Abstract
Sacral insufficiency fractures are a common source of back pain in the elderly and are associated with significant morbidity due to poor recognition and delays in diagnosis. Previous treatment modalities have centered primarily on bed rest, oral analgesia, early mobilization and physical therapy. However, in recent years sacroplasty has emerged as a viable treatment option for sacral insufficiency fractures. Earlier recovery with sacroplasty, reduced incidence of deep venous thrombosis, and earlier return to activities of daily living are some of the features that made this treatment modality more appealing than traditional conservative management. We undertook a systematic review of the literature to examine the efficacy of sacroplasty for treatment of sacral insufficiency fractures in the elderly population. Thirty-one articles were included in this study for final analysis. Cement extravasation was the most commonly reported complication; however, it was not found to have clinical significance in the majority of studies that reported this outcome. Two studies reported S1 radicular pain after the procedure while only one study reported a patient with persistent pain requiring reoperation (1/8 incidence, 12.5%). The mean reduction in pain score from pre-procedure to latest follow-up post-procedure [reported as visual analog scale (VAS)] was 5.8+1.3 for those studies that reported this figure. Overall, sacroplasty is a safe and effective procedure associated with low complication rate and consistent pain relief in patients with sacral insufficiency fractures.
Keywords: Sacroplasty, insufficiency fracture
Introduction
Percutaneous sacroplasty has become an increasingly common treatment modality for sacral insufficiency fractures in the elderly population. Sacral insufficiency fractures are typically the result of an increased amount of stress on osteoporotic bone in geriatric individuals. As the bone quality in elderly osteoporotic individuals has deteriorated to level that is inadequate to handle the stress of weight bearing activity, insufficiency fractures result. Patients may have debilitating symptoms as a result, ranging from low back pain to thigh and hip discomfort (1). This can interfere with ambulation as well, adding to the morbidity of these injuries.
Generally, described treatment for sacral insufficiency fractures has been conservative, with oral analgesics, bed rest, and physical therapy. However, individuals treated conservatively often encounter problems with impaired mobility, lack of pain relief, and decreased ability to return to pre-injury level of function (2). Prolonged bed rest and immobility is associated with the development of DVT, pneumonia, pulmonary embolism and muscle atrophy (3). Furthermore, sacroplasty offers the potential of earlier return to activities of daily living, decreased pain long-term and minimized detrimental effects from prolonged immobilization.
Sacroplasty, first described by Garant in 2002, was developed as a method of reducing the complications associated with nonoperative management of sacral insufficiency fractures. This technique involves the injection of polymethylmethacrylate (PMMA) into the sacrum to provide structural integrity. Its development followed the success of vertebroplasty for treatment of vertebral compression fractures with the similar goals of earlier symptom resolution and return to pre-injury function (4).
Numerous studies have reported the use of percutaneous sacroplasty for treatment of sacral insufficiency fractures, though a comprehensive review of their efficacy and complications has not recently been compiled. Given that it is a relatively new surgical technique that is being more frequently performed for treatment of sacral insufficiency fractures, we aim to evaluate the outcomes of this technique through a systematic review of the literature.
Methods
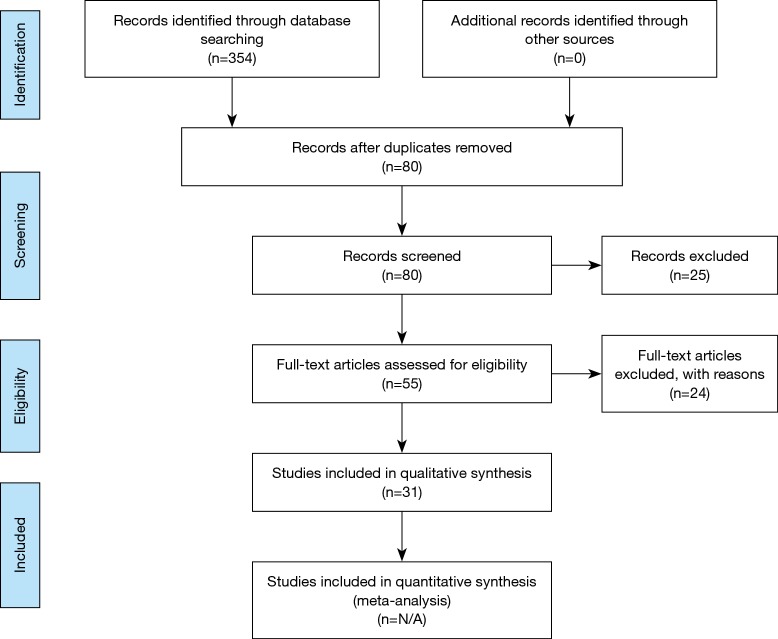
A search of three databases (PubMed, SCOPUS, and Ovid) was conducted to find articles that were relevant to this inquiry. The following keywords were used during literature searches: sacroplasty, sacral insufficiency fracture, sacroplasty complications, and sacroplasty cement augmentation. Biomechanical studies were excluded from the search, as were articles not in the English language. Studies that met the inclusion criteria consisted of prospective studies, retrospective studies, and case series. Case reports, review articles that tangentially mentioned sacroplasty, and biomechanical studies were excluded. One study included patients who underwent sacroplasty for insufficiency fractures with both osteoporotic and oncologic etiologies (5). The study was included in this report. All studies that met the inclusion criteria were subsequently evaluated for number of patients in the study, duration of follow-up, outcome measure(s), and type and frequency of complications encountered (Figure 1).
Figure 1.
Summary of search methodology per PRISMA guidelines.
Results
The initial literature searches of three databases returned 284 articles after all duplicates were removed. Once the exclusion criteria were applied, 31 articles were found to meet inclusion criteria for analysis in our study. Of these studies, 8 were prospective studies, 11 were retrospective cohort studies, and 12 were case series that each included a minimum of 3 patients. The largest sample size was in a study by Kortman et al. published in 2012 that included 243 patients (6).
A reflection of potential biases in constituent studies is an essential part of any literature review. The case series that are included in this study are inherently subject to selection bias given their design. They also lack generalizability to the general population as well as a comparison group for analysis. The retrospective studies have inherent flaws, such as confounding and sampling bias. While several prospective studies were also included in this review, they lack control groups of patients with insufficiency fractures treated conservatively.
Typical follow-up for most patients in the included studies ranged from one month to one year, with the largest follow-up being from a study by Frey et al. that followed patients out up until 10 years (7). Several studies assessed patients at multiple time intervals immediately following surgery to assess their pain scores during a given time period. Furthermore, the majority of studies reviewed utilized the visual analog scale (VAS) as the primary clinical outcome measure following the procedure. Two of the included studies (8,9) did not report their outcome measure for pain while three studies utilized a questionnaire or a unique pain scale (10-12). Furthermore, every study included in analysis reported a mean reduction in VAS at follow-up as shown in Table 1. The mean reduction in VAS at latest follow-up for those studies that reported this figure was 5.8+1.3. However, this figure should be viewed in the context that each of the included studies had variable lengths of follow-up ranging from one month to one year. This figure also does not include the eight studies in this analysis that either used a numerical pain scale other than VAS, reported subjective measures of pain, or a questionnaire.
Table 1. Articles utilized in study including measure of outcome and reported complications.
| First author | Year | Type of study | Sample size | Complications observed | Outcome measure | Mean reduction in VAS at follow-up |
|---|---|---|---|---|---|---|
| Yang | 2018 | Prospective cohort | 45 | Cement leakage | VAS | 5.0 |
| Frey | 2017 | Prospective cohort | 210 | Cement leakage, S1 radicular pain | VAS | 4.7 |
| Andresen | 2017 | Prospective cohort | 23 | Cement leakage, pneumonia | VAS | 5.6 |
| Heo | 2017 | Retrospective cohort | 68 | None | VAS | 6.2 |
| Andresen | 2017 | Prospective cohort | 40 | None | VAS, FDS | 6.6 |
| Choi | 2017 | Prospective cohort | 17 | Cement leakage | VAS | 4.3 |
| Prokop | 2016 | Case series | 46 | Cement leakage | VAS, ODI | 6.2 |
| Onen | 2015 | Retrospective cohort | 15 | Cement leakage | VAS | 5.9 |
| Andresen | 2015 | Retrospective cohort | 20 | Unknown | VAS | 6.7 |
| Moussazadeh | 2015 | Retrospective cohort | 25 | Repeat procedures, cement leakage | VAS | 4.1 |
| Hasan | 2015 | Case series | 8 | None | VAS | 5.6 |
| Gupta | 2014 | Retrospective cohort | 53 | Vertebral augmentation | VAS, FMS | 9.5 |
| Talmadge | 2014 | Prospective cohort | 18 | None | VAS | 7.0 |
| Andresen | 2013 | Prospective cohort | 10 | Unknown | VAS | 6.4 |
| Eichler | 2013 | Prospective cohort | 12 | None | VAS | 6.0 |
| Pereira | 2013 | Retrospective cohort | 58 | Cement leakage | VAS | 3.1 |
| Dougherty | 2013 | Retrospective cohort | 57 | Cement leakage | Numeric pain scale | N/A |
| Klingler | 2013 | Case series | 4 | Cement leakage | VAS | 7.0 |
| Kortman | 2012 | Retrospective cohort | 243 | Cement leakage | VAS | 6.4 |
| Shah | 2012 | Case series | 11 | None | Numeric pain scale | N/A |
| Trouvin | 2012 | Retrospective cohort | 6 | Unknown | VAS | 7.6 |
| Naderi | 2012 | Case series | 3 | None | VAS | 6.2 |
| Kang | 2009 | Retrospective cohort | 5 | Compression fracture | Numeric pain scale | N/A |
| Kamel | 2009 | Case series | 19 | None | VAS | 6.4 |
| Kang | 2009 | Case series | 8 | Persistent pain requiring reoperation | VAS | Not reported |
| Frey | 2008 | Prospective cohort | 52 | S1 radicular pain | VAS | 4.7 |
| Whitlow | 2007 | Case series | 12 | Unknown | Questionnaire | N/A |
| Frey | 2007 | Prospective cohort | 37 | S1 radicular pain | VAS | 7.0 |
| Strub | 2007 | Case series | 13 | None | VAS | Not reported |
| Butler | 2004 | Case series | 4 | Cement leakage | Subjective | N/A |
| Pommersheim | 2003 | Case series | 3 | None | Subjective | N/A |
VAS, visual analog scale; ODI, Oswestry disability index; FMS, functional mobility scale.
Of the limited number of complications reported, the most common was cement extravasation. Nine of the included studies reported this in at least one patient in the cohort of individuals analyzed. However, most extravasation events reported were clinically insignificant. Cement leakage was reported to occur in the S1 foramen, the vertebral canal, the sacroiliac joint, or anterior to the sacrum (6,8,12-14). One study reported the development of S1 radicular pain postoperatively that required a nerve root injection before subsequent relief (7). Moussazadeh et al. reported that 6 repeat procedures were performed in their study, one of which was for persistent pain that subsequently resolved while four were for progressive fracture (15).
All studies that measured pain via VAS reported statistically significant improved pain level at the latest follow-up period. Most studies had a one year follow up time. Only two of the studies included had patients with persistent pain that required reoperation. Furthermore, Frey et al. found that patients who underwent percutaneous sacroplasty had decreased use of opioid and non-opioid pain medication in the postoperative period compared to pre-operatively (7). Frey et al. published a ten-year analysis of prospective patients treated with percutaneous sacroplasty in 2017 that reflected their data collected since 2007, and their results which were also published in 2008 and 2007 with the same patient group were included separately in Table 1 to demonstrate their ongoing patient follow-up with this patient cohort.
Discussion
As a weight-bearing structure in the pelvis, the sacrum is critical for transmitting forces that occur along the spinal axis (6). More specifically, it functions as a structure through which forces from the lower extremities are transmitted and dissipated to enhance stability of the pelvic girdle. Given its crucial role in stability of the pelvis during weight-bearing, individuals who sustain sacral insufficiency fractures often have debilitating pain and difficulty ambulating. Insufficiency fractures due to osteoporosis extend in a cranial-caudal direction in parallel with the vector of force transmission through the sacral ala. Though various classification systems have been developed for sacral fractures, the Denis classification system is the most commonly utilized and divides fractures into 3 zones. Zone 1 fractures are lateral to the neural foramina, zone 2 fractures occur through the foramina, and zone 3 injuries occur medial to the foramina and involve the spinal canal.
Use of sacroplasty for treatment of insufficiency fractures was initially described by Garant in 2002. The procedure involves injection of PMMA into the cancellous bone usually at the level of S1 and S2, as they are most commonly fractured and also provide the greatest amount of stability to the sacrum (1). Denis zone I fractures of the sacral ala can safely be treated with PMMA while zone III fractures carry the risk of cement extravasation through the foramen and injury to the S1 nerve root. However, in our systematic review multiple studies reported cement leakage that had little or no clinical consequence, while only two studies reported having a patient with S1 radicular pain that subsequently resolved (6,7).
Most studies performed sacroplasty under intravenous sedation or under general anesthesia with the use of intraoperative fluoroscopy or CT. Though the articles included in this systematic review highlighted several different methods of conducting the procedure, a common approach included having the patient in prone position and using image guidance under fluoroscopy or CT to insert 13-gauge needles between the sacral foramen and sacroiliac joint at a 45-degree angle. After ensuring that the needles are inserted at the proper trajectory under the lateral view while maintaining a 45-degree angle, PMMA was injected into each sacral ala while ensuring that it did not extravasate to the nerve roots. A long-axis or short-axis technique was utilized to place the needle at the fracture site (16). The short-axis approach can be performed with fluoroscopy alone. This involves inserting the needle in a posterior-anterior direction directly to the sacral ala at the fracture site. The long-axis approach entails insertion of the needle in a caudal-cephalad direction to access the fracture site and necessitates the use of intraoperative CT scan. While the amount of PMMA injected at the fracture site varied, most groups reported injecting 4–6 mL. Furthermore, this technique does have an element of operator dependence and while most articles did not quantify average duration of procedure, some studies mentioned that most procedures took approximately 30 minutes to perform (1,6,8,17,18).
The studies exhibited varying rates of success in terms of pain relief with 68–94% reduction in VAS scores at the final follow-up visits (7,9). Several studies either did not report a scale for pain relief and reported subjective improvement or utilized their own questionnaire which was subsequently scored (8-11). Many of the included studies found that pain relief improved during subsequent follow-ups, such as Frey et al. who reported a 56% decrease in mean VAS from preoperative to immediate post-op but a 94% decrease from preoperative scores at 10 years (1). Only three of the studies included in this systematic review did not report a numerical scoring system such as VAS for quantifying patients’ pain score and instead used a questionnaire to report if patients had subjective relief. While there are numerous potential complications of sacroplasty including dural leak, hemorrhage, infection, damage to nerve roots, or injury to the lumbosacral plexus, our review did not find evidence of these more serious complications in the literature (15).
Overall, sacroplasty has been shown to be an effective procedure in terms of pain relief. Early return to function and substantial pain relief distinguished this treatment modality from traditional methods of conservative management with physical therapy and oral analgesics. Many studies reviewed, however, were retrospective. Only Frey et al. included a control group of 34 patients that underwent non-surgical management. Future prospective studies including a larger sample undergoing conservative management may allow for better comparison of these two treatment modalities. Only Gupta et al. used the functional mobility scale (FMS) to quantify the effect of sacroplasty on patient mobility, though others reported subjective improvements in patient mobility in their cohorts (17). While many of the studies did not report opioid usage before and after the procedures, those that did demonstrated that patients had decreased usage following surgery. Given the current opioid epidemic and emphasis on limited use of opioid medications for pain relief, sacroplasty may have a role contributing to decreased utilization of opioid medications in this patient population as well. Mobility and ambulation are also critical factors in assessing clinical outcome of sacral insufficiency fractures, and future studies should also assess improvements in patient mobility with sacroplasty.
Conclusions
Our systematic review finds that sacroplasty is a viable, safe, and effective option for treatment of sacral insufficiency fractures. Given the alternative conservative treatment of immobilization with pharmacotherapy for pain relief, sacroplasty offers patients a more immediate return to pre-injury level of function. Furthermore, it may reduce many of the comorbidities such as persistent pain, respiratory complications and muscle atrophy that can present with prolonged bedrest and conservative management of these injuries. We conclude that sacroplasty is effective for the treatment of sacral insufficiency fractures and is associated with minimal complications (10,13,19-31).
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Frey ME, Depalma MJ, Cifu DX, et al. Efficacy and safety of percutaneous sacroplasty for painful osteoporotic sacral insufficiency fractures: a prospective, multicenter trial. Spine 2007;32:1635-40. 10.1097/BRS.0b013e318074d4e1 [DOI] [PubMed] [Google Scholar]
- 2.Bayley E, Srinivas S, Boszczyk BM. Clinical outcomes of sacroplasty in sacral insufficiency fractures: a review of the literature. Eur Spine J 2009;18:1266-71. 10.1007/s00586-009-1048-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson R, Seibly J. Combined Sacroplasty and Iliosacral Fixation Using Triangular Titanium Implants for the Treatment of Sacral Insufficiency Fractures with Concomitant Sacral Instability. Cureus 2017;9:e1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garant M. Sacroplasty: a new treatment for sacral insufficiency fracture. J Vasc Interv Radiol 2002;13:1265-7. 10.1016/S1051-0443(07)61976-9 [DOI] [PubMed] [Google Scholar]
- 5.Pereira LP, Clarençon F, Cormier E, et al. Safety and effectiveness of percutaneous sacroplasty: a single-centre experience in 58 consecutive patients with tumours or osteoporotic insufficient fractures treated under fluoroscopic guidance. Eur Radiol 2013;23:2764-72. 10.1007/s00330-013-2881-3 [DOI] [PubMed] [Google Scholar]
- 6.Kortman K, Ortiz O, Miller T, et al. Multicenter study to assess the efficacy and safety of sacroplasty in patients with osteoporotic sacral insufficiency fractures or pathologic sacral lesions. J Neurointerv Surg 2013;5:461-6. 10.1136/neurintsurg-2012-010347 [DOI] [PubMed] [Google Scholar]
- 7.Frey ME, Depalma MJ, Cifu DX, et al. Percutaneous sacroplasty for osteoporotic sacral insufficiency fractures: a prospective, multicenter, observational pilot study. Spine J 2008;8:367-73. 10.1016/j.spinee.2007.05.011 [DOI] [PubMed] [Google Scholar]
- 8.Butler CL, Given CA, Michel SJ, et al. Percutaneous sacroplasty for the treatment of sacral insufficiency fractures. AJR Am J Roentgenol 2005;184:1956-9. 10.2214/ajr.184.6.01841956 [DOI] [PubMed] [Google Scholar]
- 9.Pommersheim W, Huang-Hellinger F, Baker M, et al. Sacroplasty: a treatment for sacral insufficiency fractures. AJNR Am J Neuroradiol 2003;24:1003-7. [PMC free article] [PubMed] [Google Scholar]
- 10.Kang SE, Lee JW, Kim JH, et al. Percutaneous sacroplasty with the use of C-arm flat-panel detector CT: technical feasibility and clinical outcome. Skeletal Radiol 2011;40:453-60. 10.1007/s00256-010-0959-4 [DOI] [PubMed] [Google Scholar]
- 11.Whitlow CT, Mussat-Whitlow BJ, Mattern CW, et al. Sacroplasty versus vertebroplasty: comparable clinical outcomes for the treatment of fracture-related pain. AJNR Am J Neuroradiol 2007;28:1266-70. 10.3174/ajnr.A0561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dougherty RW, Mcdonald JS, Cho YW, et al. Percutaneous sacroplasty using CT guidance for pain palliation in sacral insufficiency fractures. J Neurointerv Surg 2014;6:57-60. 10.1136/neurintsurg-2012-010599 [DOI] [PubMed] [Google Scholar]
- 13.Onen MR, Yuvruk E, Naderi S. Reliability and effectiveness of percutaneous sacroplasty in sacral insufficiency fractures. J Clin Neurosci 2015;22:1601-8. 10.1016/j.jocn.2015.03.039 [DOI] [PubMed] [Google Scholar]
- 14.Andresen Radmer S, Wollny M, et al. Treatment of Denis 1, 2 and 3 insufficiency fracture zones of the os sacrum: Individual approaches adapted to the course of the fracture in CT-assisted balloon sacroplasty. Osteologie 2012;21:168-73. 10.1055/s-0037-1621680 [DOI] [Google Scholar]
- 15.Moussazadeh N, Laufer I, Werner T, et al. Sacroplasty for cancer-associated insufficiency fractures. Neurosurgery 2015;76:446-50. 10.1227/NEU.0000000000000658 [DOI] [PubMed] [Google Scholar]
- 16.Gupta AC, Yoo AJ, Stone J, et al. Percutaneous sacroplasty. J Neurointerv Surg 2012;4:385-9. 10.1136/neurintsurg-2011-010136 [DOI] [PubMed] [Google Scholar]
- 17.Gupta AC, Chandra RV, Yoo AJ, et al. Safety and effectiveness of sacroplasty: a large single-center experience. AJNR Am J Neuroradiol 2014;35:2202-6. 10.3174/ajnr.A4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andresen R, Radmer S, Lüdtke CW, et al. Balloon sacroplasty as a palliative pain treatment in patients with metastasis-induced bone destruction and pathological fractures. Rofo 2014;186:881-6. 10.1055/s-0033-1356418 [DOI] [PubMed] [Google Scholar]
- 19.Frey ME, Warner C, Thomas SM, et al. Sacroplasty: A Ten-Year Analysis of Prospective Patients Treated with Percutaneous Sacroplasty: Literature Review and Technical Considerations. Pain Physician 2017;20:E1063-72. [PubMed] [Google Scholar]
- 20.Yang SC, Tsai TT, Chen HS, et al. Comparison of sacroplasty with or without balloon assistance for the treatment of sacral insufficiency fractures. J Orthop Surg (Hong Kong) 2018;26:2309499018782575. 10.1177/2309499018782575 [DOI] [PubMed] [Google Scholar]
- 21.Andresen R, Radmer S, Wollny M, et al. CT-guided cement sacroplasty (CSP) as pain therapy in non-dislocated insufficiency fractures. Eur J Orthop Surg Traumatol 2017;27:1045-50. 10.1007/s00590-017-2001-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heo DH, Park CK. Percutaneous Sacroplasty for Non-neoplastic Osteoporotic Sacral Insufficiency Fractures. Pain Physician 2017;20:89-94. [PubMed] [Google Scholar]
- 23.Andresen R, Radmer S, Andresen JR, et al. Comparison of the 18-month outcome after the treatment of osteoporotic insufficiency fractures by means of balloon sacroplasty (BSP) and radiofrequency sacroplasty (RFS) in comparison: a prospective randomised study. Eur Spine J 2017;26:3235-40. 10.1007/s00586-016-4935-0 [DOI] [PubMed] [Google Scholar]
- 24.Choi KC, Shin SH, Lee DC, et al. Effects of Percutaneous Sacroplasty on Pain and Mobility in Sacral Insufficiency Fracture. J Korean Neurosurg Soc 2017;60:60-6. 10.3340/jkns.2016.0505.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan MS. Sacroplasty for sacral insufficiency fractures. Egyptian Journal of Radiology and Nuclear Medicine 2015;46:987-91. 10.1016/j.ejrnm.2015.06.003 [DOI] [Google Scholar]
- 26.Talmadge J, Smith K, Dykes T, et al. Clinical impact of sacroplasty on patient mobility. J Vasc Interv Radiol 2014;25:911-5. 10.1016/j.jvir.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 27.Eichler K, Zangos S, Mack MG, et al. Outcome of long-axis percutaneous sacroplasty for the treatment of sacral insufficiency fractures with a radiofrequency-induced, high-viscosity bone cement. Skeletal Radiol 2014;43:493-8. 10.1007/s00256-013-1811-4 [DOI] [PubMed] [Google Scholar]
- 28.Klingler JH, Kluge P, Sircar R, et al. First experience using navigation-guided radiofrequency kyphoplasty for sacroplasty in sacral insufficiency fractures. Rofo 2013;185:733-40. 10.1055/s-0033-1335582 [DOI] [PubMed] [Google Scholar]
- 29.Shah RV. Sacral kyphoplasty for the treatment of painful sacral insufficiency fractures and metastases. Spine J 2012;12:113-20. 10.1016/j.spinee.2012.01.019 [DOI] [PubMed] [Google Scholar]
- 30.Trouvin AP, Alcaix D, Somon T, et al. Analgesic effect of sacroplasty in osteoporotic sacral fractures: a study of six cases. Joint Bone Spine 2012;79:500-3. 10.1016/j.jbspin.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 31.Naderi S, Ilaslan H, Aslan A, et al. Sacroplasty: report of three cases. Turk Neurosurg 2010;20:418-22. [DOI] [PubMed] [Google Scholar]