Abstract
Background
Lumbar spinal stenosis (LSS) results in significant pain and disability. As spine healthcare providers, monitoring patient’s outcomes is of the highest importance, and guides everything we do. However, a large amount of our data has been based solely on subjective, single time-point outcome tools limited by their subjective nature.
Methods
We herein propose a novel, simple objective scoring system, the Gait Posture index (GPi). Four key objective health metrics, which can be measured using wearable devices have been identified to correlate with health status: (I) step count; (II) gait velocity; (III) step length; (IV) posture. An algorithm combining the above metrics was established to ‘score’ patient’s ambulation from 0 (bed bound)–100 (excellent mobility and gait function). Thirteen surgical patients were assigned to the GPi scoring algorithm and compared with traditional subjective scoring systems Oswestry Disability Index (ODI) and Patient Satisfaction Index (PSI) as a proof of concept and confirmation of validity.
Results
At 3 months, 11 out of 13 patients following decompression for LSS had an improvement with their GPi 20.79±17.44, P=0.001. In addition, Pearson correlation revealed positive correlation between change in GPi with change in ODI (r=0.682, n=13, P=0.01) and negative correlation between change in GPi with PSI (r=−0.618, n=13, P=0.024).
Conclusions
The GPi algorithm correlates accurately with preoperative and post-operative mobility which are comparable to traditional subjective scoring systems. GPi affords the health care provider with a relevant measure of patient outcome, and real-time recovery dynamics following decompression for LSS.
Keywords: Lumbar spinal stenosis (LSS), Gait Posture index (GPi), step count, step length, posture, gait velocity, spine
Introduction
Spinal pathologies including degenerative, cancer and trauma constitute a large proportion of neurosurgical and orthopaedic procedures. Lumbar spinal stenosis (LSS) is a common degenerative disorder of the aging spine, and a significant cause of pain and disability. Poor walking tolerance and intermittent claudication tends to be relieved by leaning forward and the seated position (1). LSS resulting in symptomatic claudication is the most common indication for spine surgery in the over 65 age group (2). Currently, the consensus for management of LSS patients who failed conservative therapies is lumbar decompression surgery and is associated with improvement in patient quality of life as recognized in several studies including the Spine Patient Outcomes Research Trial (SPORT) study (3,4). The efficacy of surgical intervention for LSS has been long debated (5), however there are various attempts to address the lack of high level evidence (6,7).
Important patient outcomes following LSS surgery include improved mobility, walking distance and a reduction of falls risk, however there are limited objective tools for assessing these outcomes (8). Clinical outcomes of decompression procedures, with or without fusion, are based largely on patient reported outcome measures (PROMs). Existing assessment tools are subjective, such as the Visual Analogue Score (VAS), the Oswestry Disability Index (ODI), and the EuroQoL-5-Dimensions (EQ-5D), which have significant limitations including bias of reporting, variance of assessment timing, patient compliance/loss to follow-up, no capacity for continuous assessment, and the subjective nature of patient self-assessment (9,10). Outcome measures of spinal interventions are typically carried out at specific timepoints: pre-operatively, 6 weeks, 3 months and 12 months, etc. Despite this current practice, a significant downside of PROMs stems from the timepoint of outcome assessment with substantial gaps of interval between these timepoints.
PROMs have been the foundation of outcomes assessment over the last 40 years (11,12). There is however an urgent need for a transition from subjective to objective assessment tools. Insurance payers, governments and hospitals demand robust and accurate data with regards to the outcomes of expensive interventions that surgeons perform, that consume significant health care resources (13,14). Smart wearable devices that capture health metrics can potentially form the basis for objective assessment following any health care intervention (9), including spinal surgery and related pathologies (15).
The association between gait deterioration and LSS has been long established and explored in the literature. Gait parameters such as cadence, step count, step length and gait velocity have been proposed to correlate with spinal pathologies including LSS (16,17). Recent development and availability of wearable technologies which comprise gait analytic sensors has provided a simplified substitute to laboratory-based gait analysis (9,15,18,19), as traditional gait analysis in formal laboratory settings are relatively time consuming, labour-intensive and equipment heavy (20,21). A recent review of relevant gait metrics and LSS (8) has revealed several key metrics that may form the basis of accurate and pertinent outcomes assessment for this common condition, with relevant metrics scorable from wearable devices. Therefore, the aim of this prospective clinical study is to evaluate gait metrics including: (I) daily step count; (II) gait velocity; (III) step length; and (IV) body posture during ambulation including sagittal and coronal alignment for patients with LSS, and develop a simple and objective score of gait assessment: the Gait Posture index (GPi).
Methods
Rationale for the GPi
The proposed GPi is a scored measure of activity and gait performance (Figure 1), based on objective data capture, with a range of 0 (poor) to 100 (excellent function). The GPi was devised to address the problems which arise from PROMs, such as compliance issues, reporting bias and inherent subjectivity. The GPi can potentially be recorded continuously with data extraction from a wearable device, based on a patient’s mobility and physical activity, giving the healthcare provider a continuous, non-biased, objective data stream of patient performance. To test the reliability of the proposed GPi, a prospective, non-randomised single surgeon series of 13 patients with LSS was collected. Preoperative and postoperative data for 3 months were collected including GPi metrics (daily step count, gait velocity, mean step length and postural score), ODI (11) and Patient Satisfaction Index (PSI) [Modified Odom’s criteria whereby 1: excellent, 2: good, 3: fair and 4: same or worse (22)]. The VAS back/leg score was specifically removed from data capture due to its highly subjective nature. All data was collected by a research team and clinic nurse.
Figure 1.
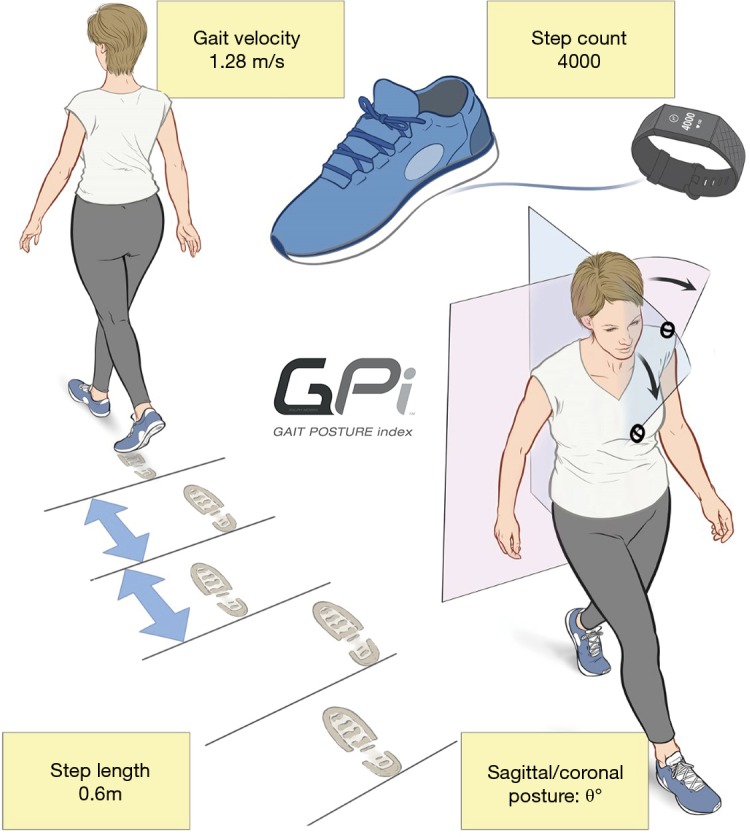
The 4 components of the Gait Posture index (GPi): (I) step count; (II) gait velocity; (III) step length; and (IV) posture.
Ethics
Approval was obtained from the South Eastern Sydney Local Health District, New South Wales, Australia (HREC 13/090). All participants provided written informed consent.
Patient recruitment
The study was a consecutive, single-surgeon prospective series. Patients were enrolled between September to December 2018 by the senior author (Ralph J. Mobbs), who performed all surgical procedures. The inclusion criteria were patients between the ages of 50 to 85 who presented with neurogenic claudication secondary to LSS over 1 or 2 levels, with less than a grade 1 spondylolisthesis on standing X-rays. All procedures were performed using a Unilateral Laminectomy for Bilateral Decompression (ULBD) (23). The exclusion criteria included infection, cancer, prior surgery at the index level, and other comorbid conditions that were believed to substantially limit activity such as hip or knee pathology, or other neurological disorders impeding walking capacity. See Table 1 for the detailed inclusion/exclusion criteria.
Table 1. Lumbar spinal stenosis/GPi study—eligibility criteria.
| Inclusion criteria |
| 50 years of age or older |
| Present with complaints of neurogenic claudication for at least 3 months. Neurogenic claudication as defined as pain, numbness and/or fatigue below the gluteal line with or without back pain (if back pain present, leg pain is greater than back pain) that is precipitated by walking and alleviated by sitting or lumbar flexion |
| Grades C or D stenosis as defined by Schizas [2010] indicating occlusion (absent CSF signal) of the central lumbar spinal canal at one or two levels on T2 weighted MRI |
| Be medically suitable for single or dual-level spinal decompression surgery |
| Have not improved with non-surgical treatment (e.g., physical therapy, injections, stretching, exercises) |
| Exclusion criteria |
| Women who are pregnant |
| Under workers compensation |
| Serious spinal pathology including cancer, infection, cauda equine syndrome, spinal fracture, inflammatory arthritis |
| Present with active Paget’s disease of the spine; |
| Previous lumbar spinal surgery at the same levels |
| Motor deficit related to lumbar compression [Medical Research Council (MRC) grades 0–4] and the motor deficit interferes with walking ability |
| Presence of known or demonstrated peripheral vascular disease-causing vascular claudication, i.e., claudication accompanied by absent foot pulse or vascular insufficiency detected with Doppler ultrasound or CT angiography |
| Presence of significant lumbar scoliosis (Cobb angle >25°) or other spinal deformities |
| Presence of lumbar instability defined as more than 4 mm or 10 degrees of angular motion (at affected or adjacent level) between flexion and extension on upright lateral radiographs (to exclude patients who might need to undergo concurrent surgical fusion) |
| Meyerding classification grade 2 or greater spondylolisthesis |
| Symptomatic hip disease with symptoms reproduced with external or internal rotation of the hip joint |
| Cognitive impairment or inadequate English language skills that interfere with patient’s ability to give fully informed consent or complete the baseline or follow-up assessments |
GPi, Gait Posture index; CSF, cerebrospinal fluid.
Gait analysis/accelerometery
Gait metrics assessed are summarised in Table 2. Physical activity performance in terms of daily step counts was assessed with a wrist-based accelerometer (MiBand2, Xiaomi, China) or patients own device if they preferred. The accelerometer was synced to the patients’ smart phone and data recorded by researcher on presentation to the clinic. The activity monitor was used to record daily step counts starting preoperatively (at least a week prior to surgery), then prospectively until 3-month mark. Parameters recorded include number of steps taken, distance travelled, and calories burnt, however step count was the primary metric reported for the current study.
Table 2. Scoring of GPi metrics.
| Metric | Range | Score |
|---|---|---|
| 1. Daily step count | 0–10,000 steps* | 0–25 |
| 2. Gait velocity | 0–1.35 m/s** | 0–25 |
| 3. Mean step length | 0–0.75 m | 0–25 |
| 4. Postural score (while ambulating) | Upright | 25 |
| <5° forward or lateral tilt | 20 | |
| 5°–15° forward or lateral tilt | 15 | |
| >15° forward or lateral tilt | 10 | |
| Requiring a walking aids (e.g., walking frame or walking stick) | 5 | |
| Unable to ambulate | 0 | |
| GPi total | 100 |
*, beyond 10,000 steps, there is no additional points awarded. **, walking velocity of 1.35 m/s is considered the upper limit of normal for health adults. GPi, Gait Posture index.
Gait velocity and mean step length was recorded preoperatively and postoperatively by a trained researcher over a given distance. Patients were required to complete an unobstructed 120 m course, or 30 m course if unable to complete the full distance, or at risk of falls. The time of completion and step count was recorded for each patient, thus yielding their gait speed (metres per second, m/s) and step lengths (metres, m). In addition to observational recording of time and step count over distance, a wearable device (TracPatch, Consensus Orthopaedics, CA, USA) was attached to the patient to confirm accuracy of these measurements. The wearable device and observational recording of gait velocity and step length was within 2% confirming precision of data collection. To negate bias, the pre and post-operation data was measured using the same wearable device for step count. No cross over device data was used.
Posture with ambulation was scored by observation. A representative picture of the patient was taken during walking on the flat, and sagittal/coronal angulation recorded. At the time of this study, there was no commercially available wearable device to accurately record sagittal or coronal body position at rest or with walking. A score was calculated based on body position with walking (Table 2).
PROMs
Clinical outcomes were measured using self-reported scores including the ODI and PSI as per Odom’s criteria, at completion of the study at 3 months postoperative (11,22). PROMs were measured preoperatively and postoperatively at each visit.
Statistics
Demographic variables including age and gender was summarized using descriptive statistics (mean ± standard deviation or percentage). The pre- and postoperative parameters were compared with a 2-tailed, paired sample t-test. A P value <0.05 was considered significant. All statistical analyses were performed using SPSS software (version 25.0, IBM). Pearson correlation analysis was performed to determine significant correlation between changes in physical activity parameters (step count, gait velocity and step length) versus change in PROMs (ODI and PSI/Odom’s score). A P value of <0.05 was considered significant.
Gait metrics identified (Table 2)
Based on available literature on relevant gait metrics that correlate with decline in function with spinal pathology and LSS, the following 4 metrics were identified as ‘key’ metrics to document and score:
Daily step count;
Mean gait velocity;
Mean step length;
Posture with ambulation.
Scoring the GPi
We herein propose a preliminary scoring algorithm for the GPi, based on gait metrics for patients with LSS. A scoring system of a total 0 (bed bound)–100 (excellent mobility and gait function) is used. Due to complexities of normative data of age, sex and height, a decision was made to report a ‘raw score’ for the current algorithm. Future modifications of the GPi may likely include a sex and aged matched scoring algorithm. Each of the 4 gait metrices were adopted and individually scored from 0 (poor) to 25 (excellent) including: (I) daily step counts up to 10,000 steps; (II) gait velocity up to 1.35 metres/second; (III) mean step length up to 0.75 metres; and (IV) postural score based on observed degree of trunk angulation in both the sagittal and coronal plane beyond 15 degrees, and scored dependant on the presence of walking aids. The total score is based on an addition of the four scores up to 100. The breakdown of each component is demonstrated in Table 2, and a summary of the metrics in Figure 1.
Drawbacks of the study
A potential bias of this study was that the treating surgeon only accepted patient’s with significant disability. The mean preoperative ODI in the current series was greater than 50 (severe disability), with the majority of surgical literature on LSS reporting mean ODI scores between 30–40 (moderate disability) (24,25). It could be argued that this cohort had a greater level of disability preoperatively than published articles on LSS, and therefore may have a greater propensity for improvement post decompression.
Results
A total of 13 patients were included in the study. Given the short recruitment, the mean follow-up was of 92±28 days (range, 50–151 days). The average age of the cohort was 69.15±11.05 (range, 51–83) years, with 2 males and 11 females. Operative levels performed include 11 single-level (1 L2/3, 3 L3/4, 7 L4/5) and 2 two-level (L3/4, L4/5) decompression. Among these 13 patients, 8 patient had a grade C stenosis and 5 patients had grade D stenosis based on the grading system for lumbar canal stenosis proposed by Schizas et al. (26).
In the preoperative period, the mean number of steps taken per day was 2,638±1,673 steps, mean gait velocity 0.8±0.33 m/s, mean step length 0.50±0.12 m and postural score 17.08±6.41. Following decompression for LSS, the number of steps at follow-up was 4,615±1,330 steps, gait velocity 1.12±0.27 m/s, mean step length 0.63±0.15 m and postural score of 23.23±3.14. There was a significant increase in the number of steps, gait velocity, step length and postural score compared to the preoperative status (Table 3).
Table 3. Statistics of pre-operative and post-operative parameters (N=13).
| Age | Pre-op steps | Post-op steps | Pre-op gait velocity (m/s) | Post-op gate velocity (m/s) | Pre-op step length (m) | Post-op step length (m) | Posture score (pre) | Posture score (post) | Pre-op GPi | Post-op GPi | Pre-op ODI | Post-op ODI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 69.15 | 2,638.46 | 4,615.38 | 0.8008 | 1.1185 | 0.5023 | 0.63 | 17.08 | 23.23 | 55.1754 | 75.9608 | 50.46 | 31.85 |
| Std. error of mean | 3.065 | 463.894 | 368.79 | 0.0915 | 0.07568 | 0.03249 | 0.0412 | 1.778 | 0.871 | 4.83755 | 3.07998 | 5.765 | 3.49 |
| Std. deviation | 11.052 | 1,672.592 | 1,329.691 | 0.32991 | 0.27288 | 0.11713 | 0.14855 | 6.409 | 3.14 | 17.44204 | 11.10504 | 20.787 | 12.582 |
| Range | 32 | 6500 | 4000 | 1.2 | 1.1 | 0.32 | 0.63 | 20 | 10 | 54.21 | 35 | 70 | 38 |
| Minimum | 51 | 500 | 3000 | 0.2 | 0.4 | 0.36 | 0.24 | 5 | 15 | 28.12 | 52.91 | 28 | 16 |
| Maximum | 83 | 7000 | 7000 | 1.4 | 1.5 | 0.68 | 0.87 | 25 | 25 | 82.33 | 87.91 | 98 | 54 |
The GPi score increased 20.79±17.44 from a preop score of 55.17±17.44, to a postop score of 75.96±11.11 (P=0.001) (Tables 3 and 4), with 11 patients increasing their GPi and 2 patients experienced a decline. Interesting, of the 2 patients that experienced a decline, one patient scored their PSI outcome a 2 (“good result”) and the other 3 (“fair result”). No patient scored a PSI of 4 (same or worse as preop). Individually, all changes in each parameter for the GPi were statistically significant (P<0.05). Using the proposed scoring algorithm of 0–25 for each gait metric, the mean step score increased by 4.94±4.87, mean gait velocity score increased by 5.74±6.60, mean step length score increased by 3.95±4.25 and mean posture score increased by 6.15±6.74.
Table 4. Paired t-test comparing post-operative outcome score to pre-operative score.
| Paired differences | |||||||
|---|---|---|---|---|---|---|---|
| Mean | Std. deviation | Std. error mean | 95% confidence interval of the difference | t | df | Sig. (2-tailed) | |
| GPi (Post) - GPi (Pre) | 20.78538 | 17.44097 | 4.83726 | 10.24591–31.32486 | 4.297 | 12 | 0.001 |
| Step score (Post) - step score (Pre) | 4.94231 | 4.86715 | 1.3499 | 2.00112–7.88349 | 3.661 | 12 | 0.003 |
| Gait velocity score (Post) - gait velocity score (Pre) | 5.74074 | 6.60292 | 1.83132 | 1.75064–9.73084 | 3.135 | 12 | 0.009 |
| Step length score (Post) - step length score (Pre) | 3.94872 | 4.25103 | 1.17902 | 1.37985–6.51759 | 3.349 | 12 | 0.006 |
| Posture score (Post) - posture score (Pre) | 6.154 | 6.743 | 1.87 | 2.079–10.229 | 3.29 | 12 | 0.006 |
| ODI (Post) - ODI (Pre) | −18.615 | 16.681 | 4.626 | −28.696 to −8.535 | −4.024 | 12 | 0.002 |
Post, post-op; Pre, pre-op; GPi, Gait Posture index.
At 3 months follow-up, there was a significant decrease in ODI scores from 50.46±20.79 to 31.85±12.58 (P=0.002) (Tables 3 and 4). Pearson correlation revealed a positive correlation between change in GPi with change in ODI whereby r=0.682, n=13, P=0.01. Negative correlations were found between change in GPi with PSI, r=−0.618, n=13, P=0.024 and change in ODI with PSI, r=−0.679, n=13, P=0.011 (Table 5) (Figure 2). Interestingly, no significance (P>0.05) were determined between correlation of (I) the degree of stenosis with change in GPi; (II) the degree of stenosis with change in ODI and (III) the degree of stenosis with pre-op GPi.
Table 5. Correlations for change in GPi (N=13).
| Change in ODI | PSI | |
|---|---|---|
| Change in GPi | 0.682* | −0.618* |
| Change in ODI | – | −0.679* |
| PSI | – | – |
*, correlation is significant at the 0.05 level (2-tailed). GPi, Gait Posture index; PSI, Patient Satisfaction Index.
Figure 2.
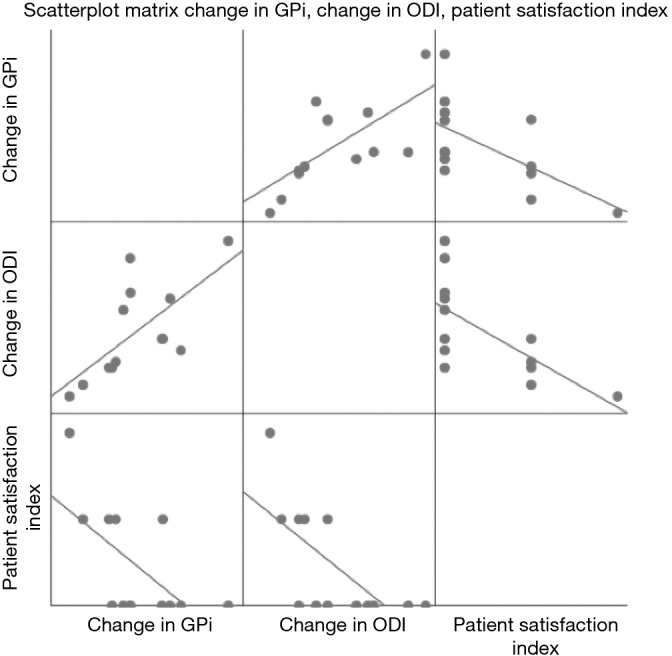
Scatterplot matrix illustrating correlation between change in GPi, change in ODI and PSI. GPi, Gait Posture index; ODI, Oswestry Disability Index; PSI, Patient Satisfaction Index.
Our preliminary results at 3 months displayed an improved GPi in 11 out of 13 patients. Of the 2 patients who had a decline in GPi, 1 patient had a wound infection while the other patient’s deterioration was not known as postoperative MRI revealed adequate central decompression of the spinal canal. Significant benefits were demonstrated in the gait velocity, mean step length and overall GPi. There was a significant improvement in patient posture at 3 months, with improvement in sagittal posture in the majority of patients. Additionally, significant correlation was observed between GPi changes, ODI changes and PSI.
Discussion
Continuous, non-biased, objective data streams will likely form the future of outcomes assessment in spinal surgery (18,27). The vast majority of outcomes data in spinal surgery are based on subjective PROMs. Validated measures such as the ODI offer a powerful tool for outcomes assessment for lumbar pathologies, however, are hindered by time, patient mental health, bias of reporting, and are subjective in nature (28).
The choice of gait metrics to define the GPi was based on a review of the literature with regards to overall health status, and specifically spinal health (8). Steps are the fundamental component of human locomotion, and daily step count is therefore a preferred metric for quantifying general physical activity (29). The strong association between steps per day and general health status has been shown in the literature (30). gait speed (velocity), has been shown to be correlate with survival among older adults in various epidemiological cohort studies and has been shown to reflect health and functional status (31-34). A reduction in step length has been shown to correlate with certain degenerative spinal pathologies and myelopathy (35). Posture is based on the coronal balance and sagittal curvatures of the spine that are arranged in order to obtain the most efficient mechanical position; the neutral posture in standing and locomotion (36). A combination of these powerful metrics was combined to generate the GPi.
Although the current study is relatively small in terms of numbers, we have demonstrated that gait metrics are a strong predictor of patient outcomes at the 3-month post-surgical time point, and our results are reflective of previous studies that have investigated gait metrics and LSS (8). The benefit with combining gait metrics into a single score such as the GPi, is to provide simplicity of reporting of multiple complex objective data streams into a single figure. Improvements of the GPi was also correlated with improvement in ODI as well as PSI, suggestive of a positive validity of the GPi.
Although not currently analysed, the GPi can potentially expand into a continuous monitoring tool using the patient’s day-to-day mobility and activity levels. This may in turn provide an objective, continuous gait analysis which is then available to multiple healthcare and non-healthcare providers who will benefit from this data. The benefits are not limited solely to assist in the provision of spinal services but also confirm benefit of the various interventions that spine providers perform. In particular, insurance providers, governments, hospitals, but most importantly patients and surgeons will be able to work together and use this data to formulate recovery plans.
The authors are conducting a prospective series of all spine patients whom present to a spine unit, with data capture of the GPi metrics of all patients (step, velocity, length, posture) to assess applicability of this score to additional spine pathologies such as acute sciatica and myelopathy.
Based on this preliminary report, the authors recommend that spine care providers use gait analysis, and tools such as the GPi, as part of their clinical evaluation to provide an objective measure of function and assessment of post intervention recovery. The use of GPi may have similar potential for other spine pathologies, such as acute disc herniations, low back pain and myelopathy, as these conditions are recognized to result in gait dysfunction.
Conclusions
Based on our findings, spine care providers are encouraged to use gait analysis as part of their routine clinical evaluation to provide an objective measure of patient function and performance post-interventions. The proposed GPi is a powerful predictor of outcome for LSS surgery. Significant improvement and positive correlation of activity outcome with conventional PROMs (ODI and Odom’s criteria) were demonstrated.
Acknowledgments
The authors would like to thank Kaitlin Rooke, Nicole Kah Mun Yoong, Jordan Perring, Christopher Katsinas and Graeme McMeekan in assisting with data collection for the study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approval was obtained from the South Eastern Sydney Local Health District, New South Wales, Australia (HREC 13/090). All participants provided written informed consent.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Genevay S, Atlas SJ. Lumbar Spinal Stenosis. Best Pract Res Clin Rheumatol 2010;24:253-65. 10.1016/j.berh.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deyo RA. Treatment of lumbar spinal stenosis: a balancing act. Spine J 2010;10:625-7. 10.1016/j.spinee.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 3.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am 2009;91:1295-304. 10.2106/JBJS.H.00913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine 2010;35:1329-38. 10.1097/BRS.0b013e3181e0f04d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machado GC, Ferreira PH, Harris IA, et al. Effectiveness of Surgery for Lumbar Spinal Stenosis: A Systematic Review and Meta-Analysis. PLoS One 2015;10:e0122800. 10.1371/journal.pone.0122800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson DB, Ferreira ML, Harris IA, et al. SUcceSS, SUrgery for Spinal Stenosis: protocol of a randomised, placebo-controlled trial. BMJ Open 2019;9:e024944. 10.1136/bmjopen-2018-024944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson DB, Mobbs RJ, Eyles J, et al. Barriers to participation in a placebo-surgical trial for lumbar spinal stenosis. Heliyon 2019;5:e01683. 10.1016/j.heliyon.2019.e01683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakravorty A, Mobbs RJ, Anderson DB, et al. The role of wearable devices and objective gait analysis for the assessment and monitoring of patients with lumbar spinal stenosis: systematic review. BMC Musculoskelet Disord 2019;20:288. 10.1186/s12891-019-2663-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mobbs RJ, Katsinas CJ, Choy WJ, et al. Objective monitoring of activity and Gait Velocity using wearable accelerometer following lumbar microdiscectomy to detect recurrent disc herniation. J Spine Surg 2018;4:792-7. 10.21037/jss.2018.12.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeVine J, Norvell DC, Ecker E, et al. Evaluating the correlation and responsiveness of patient-reported pain with function and quality-of-life outcomes after spine surgery. Spine (Phila Pa 1976) 2011;36:S69-74. 10.1097/BRS.0b013e31822ef6de [DOI] [PubMed] [Google Scholar]
- 11.Fairbank JC, Couper J, Davies JB, et al. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271-3. [PubMed] [Google Scholar]
- 12.Zanoli G, Strömqvist B, Jönsson B. Visual Analog Scales for Interpretation of Back and Leg Pain Intensity in Patients Operated for Degenerative Lumbar Spine Disorders. Spine 2001;26:2375-80. 10.1097/00007632-200111010-00015 [DOI] [PubMed] [Google Scholar]
- 13.Machado G, Lin C, Harris I. Spinal fusion surgery for lower back pain: it’s costly and there’s little evidence it’ll work. The Conversation. 2018. [Google Scholar]
- 14.NSW Health. Cost of care in NSW Hospitals. NSW Government, Health. 2016. Available online: https://www.health.nsw.gov.au/Hospitals/Going_To_hospital/cost-of-care/Pages/default.aspx. Accessed 22/6/2019.
- 15.Mobbs RJ, Phan K, Maharaj M, et al. Physical Activity Measured with Accelerometer and Self-Rated Disability in Lumbar Spine Surgery: A Prospective Study. Global Spine J 2016;6:459-64. 10.1055/s-0035-1565259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whittle MW. Clinical gait analysis: A review. Hum Mov Sci 1996;15:369-87. 10.1016/0167-9457(96)00006-1 [DOI] [Google Scholar]
- 17.Nagai K, Aoyama T, Yamada M, et al. Quantification of Changes in Gait Characteristics Associated With Intermittent Claudication in Patients With Lumbar Spinal Stenosis. J Spinal Disord Tech 2014;27:E136-42. 10.1097/BSD.0b013e3182a2656b [DOI] [PubMed] [Google Scholar]
- 18.Mobbs R. Wearables in spine surgery: Beginnings, research and real-world applications. Spinal News Int 2017; Available online: https://spinalnewsinternational.com/wearables
- 19.Simpson L, Maharaj MM, Mobbs RJ. The role of wearables in spinal posture analysis: a systematic review. BMC Musculoskelet Disord 2019;20:55. 10.1186/s12891-019-2430-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanlon M, Anderson R. Real-time gait event detection using wearable sensors. Gait Posture 2009;30:523-7. 10.1016/j.gaitpost.2009.07.128 [DOI] [PubMed] [Google Scholar]
- 21.Maffiuletti NA, Gorelick M, Kramers-de Quervain I, et al. Concurrent validity and intrasession reliability of the IDEEA accelerometry system for the quantification of spatiotemporal gait parameters. Gait Posture 2008;27:160-3. 10.1016/j.gaitpost.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 22.Odom GL, Finney W, Woodhall B. Cervical disk lesions. J Am Med Assoc 1958;166:23-8. 10.1001/jama.1958.02990010025006 [DOI] [PubMed] [Google Scholar]
- 23.Mobbs R, Phan K. Minimally Invasive Unilateral Laminectomy for Bilateral Decompression. JBJS Essent Surg Tech 2017;7:e9. 10.2106/JBJS.ST.16.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slätis P, Malmivaara A, Heliövaara M, et al. Long-term results of surgery for lumbar spinal stenosis: a randomised controlled trial. Eur Spine J 2011;20:1174-81. 10.1007/s00586-010-1652-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deyo RA, Gray DT, Kreuter W, et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976) 2005;30:1441-5; discussion 1446-7. 10.1097/01.brs.0000166503.37969.8a [DOI] [PubMed] [Google Scholar]
- 26.Schizas C, Theumann N, Burn A, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine (Phila Pa 1976) 2010;35:1919-24. 10.1097/BRS.0b013e3181d359bd [DOI] [PubMed] [Google Scholar]
- 27.Rehan K. Wearing Your Spine Health on Your Sleeve. Can "wearables" update your spine specialist instantly on your recovery? SpineUniverse, 2017. [Google Scholar]
- 28.Stienen MN, Smoll NR, Joswig H, et al. Influence of the mental health status on a new measure of objective functional impairment in lumbar degenerative disc disease. Spine J 2017;17:807-13. 10.1016/j.spinee.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 29.Bassett DR, Jr, Toth LP, LaMunion SR, et al. Step Counting: A Review of Measurement Considerations and Health-Related Applications. Sports Med 2017;47:1303-15. 10.1007/s40279-016-0663-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazaki R, Ayabe M, Ishii K, et al. Longitudinal association between the daily step count and the functional age in older adults participating in a 2.5-year pedometer-based walking program: The YURIN study. Anti-Aging Medicine 2013;10:60-9. [Google Scholar]
- 31.Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. J Am Geriatr Soc 2009;57:251-9. 10.1111/j.1532-5415.2008.02126.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc 2005;53:1675-80. 10.1111/j.1532-5415.2005.53501.x [DOI] [PubMed] [Google Scholar]
- 33.Studenski S, Perera S, Patel K, et al. Gait Speed and Survival in Older Adults. JAMA 2011;305:50-8. 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cesari M. Role of Gait Speed in the Assessment of Older Patients. JAMA 2011;305:93-4. 10.1001/jama.2010.1970 [DOI] [PubMed] [Google Scholar]
- 35.Haddas R, Ju KL, Belanger T, et al. The use of gait analysis in the assessment of patients afflicted with spinal disorders. Eur Spine J 2018;27:1712-23. 10.1007/s00586-018-5569-1 [DOI] [PubMed] [Google Scholar]
- 36.Araújo F, Lucas R. What do we know about the determinants of sagittal standing posture? OA Musculoskeletal Medicine 2014;17:15. [Google Scholar]


