Abstract
We present a 26-year-old female with HbSC disease who presented to the emergency department multiple times with pain and shortness of breath, eventually developing unresponsiveness and a brief episode of pulseless electrical activity. She was admitted to the intensive care unit with multisystem organ failure and found to have diffuse ischemic strokes. Infectious workup revealed disseminated anaplasmosis and babesiosis, which had likely caused sickle cell crisis, atypical hemolytic-uremic syndrome, and ischemic brain injury. She was started on eculizumab therapy as well as antimicrobial therapy with doxycycline, clindamycin, and atovaquone. The patient was given tracheostomy and a percutaneous feeding tube. Unfortunately, she did not have significant neurologic recovery after prolonged hospital stay and was discharged to a skilled nursing facility with significant neurologic burden.
Keywords: Stroke, Sickle cell, Anaplasmosis, Babesiosis, HbSC
Introduction
Sickle cell crisis is a known complication of patients with sickle cell anemia which can be caused by various physiologic insults, including acute infection. Potentially devastating complications of sickle cell crisis include ischemic stroke and multisystem organ failure [1]. We present a case of diffuse ischemic strokes in a patient undergoing sickle cell crisis, likely caused by disseminated infection with tick-borne entity Anaplasma phagocytophilum.
Case Report
A 26-year-old female with sickle cell trait presented to the emergency department with altered mental status. She had recovered from a viral illness 2 weeks earlier, but within the past week she had presented to the emergency department on three occasions for shortness of breath and diffuse pain that remained unexplained. Shortly after her fourth presentation, the patient became acutely unresponsive and experienced a 30-s pulseless electrical activity arrest. Return of spontaneous circulation was achieved after 10 min and the patient was transferred to a higher acuity facility.
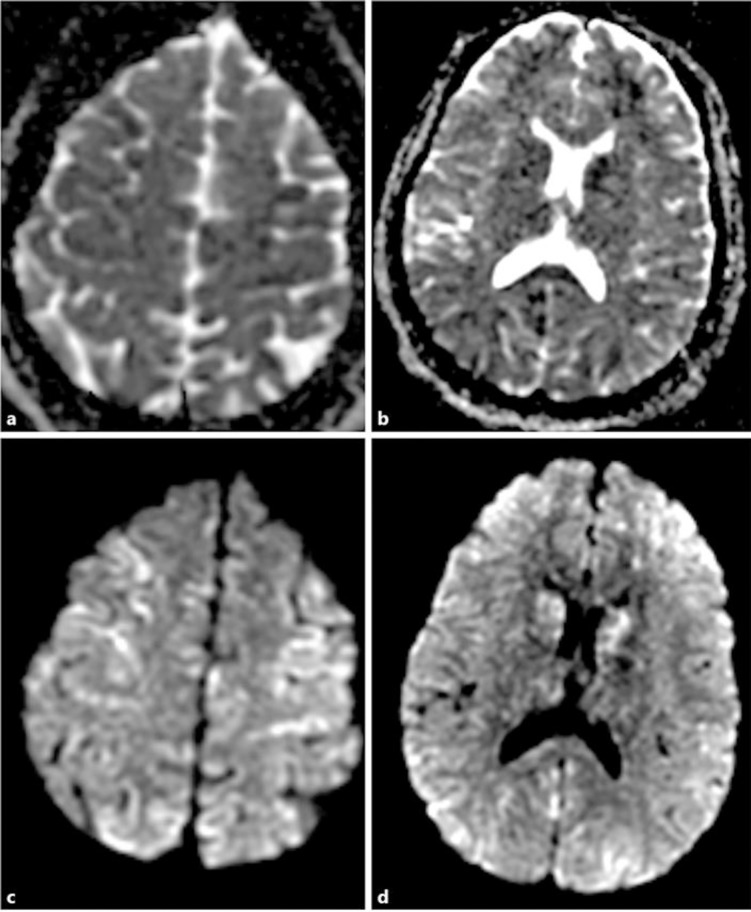
Upon admission to the intensive care unit she was found to have multisystem organ failure including acute respiratory distress syndrome, acute renal failure, and bone marrow failure with hemoglobin (5.7 g/dL), platelets (57 K/μL), and leukocytes (WBC 27.33 K/μL, neutrophil-predominant). An MRI of the brain with and without gadolinium was performed the next day and revealed diffuse scattered areas of restricted diffusion throughout the cerebellum and bilateral cortical grey matter (Fig. 1) concerning for a microangiopathic process versus anoxic brain injury. Transcranial Doppler on hospital day 7 showed increased velocity of the left middle cerebral artery and increased pulsatility indices diffusely. Echocardiography showed global hypokinesis with a left ventricular ejection fraction of 40′. Hematology was consulted due to concern for sickle cell crisis as well as thrombotic thrombocytopenic purpura due to schistocytes seen on a peripheral blood smear (2–4/HPF) in addition to the above findings. She was started on broad-spectrum antimicrobials with concern for encapsulated organisms causing septic and cardiogenic shock, as well as plasma exchange and methylprednisolone for empiric treatment of TTP.
Fig. 1.
a–d Diffusion-weighted MRI showing extensive areas of acute and subacute ischemia scattered throughout both cerebral hemispheres, predominantly lining the gyri.
After 5 days of hospitalization, the results of hemoglobin electrophoresis confirmed HbSC disease. Hematologic workup also showed that ADAMSTS activity was >10′, ruling out TTP. A peripheral blood smear was done which showed schistocytes as well as rare neutrophil-containing morulae suggestive of human granulocytic anaplasmosis (HGA) (Fig. 2). A bone marrow biopsy showed normocellular marrow without excess blasts as well as trophozoites in multiple RBCs consistent with babesiosis. The patient was started on clindamycin and atovaquone for Babesia and on doxycycline for Anaplasma. It was determined that the patient had anaplasmosis-induced sickle cell crisis with Babesia coinfection. PCR for anaplasmosis and babesiosis was found to be negative. Treatment for babesiosis was stopped due to lack of clinical evidence for babesia infection and a negative PCR, but continued for anaplasmosis due likely false negativity related to antimicrobial therapy [2]. Upon neurologic examination on hospital day 6, the patient's eyes were open spontaneously, but she exhibiting decerebrate posturing and was not following commands. On day 11 of her hospitalization, eculizumab treatment was begun due to worsening renal function with suspected atypical hemolytic uremic syndrome, and was continued for 3 weeks until clinical improvement. After 31 days in the hospital, she was transferred out of intensive care to the internal medicine service. Care was gradually deescalated without any additional major complications, and after a total of 7 weeks of hospitalization, the patient's mental status still had not improved and prognosis was thought to be poor based on anoxic brain injury and multisystem organ failure. She was discharged to a skilled nursing facility with a tracheostomy and percutaneous endoscopic gastrostomy.
Fig. 2.
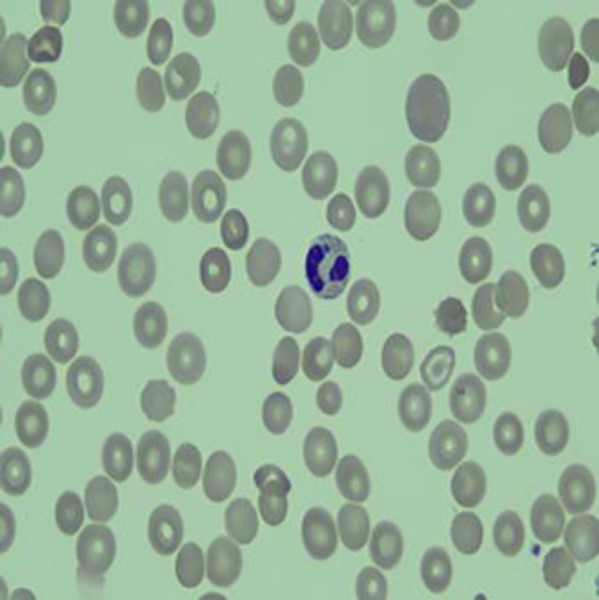
Peripheral blood smear showing a neutrophil-containing morula suggestive of anaplasmosis.
Discussion
Ischemic stroke is a known complication of sickle cell anemia and sickle cell crisis. Patients with HbSC disease, such as this patient, are at a substantially lower risk than those with HbSS, with an incidence of a cerebrovascular accident of 0.15/100 person-years [1]. Ischemic stroke is typically due to a chronic vasculopathy rather than acute thrombotic event, but the risk is increased at times of sickle cell crisis. The risk is higher with increased HbS concentrations and classically associated with increased velocities on transcranial Doppler [1, 3]. The goal of preventative treatment is to transfuse until HbS is <30′ [3, 4]. Although this patient also had a 30-s PEA arrest, the appearance of the MRI as well as the significant diffusion restriction within only a few hours of this event were felt to be more likely secondary to the sickle cell crisis.
Babesiosis is a rarely reported precipitant of crisis in sickle cell patients, most often acquired through blood transfusions [5]. No reports of anaplasmosis infection causing sickle cell crisis were found in a literature search. This patient came from a tick-endemic region where tick-borne illnesses are common. Both Anaplasma and Babesia are transmitted by the Ixodes scapularis tick, and 10′ of patients with HGA will have serologic evidence of coinfection with either Lyme or babesiosis, as was the case with this patient [6]. PCR was found to be negative for anaplasmosis, but sensitivity is decreased after initiation of antibiotics, and a negative PCR does not rule out anaplasmosis [2]. Little data exist regarding the susceptibility of hyposplenic patients or sickle cell patients to infection with Anaplasma [6]. However, these patient populations are well known to be more susceptible to infections, as splenic macrophages are key in clearing infected erythrocytes from the circulation. Complications of severe disease include severe hemolytic anemia, acute respiratory distress syndrome, disseminated intravascular coagulation, congestive heart failure, and coma. While most HGA infections will resolve after a viral-like illness, up to 3′ of cases may result in life-threatening complications and 1′ in death [7].
This is a unique neurologic case presentation of sickle cell crisis incited by disseminated anaplasmosis and babesiosis. Patients with functional hyposplenism, such as those with sickle cell anemia, are at increased risk of severe infections. Early signs of HGA typically occur within a week of infection and include fever, headache, leukopenia, thrombocytopenia, and elevated liver enzymes [6, 7]. In case of nonspecific findings in sickle cell patients who live in tick-endemic areas, a workup should be considered due to potential rapid progression to sickle crisis and multiple organ failure. Due to this risk, doxycycline may be initiated on clinical suspicion alone in this patient population [7].
Statement of Ethics
Due to the nature of the disease, the patient was unable to give informed consent for publication of this case report. This was discussed and oral consent was given by the patient's parents while she was in hospital. Unfortunately, after discharge the patient and the family were lost to follow-up, and attempts to contact them for formal written consent were unsuccessful. No personal or identifying information was used in this case report to preserve the anonymity and privacy of patient.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors have no funding sources to declare.
Author Contributions
Dr. Herbst and Mr. Crissinger performed the chart review and primary composition of the paper. Additionally, both performed the literature review for background medical science and evaluation for precedent cases. Dr. Baldwin provided supervision, writing, and editing assistance as well as patient care during the encounter.
Acknowledgement
We would like to acknowledge Dr. Sayed Kazmi for assistance with acquisition and interpretation of pathology slides.
References
- 1.Webb J, Kwiatkowski JL. Stroke in patients with sickle cell disease. Expert Rev Hematol. 2013 Jun;6((3)):301–16. doi: 10.1586/ehm.13.25. [DOI] [PubMed] [Google Scholar]
- 2.Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis — United States: A Practical Guide for Health Care and Public Health Professionals CDC MMWR, Recommendations and Reports. 2016;((65)):2. doi: 10.15585/mmwr.rr6502a1. [DOI] [PubMed] [Google Scholar]
- 3.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010 Dec;376((9757)):2018–31. doi: 10.1016/S0140-6736(10)61029-X. 10.1016/s0140-6736(10)61029-x. [DOI] [PubMed] [Google Scholar]
- 4.Azar S, Wong TE. Sickle Cell Disease: A Brief Update. Med Clin North Am. 2017 Mar;101((2)):375–93. doi: 10.1016/j.mcna.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Karkoska K, Louie J, Appiah-Kubi AO, Wolfe L, Rubin L, Rajan S, et al. Transfusion-transmitted babesiosis leading to severe hemolysis in two patients with sickle cell anemia. Pediatr Blood Cancer. 2018 Jan;65((1)):e26734. doi: 10.1002/pbc.26734. [DOI] [PubMed] [Google Scholar]
- 6.Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010 Mar;30((1)):261–92. doi: 10.1016/j.cll.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakken JS, Dumler S. Human granulocytic anaplasmosis. Infect Dis Clin North Am. 2008 Sep;22((3)):433–48. doi: 10.1016/j.idc.2008.03.011. [DOI] [PubMed] [Google Scholar]