Abstract
The mechanism of action of treatment of either curcumin or capsaicin or in combination on LPS (Lipopolysaccharide) induced inflammatory gene expression in peripheral blood mononuclear cells (PBMCs) was investigated using RT-PCR and in silico docking methods. RT-PCR analysis has shown that the curcumin and capsaicin significantly reduced LPS induced over expression of COX-2, IL-6 and TGF-β in PBMCs. Whereas combined molecules demonstrated synergistic response on the reduction of COX-2, IL-6 and TGF-β over expression in LPS induced PBMCs as compared to individual molecules. Further, The docking of curcumin and capsaicin at the active pockets of COX-2, IL-6 and TGF-β has shown − 3.90, − 4.49 and − 5.61 kcal/mol binding energy for curcumin and − 3.80, − 4.78 and − 5.76 kcal/mol binding energy for capsaicin, while multiple ligand simultaneous docking (MLSD) of both molecules has shown higher binding energy of − 4.24, − 5.35 and − 5.83 kcal/mol respectively. This has demonstrated the efficacy of combined curcumin and capsaicin against the LPS induced expression of pro-inflammatory cytokines in PBMCs. These results attributed the coordinated positive modulation on biochemical and molecular cellular process by combined curcumin and capsaicin as compared to individual molecules.
Keywords: Curcumin, Capsaicin, PBMC cells, Proinflammatory cytokine, MLSD
Introduction
PBMCs generally known as monocytes are the important cellular mediators of innate immune system responsible for defense against diverse pathogens (Parihar et al. 2010). Monocytes act as sensors in the blood circulation characteristically generating rapid and sensitive inflammatory response for LPS through the activation of Toll like receptor (TLR) signaling (Sweet and Hume, 1996; Netea et al. 2009). Among the members of mammalian TLR family; TLR4 is found to be functionally characterized cellular receptor for bacterial LPS. As LPS binds to LPS binding protein (LBP) to deliver to the cell surface receptor cluster of differentiation 14 (CD14) to form CD14-LPS-LBP complex (Barton and Medzhitov 2003) and this complex is transferred to TLR4-MD2 complex results in the formation of the receptor complex to initiate TLR4 signaling (Triantafilou and Triantafilou, 2002). Two pathways of TLR4 downstream are activated by LPS binding through myeloid differentiation primary response gene (MyD) 88-dependent and -independent pathways, which culminate in the activation of nuclear factor-κB (NF-κB) and production of cytokines (Barton and Medzhitov 2003). The MyD88-dependent pathway recruit signal transduction intermediates for the activation of the canonical inhibitor- κB (IκB) kinase (IKK) and mitogen-activated protein kinase (MAPK) thereby facilitating the nuclear translocation of Activator Protein 1 (AP-1) and NF-κB DNA binding of transcription factors, results in the activation of NF-κB pathway and release of cytokines (Medzhitov et al. 1998; Medzhitov and Kagan 2006). The MyD88-independent pathway recruits signal transduction intermediate for the activation of transcription factor interferon regulatory factor 3 (IRF3) and the late-phase activation of IKKs and MAPK leading to further activation of nuclear translocation factors AP-1 and NF-κB. NF-κB together with interferon regulatory factor 3 (IRF3) activates the transcription of target genes, resulting in the release of cytokines (Akira et al. 2003; Medzhitov and Kagan 2006).
Pro-inflammatory cytokines including cycloxygenase-2 (COX-2), interleukin 6 (IL-6) and tumor growth factor-β (TGF-β) were produced in response to LPS by monocytes are involved in the up-regulation of inflammatory reactions. IL-6 is a pleiotropic mediator known to augment T-lymphocyte response by promoting differentiation and maturation of B lymphocytes, stimulate haematopoiesis and induce the production of acute phase proteins (Le and Vilcek 1990; Snick 1990). While, TGF-β drives the differentiation of T helper 17 (Th17) cells mediated through IL-6 promotes inflammation, cancer and augments autoimmune conditions (Korn et al. 2009; Li and Flavell 2008; Brain and Moses 2010). The mitogenic or pro-inflammatory agents, including cytokines such as IL-1β, TNF-α and growth factors TGF-β, EGF, PDGF, and FGF are known to be involved in activating COX-2 expression in all types of tumour cells (Shrihari 2017). This COX-2 is more important source of prostanoid formation during inflammation and proliferative diseases such as cancer (Dubois et al. 1998).
Curcumin, the yellow coloring principle of turmeric (Curcuma longa) and capsaicin the pungent principle of red pepper (Capsicum annuum) have been well documented for their anti-inflammatory property (Manjunatha and Srinivasan 2006). Curcumin is a highly pleiotropic molecule that interacts with multiple molecular targets and potentially inhibits LPS induced inflammation mediated through ROS/TLR4-MAPK/NF-κB pathway in rat vascular smooth muscle cells, RAW 264.7 cells (Chen et al. 2008; Meng et al. 2013) and suppressed induction of proinflammatory transcription factors NF-κB, AP-1, signal transducer, activators of STAT proteins and Wnt/β-catenin; it also down regulated the expression of NF-κB regulated gene products such as TNF-α, IL-1, IL-6, IL-8, MCP-1, iNOS, MMP-2 and MMP-9 in in vivo model (Zhou et al. 2011; Yao et al. 2004). It has been reported that curcumin modulate the expression of proinflammatory enzymes that mediate the production of prostaglandins (COX-2), leukotrines (lipoxygenase), adhesion molecules and MMPs (Gupta et al. 2011; Noorafshan and Esfahani 2013). Capsaicin is reported to inhibit the LPS induced NO and/or PGE2 production by interrupting IκB-α degradation, phosphorylation and activation of NF-κB and AP-1 signaling pathways in macrophages (Chen et al. 2003; Shin et al. 2013; Kim et al. 2003). Further reports have shown that capsaicin interfere in TNF-α mRNA transcription and exerts inhibition of TNF-α release from macrophages and attenuates the expression of proinflammatory cytokines TNF-α, IL-6, NO and tissue malondialdehyde (MDA) in vivo (Aggarwal et al. 2015). Kobayashi et al. (2012) revealed that capsaicin prevents the COX-2, membrane-bound PGE synthase-1 and PGE production in vitro and in vivo, respectively.
In view of diverse and overlapping anti-inflammatory potential of curcumin and capsaicin, it has become increasingly important to investigate and understand the potency of combined curcumin and capsaicin against their individual molecules action in LPS induced proinflammatory gene expression in PBMCs. Therefore, the aim of the present study is to evaluate the influence of curucmin and capsaicin on the reduction of pro-inflammatory cytokines in PBMCs.
Materials and methods
Chemicals
Curcumin, capsaicin, LPS, Ficoll-Hypaque, HEPES- buffered RPMI-1640 medium, and Trizol reagent were obtained from Sigma-Aldrich (USA). Antibiotic and antimycotic solution, fetal bovine serum (FBS) and all other cell culture chemicals were purchased from Hi-Media Chemical Laboratories (Mumbai, India). cDNA kit was procured from Invitrogen (USA) and PCR kit was purchased from Sisco Research Laboratories (Mumbai, India). All other chemicals and solvents used were of analytical grade.
Cell preparation
Isolation
PBMCs were isolated according to the method of Yoshiaki et al. (1999) with slight modifications. 10 ml of blood was drawn from healthy volunteer and added into a tube containing 0.5 M EDTA. Diluted (1:1 dilution with PBS) 10 ml blood was carefully layered over 10 ml of Ficoll gradient, and centrifuged at 1600 rpm for 30 min at 4 °C. The centrifuged sample has different layers from top to bottom as in the following order; Plasma-Platelets-PBMC-Ficoll-red blood cells (with granulocytes). The upper plasma and platelets layer was discarded and the buffy coat of PBMCs is carefully aspirated and transferred to a fresh tube. Then, PBS was added to PBMCs to make up the volume to 50 ml and centrifuged at 1200 rpm for 10 min. at 18 °C. The supernatant fraction was discarded, and the pellet was re-suspended in PBS again centrifuged (3000 rpm for 10 min at 18 °C) to obtain cell pellet and this process was repeated for three times. The pellet was mixed in RPMI- 1640 medium and seeded in 96 well micro-titer plates.
Cell culture and stimulation
The PBMCs were added to HEPES buffered RPMI-1640 medium supplemented with 10% heat inactivated FBS, 100 U/ml penicillin and 100 µg/ml streptomycin and incubated at 37 °C in 5% CO2 incubator for 24 h. Sequential plastic adherence technique (Yoshiaki et al. 1999) was used to isolate PBMCs and these cells (4 × 105 cells/well) were seeded in a tissue culture grade 96-well plate containing 100 μl of culture medium pre-treated with curcumin (10 μM), capsaicin (10 μM) and their combination (2.5 μM each) for 1 h and later 1 μg/ml of LPS was added to all groups except normal control and incubated for 24 h under optimum conditions. In the case of combination treatment, 2.5 and 5 μM dose of each compound was subjected for screening their potency on cell viability. Based on the cell viability, 2.5 μM of each compound was selected for combination treatments.
Further, PBMCs incubated with curcumin or capsaicin or their combination suspended in 0.5% DMSO. And parallel normal control was maintained using 0.5% DMSO alone as vehicle control. PBMCs were harvested and quadruplicate haemocytometer was used to count the total number of cells. Trypan blue dye exclusion method (Warren 1997) was used to evaluate the percentage of cell viability. The viability of cells treated with 0.5% DMSO as vehicle control was considered as 100% and each compound was freshly prepared using nitrogen environment in a known volume of 0.5% DMSO.
Nitric oxide assay
The nitric oxide assay was performed as described previously by Granger et al. (1996). PBMCs were pretreated separately either with curcumin or capsaicin and their combination for 1 h, then treated with LPS and incubated for 24 h, and nitrite level was measured as an indicator of NO production using Griess reagent (1% sulphanilamide and 0.1% N-(L-naphthyl)-ethylene diamine dihydrochloride, dissolved in 2.5% H3PO4). Briefly, an equal volume of Griess reagent mixed with cell culture supernatant and incubated at room temperature for 10 min. followed by absorbance was measured at 550 nm. The amount of nitrite in the samples was calculated from a standard curve of sodium nitrite.
Percentage of nitrite inhibition by curcumin, capsaicin and their combination was quantified as per the following formula
Total RNA isolation
Parallel normal control and LPS induced PBMCs were pretreated with curcumin, capsaicin and their combination was subjected for total RNA extraction using Trizol reagent as per the manufacturers protocol (Sigma, USA). Quantification of total RNA was performed using NanoDrop spectrophotometer (ND-1000, USA). Total RNA was stored at − 80 °C until further analysis.
Reverse transcription—polymerase chain reaction (RT-PCR)
cDNA was synthesized from 0.5 μg of total RNA using SuperScript® III first- strand synthesis system (Invitrogen, USA) for RT-PCR protocol provided with specific primers (Table 1). The amplification course performed consists of 35 cycles in 50 μl reaction mixture. Specific primers for COX-2, IL-6 and TGF-β were used for synthesizing cDNA from RNA and polymerized cDNA was separated by agarose gel electrophoresis and visualized by ethidium bromide staining. Band thickness was analyzed using densitometry scanning technique.
Table 1.
PBMCs specific PCR primer sequences
| Molecule | Forward primer | Reverse primer |
|---|---|---|
| β - Actin | 5′GGACTTCGAGCAAGAGATGG3′ | 5′AGCACTGTGTTGGCGTACAG3′ |
| COX-2 | 5′TGAGCATCTACGGTTTGCTG3′ | 5′TGCTTGTCTGGAACAACTGC3′ |
| IL-6 | 5′AGGAGACTTGCCTGGTGAAA3′ | 5′CAGGGGTGGTTATTGCATCT3′ |
| TGF-β | 5′CGGATCAGCGTTTATCAGGT3′ | 5′AACTTGGGGTTGATGCTCTG3′ |
Molecular docking studies
Single ligand docking
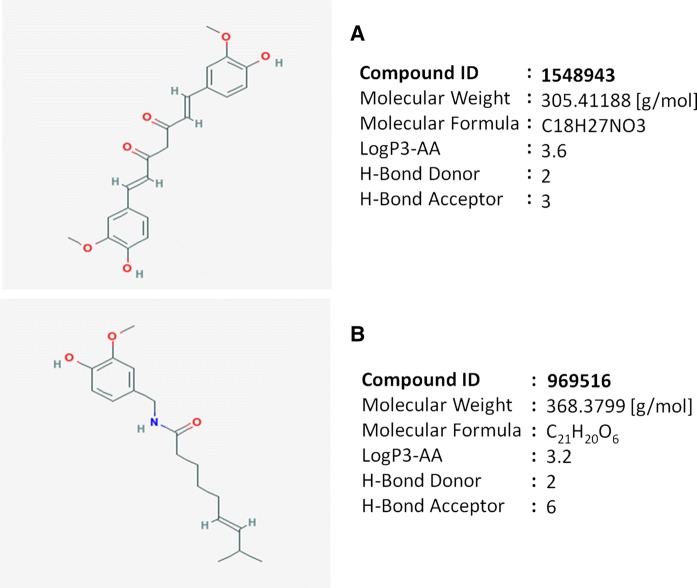
The crystallographic protein structures of COX-2, IL-6 and TGF-β were selected for docking studies. A Lamarckian genetic algorithm (LGA) was employed to study the appropriate binding modes of ligand and protein interaction using Autodock 4.2. The crystal structure of COX-2 (2.1 Å; PDB ID: 6COX), IL-6 (1.9 Å; PDB ID: 1ALU) and TGF-β (3 Å; PDB ID: 3kfd), were retrieved from RCSB protein data bank. Energy minimization was done using SPDBV software and edited by removing the water molecules and hetero atoms while non-polar hydrogen atoms were added, followed by Gasteiger charger calculation using Autodock tools. The structure of curcumin (PID: 969516) and capsaicin (PID: 1548943) ligands in.mol format were retrieved from PubChem and 3D co-ordinates for ligands were generated using PRODRG server (Gasteiger and Marsili 1980; Ghose and Crippen 1987).
Multiple ligand simultaneous docking (MLSD)
MLSD provide cumulative interaction of multiple ligands with a protein molecule. It is employed to implicitly understand the simultaneous interaction of curcumin and capsaicin with an active pocket of protein molecules compared with single ligand docking to protein molecules (Li and Li, 2010) by editing.dpf file. Docking parameter files of both the ligands and protein were merged in a single file to run MLSD. Docking started with the initialization of population, each ligand was randomly initialized with a set of state variables attaining specific configuration, ligand center, torsion tree and a group of atomic coordinates. Energy minimization was done using standard LGA procedure and the pseudo-Solis and Wets methods. After generation of each genetic operation, MLSD program maps the genotype of a ligand back to phenotype so that each ligand has its own phenotype and coordinates (Li and Li 2010). The interface residues of docked complex with ligands were obtained from Ligplot.
Statistical analysis
All the experiments were performed in triplicates (n = 3) and results were expressed as mean ± SEM. Statistical analysis was performed using one-way ANOVA followed by Dunnett’s multiple comparison tests. Data was computed for statistical analysis using GraphPad prism 5 (San Deigo, CA).
Results
Effect of curcumin, capsaicin and their combination on nitrite formation in LPS induced PBMCs
The LPS induced PBMCs have shown increased nitrite formaiton compared to parallel normal control cells. However the PBMCs treated with curcumin, capsaicin and their combination reduced the accumulation of nitrite levels by 54, 54 and 50% compared to LPS alone treated PBMCs. Normal PBMCs produced 10 μM nitrite, while LPS (1 μg/ml) induced cells have shown higher level of nitrite formation (22 μM). However, PBMCs pretreated with curcumin, capsaicin and their combination have shown significant reduction (12 μM) in nitrite formation in PBMCs, reaffirming higher benefits of combined molecules (11 μM) than individual molecules (Fig. 1).
Fig. 1.
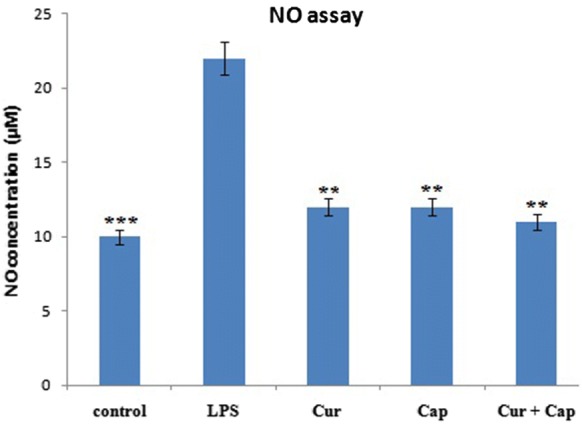
Effect of LPS on production of NO in PBMCs pre-treated either with or without (control) curcumin or capsaicin or combination of them. Values are expressed as Mean ± SEM (n = 3). Significant difference *p < 0.05, **p < 0.01 and ***p < 0.001 when compared with LPS-induced group (positive control)
Effect of curcumin, capsaicin and their combination on the over-expression of COX-2, IL-6 and TGF-β mRNA in LPS-induced PBMCs
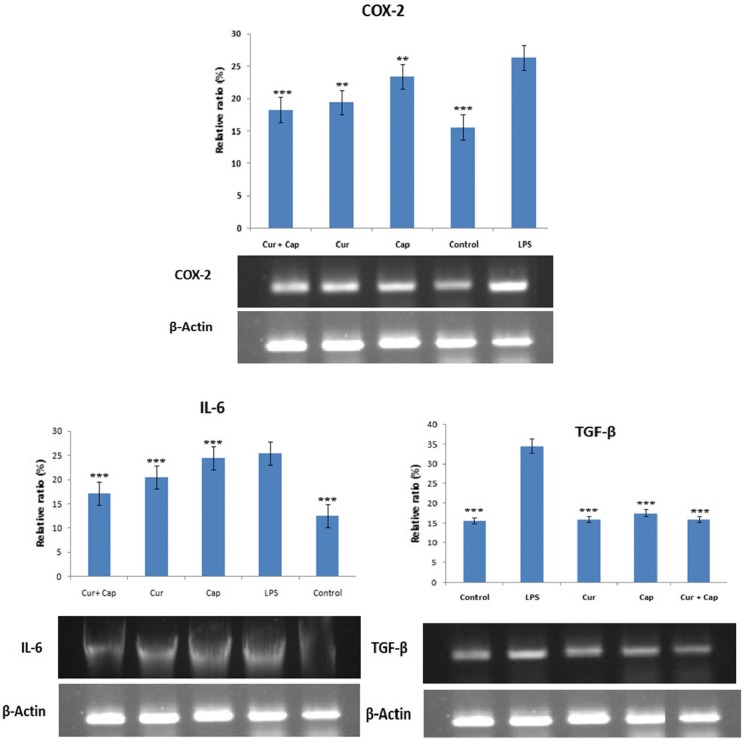
The beneficial potency of curcumin, capsaicin and their combination on the expression of COX-2, IL-6 and TGF-β levels in LPS induced PBMCs examined using RT-PCR as shown in Fig. 2. LPS induced PBMCs exhibited higher expression of COX-2, IL-6 and TGF-β (1.7, 2.0 and 2.2 folds) when compared to normal control cells respectively. However, cells pretreated with curcumin (10 μM) had shown significant decrease in the expression of COX-2, IL-6 and TGF-β by 1.4, 1.0 and 2.15 fold while 1.0, 1.25 and 2.0 fold in capsaicin (10 μM) pretreated cells. PBMCs pretreated with combined curcumin (2.5 μM) and capsaicin (2.5 μM) have shown 1.5, 1.5 and 2.2 fold decrease in the expression of COX-2, IL-6 and TGF-β, respectively. And the lower dosage of combined molecule activity was found to be equivalent (p < 0.0001) to the individual curcumin and capsaicin indicating the synergistic activity (Fig. 3).
Fig. 2.
Influence of curcumin and capsaicin, and their combination on attenuation of LPS-induced expression of COX-2, IL-6 and TGF-β in PBMCs. COX-2, IL-6 and TGF-β mRNA levels were measured by RT-PCR. COX-2, IL-6 and TGF-β mRNA in LPS alone treated cells was set as 100%. Values are expressed as Mean ± SEM (n = 3). Significant difference *p < 0.05, **p < 0.01 and ***p < 0.001 when compared with LPS-induced group (positive control)
Fig. 3.
Structure and physico-chemical properties of ligands A curcumin and B capsaicin
Molecular docking of curcumin, capsaicin and their combination at the active pocket of COX-2
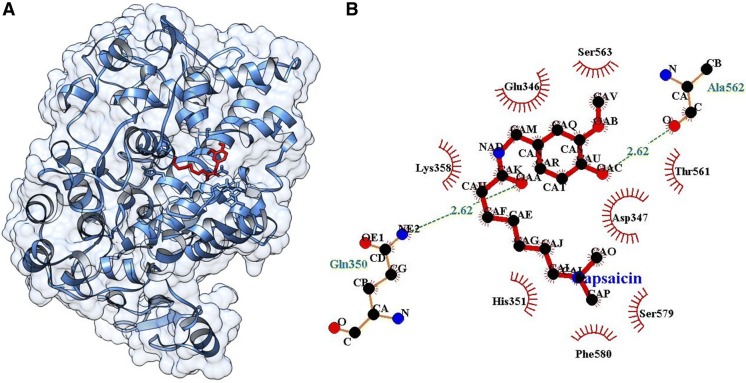
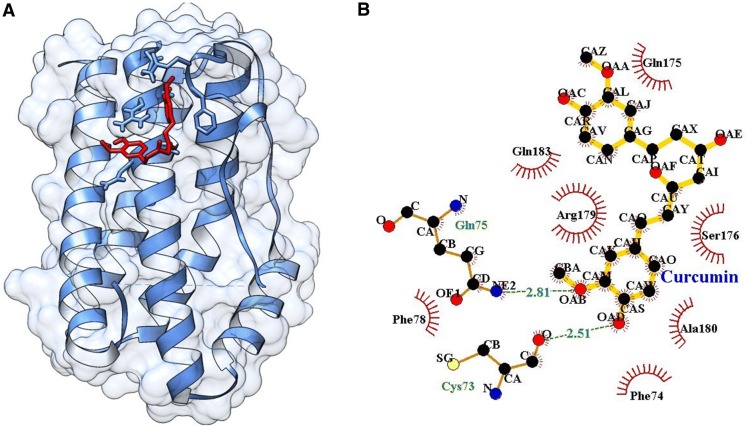
Single ligand docking of curcumin and capsaicin at the active pocket of COX-2 as shown the binding energy of − 3.90 and − 3.80 kcal/mol. Where, curcumin forms seven hydrogen bonds with His351, Gln565, Gln350, Arg109, Leu359 and Asp347 residues at the active pocket of COX-2 (Fig. 4), while capsaicin forms two hydrogen bonds with Ala562 and Gln350 (Fig. 5). In addition, MLSD of combined curcumin and capsaicin has shown the binding energy of − 4.24 kcal/mol and docking energy of − 10.82 kJ/mol demonstrating prominent interaction when compared with single ligand docking (Table 2).
Fig. 4.
A Docking of curcumin at the active pocket of COX-2, B 2D representation of the interaction of curcumin with COX-2
Fig. 5.
A Docking of capsaicin at the active pocket of COX-2, B 2D representation of the interaction of capsaicin with COX-2
Table 2.
Docking of curcumin, capsaicin and their combined form at the active pocket of protein molecules
| Protein | Ligand | DE | BE | IE | TE | IC | USE | H-Bonds | Bonding amino acid |
|---|---|---|---|---|---|---|---|---|---|
| COX-2 | Cur | − 10.366 | − 3.90 | − 2.89 | 3.58 | 1.39 mM | − 2.89 | 7 |
(2)His351, Gln565, Gln350, Arg109, Leu359, Asp347 |
| Cap | − 8.757 | − 3.80 | − 1.67 | 3.28 | 1.63 mM | − 1.67 | 2 | Ala562, Gln350 | |
| Cur + Cap | − 10.823 | − 4.24 | − 3.00 | 3.58 | 775.25 µM | − 3.00 | – | – | |
| IL-6 | Cur | − 10.856 | − 4.49 | − 2.79 | 3.58 | 511.14 µM | − 2.79 | 2 | Gln75, Cys73 |
| Cap | − 9.634 | − 4.78 | − 1.57 | 3.28 | 311.41 µM | − 1.57 | 3 | Gln183, Cys73, Ser176 | |
| Cur + Cap | − 10.189 | − 5.35 | − 1.55 | 3.28 | 118.98 µM | − 1.55 | – | – | |
| TGF-β | Cur | − 11.895 | − 5.61 | − 2.70 | 3.58 | 76.68 µM | − 2.70 | 4 | (2)Arg25, (2)Lys37 |
| Cap | − 11.243 | − 5.76 | − 1.90 | 3.58 | 60.02 µM | − 1.90 | 4 | (2)Arg25, (2)Tyr91 | |
| Cur + Cap | − 9.439 | − 5.83 | − 0.33 | 3.28 | 53.42 µM | − 0.33 | – | – |
Cur curcumin, Cap capsaicin, Cur + Cap curcumin + capsaicin, DE docking energy (kJ/mol), BE binding energy (kcal/mol), IC inhibitory constant, IE internal energy, TE torsional energy (kcal/mol), USE unbound system’s energy (Kcal/mol), Cluster RMSD: 0.00
Molecular docking of curcumin, capsaicin and their combination at the active pocket of IL-6
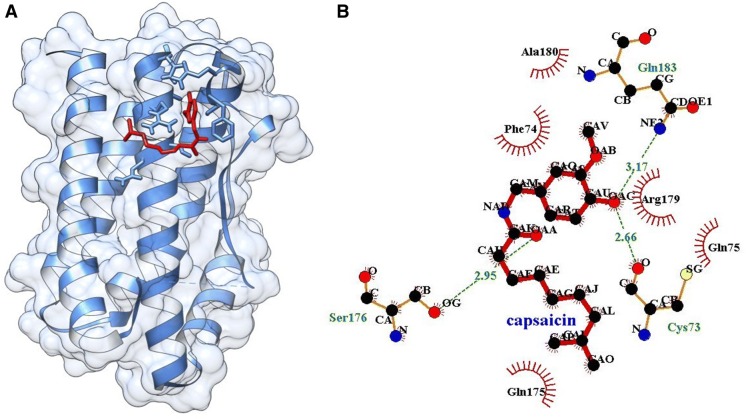
Single ligand docking of curcumin and capsaicin at the active pocket of IL-6 showing the binding energy of − 4.49 and − 4.78 kcal/mol. Curcumin forms two hydrogen bond with Gly75 and Cys73 (Fig. 6) and capsaicin forms three hydrogen bond with Gln183, Cys73 and Ser176 at the active pocket of IL-6 (Fig. 7). In addition, MLSD of combined curcumin and capsaicin has shown the binding energy of − 5.35 kcal/mol and docking energy − 10.189 kJ/mol showing prominent interaction when compared to single ligand docking (Table 2).
Fig. 6.
A Docking of curcumin at the active pocket of IL-6, B 2D representation of the interaction of curcumin with IL-6
Fig. 7.
A Docking of capsaicin at the active pocket of IL-6, B 2D representation of the interaction of capsaicin with IL-6
Molecular docking of curcumin, capsaicin and their combination at the active pocket of TGF-β
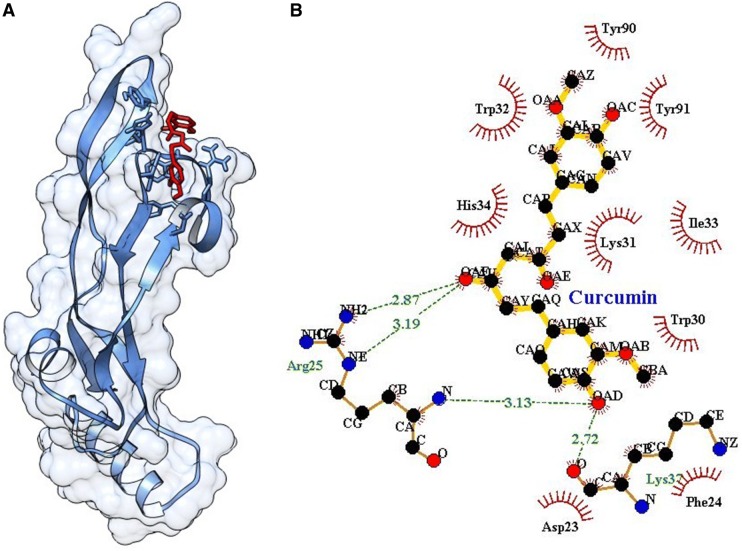
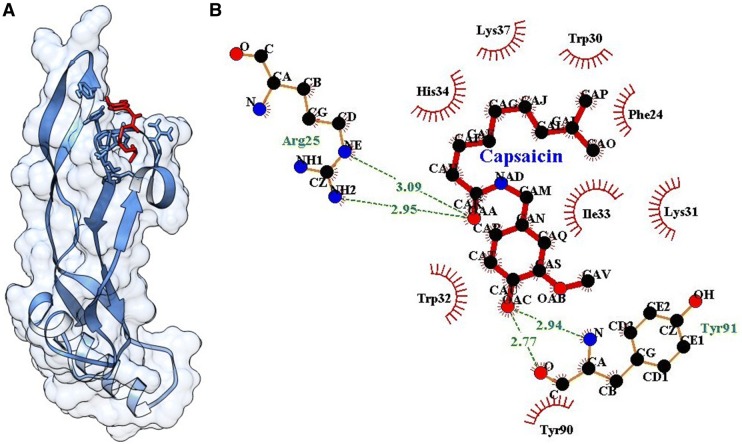
Single ligand docking of curcumin and capsaicin at the active pocket of TGF-β as shown the binding energy of − 5.61 and − 5.76 kcal/mol. Curcumin forms four hydrogen bonds with (2) Arg25 and (2) Lys37 (Fig. 8). Similarly, capsaicin forms four hydrogen bonds with Arg25 (2 hydrogen bonds) and Tyr91 (2 hydrogen bonds) (Fig. 9). The MLSD of combined curcumin and capsaicin as shown the binding energy of − 5.83 kcal/mol and docking energy − 9.439 kJ/mol showing prominent interaction when compared with single ligand docking (Table 2).
Fig. 8.
A Docking of curcumin at the active pocket of TGF-β, B 2D representation of the interaction of curcumin with TGF-β
Fig. 9.
A Docking of capsaicin at the active pocket of TGF-β, B 2D representation of the interaction of capsaicin with TGF-β
Discussion
Earlier literature revealed that the curcumin and capsaicin have shown to inhibit the production of pro-inflammatory cytokines in cell lines including RAW 264.7 macrophages and human PBMCs in vitro (Guimaraes et al. 2013; Rahardjo et al. 2014; Park et al. 2004). Currently there are fewer reports available regarding the intervention of combined major spice principles curcumin and capsaicin against LPS induced proinflammatory cytokine expression in human PBMCs. Nevertheless, our earlier studies reported that protective effect was found to be more in combined curcumin and capsaicin than the individuals against carrageenan-induced paw inflammation in rat model (Manjunatha and Srinivasan 2006). Likewise, a recent study from our laboratory reported that down regulation of LPS-induced TRAF6 expression in human PBMCs and beneficial effect was found to be higher in combined form in human PBMCs than individual molecules (Bharath et al. 2017). Thus, the present study was explicitly planned to elucidate the beneficial effect of individual and combination of curcumin and capsaicin on the LPS induced expression of COX-2, IL-6 and TGF-β in human PBMCs.
Human PBMCs/monocytes are involved in innate and adaptive immune system, has obligated us to understand this phenomenon in the field of nutrigenomics since they reflect the effects of dietary interventions at genetic level (Mello et al. 2012). LPS is the primary activator of macrophages and monocytes, which are known to activate inflammatory mediators such as nitric oxide, free radicals together with several cytokines including TNF-α, IL-6 and IL-1β (Grahames et al. 1999; Mehta et al. 2001; Van 1990).
Monocytes play a vital role in host’s defense against bacterial infection as they respond rapidly to microbial infection by secreting inflammatory mediators, such as cytokines, chemokines, antimicrobial factors, including nitric oxide (Serbina et al. 2008; Guha and Mackman 2001). Production of excessive nitric oxide has been implicated in many inflammatory diseases such as hypertension, arteriosclerosis, ischemic reperfusion and septic shock (Pacher et al. 2007; Terao 2009). Therefore, inhibition of NO by dietary components is one of the alternative strategies to prevent inflammation and its associated health problems. Treatment of curcumin and capsaicin at 10 µM showed significant down regulation of NO production in LPS-induced PBMCs. Similarly, earlier report of Zhao et al. (2014) and Kim et al. (2003) has shown that the cells pretreated with curcumin and capsaicin strongly inhibited the LPS-induced overproduction of nitric oxide in macrophages. However, PBMCs pretreated with combined curcumin and capsaicin has shown synergistic down regulation of NO production in LPS induced PBMCs as compared to individual molecules. This result is in agreement with the earlier report of Thriveni et al. (2018) Where curcumin, capsaicin and their combinations decreased LPS-induced NO production in liver and kidneys, and the fold decrease being higher in combined molecules in vivo.
LPS induces over expression of COX-2 including pro-inflammatory cytokines TGF-β and IL-6 in PBMCs, which are considered as key factors in the pathogenesis of many inflammatory diseases. Thus, down regulation of COX-2, IL-6 and TGF-β gene expression is paramount to prevent exacerbation of inflammation mediated diseases. In the current study, we observed that LPS induced over expression of COX-2 has been down-regulated in the following order; curcumin + capsaicin > curcumin > capsaicin > control (Fig. 2). These results are in agreement with earlier reports of Guimaraesa et al. (2013) on significant inhibition of LPS-induced over expression of COX-2 in RAW 264.7 macrophages by curcumin and capsaicin. In our study, combined curcumin and capsaicin has shown synergistic down-regulation of COX-2 expression in LPS induced PBMCs.
Further, IL-6 and TGF-β could be produced by macrophages and monocytes at inflammatory sites in response to induced inflammation (Gabay 2006). TGF- β in association with IL-6 drives the differentiation of T helper 17 (Th17) cells, activate the production of acute phase protein markers (Castell et al. 1989) promoting the inflammation eventually augmenting autoimmune conditions (Korn et al. 2009). Normally, IL-6 and TGF-β were found to increase in LPS induced monocytes (Choi et al. 2017). Similar pattern of elevated levels of IL-6 and TGF-β were found in positive control PBMCs. Curcumin and capsaicin reduced LPS-induced elevation of IL-6 and TGF-β mRNA levels in PBMCs and it was found to be in agreement with the previous reports (Tang 2015; Ma et al. 2017; Choi et al. 2017), where the investigators have shown inhibition of IL-6 production in LPS-induced human THP-1 cells by curcumin and capsaicin. In a separate study, capsaicin has been found to inhibit the DMN-induced over expression of TGF-β1 in vivo. Gaedeke et al. (2004) have shown modulation of TGF-β profibrotic actions in renal fibroblast through down-regulation of TGF-β receptor by curcumin. However, combined curcumin and capsaicin has shown down-regulation of IL-6 and TGF-β expression synergistic in LPS-induced PBMCs as compared to individual treatment with higher doses/concentration in both curcumin and capsaicin.
In drug-target discovery, docking is vital and has been applied as an additional experimental technique to analyze the molecular recognition and association between ligands and proteins which play a significant role in bio-molecular processes like signal transduction. Moreover, it predicts the nature of binding interactions between protein and ligand molecule to form a stable complex (Lengauer and Rarey 1996; Nienhaus 2005).
In this study, docking receptor is an prioritized factor deregulating the expression of few important genes responsible for the enhancement and progression of LPS induced inflammatory pathways. The outcome explicates the interacting mode of curcumin, capsaicin and their combined form with the active amino acid residues at the binding site in the protein molecule. The active groove of target proteins COX-2, IL-6 and TGF-β were identified according to Padhye et al. (2009), Fontaine et al. (1993) and Radaev et al. (2010) respectively. Curcumin and capsaicin individually exhibited a better binding interaction with protein molecules however the binding interaction was found to be strong in combination. This finding underlines the fact that docking simulation could be pre-requisite for searching the combinational anti-inflammatory agents.
Conclusion
The present study demonstrates the combined effect of curcumin and capsaicin on lowering COX-2, IL-6 and TGF-β over expression in LPS induced PBMCs. The anti-inflammatory activity of curcumin is associated with the down-regulation/inhibition of inflammatory mRNA expression and protein expression. While the capsaicin anti-inflammatory activity was directly related to the inhibition of enzyme activity. The enhanced anti-inflammatory activity of combined curcumin and capsaicin might be due to the co-ordination between curcumin and capsaicin. This was further substantiated by in silico molecular docking, where combined curcumin and capsaicin has shown higher binding efficacy with COX-2, IL-6 and TGF-β protein molecule than individuals.
Acknowledgement
HM and TV acknowledge the financial assistance (SB/EMEQ-343/2013) provided by Department of Science and Technology-Science and Engineering Research Board (DST-SERB) Govt. of India, New Delhi.
Compliance with ethical standards
Conflict of interests
Authors declare that they have no conflict of interests.
Ethical approval
This article does not contain any studies with human participants or animal performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thriveni Vasanthkumar, Email: thriveni.vv@gmail.com.
Manjunatha Hanumanthappa, Phone: +91 8762661348, Email: manjunatha75@gmail.com.
Rangaswamy Lakshminarayana, Email: rlnarn21@gmail.com.
References
- Aggarwal BB, Prasad S, Sung B, Gupta SC. Antiinflammatory lifestyle and spices how are they linked? In: Talwar GP, Seyed EH, Sarin SK, editors. Textbook of biochemistry, biotechnology, allied and molecular medicine. 4. PHI learning pvt ltd: Delhi; 2015. pp. 1424–1433. [Google Scholar]
- Akira S, Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- American type culture collection. MTT Cell Proliferation Assay Instruction Guide—ATCC, VA, USA. http://www.atcc.org
- Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- Bharath BR, Thriveni V, Manjunatha H, Bharath C. Interaction of curcumin and capsaicin with LPS induced TRAF6 expression in peripheral blood mononuclear cells. Med Chem Res. 2017;26:2399–2409. [Google Scholar]
- Brain B, Moses HL. Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev. 2010;21:49–59. doi: 10.1016/j.cytogfr.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castell JV, Gomez-Lechon MJ, David M, Andus T, Geiger T, Tryllengue R, Fara R, Heinrich PC. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989;242:237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- Chen CW, Lee ST, Wu WT, Fu WM, Ho FM, Lin WW. Signal transduction for inhibition of inducible nitric oxide synthase and cyclooxygenase-2 induction by capsaicin and related analogs in macrophages. Br J Pharmacol. 2003;140:1077–1087. doi: 10.1038/sj.bjp.0705533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Nie M, Fan MW, Bian Z. Anti-inflammatory activity of curcumin in macrophages stimulated by lipopolysaccharides from Porphyromonas gingivalis. Pharmacology. 2008;82:264–269. doi: 10.1159/000161127. [DOI] [PubMed] [Google Scholar]
- Choi JH, Jin SW, Choi CY, Kim HG, Lee GH, Kim YA, Chung YC, Jeong HG. Capsaicin inhibits dimethylnitrosamine-induced hepatic fibrosis by inhibiting the TGF-β1/Smad pathway via peroxisome proliferator-activated receptor gamma activation. J Agric Food Chem. 2017;65:317–326. doi: 10.1021/acs.jafc.6b04805. [DOI] [PubMed] [Google Scholar]
- Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- Fontaine V, Savino R, Arcone R, Wit LD, Brakenhoff JPJ, Content J, Ciliberto G. Involvement of the Arg179 in the active site of human IL-6. Eur J Biochem. 1993;211:749–755. doi: 10.1111/j.1432-1033.1993.tb17605.x. [DOI] [PubMed] [Google Scholar]
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8(Suppl 2):S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedeke J, Noble NA, Border WA. Curcumin blocks multiple sites of the TGF-beta signaling cascade in renal cells. Kidney Int. 2004;66:112–120. doi: 10.1111/j.1523-1755.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- Gasteiger J, Marsili M. Iterative partial equalization of orbital electronegativity-a rapid access to atomic charges. Tetrahedron. 1980;36:3219–3288. [Google Scholar]
- Ghose AK, Crippen GM. Atomic physicochemical parameters for three-dimensional-structure-directed quantitative structure–activity relationships. 2. Modeling dispersive and hydrophobic interactions. J Chem Inf Comput Sci. 1987;27:21–35. doi: 10.1021/ci00053a005. [DOI] [PubMed] [Google Scholar]
- Grahames CB, Michel AD, Chessell IP, Humphrey PP. Pharmacological characterization of ATP- and LPS-induced IL-1beta release in human monocytes. Br J Pharmacol. 1999;127:1915–1921. doi: 10.1038/sj.bjp.0702732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DL, Taintor RR, Boockvar KS, Hibbs JB., Jr Measurement of nitrate and nitrite in biological samples using nitrate reductase and griess reaction. Methods Enzymol. 1996;268:142–151. doi: 10.1016/s0076-6879(96)68016-1. [DOI] [PubMed] [Google Scholar]
- Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Guimaraes MR, Leite FRM, Spolidorio LC, Kirkwood KL, Rossa C. Curcumin abrogates LPS-induced proinflammatory cytokines in RAW 264.7 macrophages. Evidence for novel mechanisms involving SOCS-1, -3 and p38 MAPK. Arch oral boil. 2013;58:1309–1317. doi: 10.1016/j.archoralbio.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Indira K, Priyadarshini Aggarwal B B. Multitarheting by curcumin as revealed by molecular studies. Nat Prod Rep. 2011;28:1937–1955. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Kawada TB, Kim S, Han IS, Choe SY, Kurata T, Yu R. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell Signal. 2003;15:299–306. doi: 10.1016/s0898-6568(02)00086-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Watanabe K, Yokoyama S, Matsumoto C, Hirata M, Tominari T, Inada M, Miyaura C. Capsaicin, a TRPV1 Ligand, Suppresses bone resorption by inhibiting the prostaglandin E production of osteoblasts, and attenuates the inflammatory bone loss induced by lipopolysaccharide. ISRN Pharmacol. 2012;2012:1–6. doi: 10.5402/2012/439860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Le J, Vilcek J. Interleukin 6: a multifunctional cytokine regulating immune reactions and the acute phase protein response. In: Rubin E, Damjanov I, editors. Pathology reviews. Totowa, NJ: Humana Press; 1990. [PubMed] [Google Scholar]
- Lengauer T, Rarey M. Computational methods for biomolecular docking. Curr Opin Struct Biol. 1996;6:402–406. doi: 10.1016/s0959-440x(96)80061-3. [DOI] [PubMed] [Google Scholar]
- Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li C. Multiple ligand simultaneous docking: orchestrated dancing of ligands in binding sites of protein. J Comput Chem. 2010;31:2014–2022. doi: 10.1002/jcc.21486. [DOI] [PubMed] [Google Scholar]
- Ma F, Liu F, Ding L, You M, Yue H, Zhou Y, Hou Y. Anti-inflammatory effects of curcumin are associated with down regulating microRNA-155 in LPS-treated macrophages and mice. Pharm Biol. 2017;55:1263–1273. doi: 10.1080/13880209.2017.1297838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunatha H, Srinivasan K. Protective effect of dietary curcumin and capsaicin on induced oxidation of low-density lipoprotein, iron-induced hepatotoxicity and carrageenan-induced inflammation in experimental rats. FEBS. 2006;273:4528–4537. doi: 10.1111/j.1742-4658.2006.05458.x. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Kagan JC. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820–3826. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- Mello VDF, Kolehmanien M, Schwab U, Pulkkinen L, Uusitupa M. Gene expression of peripheral blood mononuclear cells as a tool in dietary intervention studies: what do we know so far? Mol Nutr. 2012;56:1160–1172. doi: 10.1002/mnfr.201100685. [DOI] [PubMed] [Google Scholar]
- Meng Z, Yan C, Deng Q, Gao DF, Niu XL. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-kappa B pathways. Acta Pharmacol Sin. 2013;34:901–911. doi: 10.1038/aps.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, Van der Meer JW, Dinarello CA. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhaus G. Protein ligand interactions: methods and applications-methods in molecular biology. New Jersey: Humana press; 2005. [Google Scholar]
- Noorafshan A, Esfahani SA. A review of therapeutic effects of curcumin. Curr Pharm Des. 2013;19:2032–2046. [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhye S, Banerjee S, Chavan D, Pandye S, Swamy KV, Ali S, Li J, Dou QP, Sarkar Fazlul H. Fluorocurcumins as Cyclooxygenase-2 Inhibitor: molecular docking, pharmacokinetics and tissue distribution in mice. Pharm Res. 2009;26:2438–2445. doi: 10.1007/s11095-009-9955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar A, Eubank TD, Doseff AI. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J Innate Immun. 2010;2:204–215. doi: 10.1159/000296507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Kawada T, Han IS, Kim BS, Goto Takahashi N, Fushiki T, Kurata T, Yu R. Capsaicin inhibits the production of tumor necrosis factor α by LPS-stimulated murine macrophages, RAW 264.7: a PPARγ ligand-like action as a novel mechanism. FEBS Lett. 2004;572:266–270. doi: 10.1016/j.febslet.2004.06.084. [DOI] [PubMed] [Google Scholar]
- Radaev S, Zou Z, Huang T, Lafer EM, Hinck AP, Sun PD. Ternary complex of transforming growth factor-β1 reveals isoform-specific ligand recognition and receptor recruitment in the superfamily. J Biol Chem. 2010;285:14806–14814. doi: 10.1074/jbc.M109.079921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahardjo B, Widjajanto E, Sujuti H, Keman K. Curcumin decreased level of proinflammatory cytokines in monocytes cultures exposed to preeclamptic plasma by affecting the transcription factors NF-κB and PPAR-γ. Biomark Genom Med. 2014;6:105–115. [Google Scholar]
- Serbina NV, Jia T, Hohl TM, Pamer EG. Monocytes-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YH, Namkoong E, Choi S, Bae JS, Jin M, Hwang SM, Arote R, Choi SY, Park K. Capsaicin regulates the NF-κB pathway in salivary gland inflammation. J Dent Res. 2013;92:547–552. doi: 10.1177/0022034513487376. [DOI] [PubMed] [Google Scholar]
- Shrihari TG. Dual rle of inflammatory mediators in cancer. Ecancermedicalscience. 2017;11:721. doi: 10.3332/ecancer.2017.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snick JV. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Sweet MJ, Hume DA. Endotoxin signal transduction in macrophages. J Leukocyte Biol. 1996;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- Tang Y. Curcumin targets multiple pathways to halt hepatic stellate cell activation: updated mechanisms in vitro and in vivo. Digest Dis Sci. 2015;60:1554–1564. doi: 10.1007/s10620-014-3487-6. [DOI] [PubMed] [Google Scholar]
- Terao J. Dietary flavonoids as antioxidants. Forum Nutr. 2009;61:87–94. doi: 10.1159/000212741. [DOI] [PubMed] [Google Scholar]
- Thriveni V, Manjunatha H, Prabhakar BT. Protective effect of dietary curcumin and capsaicin on LPS-induced inflammation in mice. Pharmacognosy J. 2018;10:725–729. [Google Scholar]
- Triantafilou K, Triantafilou M. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;23:301–304. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Warren S. Current protocols in immunology. New York: Wiley; 1997. [Google Scholar]
- Yao QH, Wang DQ, Cui CC, Yuan ZY, Chen SB, Yao XW, et al. Curcumin ameliorates left ventricular function in rabbits with pressure overload: inhibition of the remodeling of the left ventricular collagen network associated with suppression of myocardial tumor necrosis factor-alpha and matrix metalloproteinase-2 expression. Biol Pharm Bull. 2004;27:198–202. doi: 10.1248/bpb.27.198. [DOI] [PubMed] [Google Scholar]
- Yoshiaki A, Shu H, Takashi H. Curcumin inhibition of inflammatory cytokine production by Human peripheral blood monocytes and Alveolar macrophages. Pharma Res. 1999;39:41–47. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- Zhao F, Gong Y, Hu Yuan LuM, Wang J, Dong J, Chen D, Chen L, Fu F, Qiu F. Curcumin and its major metabolites inhibit the inflammatory response induced by lipopolysaccharide: translocation of nuclear factor-kB as potential target. Mol Med Rep. 2014;11:3087–3093. doi: 10.3892/mmr.2014.3079. [DOI] [PubMed] [Google Scholar]
- Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets. 2011;12:332–347. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]