Abstract
Oleanolic acid (OA), a potential drug for diabetic nephropathy (DN) treatment was found to downregulate the expression of microRNA (miR). The research aimed to investigate the effect of OA on autophagy mediated through miR-142-5p targeted PTEN signal. NRK-52E cells were cultured under normal or high glucose condition. DN model were induced by intravenous injection with streptozotocin (55 mg/kg). Renal fibrosis mice were detected by hematoxylin and eosin (HE) staining, Masson staining and immunohistochemistry assay. TargetScan and dual-luciferase reporter assay system was used to detect the target of miR-142-5p. Expression levels of microRNA and proteins were analyzed by real-time PCR and western blotting. Autophagy was decreased in the progression of renal fibrosis in diabetic nephropathy mice (in vivo) and in high glucose-induced NRK-52E cells (rat kidney epithelial cells) (in vitro) as the expression ofLC-3I and LC-3II (indicators of autophagy) were decreased mice MiR-142-5p was unregulated and PTEN was down-regulated in kidney mice and high glucose-induced NRK-52E cells. Targetscan prediction revealed that PTEN was a target of miR-142-5p. OA restricted HG-induced NRK-52E cell fibrosis through inhibition of miR-142-5p to promote PTEN expression and autophagy levels. To sum up, the research indicated that OA promoted autophagy through inhibition of PI3K/AKT/mTOR pathway. OA alleviated diabetic renal fibrosis by increasing autophagy through regulation of miR-142-5p/PTEN via PI3K/AKT/mTOR pathway in NRK-52E cells.
Keywords: Oleanolic acid, Diabetic nephropathy, miR-142, Autophagy
Introduction
Diabetic nephropathy (DN) is one of the most common clinical complications of diabetes that often causes failure, and eventually leads to death. With the incidence rising year by year and lack of efficient treatments, DN has become a global public burden. By 2025, the estimated cases of diabetic patients worldwide will reach up to 370 million, 30% of which would deteriorate into DN (Kainz 2015). The pathological phenotypes of DN includes mesangial cell proliferation, vascular mesangial expansion, and accumulation of abnormal extracellular matrix (ECM), resulting in glomerular sclerosis, fibrosis and eventually chronic renal failure, which ultimately endanger life span of patients (Lit and Meyer 2006).
Autophagy, a lysosomal-dependent degradation pathway, plays a crucial role in removing damaged cytoplasmic organelles to maintain intracellular homeostasis during physiological and pathological processes, including DN (Ding and Choi 2015). Many studies have reported autophagy was inhibited in some obesity-related metabolic diseases and was reported to play a renoprotective role in kidney injury. During DN, cytoprotective function is carried out through autophagy, which has been considered as a significant therapeutic target in DN treatment (Tanaka et al. 2012). miRNAs, 20–25 nucleotide non-coding RNAs that regulate mRNA translation, is related with autophagy in DN (Delić et al. 2016). Recently, researchers discovered resveratrol could ameliorate DN via activation of autophagy by regulating miRNA-18a-5p expression (Xu 2017). miR-142-5p is a maker of gestational diabetes mellitus (Zhu and Leung 2015). Phosphatase and tensin homolog (PTEN), a widely expressed protein in humans, was confirmed to be a downstream target gene of miR-142 with great importance in autophagy process in context of DN (Bai et al. 2017). Accumulating research indicated autophagy and miR-142 played a vital role in DN (Xia et al. 2018).
Widely distributed in food and plants, oleanolic acid (OA) is a naturally occurring well-known pentacyclic triterpenoid. Recent studies have shown that OA improves the metabolic syndrome and possesses anti-diabetic effects on animal models (Pollier and Goossens 2012). In streptomycin-induced diabetic mouse models, OA significantly reduced blood glucose levels, improved dysfunction of insulin signaling, and restored glycogenase levels and activities in muscle and liver tissues (Ngubane et al. 2011). In addition, OA exhibited hypolipidemic effects on high-fat diet-induced hyperlipidemic mice by regulating miRNA expression (Chen et al. 2017). In this study, we reported OA alleviated diabetic renal fibrosis by activation of autophagy through miR-142-5p/PTEN via PI3K/AKT/mTOR pathway in NRK-52E cells. Our study was consistent with previous research, further indicating OA functions as a potential drug candidate for DN therapeutics in vitro and in vivo.
Materials and methods
Animals
C57BL/KsJ-db/db mice carrying a mutation in the leptin receptor gene were purchased from Shanxi Said High Tech Co., Ltd. (Shanxi, China). Mice were housed in a climate-controlled animal facility at a temperature of 20–25 °C in the animal center of PLA 81st Hospital. All animal experiments were approved by the Ethics Committee of PLA 81st Hospital. 25 week-old male mice were randomly divided into two groups: ① Normal control group (NC group, n = 10) and ② Diabetic nephropathy group (DN group, n = 10). All mice had free access to food and water throughout the whole experiment. DN mouse models were induced by tail vein injection with streptozotocin (STZ; Sigma, MO, USA) at 55 mg/kg, while the NC groups mice were injected with the same amount of vehicle. The level of blood glucose (BG) was analyzed 48 h post-injection. In this study, concentration of BG higher than 16.7 mM was considered as diabetics, indicating DN model was successfully established. Femoral arterial blood and 24 h-urine were collected at the 10th week for urine protein and biochemical indexes quantification using a 1650 automatic biochemical analyzer (Beckman Instruments, CA, USA). All animals were anesthetized with chloral hydrate, the blood samples were collected from abdominal aorta until the animal died. Then kidney samples were collected. The kidney index (KI) was calculated as followed:
Cell culture
NRK-52E cells (rat kidney epithelial cells, ATCC® CRL-1571) were cultured in DMEM medium (Gibco, CA, USA) supplemented with 5% fetal bovine serum (FBS; Gibco, CA, USA) with glucose (NG, 5.5 mM glucose or HG, 30 mM glucose) in at 37 °C in the 5% CO2 humidified atmosphere.
Histology and immunohistochemistry
Hematoxylin and eosin (HE) staining was utilized to examine the renal tissue paraffin sections while the renal tissue fibrosis was assessed by Masson staining. Immunohistochemistry assay against Col IV protein by anti-Col IV antibody (Sigma) was performed using biotin–streptavidin–peroxidase method (ZSBIO, Beijing, China) according to the manufacturer’s instruction.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNAs were isolated from renal tissues or NRK-52E cells by Trizol (Invitrogen, CA, USA). For cDNA synthesis, Bulge-Loop™ miRNA RT-PCR primer kits (RiboBio, China) and First Strand cDNA Synthesis kit (Takara, Japan) were used according to the manufactures’ instructions. Real-time PCR was carried out using SYBR Green SuperMix (Bio-Rad) kit to analyze expression of miRNA and PTEN. Thermo cycling conditions were set up as following: ① 95 °C for 30 s, ② 35 cycles of 95 °C for 10 s and 60 °C for 25 s. Primers were designed referred to our previously published study (Xu and Wang 2018). Relative expression levels of miR-142-5p (normalized to U6) and PTEN (normalized to GAPDH) were calculated using the 2−△△Cq method.
Western blotting
Total proteins from the renal tissues orNRK-52E cells were extracted using lysis buffer (50 mM Tris (pH = 7.4), 150 mM NaCl, 1% TritonX-100, 1% sodium deoxycholate, 0.1% SDS). 20 μg samples were subjected to electrophoresis using SDS-PAGE gradient gel (4–15%, Bio-rad) and then transferred onto PVDF membrane. Non-specific membrane binding was blocked with 5% BSA in 1xPBST for 1 h. Membrane was then incubated with primary antibodies (at a dilution of 1:1000) at room temperature for 3 h as follows: PTEN (CST #9188), Col IV (Abcam, ab6586), LC3 (CST, #3868), p62 (CST, #39749), Akt (CST #4685), p-Akt (CST #9611), mTOR (CST #2983), p-mTOR (CST #5536), and β-actin (CST #4970). Subsequently, for detection, HRP-conjugated secondary antibodies were incubated with membranes for 1 h. Antigen–antibody complex were visualized using enhanced chemiluminescence kit (Fujifilm, Tokyo, Japan).
Luciferase reporter assay
The pMIR-REPORT plasmid (Invitrogen) containing putative miR-142-5p binding sequence or the mismatch sequence in PTEN 3′UTR was cloned, and then the predicted mutation site was introduced by site-directed mutagenesis. 5 × 104 NRK-52E cells were seeded into a 24-well plate, followed by co-transfection with 50 nM of miR-142-5p mimics or NC mimics and 100 ng of the Wild-type (WT) PTEN 3′UTR or Mutant (MUT) PTEN 3′UTR. Luciferase activity was examined 24 h post-transfection by dual-luciferase reporter assay system (Promega, USA).
Statistical analysis
The statistical analysis was performed by Student’s 2-tailed t-test for two groups or One-way ANOVA for more than two groups followed by Dunnett’s test. Data were shown as mean ± SD from at least three independent experiments. p < 0.05 was considered statistically significant in this study (*p < 0.05, **p < 0.01, ***p < 0.001).
Results
Autophagy was decreased in the progression of renal fibrosis in diabetic nephropathy mice
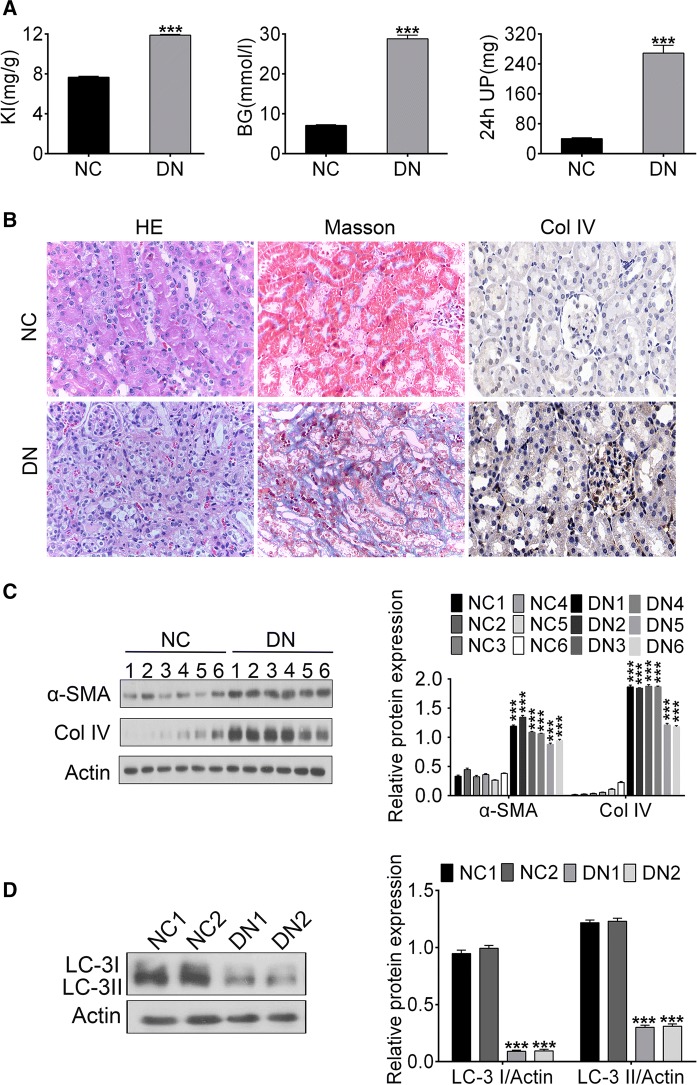
As one of the most important clinical manifestations in DN patients, proteinuria is reported to be induced by high blood glucose. In addition, kidney index (KI) was adopted as an indicator of renal function in this study. Our results showed that KI, blood glucose (BG), and 24 h urinary protein (UP) were increased in DN mice compared to negative control (***p<0.001) (Fig. 1a), indicating DN mice models were established successfully.
Fig. 1.
Autophagy level in the progression of DN. a The concentration or content of KI, BG and 24 h UP of control and DN mice. b HE staining, Masson staining and Col IV immumohistochemical staining of nephridial tissue of control and DN mice. c The expression of α-SMA and Col IV of nephridial tissues by Western blotting. d The expression of LC3-I and LC3-II of nephridial tissues by Western blotting. (***p < 0.001 vs. control or NC group)
HE staining demonstrated mild vacuolar degeneration, renal tubular dilatation and macrophage infiltration of renal interstitium were presented in the renal tubule epithelial cells in DN mice but not in NC mice (Fig. 1b). According to Masson staining, large numbers of fibroblasts were proliferated and collagen fibers (blue) formed in the renal interstitial with diffuse inflammatory cell infiltration (Fig. 1b). IHC showed that the Col IV was upregulated in DN rat renal tissues (Fig. 1b). Western blotting results revealed expression of Col IV or α-SMA were elevated significantly (**p < 0.01), while LC3-I and LC3-II, indicators of autophagy, were downregulated (*p < 0.05) in the renal tissues in DN mice (Fig. 1c, d). Taken together, our studies reveal that the decreased level of autophagy may be associated with renal fibrosis in the diabetic nephropathy mice.
Autophagy was inhibited upon high glucose environment in NRK-52E cells
Kidney epithelial cell line NRK-52E cells were treated with high glucose to establish an in vitro model. Western blotting results indicated the expression of Col IV protein in the HG group was increased compared with that in NG group (Fig. 2a). To further study the effect of autophagy in HG-induced NRK-52E cells, cultured cells were treated with 100 nM of autophagy agonist rapamycin (RAPA), or DMSO as negative control for 48 h. In HG-treated cells, expression levels of both LC3-I and LC3-II were decreased (Fig. 2b). In contrast, the expression of p62 and Col IV were increased (Fig. 2c). Interestingly, RAPA inversed the effects induced by high glucose (***p < 0.001). Collectively, results obtained from this section demonstrate that autophagy might inhibit the expression of HG-induced Col IV.
Fig. 2.
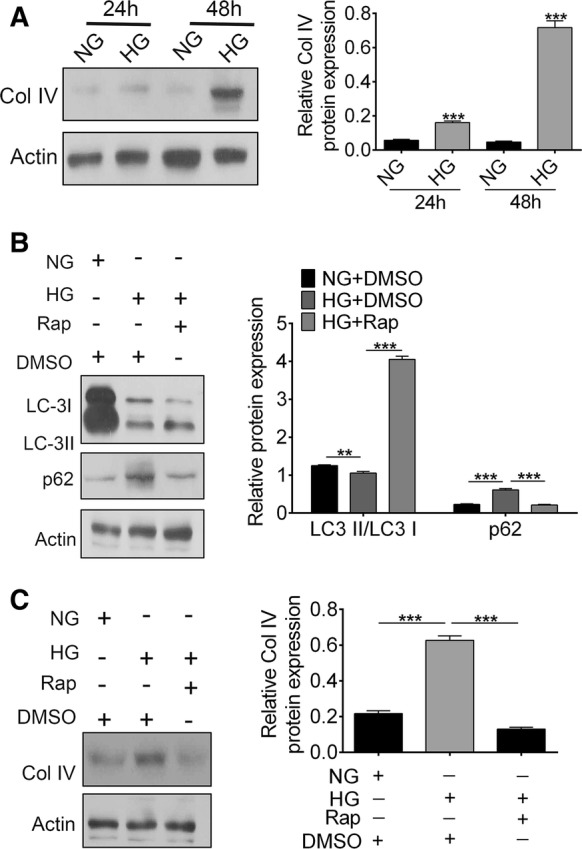
Effects of HG on autophagy in NRK-52E cells. a The expression of Col IV in with/without HG treated NRK-52E cells at 24 h and 48 h. b The expression of LC3-I, LC3-II and p62 of NRK-52E cells treated with or without RAPA upon HG. c The expression of Col IV of NRK-52E cells treated with or without RAPA upon HG. (***p < 0.001 vs. control or NC group)
miR-142-5p was up-regulated and PTEN was downregulated in DN model in vitro and in vivo
Real-time PCR and Western blotting analysis were performed to quantify the mRNA and protein expression level. Our results showed the expression of miR-142-5p were significantly higher in renal tissues of DN mice and in HG-induced NRK-52E cells than that in control (***p < 0.001) (Fig. 3a). Levels of PTEN mRNA and protein in renal tissues in DN mice and in HG-induced NRK-52E cells were lower than control groups (Fig. 3b, c). These results suggest miR-142-5p was up-regulated while the PTEN was down-regulated in renal tissues of DN mice and in HG-induced NRK-52E cells.
Fig. 3.
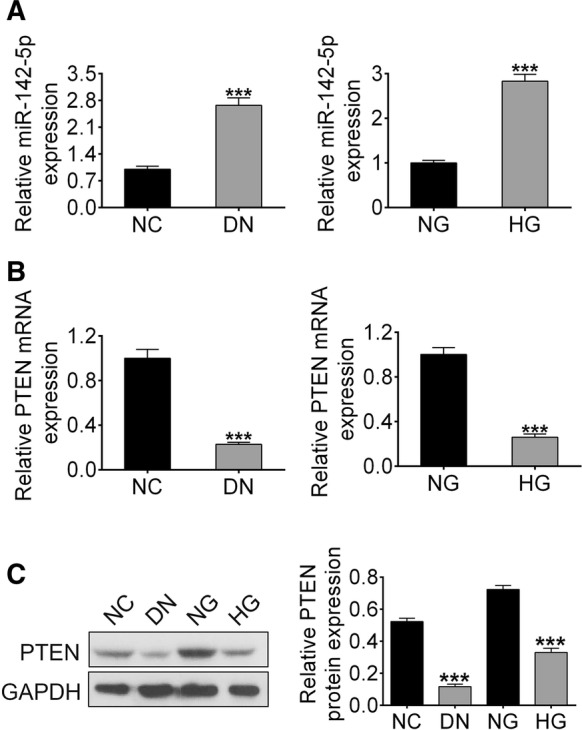
Expression levels of miR-142-5p and PTEN in DN mice or HG treated NRK-52E cells. a miR-142-5p expression analyzed by RT-PCR; b PTEN expression analyzed by RT-PCR; c the level of PTEN protein analyzed by Western blotting. (***p < 0.001 vs. control group)
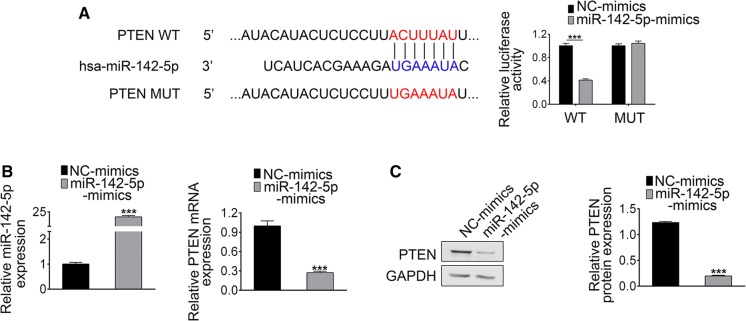
PTEN was a downstream target of miR-142-5p
The potential target genes of miR-142-5p were predicted by TargetScan (http://www.targetscan.org). PTEN was found to be a potential target for miR-142-5p (Fig. 4a). To further verify our bioinformatics results, miR-142-5p mimics or miR-NC and either WT-PTEN or MUT-PTEN (sequence in Fig. 4a) were co-transfected into NRK-52E cells. Luciferase activity of WT-PTEN was reduced in miR-142-5p mimic group compared to that of NC. However, there were no significant differences between miR-142-5p mimic and NC group in cells expressing MUT-PTEN. Transfection efficiency and the expression of PTEN were analyzed by RT-PCR (Fig. 4b). Expression level of miR-142-5p was elevated in miR-142-5p mimic group, accompanied by decreasing expression of PTEN (Fig. 4c). These results confirm that PTEN was a direct target of miR-142-5p.
Fig. 4.
Relationship between miR-142-4p and PTEN. a PTEN 3′UTR was predicted to be a target of miR-142-5p and the MUT sequence of PTEN. b the expression of miR-142-5p and PTEN in NC or miR-142-5p mimic group analyzed by RT-PCR. c the expression PTEN in NC or miR-142-5p mimic group analyzed by Western blotting. (***p < 0.001 vs. control group)
Oleanolic acid downregulated miR-142-5p and upregulated PTEN in HG-induced NRK-52E cells
To investigate the effects of OA on DN, cells were incubated with HG with/without 10 μM OA treatment for 48 h, then expression levels of miR-142-5p and PTEN were analyzed. As shown in Fig. 5a, OA inhibited miR-142-5p expression upon HG induction. Reversely, expression level of PTEN (Fig. 5b) was increased significantly upon OA treatment.
Fig. 5.
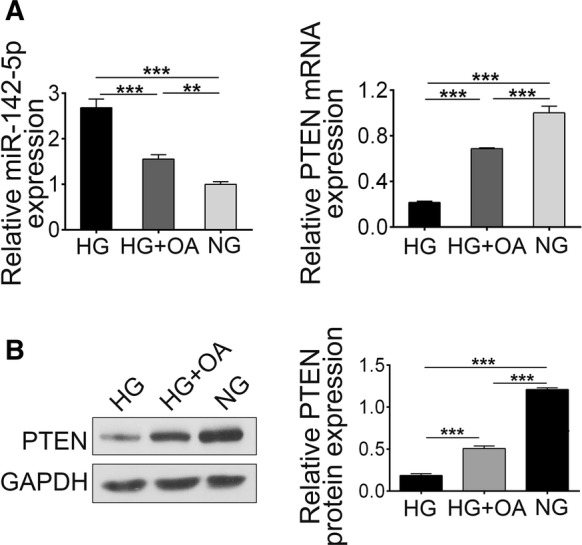
Expression level of miR-142-5p and PTEN with or without OA treatment upon HG. a the level of miR-142-5p and PTEN analyzed by RT-PCR. b The level of PTEN analyzed by Western blotting. (***p < 0.001)
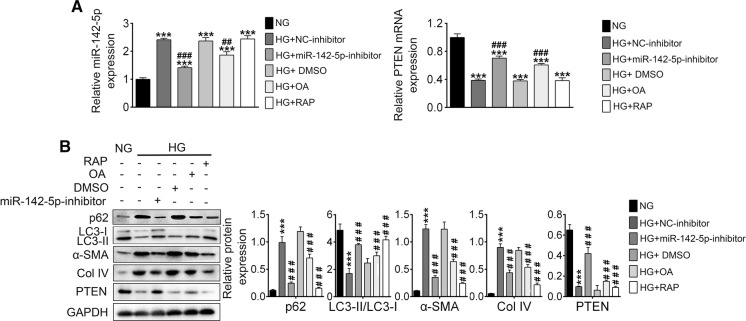
OA inhibited HG-induced fibrosis by downregulation of miR-142-5p, up-regulation of PTEN and thus enhancing autophagy
To clarify the relationship between fibrosis and autophagy as well as the role miR-142-5p played, miR-142-5p inhibitor or NC was transfected into NRK-52E cells and treated with/without OA upon HG. As shown in Fig. 6a, miR-142-5p level was increased upon HG (NG vs. HG + NC-inhibitor), which could be inhibited by OA (HG + OA vs. HG + NC-inhibitor) or miR-142-5p inhibitor (HG + miR-142-5p inhibitor vs. HG + NC-inhibitor). Furthermore, RAPA exhibited little effect on miR-142-5p expression (HG + RAPA vs. HG + NC-inhibitor), indicating autophagy alone could inhibit DN. PTEN expression of different groups was negatively related with miR-142-5p. Furthermore, expression levels of PTEN and p62 were increased, LC3-I and LC3-II decreased, and fibrosis proteins (α-SMA and Col IV) was upregulated, suggesting autophagy was inhibited and fibrosis was promoted (Fig. 6b). The effects of HG could be inversed by miR-142-5p inhibitor, OA and RAPA, which further confirm OA inhibited the HG-induced fibrosis by downregulating miR-142-5p, up-regulating PTEN and thus enhancing autophagy.
Fig. 6.
OA promoted autophagy by inhibition of PI3K/AKT/mTOR. a The level of miR-142-5p and PTEN in different group analyzed by RT-PCR; b autophagy or fibrosis associated proteins expression analyzed by Western blotting. (***p < 0.001 vs. NG group, ###p < 0.01 vs. HG + NC-inhibitor group, ###p < 0.001 vs. HG + NC-inhibitor group)
Oleanolic acid promoted autophagy by inhibition of PI3K/AKT/mTOR pathway
The role of the PI3K/AKT/mTOR pathway in autophagy has been well recognized. With the aim to investigate the mechanisms of OA-promoted autophagy, NG or HG-induced NRK-52E cells were treated with OA or an inhibitor of PI3K named LY294002. Expression levels of PTEN, PI3K, pAKT, tAKT and pmTOR were detected by western blotting. Results (Fig. 7) showed protein level of PTEN was decreased in HG group, and which could be inversed by OA treatment. Expressions of PI3K, pAKT, and pmTOR were increased in HG group, suggesting activation of PI3K/AKT/mTOR signaling. Both OA or LYZ294002 inhibited the expression of PI3K, pAKT, and pmTOR, illustrating OA might promote autophagy by inhibition of PI3K/AKT/mTOR pathway.
Fig. 7.
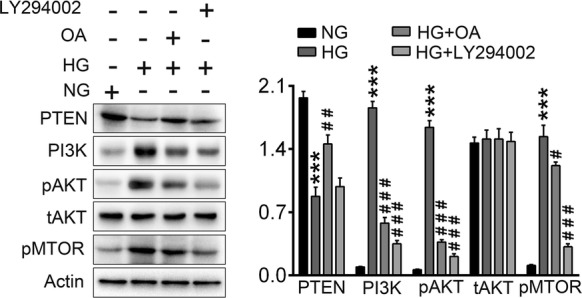
Western blot of PTEN and PI3K/AKT signaling pathway proteins. (***p < 0.001 vs. NG group, ###p < 0.01 vs. HG group, ###p < 0.001 vs. HG group)
Discussion
DN is considered as one of the most serious chronic complications caused by diabetes. The clinical manifestations of DN were progressive renal impairment and severe renal failure, which is the leading causes of death for diabetic patients. The main strategy of therapy includes the good management of blood sugar, blood pressure, blood lipids, lifestyle, and application of angiotensin-converting enzyme inhibitor/angiotensin receptor antagonist drugs. However, the function of drug is limited (Lim 2014). Interestingly, many clinical and experimental studies confirmed Chinese Materia Medica had achieved good effects in treating diabetes and DN (Qi 2003).
Oleanolic acid, a natural compound firstly isolated from Olea europaea, is abundant in olive oil (Pollier and Goossens 2012). A large number of studies have shown that OA exhibits potent effects on diabetes through regulation of various metabolic pathways (Li et al. 2018). OA not only regulates the absorption of exogenous glucose and the production of endogenous glucose, but also improves the function of islet β-cells, improve insulin resistance and diabetic complications (Mukundwa et al. 2016). OA has been reported to ameliorate DN through reduction of oxidative stress, endoplasmic reticulum stress and glomerular hyperplasia (Castellano et al. 2013). Whereas, the precise mechanisms of OA-mediated protective functions in DN remain unclear. Previous studies have indicated insufficiency of autophagy in renal cell contributed to pathogenesis of DN. Therefore, regulation of autophagy may be an efficient therapeutic approach for suppression of DN (Tanaka et al. 2012). This study showed that decreased level of autophagy was associated with renal fibrosis in DN mice and HG-induced NRK-52E cells, which is consistent with previously published results. PTEN was reported to regulate various cellular processes, including proliferation, apoptosis, metabolism, and inhibit the activity of Akt (Mitchell 2012). Researchers found that PTEN regulated renal extracellular matrix production through activated Akt and attenuated DN in diabetes mellitus (Zhu et al. 2016). miR-142-5p, targets on PTEN, a key regulator of autophagy, was elevated while PTEN was decreased in renal tissues of DN mice and HG-induced NRK-52E cells. OA inhibited miR-142-5p level and upregulated PTEN in HG-induced NRK-52E cells. Here, the mechanism of OA regulating miR-142 synthesis was not been studied. In the view of biogenesis of miRNA, OA may affect the transcription and processing of pre-miR-142-5p, or transport of pre-miR-142-5p from nucleus to cytoplasm. Further studies showed that OA restricted HG-induced NRK-52E cell fibrosis through inhibition of autophagy-related miR-142-5p.
PI3K/AKT/mTOR pathway, an intracellular signaling pathway partly regulated by PTEN, associated with cellular proliferation, quiescence, cancer, and longevity (Carnero et al. 2008). Many studies reported activation of PI3K/AKT/mTOR pathway is very crucial during DN pathogenesis. Decreased expression of PTEN and activation of PI3K/Akt/mTOR pathway promote the development of DN (Vasiliki 2013). Our results indicated expression of PTEN was significantly decreased in NRK-52E cells upon HG. Similarly, PTEN expression was up-regulated and PI3K/AKT/mTOR pathway was inhibited under OA treatment. Accumulating evidences demonstrate micro-RNAs are of great importance in the activation of the PI3K/Akt/mTOR signaling (Du 2015). This study suggests miRNA-142-5p targets PTEN to activate the PI3K/Akt/mTOR signaling, thus contributing to the development of DN. OA treatment significantly inhibited miRNA-142-5p level and promote PTEN expression in a HG-induced model, leading to the inhibition of PI3K/Akt/mTOR pathway.
In summary, this study demonstrated by inhibition of miRNA-142-5p expression, OA up-regulated expression level of PTEN, promoted autophagy, and inhibited PI3K/Akt/mTOR signaling pathway in the HG-induced NRK-52E cells, which confirmed the potential of utilization of OA as an anti-diabetic candidate. However, the in vivo effects of OA on the regulation of miR-142-5p need further research. This study provides novel insides into therapeutic approach targeting miR-142-5p and autophagy for DN treatment from bench to clinic.
Acknowledgements
Not applicable.
Funding
None.
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests, and all authors should confirm its accuracy.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bai X, Zhou Y, Yang M. MicroRNA-142-5p induces cancer stem cell-like properties of cutaneous squamous cell carcinoma via inhibiting PTEN. J Cell Biochemis. 2017;119:2179–2188. doi: 10.1002/jcb.26379. [DOI] [PubMed] [Google Scholar]
- Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–198. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Angeles G, Teresa D, Mirela R, Cayuela JA. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes. 2013;62:1791–1799. doi: 10.2337/db12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wen X, Zhang W, Wang C, Liu J, Liu C. Hypolipidemic effect of oleanolic acid is mediated by the miR-98-5p/PGC-1β axis in high-fat diet-induced hyperlipidemic mice. FASEB J. 2017;31:1085. doi: 10.1096/fj.201601022R. [DOI] [PubMed] [Google Scholar]
- Delić D, et al. Urinary exosomal miRNA signature in type II diabetic nephropathy patients. PLoS ONE. 2016;11:e0150154. doi: 10.1371/journal.pone.0150154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Choi ME. Autophagy in diabetic nephropathy. J Endocrinol. 2015;224:R15. doi: 10.1530/JOE-14-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, et al. MicroRNA-451 regulates stemness of side population cells via PI3K/Akt/mTOR signaling pathway in multiple myeloma. Oncotarget. 2015;6:14993–15007. doi: 10.18632/oncotarget.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainz A, et al. Prediction of prevalence of chronic kidney disease in diabetic patients in countries of the European Union up to 2025. Nephrol Dial Transplant. 2015;30:iv113. doi: 10.1093/ndt/gfv073. [DOI] [PubMed] [Google Scholar]
- Li XU, Wang C. Research progress and prospect of oleanolic acid in treatment of diabetes. Chinese J Exp Trad Med Formul. 2018;24:228–234. [Google Scholar]
- Lim AKH. Diabetic nephropathy—complications and treatment. Int J Nephrol Renovasc Dis. 2014;7:361–381. doi: 10.2147/IJNRD.S40172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lit YZ, Meyer T. Managing diabetic nephropathy: recent studies. Curr Opin Nephrol Hypertens. 2006;15:111–116. doi: 10.1097/01.mnh.0000214768.21911.62. [DOI] [PubMed] [Google Scholar]
- Mitchell F. Diabetes: PTEN mutations increase insulin sensitivity and obesity. Nat Rev Endocrinol. 2012;8:698. doi: 10.1038/nrendo.2012.186. [DOI] [PubMed] [Google Scholar]
- Mukundwa A, Langa SO, Mukaratirwa S, Masola B. In vivo effects of diabetes, insulin and oleanolic acid on enzymes of glycogen metabolism in the skin of streptozotocin-induced diabetic male Sprague-Dawley rats. Biochem Biophys Res Commun. 2016;471:315–319. doi: 10.1016/j.bbrc.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Ngubane PS, Bubuya M, Musabayane CT. The effects of Syzygium aromaticum-derived oleanolic acid on glycogenic enzymes in streptozotocin-induced diabetic rats. Ren Fail. 2011;33:434–439. doi: 10.3109/0886022X.2011.568147. [DOI] [PubMed] [Google Scholar]
- Pollier J, Goossens A. Oleanolic acid. Phytochemistry. 2012;77:10–15. doi: 10.1016/j.phytochem.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Qi W. The present situation of TCM treatment for diabetes and its researches. J Trad Chin Med. 2003;23:67–73. [PubMed] [Google Scholar]
- Tanaka Y, Kume S, Kitada M, Kanasaki K, Uzu T, Maegawa H, Koya D. Autophagy as a therapeutic target in diabetic nephropathy. J Diabetes Res. 2012;2012:628978. doi: 10.1155/2012/628978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliki M, et al. Losartan affects glomerular AKT and mTOR phosphorylation in an experimental model of type 1 diabetic nephropathy. J Histochem Cytochemis. 2013;61:433–443. doi: 10.1369/0022155413482925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C, Liang S, He Z, Zhu X, Chen R, Chen J. Metformin, a first-line drug for type 2 diabetes mellitus, disrupts the MALAT1/miR-142-3p sponge to decrease invasion and migration in cervical cancer cells. Eur J Pharmacol. 2018;830:59–67. doi: 10.1016/j.ejphar.2018.04.027. [DOI] [PubMed] [Google Scholar]
- Xu W, Wang W. MicroRNA-142-5p modulates breast cancer cell proliferation and apoptosis by targeting phosphatase and tensin homolog. Mol Med Rep. 2018;17:7529–7536. doi: 10.3892/mmr.2018.8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XH, et al. Resveratrol transcriptionally regulates miRNA-18a-5p expression ameliorating diabetic nephropathy via increasing autophagy. Eur Rev Med Pharmacol Sci. 2017;21:4952–4965. [PubMed] [Google Scholar]
- Zhu H, Leung SW. Identification of microRNA biomarkers in type 2 diabetes: a meta-analysis of controlled profiling studies. Diabetologia. 2015;58:900–911. doi: 10.1007/s00125-015-3510-2. [DOI] [PubMed] [Google Scholar]
- Zhu L, Zhao S, Liu S, Liu Q, Li F, Hao J. PTEN regulates renal extracellular matrix deposit via increased CTGF in diabetes mellitus. J Cell Biochem. 2016;117:1187–1198. doi: 10.1002/jcb.25402. [DOI] [PubMed] [Google Scholar]