209. doi: 10.1152/ajprenal.90649.2008. [DOI] [PubMed] [Google Scholar]
- McClurkin C, Jr, Phan SH, Hsu CH, Patel SR, Spicker JK, Kshirsagar AM, Yuan WY, Wiggins RC. Moderate protection of renal function and reduction of fibrosis by colchicine in a model of anti–GBM disease in the rabbit. J Am Soc Nephrol. 1990;1:257–265. doi: 10.1681/ASN.V13257. [DOI] [PubMed] [Google Scholar]
- Monick M, Staber J, Thomas K, Hunninghake G. Respiratory syncytial virus infection results in activation of multiple protein kinase C isoforms leading to activation of mitogen-activated protein kinase. J Immunol. 2001;166:2681–2687. doi: 10.4049/jimmunol.166.4.2681. [DOI] [PubMed] [Google Scholar]
- Nuki G. Colchicine: its mechanism of action and efficacy in crystal–induced inflammation. Curr Rheumatol Rep. 2008;10:218–227. doi: 10.1007/s11926-008-0036-3. [DOI] [PubMed] [Google Scholar]
- Rodríguez L, Cerbón-Ambriz J, Muñoz ML. Effects of colchicine and colchiceine in a biochemical model of liver injury and fibrosis. Arch Med Res. 1998;29:109–116. [PubMed] [Google Scholar]
- Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol. 2005;79:3350–3357. doi: 10.1128/JVI.79.6.3350-3357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- Slobodnick A, Shah B, Pillinger MH, Krasnokutsky S. Colchicine: old and new. Am J Med. 2015;128:461–470. doi: 10.1016/j.amjmed.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Peters N, Schwarze J. Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. J Immunol. 2006;177:6263–6270. doi: 10.4049/jimmunol.177.9.6263. [DOI] [PubMed] [Google Scholar]
- Wang YCYX, Yang X, Xing LH, Kong WZ. Effects of SAHA on proliferation and apoptosis of hepatocellular carcinoma cells and hepatitis B virus replication. World J Gastroenterol. 2013;19:5159–5164. doi: 10.3748/wjg.v19.i31.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng R, Li C, Li N, Wei L, Cui Y. The role of cytokines and chemokines in severe respiratory syncytial virus infection and subsequent asthma. Cytokine. 2011;53:1–7. doi: 10.1016/j.cyto.2010.09.011. [DOI] [PubMed] [Google Scholar]