Abstract
Acrodermatitis enteropathica (AcE) is a rare, autosomal recessive inherited disorder caused by mutation of the SLC39A4 gene coding for zinc transport protein (ZIP 4). The disease appears during childhood especially in breastfeeding or post-breastfeeding infant. Eczema herpeticum refers to a disseminated skin infection of herpes simplex virus that usually leads to vesicular eruptions commonly seen on a background of atopic dermatitis (AD). We describe an 11-year-old boy with periorificial erosions in periorbital, perinasal, perioral, perineal, and gluteal areas, accompanied with itchy vesicles, some covered with hemorrhagic crusts. A clinical diagnosis of AcE and eczema herpeticum with AD was supported by typical lesions and acute and chronic eczematous changes found mainly in the flexural aspects of extremities, which is diagnostic of AD. Laboratory findings showed anti HSV1 IgG (23.43) and high levels of IgE (478.9 IU/L). There was no multinucleated giant cell in the Tzanck test. Skin histology was compatible with AcE. Direct immunofluorescent examination showed no deposits of IgG, IgM, IgA, or complement. Complete resolution occurred within 2 weeks of acyclovir and oral zinc supplementation.
Keywords: Acrodermatitis enteropathica, Eczema herpeticum, Atopic dermatitis
Introduction
Acrodermatitis enteropathica (AcE; MIM #20110) is a rare, autosomal recessive inherited disorder caused by mutation of the SLC39A4 gene responsible for zinc transport protein (ZIP 4) [1, 2]. The mutation results in abnormal zinc-bearing molecules with impaired zinc absorption in the intestine [1]. The disease may appear during childhood, especially in breastfeeding or post-breastfeeding infant (transient neonatal zinc deficiency; MIM #608118), as a consequence of a low milk zinc concentration in their nursing mothers. The acquired AcE can occur in patients who have undergone gastrectomy, parenteral nutrition, with celiac disease, or inflammatory bowel disease [3, 4]. The clinical symptoms vary depending on the age of onset [5, 6]. Moderate to severe zinc deficiency usually presents with eczematous to vesiculobullous, pustular, and erosive lesions, whereas psoriasiform lesions are seen in mild or chronic zinc deficiency [7, 8]. The prevalence of AcE is estimated to occur 1–9 in 1,000,000, with a global incidence rate of 1:500,000 newborns worldwide without gender and racial tendencies [9].
Eczema herpeticum (EH) generally refers to widespread clusters of umbilicated vesicles and pustules that evolve into crusted skin erosions, occurring in patients with an underlying cutaneous disease [10]. Herpes simplex virus is considered as the main causative agent. The most frequently affected sites are the face/head, trunk, and neck. In some cases, it may progress to fulminating, life-threatening infection and can have severe sequelae [10]. Although it commonly appears on a background of atopic dermatitis (AD), it has been described in association with other skin conditions such as ichthyosis vulgaris, bullous pemphigoid, dyskeratosis follicularis (Darier disease), mycosis fungoides, and contact dermatitis [11, 12]. The association with AcE, to the best of our knowledge, has not yet been described.
Case Report
An 11-year-old boy presented with a 2-week history of a diffuse eruption of pruritic, umbilicated, erythematous vesicles, with erosions and some covered with hemorrhagic crusts, in almost his entire body. According to his parents, the initial lesions were discrete and seen only in perineal and gluteal regions, starting at the age of 7 months, about 1 month after he was weaned from breastfeeding. The disease partially ameliorated with topical treatment given by the general physician, but with multiple relapsing courses. There were no history of diarrhea or psychiatric complaints. His family history revealed apparently a similar disease in his paternal uncle and cousin.
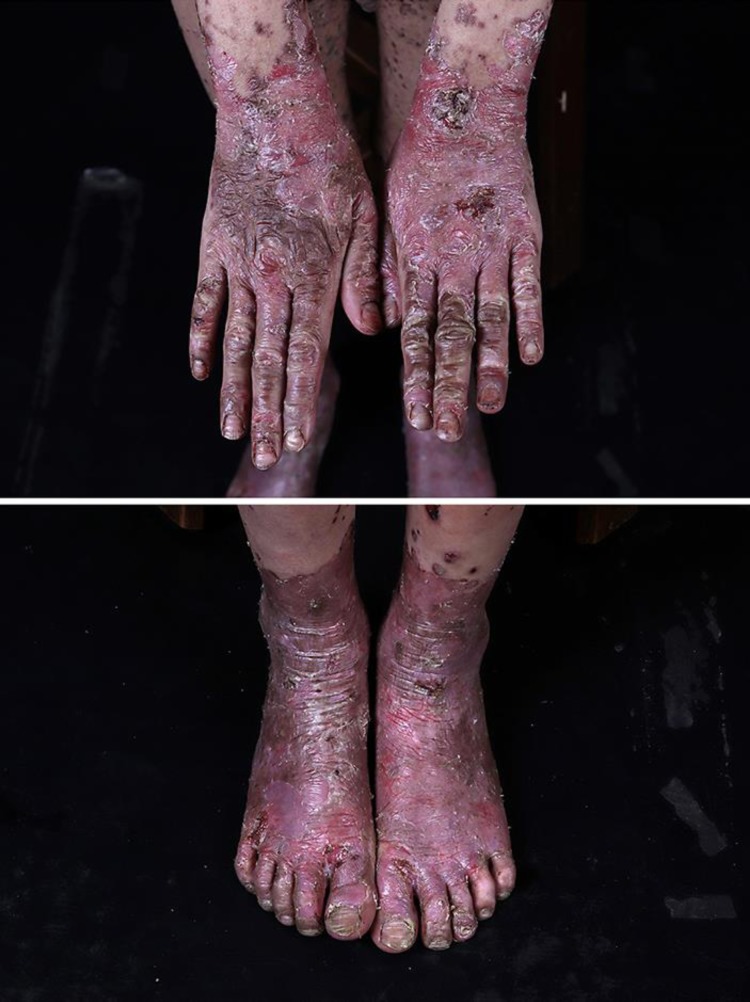
Physical examination showed normal height, but the weight for age below the 50th percentile. He was afebrile, and with no lymphadenopathy. The hair was thin and sparse, with areas of alopecia and diminished eyebrows and eyelashes. Skin examination revealed some erosions predominantly in the periorificial areas (Fig. 1), as well as multiple erythematous vesicles with erosions and partly hemorrhagic crusts in almost his entire body (Fig. 2). Acute and chronic eczematous changes were found mainly in the flexural aspects of the extremities, such as antecubital, popliteal, and carpotarsal sites, which is typical of AD. There was opaque change of all finger- and toenails with multiple Beau lines and perionychia (Fig. 3).
Fig. 1.
The eyebrows and the eyelashes were diminished. Some erosions predominantly on the periorificial.
Fig. 2.
Erythematous vesicles with erosion and some covered with hemorrhagic crusts in almost the entire body. Acute and chronic eczematous changes can be observed mainly in the flexural aspects of the extremities, typical of AD.
Fig. 3.
Opaque change of all finger and toe nails with multiple Beau lines and perionychia.
Cytologic analysis of an unroofed vesicle showed no giant, ballooned keratinocytes or multinucleated giant cell suggestive of herpes virus infection. Laboratory tests showed an increase in neutrophils of 68.2% (normal 35–65%), levels of IgE 478.9 kUI/L (normal <100 kUI/L), anti HSV1 IgG 23.43 (normal <9), anti HSV2 IgG 5.81 (normal <9). Blood and hair zinc level were 58.7 μg/dL and 84.9 μg/dL, respectively (normal 80–120 μg/dL).
Histology from a crusted papule on the right waist showed epidermal basket weave hyperorthokeratosis, hypogranulosis, spongiosis, and lymphocytic exocytosis to the stratum corneum. Necrosis with inflammatory cells, including polymorphonuclear cells, lymphocytes, and eosinophils, with a subepidermal bulla, was also found. Together with fibrosis and collagenization in the dermis, AcE was suspected (Fig. 4). Direct immunofluorescence (DIF) examination showed no deposits of IgG, IgM, IgA, or complement, excluding the possibility of autoimmune bullous dermatoses.
Fig. 4.
Epidermal basket weave hyperorthokeratosis, hypogranulosis, spongiosis, and lymphocytic exocytosis to the stratum corneum. Necrosis with inflammatory cells including polymorphonuclear cells and lymphocytes, and eosinophils, with a sub-epidermal bullae. Dermis fibrosis and collagenization (H&E; a 40×, b 200×).
Based on the diagnosis of AcE concurrent with EH in the presence of AD, treatment was started with elemental zinc in the form of zinc sulphate orally at a dose of 50 mg twice daily (2 mg/kg body weight/day), acyclovir tablets 400 mg three times daily for 7 days, and moisturizer twice daily, skin lesions showed dramatic improvement within 2 weeks.
Discussion
The characteristic clinical triad of AcE includes periorificial and acral dermatitis, diarrhea, and alopecia, only 20% of the cases show all three manifestations simultaneously [9]. It usually appears in children at 1–2 weeks after weaning, with acral and periorificial, symmetrical, eczematous, plaques that become vesicular, bullous, pustular, or erosive with characteristic crusting at the edges [5]. Plasma zinc levels help to confirm the diagnosis [9]. In our patient, the blood zinc level was still low (<70 μg/dL), even after 2 weeks of given zinc supplementation. Although the proof of genetic mutations was missing in the current case, the clinical diagnosis was supported by the positive family history with apparently similar disease in his paternal uncle and cousin indicating a genetic background, a low weight for age below the 50th percentile suggesting a nutritional deficiency, and rapid improvement of skin changes with zinc supplementation. We supposed a heterogeneity in genetic mutations precipitated by nutritional deficiency led to the long-term chronic recurrent course of the disease in our patient. A serum value of albumin, which can indicate the nutritional status, while a low level of serum alkaline phosphatase, a zinc-dependent metalloenzyme, which may support the diagnosis of zinc deficiency, were not measured. It is to note that serum alkaline phosphatase activity is a moderately sensitive indicator of zinc status, although not a particularly early marker of deficiency. Its activity remains near normal in mild cases until profound and prolonged deficiency exists [13].
The diagnosis of EH in our patient is made primarily based on clinical findings, elevated viral serology, and the rapid therapeutic effectiveness to oral acyclovir. The Tzanck smear, viral cultures, skin biopsy, or detection of viral DNA by polymerase chain reaction can be helpful in doubtful cases. Antiviral therapy should be started as soon as possible on diagnosis to reduce morbidity and mortality. Eczema herpeticum represent a dermatology emergency with potentially life-threatening complications [10], which can be minimized by early diagnosis and systemic antiviral treatment.
The current case is unique in the coexistence of AD, AcE, and EH. Acrodermatitis enteropathica can simulate AD [14], but complication with herpes simplex infection is unknown. On the other hand, abnormal immune responses in patients with AcE with elevated serum IgE level and positive rheumatoid factor have been reported, but the attribution to zinc deficiency remains unclear [15]. Association between AcE and food allergy with elevation of specific IgE has been described [16].
The prevalence of nail changes in AcE is unclear. In our case, the AD involving the hands/fingers, and the disseminated herpes infection might also contribute to the extensive nail changes. Our patient was treated with zinc sulfate in combination with acyclovir, which led to significant improvement of skin lesions in 2 weeks. There is no clear consensus or recommendation on the exact dose and how long zinc should be given for AcE. It is important to sustain high-dose zinc supplementation and regular monitoring of the affected patients for signs of deficiency [7, 17].
Conclusion
Atopic dermatitis is a common dermatosis in children, but AcE and EH are rare. In children with AD displaying periorificial and acral involvement recalcitrant to topical steroid, AcE should be considered as differential diagnosis. Rapid dissemination of vesiculo-crusted lesions may indicate a superimposed viral infection such as in the scenario of EH.
Statement of Ethics
The parents have given informed consent on having the patients data and pictures published.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was obtained.
Author Contributions
All authors have made substantial contributions to all of the following: (1) the conception and design of the review, analysis, and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, and (3) final approval of the version to be submitted.
References
- 1.Küry S, Dréno B, Bézieau S, Giraudet S, Kharfi M, Kamoun R, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002 Jul;31((3)):239–40. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- 2.Nakano A, Nakano H, Nomura K, Toyomaki Y, Hanada K. Novel SLC39A4 mutations in acrodermatitis enteropathica. J Invest Dermatol. 2003 Jun;120((6)):963–6. doi: 10.1046/j.1523-1747.2003.12243.x. [DOI] [PubMed] [Google Scholar]
- 3.Wu D, Fung MA, Kiuru M, Sharon VR. Acquired bullous acrodermatitis enteropathica as a histologic mimic of pemphigus foliaceus in a patient on parenteral nutrition. Dermatol Online J. 2017 Jul;23((7)):1–4. [PubMed] [Google Scholar]
- 4.Rana J, Plovanich M, Wallace EB, Yang C, Canales AL, Mostaghimi A. Acquired acrodermatitis enteropathica after gastric bypass surgery responsive to IV supplementation. Dermatol Online J. 2016 Nov;22((11)):13030/qt50v2f3mb. [PubMed] [Google Scholar]
- 5.Kaur S, Thami GP, Kanwar AJ. Acrodermatitis enteropathica in a full-term breast-fed infant. Indian J Pediatr. 2002 Jul;69((7)):631–3. doi: 10.1007/BF02722694. [DOI] [PubMed] [Google Scholar]
- 6.Nistor N, Ciontu L, Frasinariu OE, Lupu VV, Ignat A, Streanga V. Acrodermatitis enteropathica: a case report. Medicine (Baltimore) 2016 May;95((20)):e3553. doi: 10.1097/MD.0000000000003553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranugha P, Sethi P, Shastry V. Acrodermatitis enteropathica: the need for sustained high dose zinc supplementation. Dermatol Online J. 2018 Dec;24((12)):24. [PubMed] [Google Scholar]
- 8.Jen M, Yan AC. Syndromes associated with nutritional deficiency and excess. Clin Dermatol. 2010 Nov-Dec;28((6)):669–85. doi: 10.1016/j.clindermatol.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 9.Jagadeesan S, Kaliyadan F. Acrodermatitis enteropathica. in StatPearls. Treasure Island (FL) 2018 [PubMed] [Google Scholar]
- 10.Sharif J, McMullen E. Dermatology emergencies: handy hints for the acute medical team. Br J Hosp Med (Lond) 2018 Jul;79((7)):378–83. doi: 10.12968/hmed.2018.79.7.378. [DOI] [PubMed] [Google Scholar]
- 11.Gogou M, Douma S, Haidopoulou K, Giannopoulos A. Herpeticum-like rash in a child with atopic dermatitis: early clinical suspicion is valuable. Sudan J Paediatr. 2018;18((2)):53–5. doi: 10.24911/SJP.106-1536167231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karray M, Souissi A. Kaposi varicelliform eruption. in StatPearls. Treasure Island (FL) 2018 [PubMed] [Google Scholar]
- 13.Maverakis E, Fung MA, Lynch PJ, Draznin M, Michael DJ, Ruben B, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007 Jan;56((1)):116–24. doi: 10.1016/j.jaad.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 14.da Matta Ain AC, dos S Valente E, Mallozi MC, Sarni RO, Furquim M, Solé D. Acrodermatitis enteropathica-like simulating severe atopic dermatitis: a case report. Allergol Immunopathol (Madr) 2008 May-Jun;36((3)):176–9. [PubMed] [Google Scholar]
- 15.Anttila PH, von Willebrand E, Simell O. Abnormal immune responses during hypozincaemia in acrodermatitis enteropathica. Acta Paediatr Scand. 1986 Nov;75((6)):988–92. doi: 10.1111/j.1651-2227.1986.tb10328.x. [DOI] [PubMed] [Google Scholar]
- 16.Martin DP, Tangsinmankong N, Sleasman JW, Day-Good NK, Wongchantara DR. Acrodermatitis enteropathica-like eruption and food allergy. Ann Allergy Asthma Immunol. 2005 Mar;94((3)):398–401. doi: 10.1016/S1081-1206(10)60994-5. [DOI] [PubMed] [Google Scholar]
- 17.Hammersen J, Has C, Galiano M, Lindner M, Rossi R, Kohlhase J, et al. Sustained need for high-dose zinc supplementation in children with acrodermatitis enteropathica. Clin Pediatr (Phila) 2018 Jan;57((1)):99–102. doi: 10.1177/0009922816685820. [DOI] [PubMed] [Google Scholar]