Summary
Monitoring the cascade or continuum of HIV services – ranging from outreach services to anti-retroviral treatment – has become increasingly important as the focus in prevention moves toward biomedical interventions, in particular, ‘Treatment as Prevention.’ The HIV continuum typically utilises clinic-based care and treatment monitoring data and helps identify gaps and inform program improvements. This paper discusses the merits of a population-based survey-informed continuum of services. Surveys provide individual-level, population-based data by sampling persons both in and outside the continuum, which facilitate the estimation of population fractions, such as the proportion of people living with HIV in care, as well as the examination of determinants for being in or outside the continuum. Survey-informed cascades of services may especially benefit key populations at increased risk for HIV infection for who social marginalisation, criminalisation, and stigma result in barriers to access and retention in services, a low social visibility, mobility, and outreach-based services can compromise clinic-based monitoring. Adding CD4+ T-cell count and viral load measurements to such surveys may provide population-level information on viral load suppression, stage of disease, treatment needs, and population-level transmission potential. While routine clinic-based reporting will remain the mainstay of monitoring, a survey-informed service cascade can address some of its limitations and offer additional insights.
Keywords: Continuum of HIV services, cascade of services, care and treatment, population-based surveys, key populations, HIV
Introduction
Recent years have seen a shift in human immunodeficiency virus infection (HIV) prevention with the advent of evidence-based biomedical interventions best epitomised by the concept of ‘Treatment as Prevention’1 defined as HIV prevention methods that use antiretroviral therapy (ART) in HIV-positive persons to decrease the chance of HIV transmission independent of CD4 T cell count. Viral load suppression through ART and the consequent immunological recovery provides the potential for near-normal life expectancy2,3 minimises HIV transmission4,5 and is integral to reaching the Joint United Nations Programme on HIV and AIDS’ (UNAIDS) goal of 90% of people living with HIV (PLHIV) knowing their serostatus, 90% of diagnosed PLHIV receiving ART, and 90% of people receiving ART achieving viral load suppression.6 The potential impact of ART on population-level health has enhanced the utility of HIV testing as a gateway to a range of clinical services, including linkage to and retention in care, ART initiation, and adherence to treatment. On a programmatic level, these serially linked events are necessary to achieve the desired outcome of sustained viral load suppression, a pivotal goal both for individual and population health. Ideally, all HIV-infected persons would get diagnosed, initiate treatment, and achieve 100% viral load suppression. However, not all HIV-infected persons may access or may stop using these services at any stage, and so the proportion of people living with HIV (PLHIV) reaching a particular stage is getting successively smaller with each step, a phenomenon often termed the treatment cascade or ‘continuum of HIV care,’ as described by Gardner et al.7 With multiple entry and drop-off points, evaluating the uptake of services through such a ‘cascade’ or ‘continuum’ is therefore an important tool for evaluating a population’s access to and uptake of HIV services as well as the associated outcomes and impacts.8,9
Graphs or tables describing the continuum are typically constructed and described using both public health and clinic-based monitoring data. This paper discusses the utility of population-based surveys as a complementary data source, especially in resource-limited settings. We examine the structure and merits of the current continuum constructed using clinic-based monitoring data, the potential for using population-based surveys to describe the continuum and the advantages and limitations of survey-informed compared to clinic-based monitoring systems. While these considerations may apply to both the general population and key populations at increased risk for HIV infection, we pay special attention to the latter, which includes sex workers (SW), men who have sex with men (MSM), transgendered persons, and people who inject drugs (PWID).
The HIV continuum of services
Commonly used steps in constructing the HIV continuum of services for different settings include determining (i) the number of diagnosed PLHIV; and (ii) the number or proportion of people linked to care, (iii) retained in care, (iv) initiating ART, (v) retained in ART, and (vi) with virologic suppression. At each stage some patients may stop utilising services for various reasons and to various degrees so that the resulting number of PLHIV with virologic suppression is often substantially smaller than the number of diagnosed PLHIV. The resulting cascade or continuum provides a powerful display of where rates of attrition are greatest and facilitates estimating the overall proportion of HIV-diagnosed patients who achieve viral load suppression. For example, data from the United States indicated that only an estimated 28% of PLHIV had achieved viral load suppression10 whereas, in South Africa and Uganda a home-based counseling and testing initiative improved viral load suppression among PLHIV from 50% to 65%.11 National governments, United Nations agencies, and large donors have developed monitoring frameworks that help populate the continuum of services through routinely reported data on key indicators from HIV service providers and national HIV programs.12 Clinic-based monitoring will remain a key data source to monitor progress towards universal access to treatment and treatment-related indicators and outcomes such as attrition, viral load suppression, HIV drug resistance levels, and mortality.
Challenges and limitations of the clinic-based service continuum
While routine, clinic-based monitoring of service delivery plays an essential role in building the continuum of services, it is also subject to some inherent limitations. The quality of clinic data may vary due to the lack of or suboptimal adherence to rigorous data protocols in many (resource-limited) settings, resulting in incomplete, inaccurate, or delayed reporting.13–15 Most clinic-based services data (using records from health care settings) typically originate from standard pre-ART and ART registers and are reported to Ministries of Health or donors on an aggregate level, leaving them unsuitable for individual-level analysis. The lack of unique identifiers and linked data systems further impedes the tracking of individuals across the health care system, challenging the distinction among persons lost to care, re-engaging in care, or transferring between clinics. Further, most clinic-based monitoring systems capture only those who access services but cannot inform about those who never enter the continuum of services or those who no longer receive care. Lastly, continuum of services describing key populations may underestimate clients in care or treatment if some clients fail to disclose their defining high-risk behaviors or if some programs do not specifically report on them, or overestimate numbers if patients enroll into care with several providers. A more intricate data issue may arise when each step in the cascade’s data analysis is dependent on the previous which may lead to artificially lower proportions of people having suppressed viral loads.16
Key populations and the continuum of services
The importance of key populations for HIV control at the population level is well recognised in concentrated epidemics and increasingly so in generalised HIV epidemics. Key populations account for the majority of HIV infections in concentrated epidemics and are at the highest risk of infection in all epidemic settings.17–22 The elevated HIV prevalence among key populations and the social and legal constraints they face suggest the need for more intense efforts to reach and provide HIV prevention, care, and treatment services for these populations compared to the general population. Modeling studies from industrialised settings23,24 suggest that extraordinarily high levels of uptake through the entire range of services, in conjunction with condom use, are needed to curb the epidemic in MSM and possibly in other key populations. These findings are also likely to hold true for key populations in sub-Saharan Africa’s generalised epidemics, warranting intensive efforts to examine service uptake among key populations.
Where programs are not tailored specifically for high-risk groups, key population members may not always be identified as such in routine clinic-based monitoring and reporting data may not indicate or disaggregate by risk behavior. Because of the criminalisation of certain high-risk behaviors in some countries,25–27 key populations may not self-identify as such when accessing services28 for fear of being reported or arrested. Such hostile legal environments are sometimes worsened by the accompanying social marginalisation, stigma, and discrimination toward key populations, which discourages individuals from disclosing to health care providers that they sell sex or inject drugs,28 for example, lest they risk poor services or denial of services.29–32 The loss of patients between each element along the continuum, the coverage reached for individual stages along the continuum, and the number and proportion of patients achieving viral suppression may therefore be markedly different for the general population and key populations, warranting analysis by population group. An added complexity is the lack or uncertainty of population size estimates for key populations, especially in resource-limited settings. While program data provide important count data, without accurate key population size estimates the relative uptake of services among key population members is unknown using clinic or service-based data alone.
The relative lack of clinic-based data for key populations makes population-based surveys in these populations a useful means to gather such data and to construct an accurate cascade that can inform planning of services. This may be more important in generalised epidemics where the focus of HIV control efforts is centered squarely on the general population and where accurate data on key populations are often sparse.
The potential of surveys to describe the continuum of services
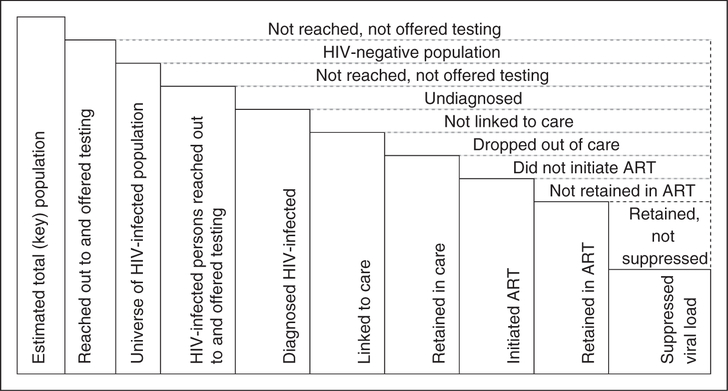
A population-based, survey-described continuum can be estimated through the use of a dedicated survey instrument to collect self-reported respondent characteristics related to each stage of the continuum. These questions may probe exposure to mobile HIV services (peer or outreach services), HIV testing and counseling, HIV-positive serostatus knowledge, linkage to and retention in care, as well as initiation and retention in ART (Figure 1). Questions may probe service utilisation both for the past – ‘Have you ever been in HIV care?’ – and present: ‘Are you currently enrolled in HIV care?’ Where feasible, surveys that include the collection of biological specimens and the measurement of biological markers of HIV infection (antibody or antigens, viral load) and treatment (ARVs) can inform respondents’ HIV serostatus, treatment eligibility (CD4 T-cell count), current treatment (ARVs), and viral load suppression. Such a survey-derived diagram of the continuum is based on population-based data as the sample includes HIV-negative and HIV-positive respondents in and outside the continuum.
Figure 1.
Schematic of a continuum of services populated through survey data.
Individual-level data analysis
Survey data are analysed on the individual level, allowing for the stratification of services along the continuum by important behavioral or demographic sub-groups that may have distinct patterns of service uptake, e.g. street versus venue-based SW, young versus old MSM, female versus male PWID, or, in the general population, by age group, residence, or gender. Clinic-based reporting data usually are analysed at the aggregate level, although the expansion of electronic medical record systems or chart abstraction may make individual-level data analysis more common and much more insightful as it then may correlate individual patient characteristics with outcomes of interest.
Capturing the population ‘outside’ the continuum
Population-based surveys that specifically target key populations use complex sampling designs such as respondent-driven sampling or time location sampling. These sampling designs may capture the entire universe of key population members, i.e. both in and outside the continuum of services, and allow for the estimation of population fractions (rather than patient fractions) for each stage in the continuum of services. Survey questions may probe reasons for having exited a particular stage of the continuum (attrition) or for never having entered or reached it. The examination of such factors can inform service providers and policy makers in their efforts to improve access to and retention in services. The reasons for being outside the continuum of services are manifold and may well differ for key and general populations, such as lack of serostatus knowledge, fear of stigma or disclosure, distance, transport costs, fees or time, poor service or stock-outs, or religious reasons. Because each of these warrant different actions their identification and characterisation is important to know.
Survey-informed key population-specific continuum of services
Specific to key populations, the social marginalisation, stigma, and discrimination that prevent them from identifying themselves or being recorded as such in many routine health care settings can more easily be overcome in the survey context. Representative population-based surveys may estimate service uptake starting outside the clinic setting to include outreach or peer-based services, thus enabling the creation of a more comprehensive continuum than what most clinic-based systems can produce. Population-based surveys may facilitate better planning for services such as HIV testing and counseling, prevention messages, and delivery of preventive interventions. Such surveys sample both HIV-uninfected and -infected people, and may refer undiagnosed HIV-infected survey respondents to care.33
Survey-based biomarker data to examine the continuum of services
Testing for HIV and often other biomarkers is already recommended and has become routine in many HIV-related surveys, including general population-based surveys such as Demographic Health Surveys and AIDS Indicator Surveys and key population surveys.34,35 In addition to HIV prevalence estimates, surveys have the potential to provide population-level estimates of viral load, the proportion of HIV-infected individuals with suppressed viral load, and the distribution of CD4 T cell counts. Viral load suppression serves as the desired endpoint of ‘Seek, Test, Treat, and Retain’ efforts.36 Summary viral load measures are useful indicators to monitor the magnitude of potential onward transmission of HIV in populations, reflecting the concept of ‘Treatment as Prevention’ on an aggregate level. Routine treatment program data are unable to monitor this endpoint at the population level because they do not capture individuals with undiagnosed HIV infection or those who exited care. Further, in some resource-limited settings regular clinic-based viral load monitoring among patients on ART is still not routine.37 Population-based surveys can provide estimates of viral load for HIV-infected persons in and outside the continuum, as e.g. demonstrated in a large survey in Swaziland.38 Linking these data with services across the continuum opens up the possibility of more accurately and objectively measuring impact at different stages. For example, viral load measures, including viral load stratification by serostatus knowledge, care status, or treatment status can be used to estimate impact of interventions. With the expansion of treatment availability and a growing number of Treatment as Prevention (TasP) pilots or test and treat demonstration projects for key and other populations, the prevalence of detectable viral load among PLHIV will become an important additional indicator alongside HIV seroprevalence and incidence.
Challenges of using survey data to describe the continuum
Table 1 provides an overview of how survey- and clinic-based monitoring data can describe the continuum. Using survey data to describe the service continuum is not without limitations or challenges.
Table 1.
Characteristics of population-based and clinic-based continuum of services.
| Characteristic | Population-based surveys | Clinic-based reporting |
|---|---|---|
| Population | Survey target population (general population or select key population) | Persons accessing services |
| Geographic scale | Defined by sampling area | Clinic, sub-national, and national level |
| Timing of data collection | Periodic | Continuous |
| Data source | Survey respondents | Routine clinic records |
| Data level | Individual level | Clinic level (typically) |
| Data accuracy | Subject to reporting bias, respondent health literacy, sampling error | Subject to clinic logbook quality and transcription errors |
| Accuracy of biomeasures | Subject to accuracy of recall (CD4+T cell count, viral load) but ameliorated if biomarkers are measured as part of survey | Subject to quality of data entry and data transcription |
| Estimating proportion accessing services | Yes | Not usually reported or difficult |
| Assessing determinants of service uptake, lack of service uptake, or exiting the continuum of services | Feasible | Not feasible (service uptake) or not always done (evaluating exits) |
| Distinguishing between exiting a service and transferring to another provider | Feasible | Often not reported, difficult to transcribe |
| Estimating (relative) size of population outside each care element | Feasible | Not feasible with clinic-based data alone |
Reporting bias.
In population-based surveys HIV-related stigma may prevent some survey respondents from revealing that they are HIV-positive or that they are already accessing HIV care and treatment services.39 Refusal of blood draws or HIV testing, if substantial, may bias HIV prevalence estimates and other estimates.40
Measurement error.
Respondent health literacy or memory of important health care events may be expected to be imperfect. For example, some respondents may have trouble distinguishing between pre-ART care and ART or accurately recall the date they enrolled in HIV care and treatment, or their last CD4 T cell count or viral load value. Both reporting bias and inaccurate recall may be partially overcome by adding relevant biomarkers to the survey, albeit at an increase in cost and complexity: HIV serology, CD4 T cell count, viral load, and ARV metabolites.
Cost.
The marginal returns of adding data measures and biomarkers to population-based surveys can be substantial. Still, longer survey interviews may increase the burden for respondents and require more staff time. Further, survey sample sizes are calculated to meet a desired precision around the most important measure, e.g. HIV prevalence. Because most survey-described stages along the continuum of services apply to PLHIV only, surveys will often require sample sizes larger than typically obtained in order to estimate these steps along the continuum with sufficient precision.
Where surveys costs are deemed too high, large-scale HIV testing and counseling campaigns may be seen as an alternative. Such campaigns provide tangible programmatic benefits (uptake of HIV serostatus knowledge, referral of PLHIV to care although with a likely reduction in representativeness).
Representativeness.
Key population surveys typically lack proper sampling frames. Investigators may resort to alternative ways of estimating sampling probabilities41,42 or construct makeshift sampling frames.43 Non-representative samples may yield biased estimates for the continuum and other variables. The limited geographic scope of key population surveys, which are often conducted in the capital or other large cities where access to services may be greater, is not conducive to the generation of national-level estimates, although key populations are concentrated in urban settings.
Timeliness.
The periodic or one-time nature of surveys does not lend itself to the production of continuous data as routine clinic-based reporting does. The importance of monitoring the service continuum at the population level therefore reinforces the call to transform one-time survey efforts into standing surveillance systems that include regularly repeated population-based surveys in key locations or nation-wide for both general and key populations.
Proposed continuum of services data elements to be measured in population-based surveys
Many population-based surveys to date already measure discrete elements of the continuum of services without being sufficiently comprehensive to display the entire continuum of services or examining determinants for being outside the continuum. Good examples include Kenya’s AIDS Indicator survey44 or South Africa’s national household-based HIV survey.45 A standardised systematic approach is warranted to measure the entire continuum through the use of a dedicated data instrument and, where feasible, biomarker collection. Such a data instrument can easily be embedded into existing questionnaires. At a minimum, instruments should address each step of the continuum of services: exposure to outreach services, HIV testing, linkage to and retention in care, and initiation and retention on ART. Additional questions may probe elements within a particular stage such as additional support services (i.e. family planning), past opportunistic infections screening and treatment (i.e., tuberculosis), or the timing of events (i.e. entry and exit) for each applicable step in the continuum. To maximise a survey’s potential, such instruments could also collect additional data on the reasons why respondents never accessed a particular service, were non-adherent in care or treatment, re-engaged service providers, or left a particular service entirely. Surveys may probe perceived poor services or denial of services due to stigma or discrimination as additional reasons for being outside the continuum. Qualitative interviews may provide further context to respondents’ perceptions and decision-making. Conversely, survey investigators may explore why respondents actually do access services. These supplementary data elements may not be available through routine clinic-based reporting, highlighting the value added with population-based surveys. The repeated conduct of such surveys at regular intervals (e.g. every 2 years) would allow the measurement of trends over time and contribute to program evaluation.
Analysis of survey-derived continuum of services data
A population-based survey capturing data on the entire continuum offers a range of possibilities during data analysis to more effectively characterise program efforts. It is important that sample sizes be large enough to allow for a meaningful analysis of the various segments of the HIV-positive population at each step of the continuum. Survey data derived through complex sampling designs (such as respondent-driven sampling) would warrant appropriate weighting to yield population estimates. Using the ultimate endpoint of viral load suppression, a complete survey data set would allow not only for the estimation of the proportion of people with suppressed viral load but also the population-attributable fraction of each step along the continuum. Subject to sufficiently large sample sizes, investigators can stratify the continuum data by categories such as age, gender, location, or behavioral characteristics, such as sex work status of MSM or location where sex is sold. Those outside the continuum constitute an important comparison group, allowing for the examination of factors associated with poor or no uptake of services (i.e. being outside the continuum of services). Population-based survey-derived findings may be compared to clinic-based descriptions of the continuum of services provided they can be matched by time and place, thus allowing a key population survey-derived continuum of services to be compared with the clinic-based uptake of services in the general population or survey findings to be compared with routine clinic-based reporting for the same key population.
Conclusion
Realising the goal of an AIDS-free generation has increased the demand for accurate measures of exposure to, uptake of, and retention in key HIV services as well as health care outcomes. While this paper focuses on the continuum of services that starts with exposure to outreach and HIV testing and counseling, and ends with treatment-related viral load suppression, similar survey-described constructs have been envisioned for other continuums of care, such as PMTCT with its endpoints of HIV-negative children at 18 months of age and mothers on treatment, the detection and treatment of HIV-related opportunistic infections like TB, or the provision of pre-exposure prophylaxis. HIV-related population-based surveys historically focused on behavioral or other risk factors, with the later addition of HIV testing. Increasing affordability, availability, and ease of biomarker testing (i.e. CD4 T cell counts, viral load) and the shift in focus to biomedical, especially treatment-based, interventions suggests an expanded role for population-based surveys to describe the service continuum, characterise who is in it and who is not, and understand why some people are left out or choose to exit at different steps. As key populations are exposed to stigma, discrimination, and criminalisation, and continue to face important barriers to accessing health and HIV services, surveys can be a key data source to help inform much-needed improvements for key population programming. These crucial advantages offered by population-based surveys ought to be exploited by investigators and program implementers, and promoted by donors and policy makers.
Acknowledgments
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the U.S. Department of Health and Human Services.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Montaner JS. Treatment as prevention–a double hattrick. Lancet 2011; 378: 208–209. [DOI] [PubMed] [Google Scholar]
- 2.Mills EJ, Bakanda C, Birungi J, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Int Med 2011; 155: 209–216. [DOI] [PubMed] [Google Scholar]
- 3.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS One 2013; 8: e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeten JM, Kahle E, Lingappa JR, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Trans Med 2011; 3: 77ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. New Eng J Med 2011; 365: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joint United Nations Programme on HIV/AIDS. 90-90-90. An ambitious treatment target to help end the AIDS epidemic. UNAIDS; 2014, http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf (accessed 24 Jan 2015). [Google Scholar]
- 7.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test- and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52: 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tassie JM, Bertagnolio S and Souteyrand Y. Integrated surveillance of HIV care in low-income and middle-income countries. Curr Opin HIV AIDS 2011; 6: 233–238. [DOI] [PubMed] [Google Scholar]
- 9.Kilmarx PH and Mutasa-Apollo T. Patching a leaky pipe: the cascade of HIV care. Curr Opin HIV AIDS 2013; 8: 59–64. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Vital signs: HIV prevention through care and treatment–United States. MMWR 2011; 60: 1618–1623. [PubMed] [Google Scholar]
- 11.Barnabas RV, van Rooyen H, Tumwesigye E, et al. Initiation of antiretroviral therapy and viral suppression after home HIV testing and counselling in KwaZulu-Natal, South Africa, and Mbarara district, Uganda: a prospective, observational intervention study. Lancet HIV 2014; 1: e68–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joint United Nations Programme on HIV/AIDS (UNAIDS). Indicator Standards and Assessment Tool, http://www.unaids.org/en/dataanalysis/datacollectionan-danalysisguidance/monitoringandevaluationguidelines/ (accessed 8 July 2013).
- 13.Makombe SD, Hochgesang M, Jahn A, et al. Assessing the quality of data aggregated by antiretroviral treatment clinics in Malawi. Bull World Health Org 2008; 86: 310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mate KS, Bennett B, Mphatswe W, et al. Challenges for routine health system data management in a large public programme to prevent mother-to-child HIV transmission in South Africa. PloS One 2009; 4: e5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nash D, Elul B, Rabkin M, et al. Strategies for more effective monitoring and evaluation systems in HIV programmatic scale-up in resource-limited settings: Implications for health systems strengthening. J Acquir Immune Def Syndr 2009; 52(Suppl 1): S58–S62. [DOI] [PubMed] [Google Scholar]
- 16.Horberg M, Hurley L, Klein D, et al. The HIV care cascade (“cascade”) measured over multiple time periods varies by time period and methode. International AIDS Conference, Melbourne, http://pag.aids2014.org/flash.aspx?pid=1419 (2014, accessed 7 Feb 2015). [Google Scholar]
- 17.Pruss-Ustun A, Wolf J, Driscoll T, et al. HIV due to female sex work: regional and global estimates. PloS One 2013; 8: e63476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathers BM, Degenhardt L, Phillips B, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet 2008; 372: 1733–1745. [DOI] [PubMed] [Google Scholar]
- 19.Beyrer C, Sullivan P, Sanchez J, et al. The increase in global HIV epidemics in MSM. AIDS 2013; 27: 2665–2678. [DOI] [PubMed] [Google Scholar]
- 20.Beyrer C, Baral SD, van Griensven F, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet 2012; 380: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baral S, Beyrer C, Muessig K, et al. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12: 538–549. [DOI] [PubMed] [Google Scholar]
- 22.Joint United Nations Programme on HIV/AIDS. UNAIDS Report on the Global AIDS Epidemic 2012. Geneva, http://www.unaids.org/en/media/unaids/content-assets/documents/epidemiology/2012/gr2012/20121120_NAIDS_Global_Report_2012_en.pdf (2012, accessed 8 July 2013). [Google Scholar]
- 23.Phillips AN, Cambiano V, Nakagawa F, et al. Increased HIV incidence in men who have sex with men despite high levels of ART-induced viral suppression: analysis of an extensively documented epidemic. PloS One 2013; 8: e55312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorensen SW, Sansom SL, Brooks JT, et al. A mathematical model of comprehensive test-and-treat services and HIV incidence among men who have sex with men in the United States. PloS One 2012; 7: e29098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.International lesbian, gay, bisexual, trans and intersex association (ILGA). State-sponsored homophobia. A world survey of laws: Criminalisation, protection and recognition of same-sex love, http://old.ilga.org/Statehomophobia/ILGA_State_Sponsored_Homophobia_2013.pdf (accessed 29 Aug 2013).
- 26.Johnson CA. Off the Map: How HIV/AIDS programming is failing same-sex practicing people in Africa. New York, 2007. International Lesbian and Gay Human Rights Commission, http://www.iglhrc.org/sites/default/files/6-1.pdf (accessed 29 Aug 2013). [Google Scholar]
- 27.UNDP, The Global Fund, and UNAIDS. Analysis of key human rights programmes in Global Fund-supported HIV programmes. 2010, http://www.undp.org/content/dam/undp/library/hivaids/Analysis%20of%20Key%0HRTS%20Programmes%20in%20GF-Supported%20HIV%20Programms%20-Exec%20Sum.pdf (accessed 29 Aug 2013).
- 28.Naughton JDA. HIV-related stigmatization in treatment settings: effects on patient comfort, risk disclosure, and treatment decisions. Dissertation, Syracuse University, http://surface.syr.edu/cgi/viewcontent.cgi?article=1171&context=psy_etd (accessed 8 October 2013). [Google Scholar]
- 29.Beyrer C, Baral S, Kerrigan D, et al. Expanding the space: inclusion of most-at-risk populations in HIV prevention, treatment, and care services. J Acquir Immune Def Syndr 2011; 57 Suppl 2: S96–S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beyrer C, Sullivan PS, Sanchez J, et al. A call to action for comprehensive HIV services for men who have sex with men. Lancet 2012; 380: 424–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baral S, Trapence G, Motimedi F, et al. HIV prevalence, risks for HIV infection, and human rights among men who have sex with men (MSM) in Malawi, Namibia, and Botswana. PloS One 2009; 4: e4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scorgie F, Nakato D, Harper E, et al. ‘We are despised in the hospitals’: sex workers’ experiences of accessing health care in four African countries. Culture Health Sexuality 2013; 15: 450–465. [DOI] [PubMed] [Google Scholar]
- 33.Solomon SS, Lucas GM, Celentano DD, et al. Beyond surveillance: a role for respondent-driven sampling in implementation science. Am J Epidemiol 2013; 178: 260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.UNAIDS/WHO Working Group on Global HIV/AIDS and STI Surveillance. Guidelines on surveillance among populations most at risk for HIV, http://www.unaids.org/en/media/unaids/contentassets/restore/20110518_Surveillance_among_most_at_risk.pdf (accessed 29 Aug 2013).
- 35.UNAIDS/WHO Working Group on Global HIV/AIDS and STI Surveillance. Guidelines for second generation HIV surveillance, http://www.unaids.org/en/media/unaids/contentassets/dataimport/publications/irc-pub01/jc370-2ndgeneration_en.pdf (2000, accessed 29 Aug 2013).
- 36.Bertozzi SM. PEPFAR/Global Fund at 10 years: Past, Present, and Future. Plenary, CROI 2104, 6 March 2014, http://www.croiwebcasts.org/st/plenary?link=nav&linkc=sesstype (accessed 26 Jan 2015). [Google Scholar]
- 37.Miller WC, Powers KA, Smith MK, et al. Community viral load as a measure for assessment of HIV treatment as prevention. Lancet Infect Dis 2013; 13: 459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conference Highlights—CROI 2013: Epidemiology and prevention. Top Antiviral Med 2013; 21: 48, at: http://www.iasusa.org/sites/default/files/tam/21-2-47.pdf (accessed 10 Oct 2013). [Google Scholar]
- 39.Floyd S, Molesworth A, Dube A, et al. Underestimation of HIV prevalence in surveys when some people already know their status, and ways to reduce the bias. AIDS 2013; 27: 233–242. [DOI] [PubMed] [Google Scholar]
- 40.Reniers G and Eaton J. Refusal bias in HIV prevalence estimates from nationally representative seroprevalence surveys. AIDS 2009; 23: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Prob 1997; 44: 174–199. [Google Scholar]
- 42.Heckathorn DD. Respondent-driven sampling II: deriving valid population estimates from chain-refferal samples of hidden populations. Soc Prob 2007; 49: 11–34. [Google Scholar]
- 43.MacKellar D, Valleroy L, Karon J, et al. The Young Men’s Survey: methods for estimating HIV seroprevalence and risk factors among young men who have sex with men. Public Health Rep 1996; 111(Suppl 1): 138–144. [PMC free article] [PubMed] [Google Scholar]
- 44.Kenya AIDS Indicator Survey 2012. JAIDS 2014; 66: S1–S137. [DOI] [PubMed] [Google Scholar]
- 45.South Africa National HIV Prevalence, Incidence, and Behaviour Survey, 2012. HSRC, http://www.hsrc.ac.za/uploads/pageContent/4565/SABSSM%20IV%20LEO%20final.pdf (2014, accessed 7 Feb 2015). [Google Scholar]