Abstract
GnRH antagonist and GnRH agonist are widely used as androgen deprivation therapy for metastatic prostate cancer. A previous report demonstrated that patients with PSA levels of >20 ng/mL using GnRH antagonists showed favorable outcomes in comparison to those using GnRH agonists. An 82-year old male patient with edema, a stony hard nodule on his prostate, and an initial PSA level of 6,717 ng/mL was referred to our hospital due to suspected prostate cancer. He received prostate needle biopsy and was diagnosed with prostate cancer with bone metastasis, with a Gleason Score of 4 + 4 = 8. He was then treated with a GnRH agonist (leuprorelin acetate) and bicalutamide from July 2015. Although his PSA level decreased to 582.0 ng/mL in December 2015, his PSA level gradually increased and CRPC developed. He indicated that he did not wish to take 2nd generation anti-androgen drugs or receive systemic chemotherapy. We introduced a GnRH antagonist (degarelix) in February 2015; his PSA level did not change and his CRPC was controlled. We herein report a case in which changing a GnRH agonist to a GnRH antagonist contributed to CRPC control.
Keywords: Degarelix, GnRH antagonist, CRPC
Introduction
Huggins et al. first reported the effectiveness of surgical castration for prostate cancer in 1941 [1]. Since then androgen deprivation therapy still plays an important role in advanced prostate cancer.
GnRH agonist treatment is associated risks in that it takes approximately 2 weeks to reduce the serum testosterone level to castration level, and it is also associated with a flare effect [2]. Degarelix is a GnRH agonist that came into clinical use in Japan in 2012. Degarelix treatment is not associated with a surge in serum testosterone and rapidly reduces the serum testosterone level to a castration level. Klotz et al. reported degarelix was more effective than a GnRH agonist in the treatment of high-risk prostate cancer patients [3]. On the other hand, degarelix is associated with a higher rate of injection site reaction than GnRH agonist drugs, including leuprorelin acetate and gosereline acetate [4].
Miller et al. reported a case in which changing from leuprorelin acetate to degarelix resulted in PSA control [5]. Until now, there have been no reports about changing from a GnRH agonist to an antagonist for the treatment of CRPC in a Japanese patient. We herein report a case in which changing a GnRH agonist to an antagonist resulted in relatively longer CRPC control.
Case Presentation
An 82-year old man with edema, a stony hard nodule on his prostate, and an initial PSA level of 6,717 ng/mL was referred to our hospital due to suspected prostate cancer. The patient was diagnosed with prostate adenocarcinoma based on prostate needle biopsy, and showed a Gleason Score of 4 + 4 = 8. CT and bone scintigraphy revealed multiple bone metastases (BSI 0.29), without organ metastasis. In July 2015, combined androgen blockade was initiated using a GnRH agonist (leuproreline acetate) and bicalutamide 80 (mg/day). In December 2015, his PSA level decreased to 582 ng/mL but re-elevated to 807 ng/mL in January 2016. We changed from leuprorelin acetate to a GnRH antagonist (degarelix) in February 2016 and bone scintigraphy showed no increase in the uptake (BSI 0.04). Thereafter, we continued degarelix monotherapy and his PSA level did not increase until February 2017. The patient died of pneumonia in April 2017 (Fig. 1).
Fig. 1.
Clinical course of patient.
Discussion
In 1941, Huggins first reported that the performance of orchiectomy controlled prostate cancer in prostate cancer patients [1]. Since then, surgical castration has changed to medical castration using GnRH agonists and GnRH antagonists, and these androgen deprivation therapies still play an important role in the treatment of advanced prostate cancer [6].
The important difference between GnRH agonists and antagonists is that GnRH agonist treatment is associated with testosterone surge and some patients show flare effects. In patients with advanced prostate cancer, these flare effects are sometimes associated with spinal cord compression, worsened pain, worsened lower urinary tract symptoms and other problems [2]. In advanced cases, anti-androgen therapy is sometimes introduced before a GnRH agonist, to avoid flare-up. Uehara et al. reported that prior anti-androgen did not prevent flare-up [7]. Thus, GnRH antagonists are needed for the treatment of advanced prostate cancer. Degarelix, a GnRH antagonist came into clinical use in Japan in 2012. For patients whose PSA levels are more than 20 ng/mL, GnRH antagonist treatment prolonged overall survival in comparison to GnRH agonist treatment [3]. Harri et al. reported a case series in which GnRH agonists and antagonists were switched in Finnish prostate cancer patients [8]. The detailed mechanisms are still unknown; however, one possibility is that GnRH antagonists also lower the serum FSH level and that this decrease in FSH might be associated with an anti-tumor effect in prostate cancer.
Due to the recent evidences in CRPC treatment, newly anti-androgen blockade medications or systematic chemotherapy are recommended treatments after the development of resistance to initial androgen deprivation therapy [9, 10]. Not all elderly CRPC patients or patients with complications receive these new drug treatments in the real-world clinical setting [11]. For these patients, switching treatment to a GnRH antagonist is less invasive therapy and sometimes shows longer efficacy [5].
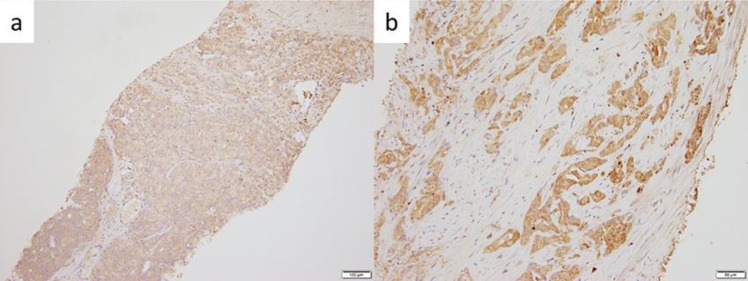
In our case, PSA control was achieved for more than one year after switching to a GnRH antagonist, following the failure of initial androgen deprivation therapy [5]. For further investigation, this case demonstrated a higher FSHR expression level in the initial prostate needle biopsy specimens (Fig. 2). There are no reports demonstrating the efficacy of switching from a GnRH agonist to an antagonist; however, the FSH receptor expression might be a clue to identifying cases in which switching treatment might be beneficial.
Fig. 2.
FSH receptor expression (a) low power field and (b) high power field.
Conclusion
We reported a case in which changing from a GnRH agonist to an antagonist contributed to CRPC control in a Japanese patient. Although new targeted drugs for CRPC have been introduced within the past decade, some elderly CRPC patients require less invasive therapy. Changing from a GnRH antagonist to an agonist might be one option for these patients.
Statement of Ethics
Informed consent was obtained from patient and all methods were followed by the ethical standards of the Declaration of Helsinki.
Consent for Publication
Written informed consent was obtained from the patient. A copy of the written consent form is available for review from the Editor-in-Chief of this journal.
Disclosure Statement
The authors declare no conflicts of interest in association with the present study.
Funding Sources
None for this research.
Author Contributions
RS, TK. are responsible for the concept and drafted the manuscript.
SC, YM, MY, HU provided the intellectual content and critically reviewed the manuscript.
Availability of Data and Material
Due to ethical restrictions, the raw data underlying this paper are available upon request from the corresponding author.
Acknowledgements
N/A.
References
- 1.Huggins C. Effect of Orchiectomy and Irradiation on Cancer of the Prostate. Ann Surg. 1942 Jun;115((6)):1192–200. doi: 10.1097/00000658-194206000-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinton TN, Woldu SL, Raj GV. Degarelix versus luteinizing hormone-releasing hormone agonists for the treatment of prostate cancer. Expert Opin Pharmacother. 2017 Jun;18((8)):825–32. doi: 10.1080/14656566.2017.1328056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klotz L, Miller K, Crawford ED, Shore N, Tombal B, Karup C, et al. Disease control outcomes from analysis of pooled individual patient data from five comparative randomised clinical trials of degarelix versus luteinising hormone-releasing hormone agonists. Eur Urol. 2014 Dec;66((6)):1101–8. doi: 10.1016/j.eururo.2013.12.063. [DOI] [PubMed] [Google Scholar]
- 4.Hosseini SA, Rajabi F, Akbari Sari A, Ayati M, Heidari S, Ghamary F. Degarelix for the treatment of advanced prostate cancer compared with GnRh-Agonists: a systematic review and meta-analysis. Med J Islam Repub Iran. 2016 Jan;30:317. [PMC free article] [PubMed] [Google Scholar]
- 5.Miller K, Simson G, Goble S, Persson BE. Efficacy of degarelix in prostate cancer patients following failure on luteinizing hormone-releasing hormone agonist treatment: results from an open-label, multicentre, uncontrolled, phase II trial (CS27) Ther Adv Urol. 2015 Jun;7((3)):105–15. doi: 10.1177/1756287215574479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. European Association of Urology EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014 Feb;65((2)):467–79. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Uehara S, Yuasa T, Fujii Y, Yano A, Yamamoto S, Masuda H, et al. Prior administration of a non-steroidal anti-androgen failed to prevent the flare-up caused by a luteinizing hormone-releasing hormone agonist in a patient with metastatic prostate cancer. BMC Res Notes. 2015 Aug;8((1)):335. doi: 10.1186/s13104-015-1297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Visapää H. Switching from an LHRH Antagonist to an LHRH Agonist: A Case Report of 10 Finnish Patients with Advanced Prostate Cancer. Oncol Ther. 2017;5((1)):119–23. doi: 10.1007/s40487-017-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. PREVAIL Investigators Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014 Jul;371((5)):424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. AFFIRM Investigators Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012 Sep;367((13)):1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 11.Thompson AL, Sarmah P, Beresford MJ, Jefferies ER. Management of metastatic prostate cancer in the elderly: identifying fitness for chemotherapy in the post-STAMPEDE world. BJU Int. 2017 Dec;120((6)):751–4. doi: 10.1111/bju.13990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to ethical restrictions, the raw data underlying this paper are available upon request from the corresponding author.