Abstract
We compared the therapeutic efficacy of facial nerve decompression (FND) and conservative treatment in patients with Bell’s palsy through a systematic review and meta-analysis. Primary database search was performed in PubMed, Medline, and Embase. After screening, 13 studies were assessed for their eligibility. Among them, seven studies employing either the House-Brackmann grading system (HBGS) or May’s classification (modified HBGS) were selected for quantitative and qualitative analysis. Based on May’s classification, the degree of recovery was classified into complete (HBGS I), fair (HBGS II–III), or failed (HBGS IV–VI) recovery. The outcomes were assessed between 6 and 12 months after surgery. The estimated pooled odds ratio (OR) and 95% confidence interval (CI) were calculated using random effects model. Cohorts were comprised of patients who underwent FND (n=202, 53.0%) and conservative treatments (n=179, 47.0%). In pooled analysis, the rate of complete recovery was significantly higher in the FND group than in the control group (OR, 2.06; 95% CI, 1.22 to 3.48; P=0.007) showing neither heterogeneity nor publication bias. Meanwhile, the rates of fair recovery (OR, 0.71; 95% CI, 0.42 to 1.21; P=0.208) and failed recovery (OR, 0.60; 95% CI, 0.22 to 1.67; P=0.327) in the FND group were similar to that in the control group. In subgroup analyses, there was no significant difference in the OR according to the operation timing and surgical approach. FND can be a possible treatment option for patients with complete Bell’s palsy, especially for complete recovery, which provide insights on decision-making and outcome prediction. However, FND should be determined carefully given the risk of small study effects and possible complications.
Keywords: Bell Palsy, Facial Nerve Decompression, Meta-Analysis
INTRODUCTION
Facial nerve palsy (FNP) is typically a paresis or paralysis of the unilateral facial muscles. Idiopathic FNP, also known as Bell’s palsy, accounts for approximately 75% of acute FNP cases [1]. Although most patients with Bell’s palsy recover from the functional nerve dysfunction following the initial insult [2], 30% of complete facial palsy patients exhibited an incomplete recovery that was closely linked to aesthetic, psychologic, and social problems [3]. In addition, complete facial palsy detected during early evaluation is known to be associated with poor prognosis [4]. Therefore, understanding of nature prognosis and timely intervention are vital to achieving optimal therapeutic outcomes, especially in the complete type of Bell’s palsy.
High-dose systemic corticosteroid administration is the key to the initial treatment of Bell’s palsy [5]. Antiviral agents may be implicated in combination with steroids to restore the facial nerve function, even though evidence on the effectiveness of combination therapy is still controversial [6]. Nonetheless, previous a large cohort study demonstrated some extent of patients showed incomplete recovery or no recovery at all, despite combination therapy with prednisolone and valganciclovir [7]. In cases involving severe nerve degeneration within 14 days after the onset of complete facial palsy, facial nerve decompression (FND) can be considered as a possible surgical option to restore facial function [3]. The outcome of treatment with FND has been shown to be variable and depends on the timing of the treatment, approach of surgery, or other accompanying factors of FNP [8,9].
A previous Cochrane review on early surgical interventions for Bell’s palsy reported that there was an insufficient evidence to demonstrate that FND was beneficial [10]. However, the aforementioned Cochrane review was based only on two studies which involved FND using a transmastoid approach. Since the publication of this Cochrane review, studies aiming to compare FND and medical treatment have emerged. Nonetheless, a recent meta-analysis showed that FND does not lead to significant improvement in facial nerve function compared to medical treatment based on the House-Brackmann grading system (HBGS) [11]. However, the results were limited by a significant higher heterogeneity across the studies (I2=89.72). Moreover, the bias related to small-study effects, such as publication bias, was not thoroughly addressed in that study. Thus, the therapeutic role of FND in the treatment of complete Bell’s palsy needs to be re-established.
We, herein, aimed to compare therapeutic efficacy between FND and conservative treatments in patients with complete Bell’s palsy through a systematic review and meta-analysis. Additionally, we performed subgroup analyses according to the timing of treatment and approach of surgery with meticulous interpretations of bias.
MATERIALS AND METHODS
This systematic review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklists. A PRISMA flow diagram was used to describe the flow of information during various phases of the systematic review [12]. This study utilized quantitative methods to examine the reasons for variation in treatment outcomes.
Search strategy
Two of the authors (SYL and YHK) independently searched PubMed, Medline, and Embase databases. Available studies in the databases from their inception to June 30, 2018 were searched. We employed the following search syntax, adapted to each database as appropriate: #1 (Bell’s palsy) OR (Bell palsy) OR (idiopathic facial paralysis) OR (idiopathic facial palsy) OR (facial paralysis) OR (facial palsy) AND #2 (decompression) OR (facial nerve decompression) OR (facial decompression) OR (transmastoid decompression) OR (middle fossa decompression).
Selection of studies
Two authors (SYL and YHK) reviewed all retrieved articles by screening the titles and abstracts. Full texts of eligible articles were subsequently evaluated to determine whether they met the inclusion criteria. Only studies which involved the following were included in the analysis: (1) participants who presented with idiopathic facial palsy (Bell’s palsy); (2) direct comparison of recovery of facial nerve function between FND and conservative treatments; and (3) the measurements of outcomes using HBGS or modified HBGS (May’s classification). Indeed, FND in the included studies was performed in patients with persistent facial palsy following medical treatment, except for the one study. Brown [13] designed a double-blind study to test the facial nerve function; thereby, 41 cases received only steroid therapy while 41 cases underwent FND without preceding medical treatment. The medical treatment includes the steroid and/or antiviral therapy, regardless of different treatment regimen in perspectives of dose and duration. The conservative treatment refers to cases of the medical administration as the same protocol of FND group followed by observation. The following publications were excluded: (1) publications which involved patients diagnosed with herpes zoster, who had facial palsy with traumatic etiology or recurrent facial palsy; (2) review articles and case reports; and (3) publications with inaccessible original articles (e.g., only abstracts were available) and/or with incomplete data; and (4) duplicate publications.
Data extraction
Two authors independently extracted data; any discrepancies were resolved by consensus between the two authors. For the meta-analysis, the following information was obtained: author, year of publication, study design, number of participants, inclusion criteria, surgical approach, treatment outcomes, evaluation period, and complications.
Outcomes criteria
We mainly focused on the degree of improvement in facial nerve function after FND compared to conservative treatment. Based on May’s evaluation method (modified HB grading), the degree of facial nerve function was classified into complete recovery (grade HB I), fair recovery (grade HB II, III), and failed recovery (grades HB IV, V, and VI) (Supplementary Table 1). The treatment outcomes between 6 months and 1 year after treatment were evaluated in this study. There were insufficient studies to enable any statistical analysis of continuous data, such as changes in electroneurography and electromyography scores. Furthermore, subgroup analyses according to surgical approach and operation timing were assessed among available studies. Specifically, operation timing was classified into early (<14 days) and delayed (≥14 days) interventions. Additionally, we analyzed the complications after FND, when available.
Data synthesis and measurement of treatment outcomes
In this study, the estimated pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using the random effects model. Selection of random effects was determined based on a conceptual understanding of the presence of population effects within enrolled studies, rather than using the statistical results of homogeneity tests. Analysis of pooled proportions was performed; cases with missing or incomplete information were excluded. For dichotomous outcome data (May’s classification), the pooled ORs and their 95% CIs were calculated. For continuous outcome data (HBGS), the standardized mean differences (SMDs) with 95% CIs were measured. All analyses were performed using the R software package ver. 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
Assessment of heterogeneity and interpretation
We calculated the I2 statistic to evaluate rates of heterogeneity across the studies. If an I2 value of >50% and a P-value of <0.10 were identified, we classified the heterogeneity of the effect size as substantial. For post-hoc analyses, either the trim- and-fill method or sensitivity analysis was used to verify the integrity of the quantitative analysis results. Moreover, publication bias was evaluated with a funnel plot if more than three studies were included.
RESULTS
Characteristics of studies included in the meta-analysis
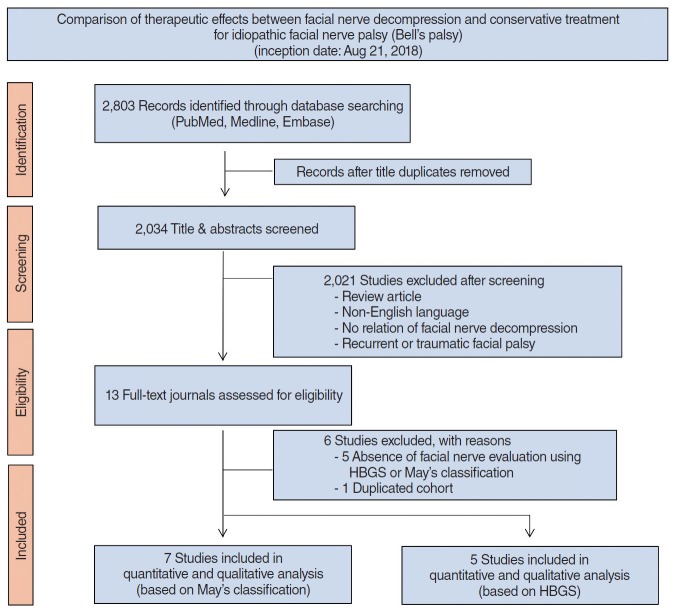
The inclusion of studies was determined using a flow diagram (Fig. 1). After identification and screening, 13 studies were assessed for eligibility. As indicated in Table 1, six studies were excluded because they used evaluation methods that were different from the HBGS or May’s classification (n=5) [9,14-17] and one study was further excluded, including presumed duplicate cohort, with subsequent studies published by the same author [18]. Ultimately, seven studies were selected for quantitative and qualitative analysis [8,13,19-22].
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram outlining the study design. HBGS, House-Brackmann grading system.
Table 1.
Characteristics of excluded studies (ordered by study year)
| Study | Reason for exclusion |
|---|---|
| Aoyagi et al. (1988) [14] | Outcome measures: the degree of facial nerve palsy recovery is evaluated by ENoG and cannot be changed to May’s classification. |
| Fisch et al. (1981) [9] | Outcome measures: the degree of facial nerve palsy recovery is also evaluated by nerve degeneration as ENoG and cannot be changed to may's classification. |
| Adour et al. (1978) [15] | Outcome measures: the degree of facial nerve palsy recovery was evaluated by a unique method of FPRP and FPRI. There was no change can be evaluated in may's classification. |
| Mcneill (1974) [17] | Outcome measures: the degree of facial nerve paralysis was divided into acceptable and unacceptable; thereby, conversion to may's classification was impossible. |
| Mechelse et al. (1971) [16] | Outcome measures: the degree of facial nerve palsy recovery was assessed by classifying the frontalis muscle, orbicularis oculi muscle orbicularis oris muscle from 0 to 5 in six stages, so that it could not be replaced with may's classification. |
| May et al. (1981) [18] | Subjects: the presumed duplicate cohort, with subsequent studies published by the same author. |
ENoG, electroneurography; FPRP, facial paralysis recovery profile; FPRI, facial paralysis recovery index.
As shown in Table 2, seven studies were included in the meta-analysis: two quasi-randomized controlled trials (RCTs), three prospective and two retrospective case-controlled studies. A total of 381 patients were involved in the meta-analysis; 202 (53.0%) underwent FND while 179 (47.0%) underwent conservative treatment (steroid and/or antiviral therapy). The selected studies were conducted between 1982 and 2017. The diagnostic criteria of FND and control group are identical. In detail, all patients enrolled in the meta-analysis were compatible with complete palsy (HBGS V and VI based on HBGS) and/or severe degeneration of facial nerve function (degeneration ratio >90% based on electrodiagnostic testing). The surgical approaches for FND were the transmastoid approach (n=5) and middle fossa approach (n=2). Additionally, according to the operation timing, early and delayed intervention group were four and three, respectively.
Table 2.
Demographics and clinical characteristics of enrolled studies
| Study/country | Study design | Age (yr, range) | No. of FND groups | No. of controls | Surgical indication | Evaluation grading | Treatment outcome |
|---|---|---|---|---|---|---|---|
| Li et al. (2016)/China [19] | Quasi-RCT | 21–62 (median, 41.3) | 25 | 13 | HBGS V or VI CMAP degeneration >95% (based on EMG) | May’s classification (modified HBGS) | Recovery of facial function, postoperative complications |
| Kim et al. (2016)/Korea [8] | Retrospective | FND: 18–76 (mean±SD, 48.5±17.4); control: 18–74 (mean±SD, 50.4±16.1) | 12 | 22 | Degeneration >90% (based on ENoG) | HBGS | Recovery of facial function, hearing threshold |
| No voluntery EMG | |||||||
| Yanagihara et al. (2001)/Japan [20] | Retrospective | FND: 16–71 (mean±SD, 34.6±14.6); control: 16–84 (mean±SD, 55.1±17.9) | 58 | 43 | HBGS V or VI CMAP degengeration >95% (based on EMG) | HBGS | Recovery of facial function Postoperative, complications |
| Gantz et al. (1999)/USA, multicenter [21] | Prospective | FND: 9–58 (mean, 32); control: 23–66 (mean, 47) | 34 | 36 | Degeneration >90% (based on ENoG) | HBGS | Recovery of facial function |
| No voluntary EMG within 2 weeks | |||||||
| Gantz et al. (1999)/USA, Iowa [21] | Prospective | FND: 20–57 (mean, 41); control: 23–66 (mean, 47) | 7 | 11 | Degeneration >90% (based on ENoG) | HBGS | Recovery of facial function, postoperative complications |
| No voluntary EMG within 2 weeks | |||||||
| May et al. (1985)/USA [22] | Prospective | NA | 25 | 13 | Complete paralysis (and) degeneration >90% (based on EMG) | HBGS | Recovery of facial function |
| Brown (1982)/Canada [13] | Quasi-RCT | NA | 41 | 41 | Complete paralysis | May’s classification (modified HBGS) | Recovery of facial function, postoperative complications |
| Unfavorable prognosis for complete recoverya) |
FND, facial nerve decompression; RCT, randomized controlled trial; HBGS, House-Brackmann grading system; CMAP, compounding muscle action potential; EMG, electromyography; SD, standard deviation; ENoG, electroneurography; NA, not available.
Facial nerve function test showed function of less than 25% on the affected side.
The recovery of facial nerve function
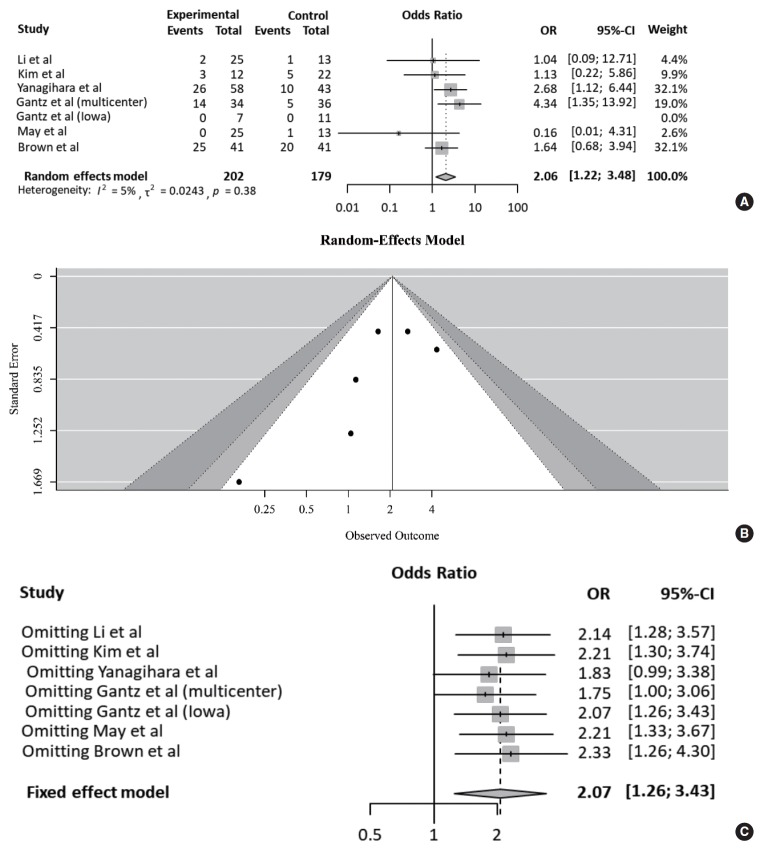
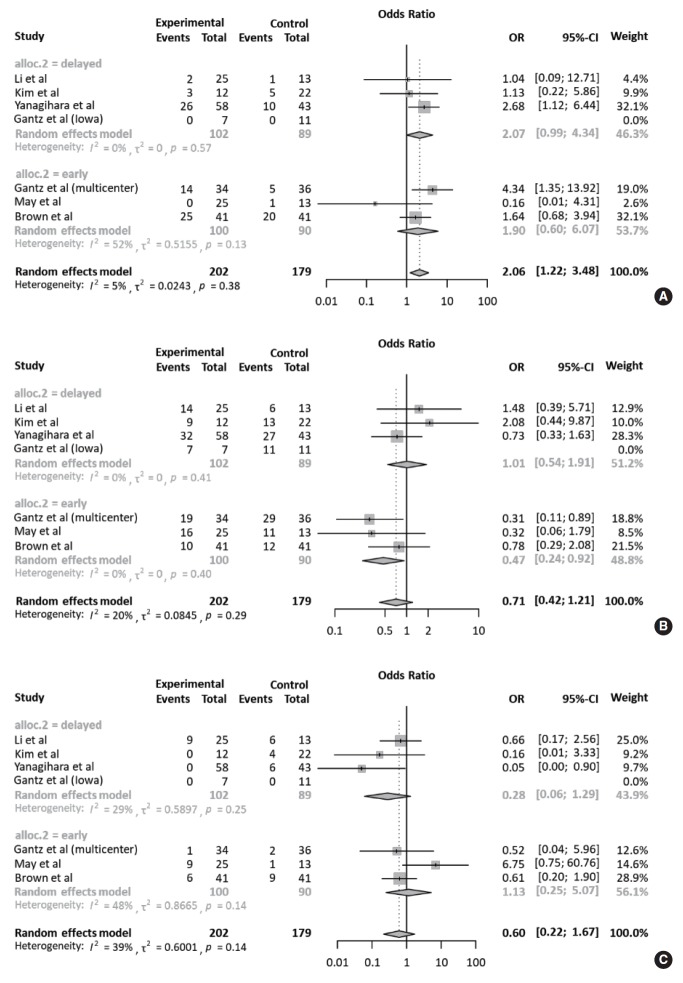
Table 3 compares the outcomes of each study according to treatment approach (FND vs. conservative treatment). All studies contained data on the degree of recovery of facial nerve function. In a pooled analysis (Fig. 2A), based on May’s classification, the complete recovery of facial function was significantly higher in the FND group than in the conservative group (OR, 2.06; 95% CI, 1.22 to 3.48; P=0.007). Neither heterogeneity nor publication bias was found in studies which reported complete recovery (Fig. 2B). Moreover, sensitivity analysis revealed similar ORs, regardless of a strategy by omitting each study (Fig. 2C).
Table 3.
Comparison of treatment outcomes between FND and conservative treatment
| Study/country | Approach | Surgical timing | Assessment timing (mo) | FND |
Control |
Side effect (FND) | ||
|---|---|---|---|---|---|---|---|---|
| Recovery of facial function HBGS | Recovery of facial function May’s classification | Recovery of facial function HBGS | Recovery of facial function May’s classification | |||||
| Li et al. (2016)/China [19] | Transmastoid approach | >2 mo (delayed) | 12 | NA | (Total=25) Complete: 2, fair: 14, fail: 9 | NA | (Total=13) Complete: 1, fair: 6, fail: 6 | SNHL: 4/25, tinnitus: 3/25 |
| Kim et al. (2016)/Korea [8] | Transmastoid approach | 42 day (21–70, delayed) | 6 (SD, 2.5) | (Total=12) I: 3, II: 6, III: 3, IV–VI: 0 | (Total=12) Complete: 3, fair: 9, fail: 0 | (Total=22) I: 5, II: 9, III: 4, IV: 3, V: 1 | (Total=22) Complete: 5, fair: 13, fail: 4 | Deterioration of hearing threshold (a mean of 9.7 dB) |
| Yanagihara et al. (2001)/Japan [20] | Transmastoid approach | >14 day (delayed) | 12 | (Total=58) I: 26, II: 15, III: 17, IV–VI: 0 | (Total=58) Complete: 26, fair: 32, fail: 0 | (Total=43) I: 10, II: 16, III: 11, IV–V: 6 | (Total=43) Complete: 10, fair, 27, fail: 6 | Transient CHL |
| Gantz et al. (1999)/USA, multicenter [21] | MFA | <14 day (early) | 7 | (Total=34) I: 14, II: 17, III: 2, IV: 1, V–VI: 0 | (Total=34) Complete: 14, fair: 19, fail: 1 | (Total=36) I: 5, II: 10, III: 19, IV: 2, V–VI: 0 | (Total=36) Complete: 5, fair: 29, fail: 2 | NA |
| Gantz et al. (1999)/USA, Iowa [21] | MFA | >14 day (delayed) | 7 | (Total=7) I: 0, II: 2, III: 5, IV–VI: 0 | (Total=7) Complete: 0, fair: 7, fail: 0 | (Total=11) I: 0, II: 4, III: 7, IV–VI: 0 | (Total=11) Complete: 0, fair: 11, fail: 0 | CHL: 1/26, CSF leakage: 1/26 |
| May et al. (1985)/USA [22] | Transmastoid approach | <14 day (early) | >6 | (Total=25) I: 0, II: 5, III: 11, IV: 9 | (Total=25) Complete: 0, fair: 16, fail: 9 | (Total=13) I: 1, II: 2, III: 9, IV: 1 | (Total=13) Complete: 1, fair: 11, fail: 1 | NA |
| Brown (1982)/Canada [13] | Transmastoid approach | <14 day (early) | 6–12 | (Total=41) Complete: 25, fair: 10, fail: 6 | (Total=41) Complete: 20, fair: 12, fail: 9 | Deafness: 6/41, persistent giddiness: 2/41 | ||
FND, facial nerve decompression; HBGS, House-Brackmann grading system; NA, not available; SNHL, sensorineural hearing loss; SD, standard deviation; CHL, conductive hearing loss; MFA, middle fossa approach; CSF, cerebrospinal fluid.
Fig. 2.
Comparison of the rate of complete recovery. (A) Forest plot comparing the rate of complete recovery between facial nerve decompression (experimental) and conservative treatment (control) using the odds ratio (OR) and 95% confidence interval (CI). Events represent the number of cases with complete recovery based on May’s classification. (B) Symmetry based on funnel plot suggesting no publication bias. (C) Sensitivity analysis.
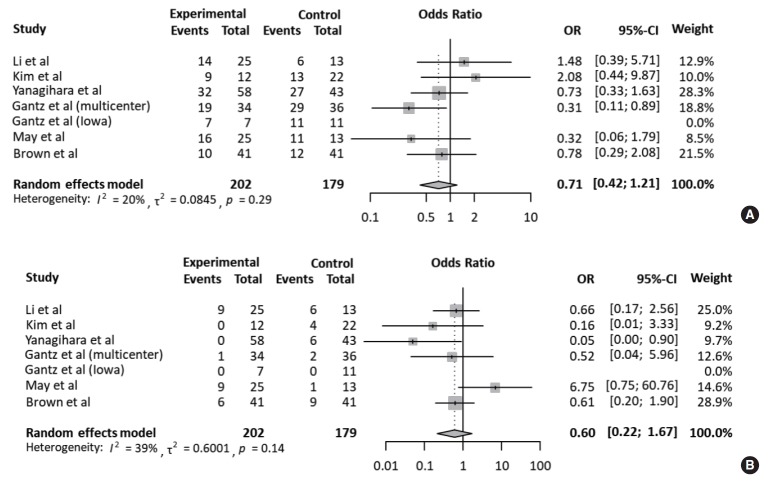
Meanwhile, the rate of fair recovery (OR, 0.71; 95% CI, 0.42 to 1.21; P=0.208) in the FND group was similar to that in the conservative group (Fig. 3A). For failed recovery, no difference in ORs was observed between the two groups (OR, 0.60; 95% CI, 0.22 to 1.67; P=0.327) (Fig. 3B). There was no substantial heterogeneity in studies which reported fair and failed recovery. Moreover, publication bias did not show a noticeable asymmetry on the funnel plot.
Fig. 3.
Comparison of the rate of fair and failed recovery. Forest plots comparing the rate of (A) fair recovery and (B) failed recovery between facial nerve decompression (experimental) and conservative treatment (control) using the odds ratio (OR) and 95% confidence interval (95% CI). Events were based on May’s classification.
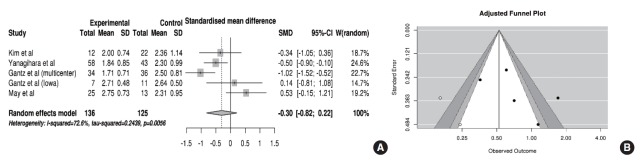
Five of eight studies reported the recovery of facial nerve function after using the HBGS. In the pooled analysis (Fig. 4A), HB scores were improved more in the FND group than in the conservative group (SMD, –0.30; 95% CI, –0.82 to 0.22; P= 0.26), but this improvement did not reach statistical significance. Specifically, a substantial amount of heterogeneity (I2 =72.6% and P=0.005) and publication bias was found (Fig. 4B). After adjustment, the trim-and-fill method revealed that the SMD for improvements in HB scores was –0.64 (95% CI, –1.20 to –0.08; P=0.025), indicating significant small-study effects which compromised the analysis using HBGS.
Fig. 4.
Comparison of the House-Brackmann grading scores. (A) Forest plot comparing the House-Brackmann grading scores between facial nerve decompression (experimental) and conservative treatment (control) based on the standardized mean difference (SMD). (B) Adjusted publication bias after applying the trim-and-fill method. SD, standard deviation; CI, confidence interval.
Subgroup analysis: operation timing
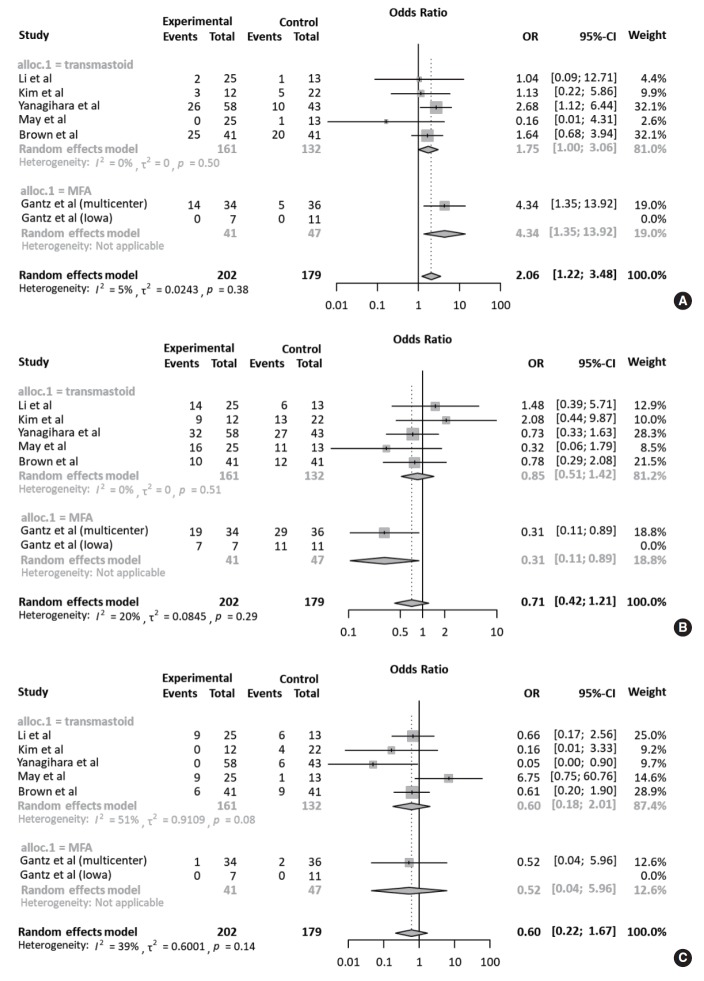
All studies were subjected to subgroup analysis according to the operation timing. Regarding complete recovery, the early intervention group (OR, 2.07; 95% CI, 0.99 to 4.34) appeared to have a higher OR than the delayed intervention group (OR, 1.90; 95% CI, 0.60 to 6.07), but there was no statistical significance (P=0.901). This tendency was also observed in the early intervention group with regards to fair and failed recovery. Collectively, based on May’s classification, there was no significant difference in ORs between the FND and conservative groups, according to operation timing (Fig. 5). For subgroup analysis of the surgical approach, based on May’s classification, the degree of recovery of facial nerve function was similar regardless of the surgical approach (Fig. 6).
Fig. 5.
Subgroup analyses according to operation timing. Forest plot comparing the recovery of facial nerve function according to the operation timing (early intervention: <14 days after onset vs. delayed intervention: >14 days after onset). (A) Complete recovery. (B) Fair recovery. (C) Failed recovery. OR, odds ratio; CI, confidence interval.
Fig. 6.
Subgroup analyses according to surgical approach. Forest plot comparing the recovery of facial nerve function according to the surgical approach (transmastoid approach vs. middle fossa approach). (A) Complete recovery. (B) Fair recovery. (C) Failed recovery. OR, odds ratio; CI, confidence interval.
Postoperative complications
Data regarding postoperative complications were available in five studies (Table 3). Specifically, hearing loss was evident among patients who underwent FND via transmastoid approach. This hearing loss ranged from transient conductive hearing loss (CHL) to deafness. Among the five studies, the incidence of hearing loss was 12.0% (11/91). In patients who underwent FND via the middle fossa approach, CHL and cerebrospinal fluid leakage were observed. Due to the lack of information on complications in studies involving conventional treatment, statistical analysis was not possible.
Quality assessment and publication bias
We assessed the risk of bias in quasi-RCTs based on the Cochrane Collaboration tool (Supplementary Table 2). Additionally, we used the Newcastle-Ottawa Scale criteria to examine the internal validity of prospective and retrospective studies (Supplementary Table 3).
DISCUSSION
We compared the therapeutic efficacy of FND with conservative treatment through a systematic review and meta-analysis based on seven eligible studies. To the best of our knowledge, our quantitative analyses were based on the largest number of studies, providing meticulous interpretations of integrity regarding potential bias. Interestingly, FND significantly enhanced the rate of complete recovery compared to conventional treatment. Although different prognostic perspectives on surgical approach and operation timing have been suggested, our subgroup analyses did not show significant differences.
Bell’s palsy, the most common cause of acute FNP, is considered idiopathic [23]. Although various mechanisms, including viral infections, vascular ischemia, and immune-mediated processes, have been suggested to contribute to Bell’s palsy [24,25], the exact pathophysiology of Bell’s palsy has not yet been clearly elucidated. In anatomical perspectives, the diameter of the meatal segment of the facial nerve is small (approximately 0.68 mm) at the point where it enters the fallopian canal and is susceptible to inflammation and edema [26]. Thus, the edematous swelling of the fallopian canal which underlies Bell’s palsy decreases the room for expansion in a rigid bony canal [27], potentially leading to severe nerve damage and even necrosis and fibrosis. More than 90% nerve degeneration within 14 days after the onset of Bell’s palsy is correlated with poor prognosis [28], indicating the need for FND. Opening the bony canal and subsequently releasing the pressure on the nerve sheath causes decompression of the nerve fibers, which can improve the circulation and minimize damage to distal nerve fibers [29,30]. Thus, FND prevents continuous nerve degeneration and restores facial nerve function.
However, the therapeutic role of FND in complete Bell’s palsy remains unclear. Although evidence on the therapeutic efficacy of FND in improving facial nerve function has increased since the Cochrane review, a recent meta-analysis reported no difference in HBGS between surgical and medical treatment. The study was limited by a significant higher of heterogeneity. Moreover, our current study revealed that the meta-analysis using HBGS was biased as documented by the trim-and-fill method. Based on this, we adopted the May’s classification, namely a modified HBGS, to enhance the statistical power and integrity. As shown in Figs. 2 and 3, Neither heterogeneity nor publication bias was found. In other words, the May’s classification in this study provides a more reliable interpretation of meta-analysis than HBGS. However, in prognosis perspective to distinguish subtle changes, there seems to be no difference between two evaluation methods when considering the May’s classification is based upon HBGS. Our results indicate that FND is closely associated with complete recovery (HBGS I). This information could be clinically significant considering that 10% of patients with complete palsy only partially recover despite combination therapy with prednisolone and valganciclovir [7].
Several studies reported improved outcomes following early intervention (within 2 weeks after onset) compared to delayed intervention (more than 2 weeks after onset) [21]. A recent meta-analysis demonstrated that middle fossa decompression within 14 days of symptom onset improved the HBGS compared to medical management [11]. Early intervention has been shown to ameliorate the risk of ischemia in the fallopian canal, which is correlated with prognosis [13]. Given that Wallerian degeneration originates from long-standing compression of the facial nerve in the fallopian canal [31], delayed intervention is likely to be associated with poor prognosis. Nonetheless, evidence on benefits of delayed decompression surgery which occurs within 90 days after onset has increased for patients who cannot afford early surgery [32]. Also, our results indicated that the therapeutic efficacy of FND became more slightly evident if those with complete Bell’s palsy underwent FND less than 14 days from the onset of symptoms, but with no statistical significance. Collectively, early intervention may lead to better outcomes, but the window of the exact operation timing to rescue the benefit from FND remains controversies.
In light of the presumed anatomical etiology of Bell’s palsy, the middle cranial fossa approach which can be used to identify and decompress the proximal labyrinthine segment of the facial nerve may be feasible compared to the transmastoid approach [11,21]. Indeed, a previous study which employed the transmastoid approach showed no significant benefit of this approach to complete facial palsy [13], suggesting that the meatal foramen may be key to the pathogenesis of Bell’s palsy. However, we did not find any difference in treatment outcomes based on surgical approach. This may be attributed to the inclusion of recent studies which showed that using the transmastoid approach resulted in either complete or fair recovery in most cases. Additionally, it remains unknown whether the surgical approach is an independent prognostic factor when considering clinical factors such as age, comorbidities, and initial status of facial nerve function [33,34]. Therefore, a surgical approach may not be an independent determinant of the prognosis after adjusting for possible confounders.
By comparing the therapeutic efficacy of FND with conservative treatment in in patients with complete Bell’s palsy following medical treatment, our results provide insights on appropriate determination of treatment strategy and timely intervention to restore the facial nerve function. Nevertheless, there are some limitations that should be addressed in future studies. First, even though our results were based on the largest number of studies, small number of randomized controlled studies limit the integrity of the analysis. Moreover, most patients in this study underwent medical treatment before undergoing FND. To be precise, the present study made a direct comparison of facial function recovery between FND following medical treatment and only medical treatment followed by observation, except for one study [13]. In other words, the therapeutic effects of steroid or antiviral therapy were not controlled in the FND group. Thus, randomized controlled studies involving a larger cohort are warranted to further support our current conclusions. Second, some of the selected studies had potential biases, as documented by the Newcastle-Ottawa Scale (Supplementary Table 2), albeit with symmetry in the funnel plot. Given the bias, evaluation with May’s classification seems to be more reliable for verifying the therapeutic role of FND than evaluation with HBGS, as shown in Fig. 5. Third, neither HBGS nor May’s classification can differentiate subtle changes in facial function, as well as limitations of interobserver agreement. These concerns have led to the proposal of additional systems, such as deep-learning based approach to measuring the precise facial function [35]. Lastly, risk factors that affect the recovery of facial nerve function, such as age and comorbidities (i.e., hypertension and diabetes), were not considered in our selected studies [36].
Taken together, FND can be a possible treatment option in patients with complete Bell’s palsy, especially for complete recovery. In subgroup analyses, the recovery of facial nerve function was similar regardless of the surgical approach. Additionally, early intervention with FND within 14 days from the onset of Bell’s palsy seems to increase the therapeutic efficacy, but the optimal time window remains unclear. Our results may be useful for decision-making and outcome prediction in patients with complete Bell’s palsy. However, FND should be determined carefully when considering the risk of bias and possible complications.
HIGHLIGHTS
• In patients with complete Bell’s palsy, facial nerve decompression (FND) leads to a higher complete recovery rate compared to conservative treatment.
• There was no significant difference in the odds ratio for fair and failed recovery.
• In subgroup analyses according to operation timing and surgical approach, no significant difference of facial function recovery was found.
• FND can be a possible treatment option for patients with complete Bell’s palsy, especially for complete recovery.
• FND should be determined carefully when considering the risk of bias and possible complications.
Acknowledgments
This study was supported by a clinical research grant from the SMG-SNU Boramae Medical Center, Seoul, Korea.
Footnotes
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization, Data curation, & Formal analysis: SYL. Funding acquisition: YHK. Methodology, Project administration, Visualization, & Writing - original draft: SYL. Writing - review & editing: all authors.
SUPPLEMENTARY MATERIALS
Supplementary material can be found via https://doi.org/10.21053/ceo.2019.00535.
The recovery of facial function and characteristics according to HBGS and May’s classification (modified HBGS)
Cochrane collaboration’s tool for assessing risk of bias
Newcastle-Ottawa scale of bias risk for the non-randomized studies
REFERENCES
- 1.Sun DQ, Andresen NS, Gantz BJ. Surgical management of acute facial palsy. Otolaryngol Clin North Am. 2018 Dec;51(6):1077–92. doi: 10.1016/j.otc.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Peitersen E. Natural history of Bell’s palsy. Acta Otolaryngol Suppl. 1992;492:122–4. doi: 10.3109/00016489209136829. [DOI] [PubMed] [Google Scholar]
- 3.Andresen NS, Sun DQ, Hansen MR. Facial nerve decompression. Curr Opin Otolaryngol Head Neck Surg. 2018 Oct;26(5):280–5. doi: 10.1097/MOO.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 4.Mantsopoulos K, Psillas G, Psychogios G, Brase C, Iro H, Constantinidis J. Predicting the long-term outcome after idiopathic facial nerve paralysis. Otol Neurotol. 2011 Jul;32(5):848–51. doi: 10.1097/MAO.0b013e31821da2c6. [DOI] [PubMed] [Google Scholar]
- 5.Holland NJ, Weiner GM. Recent developments in Bell’s palsy. BMJ. 2004 Sep;329(7465):553–7. doi: 10.1136/bmj.329.7465.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adour KK. Combination treatment with acyclovir and prednisone for Bell palsy. Arch Otolaryngol Head Neck Surg. 1998 Jul;124(7):824. doi: 10.1001/archotol.124.7.824. [DOI] [PubMed] [Google Scholar]
- 7.Peitersen E. Bell’s palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol Suppl. 2002;(549):4–30. [PubMed] [Google Scholar]
- 8.Kim SH, Jung J, Lee JH, Byun JY, Park MS, Yeo SG. Delayed facial nerve decompression for Bell’s palsy. Eur Arch Otorhinolaryngol. 2016 Jul;273(7):1755–60. doi: 10.1007/s00405-015-3762-y. [DOI] [PubMed] [Google Scholar]
- 9.Fisch U. Surgery for Bell’s palsy. Arch Otolaryngol. 1981 Jan;107(1):1–11. doi: 10.1001/archotol.1981.00790370003001. [DOI] [PubMed] [Google Scholar]
- 10.McAllister K, Walker D, Donnan PT, Swan I. Surgical interventions for the early management of Bell’s palsy. Cochrane Database Syst Rev. 2013 Oct;(10):CD007468. doi: 10.1002/14651858.CD007468.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Casazza GC, Schwartz SR, Gurgel RK. Systematic review of facial nerve outcomes after middle fossa decompression and transmastoid decompression for Bell’s palsy with complete facial paralysis. Otol Neurotol. 2018 Dec;39(10):1311–8. doi: 10.1097/MAO.0000000000001979. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009 Jul;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown JS. Bell’s palsy: a 5 year review of 174 consecutive cases: an attempted double blind study. Laryngoscope. 1982 Dec;92(12):1369–73. doi: 10.1288/00005537-198212000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Aoyagi M, Koike Y, Ichige A. Results of facial nerve decompression. Acta Otolaryngol Suppl. 1988;446:101–5. doi: 10.3109/00016488709121850. [DOI] [PubMed] [Google Scholar]
- 15.Adour KK, Byl FM, Hilsinger RL, Jr, Kahn ZM, Sheldon MI. The true nature of Bell’s palsy: analysis of 1,000 consecutive patients. Laryngoscope. 1978 May;88(5):787–801. doi: 10.1002/lary.1978.88.5.787. [DOI] [PubMed] [Google Scholar]
- 16.Mechelse K, Goor G, Huizing EH, Hammelburg E, van Bolhuis AH, Staal A, et al. Bell’s palsy: prognostic criteria and evaluation of surgical decompression. Lancet. 1971 Jul;2(7715):57–9. doi: 10.1016/s0140-6736(71)92041-1. [DOI] [PubMed] [Google Scholar]
- 17.McNeill R. Facial nerve decompression. J Laryngol Otol. 1974 May;88(5):445–55. doi: 10.1017/s0022215100078919. [DOI] [PubMed] [Google Scholar]
- 18.May M, Blumenthal F, Taylor FH. Bell’s palsy: surgery based upon prognostic indicators and results. Laryngoscope. 1981 Dec;91(12):2092–103. doi: 10.1288/00005537-198112000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Sheng Y, Feng GD, Wu HY, Gao ZQ. Delayed surgical management is not effective for severe Bell’s palsy after two months of onset. Int J Neurosci. 2016 Nov;126(11):989–95. doi: 10.3109/00207454.2015.1092144. [DOI] [PubMed] [Google Scholar]
- 20.Yanagihara N, Hato N, Murakami S, Honda N. Transmastoid decompression as a treatment of Bell palsy. Otolaryngol Head Neck Surg. 2001 Mar;124(3):282–6. doi: 10.1067/mhn.2001.112309. [DOI] [PubMed] [Google Scholar]
- 21.Gantz BJ, Rubinstein JT, Gidley P, Woodworth GG. Surgical management of Bell’s palsy. Laryngoscope. 1999 Aug;109(8):1177–88. doi: 10.1097/00005537-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 22.May M, Klein SR, Taylor FH. Idiopathic (Bell’s) facial palsy: natural history defies steroid or surgical treatment. Laryngoscope. 1985 Apr;95(4):406–9. doi: 10.1288/00005537-198504000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Mattox DE. Clinical disorders of the facial nerve. In: Flint PW, editor. Otolaryngology head and neck surgery review. 3rd ed. St. Louis (MO): Mosby-Year Book; 1998. pp. 2767–84. [Google Scholar]
- 24.Greco A, Gallo A, Fusconi M, Marinelli C, Macri GF, de Vincentiis M. Bell’s palsy and autoimmunity. Autoimmun Rev. 2012 Dec;12(2):323–8. doi: 10.1016/j.autrev.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Paolino E, Granieri E, Tola MR, Panarelli MA, Carreras M. Predisposing factors in Bell’s palsy: a case-control study. J Neurol. 1985;232(6):363–5. doi: 10.1007/BF00313837. [DOI] [PubMed] [Google Scholar]
- 26.Jain S, Kumar S. Bell’s palsy: a need for paradigm shift. Ann Otol Neurotol. 2018;1(01):034–9. [Google Scholar]
- 27.Owusu JA, Boahene KD. Bell’s palsy. In: Kountakis SE, editor. Encyclopedia of otolaryngology, head and neck surgery. Berlin, Heidelberg: Springer; 2013. pp. 256–8. [Google Scholar]
- 28.Baugh RF, Basura GJ, Ishii LE, Schwartz SR, Drumheller CM, Burkholder R, et al. Clinical practice guideline: Bell’s palsy. Otolaryngol Head Neck Surg. 2013 Nov;149(3 Suppl):S1–27. doi: 10.1177/0194599813505967. [DOI] [PubMed] [Google Scholar]
- 29.Pulec JL. Early decompression of the facial nerve in Bell’s palsy. Ann Otol Rhinol Laryngol. 1981 Nov-Dec;90(6 Pt 1):570–7. doi: 10.1177/000348948109000612. [DOI] [PubMed] [Google Scholar]
- 30.Gelberman RH, Eaton RG, Urbaniak JR. Peripheral nerve compression. Instr Course Lect. 1994;43:31–53. [PubMed] [Google Scholar]
- 31.Jackson CG, von Doersten PG. The facial nerve: current trends in diagnosis, treatment, and rehabilitation. Med Clin North Am. 1999 Jan;83(1):179–95. doi: 10.1016/s0025-7125(05)70096-1. [DOI] [PubMed] [Google Scholar]
- 32.Berania I, Awad M, Saliba I, Dufour JJ, Nader ME. Delayed facial nerve decompression for severe refractory cases of Bell’s palsy: a 25-year experience. J Otolaryngol Head Neck Surg. 2018 Jan;47(1):1. doi: 10.1186/s40463-017-0250-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillman GS, Schaitkin BM, May M, Klein SR. Bell’s palsy in pregnancy: a study of recovery outcomes. Otolaryngol Head Neck Surg. 2002 Jan;126(1):26–30. doi: 10.1067/mhn.2002.121321. [DOI] [PubMed] [Google Scholar]
- 34.Lee DH, Chae SY, Park YS, Yeo SW. Prognostic value of electroneurography in Bell’s palsy and Ramsay-Hunt’s syndrome. Clin Otolaryngol. 2006 Apr;31(2):144–8. doi: 10.1111/j.1749-4486.2006.01165.x. [DOI] [PubMed] [Google Scholar]
- 35.Brenner MJ, Neely JG. Approaches to grading facial nerve function. Semin Plast Surg. 2004 Feb;18(1):13–22. doi: 10.1055/s-2004-823119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takemoto N, Horii A, Sakata Y, Inohara H. Prognostic factors of peripheral facial palsy: multivariate analysis followed by receiver operating characteristic and Kaplan-Meier analyses. Otol Neurotol. 2011 Aug;32(6):1031–6. doi: 10.1097/MAO.0b013e31822558de. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The recovery of facial function and characteristics according to HBGS and May’s classification (modified HBGS)
Cochrane collaboration’s tool for assessing risk of bias
Newcastle-Ottawa scale of bias risk for the non-randomized studies