Abstract
Extracellular vesicles (EV) can transfer cellular molecules for specific intercellular communication with potential relevance in pathological conditions. We searched for the presence in plasma from coronary artery disease (CAD) patients of EV containing the adenosine A2A receptor (A2AR), a signalling receptor associated with myocardial ischaemia and whose expression is related to homocysteine (HCy) metabolism. Using protein organic solvent precipitation for plasma EV preparation and Western blotting for protein identification, we found that plasma from CAD patients contained various amounts of EV with ubiquitin bound to A2AR. Interestingly, the presence of ubiquitinated A2AR in EV from patients was dependent on hyperhomocysteinemia, the amount being inversely proportional to A2AR expression in peripheral mononuclear cells in patients with the highest levels of HCy. CEM, a human T cell line, was also found to released EV containing various amounts of ubiquitinated A2AR in stimulated conditions depending on the hypoxic status and HCy level of culture medium. Together, these data show that ubiquitinated A2AR‐containing EV circulate in the plasma of CAD patients and that this presence is related to hyperhomocysteinemia. A2AR in plasma EV could be a useful tool for diagnosis and a promising drug for the treatment of CAD.
Keywords: adenosine A2A receptor, coronary artery disease, extracellular vesicles, homocysteine, ubiquitin
1. INTRODUCTION
Extracellular vesicles (EV) such as exosomes and microvesicles are bi‐lipid membranous vesicles with endocytic origin that are released by many cell types including immune, endothelial and mesenchymal stem cells, erythrocytes and platelets.1 EV participate in intercellular communication by carrying and delivering cargo including proteins, lipids, miRNA and mRNA specific to the type of cell from which they originate.2 EV are key mediators of a process now thought to be a form of intercellular signalling that impacts the physiology of cells, tissues and organs.3 EV are released constitutively or after stimulation and taken up by other cells via membrane fusion or ligand‐receptor interactions.4 Due to their ability to trap their cargo and circulate freely in body fluids, EV are natural sources of non‐invasive diagnostic and prognostic biomarkers that may also be used as vehicles of targeted therapy for tumour progression, neurodegeneration, autoimmune disorders and other human diseases.5 In cardiovascular disease, EV represent one of the most intensely studied and rapidly growing areas of research.6, 7 EV were shown to exert diverse and sometimes discordant biological effects in different studies related to cardiovascular disease. For example, EV can play an atheroprotective or atherogenic role in several conditions accompanying atherosclerosis.8
Adenosine greatly impacts the cardiovascular system via four specific G protein‐coupled receptors, named respectively A1, A2A, A2B and A3. Among them, the A2A receptor (A2AR) is strongly expressed in coronary cells and its activation increases coronary blood flow,9 partly through the production of cAMP in target cells.10 A2AR from patients with coronary artery disease (CAD) is poorly expressed and, consequently, produces low level of cAMP, two characteristics that are associated with myocardial ischaemia, as documented by positive exercise stress testing or reduced flow reserve.11, 12, 13 The down‐regulation of A2AR expression in CAD patients is related to the homocysteine (HCy) metabolism via its degradation product H2S.14 A2AR expressed on peripheral blood mononuclear cells (PBMC) of CAD patients reflect coronary tissue expression showing the systemic nature of the adenosinergic signalling.15
Circulating EV can be considered as a reserve of functional G protein‐coupled receptors as previously suggested from data obtained on a mouse model of heart cellular stress for angiotensin II type 1 receptor.16 Taking into account the major role of A2AR in cardiovascular disease and the potential contribution of circulating EV in delivering cell receptor from donor to target cells, we searched for the presence of A2AR in EV from plasma of patients with CAD and culture supernatant of human lymphoblastoid T cells cultured in CAD‐like conditions.
2. MATERIALS AND METHODS
2.1. Human materials
Fourteen patients (11 men and three women, 56‐58 years old) with angiographically documented CAD were included in this pilot study (Table 1). The first group consisted of eight patients selected blind and the second group was six patients with moderate hyperhomocysteinemia. Controls were eight healthy individuals (six men and two women, 56‐64 years old) with a normal level of HCy (Table 1) recruited from the research laboratory or hospital staff, without medical treatment or history of cardiovascular disease. The study was conducted in compliance with the principles of the Declaration of Helsinki and approved by the Ethics Committee for Human Research of our University Hospital. All participants provided written informed consent to participate.
Table 1.
HCy levels of healthy individuals and CAD patients
| Healthy individuals | Unselected CAD patients | CAD patients with moderate hyperhomocysteinemia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref | Age | Sex | HCy | Ref | Age | Sex | HCy | Ref | Age | Sex | HCy |
| A | 59 | M | 10.7 ± 0.2 | 1 | 56 | F | 13.5 ± 0.5 | 9 | 57 | M | 31.4 ± 1.6 |
| B | 60 | M | 9.3 ± 0.1 | 2 | 60 | M | 14.8 ± 1.4 | 10 | 59 | M | 24.1 ± 0.9 |
| C | 56 | M | 9.2 ± 0.3 | 3 | 56 | M | 13.1 ± 0.8 | 11 | 63 | M | 38.7 ± 2.1 |
| D | 57 | F | 8.7 ± 0.5 | 4 | 61 | M | 32.1 ± 2.3 | 12 | 57 | M | 27.7 ± 1.3 |
| E | 64 | M | 8.9 ± 0.7 | 5 | 57 | M | 16.7 ± 0.7 | 13 | 62 | M | 32.5 ± 1.5 |
| F | 59 | F | 10.9 ± 0.7 | 6 | 68 | F | 24.1 ± 1.2 | 14 | 65 | F | 23.8 ± 1.9 |
| G | 63 | M | 9.1 ± 0.6 | 7 | 57 | M | 11.1 ± 0.9 | ||||
| H | 56 | M | 10.4 ± 1.0 | 8 | 63 | M | 18.6 ± 1.2 | ||||
Age (Y), Sex (Male/Female), HCy (µmol/L); Mean ± SD. Normal level of HCy: <12 µmol/L; Mild Hyperhomocysteinemia: 12‐20 µmol/L; Moderate Hyperhomocysteinemia: 21‐100 µmol/L.
Abbreviation: Ref: Reference Letter for Healthy Subjects and Reference Number for CAD patients.
Plasma and PBMC were obtained from blood collected by venipuncture at the brachial vein in citrate tube and cell preparation tube (CTP) for the separation of mononuclear cells (Vacutainer, Becton‐Dickinson), respectively, according to the manufacturer's instructions.
Extracellular vesicles were isolated from fresh plasma by a simple, rapid and reliable solvent‐based protein precipitation method as previously described.17 Briefly, 200 µL of plasma was mixed with 800 µL cold acetone (−20°C) in 1.5 mL conical tube and centrifuged at 3000 × g for 1 minute. After centrifugation, 500 µL of EV‐containing supernatant was freeze at −20°C and dried in a Savant SpeedVac concentrator (Thermo Fisher Scientific).
Extracellular vesicles and PBMC were analysed by Western blotting as previously reported.11, 12, 13, 14, 15 Briefly, freeze‐dried EV corresponding to 100 µL of plasma and cell pellets corresponding to 0.25 × 106 PBMC were solubilized in 15 µL Laemmli sample buffer (BioRad) with 5% SDS, 5% 2‐mercaptoethanol and a complete set of protease inhibitors (Roche), heated for 5 minutes at 95°C and loaded on 12% SDS‐PAGE minigel (BioRad). Prestained molecular weight markers (BioRad) have always been loaded onto the gel. After electrophoresis, separated proteins were blotted onto nitrocellulose membrane. Protein detection was conducted using an appropriate primary antibody: anti‐CD63 and anti‐CD9 (EXOAB‐KIT, Ozyme), anti‐ubiquitin (clone 6C1, Sigma‐Aldrich) and anti‐A2AR (Adonis,18 CliniSciences). Blots were then revealed using phosphatase alkaline‐labelled secondary antibody by a BCIP®/NBT‐Purple liquid substrate system for membranes, (B3679, Sigma‐Aldrich). Densitometric quantification of the blot was performed using the ImageJ software (https://imagej. nih.gov). Briefly, a scanned blot image (in grey scale and TIFF format) was imported into ImageJ, areas of interest were selected and plots of pick profiles were generated. Lines were drawn to select the peaks of interest, and the peak areas were integrated and converted into pixel intensities. Results were given as per cent of total pixels of all the relevant bands on a same migration line on the blot. The protein load was controlled by the reproducibility of the results, Western blots being done in triplicate.
2.2. Homocysteine assay
Total HCy from the human plasma was quantified with the liquid chromatography‐tandem mass spectrometry Clinmass® apparatus using the dedicated kit (Homocysteine in plasma/serum, Recipe) according to the manufacturer's instructions. Results (in µmol/L) are the mean ± SD of duplicates.
2.3. CEM T cell line
CEM, a human lymphoblastoid T cell line19 was cultured in RPMI 1640 medium supplemented with 2 mmol/L l‐glutamine, 10% foetal calf serum (cleaned for cell debris and aggregates by ultracentrifugation and filtration through a 0.22 µm sterile membrane) and 100 U/mL penicillin + 100 µg/mL streptomycin at 37°C under 5% CO2. Cells were seeded in 75‐cm2 flasks (0.5 × 106 cells/mL, 50 mL/flask) and stimulated using phorbol myristate acetate (PMA, 50 ng/mL) and phytohemagglutinin (PHA, 5 µg/mL) for 24 hours in control condition. As previously reported in this cellular model, hypoxia condition was achieved by adding 100 µmol/L CoCl2 to the culture medium and the effect of HCy on cells in hypoxia was obtained by adding 200 µmol/L HCy just prior to the addition of CoCl2.14
After 24 hours incubation, cells cultured in the three conditions (Control, Hypoxia, Hypoxia + HCy) were separated from supernatants by centrifugation at 3000 × g for 15 minutes. Cells were rinsed in phosphate‐buffered saline, pH 7.3, counted and aliquoted for A2AR expression assay. EV were prepared from the culture supernatant by a dedicated method using the ExoQuick‐TC reagent (System Biosciences) according to the manufacturer's instructions. Briefly, culture supernatant (10 mL) and 2 mL reagent were mixed well by inverting into a 15 mL tube. After overnight incubation at 4°C, the mixture was centrifuged at 1500 × g for 30 minutes and the supernatant was discarded. Residual supernatant was removed by additional centrifugation and careful aspiration to not disturb the pelleted material. EV was solubilized in 100 µL Laemmli sample buffer (BioRad) with 5% SDS, 5% 2‐mercaptoethanol and a complete set of protease inhibitors (Roche) and heated for 5 minutes at 95°C. EV from culture supernatant and CEM T cells were analysed by Western blotting as described above for the human material.
Cell viability was monitored using the 3‐(4,5‐dimethyl‐2‐thiazolyl)‐2,5‐diphenyl‐2H‐tetrazolium bromide (MTT) assay as previously described.14 MTT (0.5 mg in 100 µL of PBS, pH 7.3) was added to 24‐well plates containing 0.5 × 106 cells/mL cultured in the three conditions (Control, Hypoxia, Hypoxia + HCy) 3 hours prior to the end of the 24 hours incubation period. After treatment, cells were pelleted (10 000 × g for 5 minutes) and supernatants were discarded. The insoluble violet formazan crystals associated with the cell pellets were dissolved into pure dimethyl sulfoxide and absorbance was measured at 550 nm. Results are the mean ± SD of duplicates.
3. RESULTS
3.1. Presence of ubiquitinated A2AR in EV of CAD patients
First, we tested plasma from four patients with CAD (Reference Numbers 1, 4, 6 and 7) for the presence of EV carrying A2AR. EV were isolated from plasma using acetone to remove proteins by precipitation leaving purified EV suspended in the liquid phase.17 Freeze‐dried EV were submitted to Western blot procedure using Adonis, a monoclonal antibody to the human A2AR 18 widely used in previous studies 11, 12, 13, 14, 15 and anti‐tetraspanin CD9 as a biomarker of EV with a low molecular weight (28 kD) migrating at high distance from the A2AR to test both in same lanes of gel. Intriguingly, we found using Adonis various amounts of a band (intensity from 0% to 68.5% pixels depending of the patient) that migrated to a higher position than expected (45 kD for cellular A2AR) between the 75 and 50 kD markers in three to four patients (Figure 1A). We found CD9 bands displaying similar intensities (from 3.3% to 64.0% pixels) to those obtained for the A2AR. The negative patient for A2AR probably contained a low level of receptors, which corresponded to a low level of EV in plasma as judged by the faint intensity of the CD9 band (3.3% pixels) and consequently had undetectable A2AR in our experimental conditions (Figure 1A).
Figure 1.
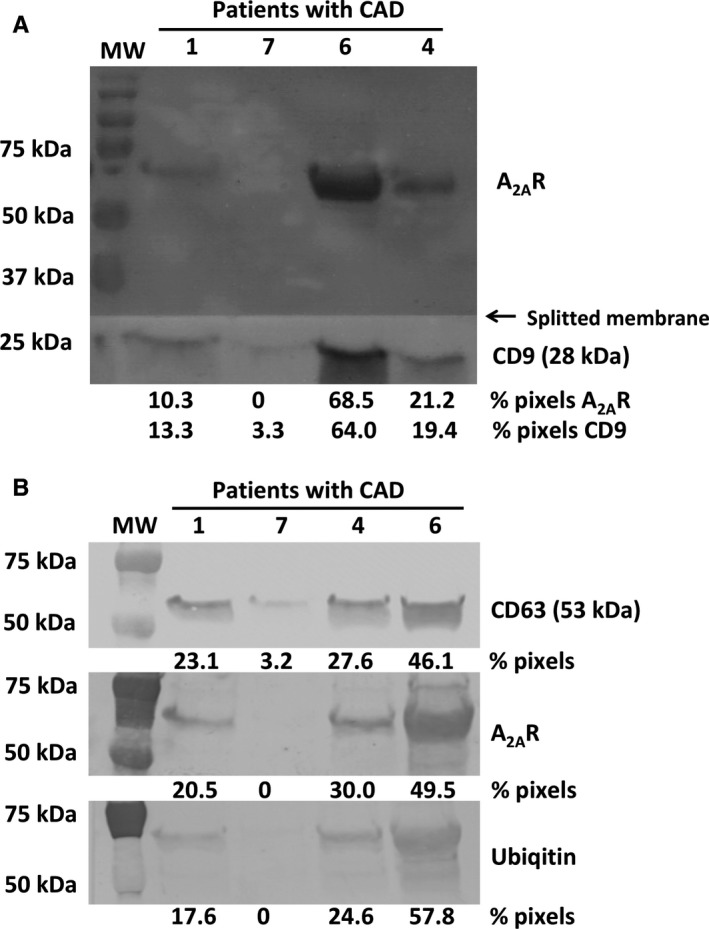
Presence of ubiquitinated A2AR in EV from plasma of CAD patients. Western blot of EV from plasma of four CAD patients was splitted in two parts and separately revealed using anti‐A2AR (upper part) and anti‐CD9 (lower part) primary antibody (A). Western blots of EV from four CAD patients were revealed using (from top to bottom) anti‐CD63, anti‐A2AR and anti‐ubiquitin primary antibody (B). Molecular weight markers are on the left. To compare, blots obtained with each primary antibody are mounted on the figure and are representative of triplicates
Next, we performed another set of Western blots using preparations of EV from the same four plasma samples. The use of a different anti‐tetraspanin marker CD63 (mol wt of 53 kD) gave a band intensity gradation similar to that obtained with CD9 and confirmed that the plasma level of A2AR in CAD patients was related to their EV content ranking patients from lowest to highest as follows: Reference Numbers 7,1, 4 and 6 (Figure 1B). Looking for a plausible post‐translational process that could have increased the molecular weight of the A2AR in the context of EV trafficking we used an anti‐ubiquitin antibody, which revealed the same heavy A2AR bands as Adonis with a similar intensity gradation between patients, as previously described (Figure 1B). Blotted bands of ubiquitinated A2AR migrated above the CD63 bands. Together, these data suggested that exosomal A2AR contained at least two molecules of ubiquitin (mol wt 8 kD) raising the molecular weight of A2AR from 45 to 61 kD.
3.2. Comparison of EV and cell expression of A2AR
Second, we examined A2AR content both in EV and PBMC from eight unselected CAD patients (Reference Numbers 1‐8) and two healthy individuals(Reference Letters A and B). To compare the A2AR level in EV from 100 µL plasma to that contained in 0.25 × 106 PBMC, Western blots were made in triplicates, each giving a similar result. Densitometric quantification of representative blots was performed and expressed as % of total pixels (Figure 2A). Intensity of the patient bands varied from 0% to 26.2% pixels in EV and from 3.6% to 17.6% pixels in PBMC. EV bands from patients 4 and 6 were among the strongest (20.4 and 24.9% pixels, respectively) when their cell counterparts from PBMC were among the weakest (3.6 and 6.4% pixels, respectively). Patients 7 as the two healthy individuals A and B gave inverted results, that is no band for A2AR from EV as compared to strongest bands for A2AR from PBMC (17.6, 25.7 and 13.9% pixels, respectively). Relative levels in the EV and PBMC compartments were almost similar for other patients, that is, low in patients 1, 2 and 3 and high in patients 5 and 8. Interestingly, patients 4 and 6 had the highest levels of HCy (32.1 ± 2.3 and 24.1 ± 1.2 µmol/L, respectively) indicating moderate hyperhomocysteinemia. The other patients had mild hyperhomocysteinemia whereas patients 7 and healthy individuals had normal level of HCy (Table 1).
Figure 2.
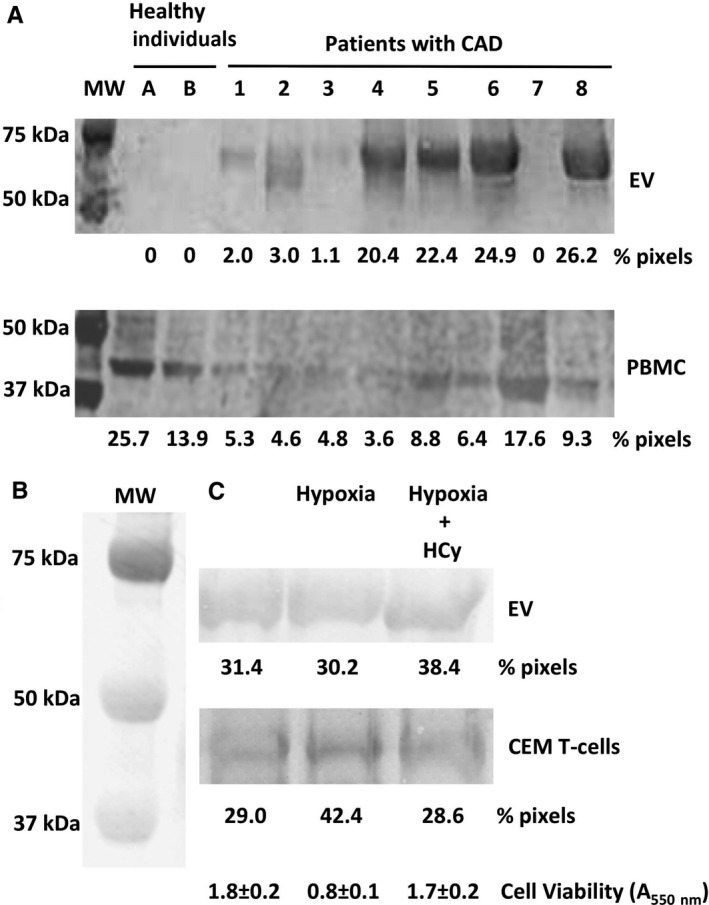
Comparison of EV and cell expression of A2AR. Western blots of EV (upper) and PBMC (lower) from eight CAD patients and two healthy individuals (A). Western blots of EV (upper) and CEM T cells (lower) from cells cultured in three conditions: Control, Hypoxia, Hypoxia + HCy (B). Blots were revealed using anti‐A2AR primary antibody. Molecular weight markers are on the left. To compare, blots are mounted on the figure and are representative of triplicates. Blotted bands were quantified, and results are given in % of total pixels. At the bottom of the figure, CEM cell viability after 24 h culture in the three conditions is given in absorbance at 550 nm (mean ± SD)
We then confirmed the presence of heavy A2AR (61 kD) in EV released by stimulated CEM T cells in culture medium and prepared using a commercial kit (Figure 2B). Western blots were performed as above using EV from 10 mL of culture supernatant and 0.25 × 106 CEM T cells. Maximal release of A2AR in EV (38.4% pixels) was observed in cells cultured in hypoxia + HCy conditions. A2AR expression in CEM T cells increased under stress hypoxia (42.4% pixels) and returned to control value in the presence of HCy (28.6% pixels). Hypoxia stress was confirmed by about fifty per cent of cell loss as compared to control (A550 nm = 0.8 ± 0.1 vs 1.8 ± 0.2), which was reversed by HCy (A550 nm = 1.7 ± 0.2) as assessed by the MTT assay.14 The highest content of A2AR in EV (38.4% pixels) matched with the lowest cellular content of A2AR (28.6% pixels) and, conversely, the lower one in EV was the highest in cells (30.2% vs 42.4% pixels, respectively).
3.3. Relationship between A2AR export in EV and hyperhomocysteinemia
Finally, to further explore the relationship between the A2AR export via EV and hyperhomocysteinemia, we compared on a same blot the A2AR content of the EV from 6 healthy individuals (Reference Letters C‐H) with normal level of HCy taken as controls with that of 6 CAD patients (Reference Numbers 9‐14) with moderate hyperhomocysteinemia (Table1). The results shown in Figure 3 confirmed the previous data. The control samples lacked A2AR, but those from CAD patients with moderate hyperhomocysteinemia all expressed the A2AR band of a pixels intensity of 6.6 to 40.4% (Figure 3A). The scatter plot in Figure 3B shows the relationship between A2AR levels in EV of CAD patients and their HCy levels greater than 20 µmol/L. The patients 10, 12 and 14 with the lowest HCy levels gave the A2AR bands with the lowest intensities and the patients 9, 11 and 13 with the highest HCy levels had the most A2AR in their EV.
Figure 3.
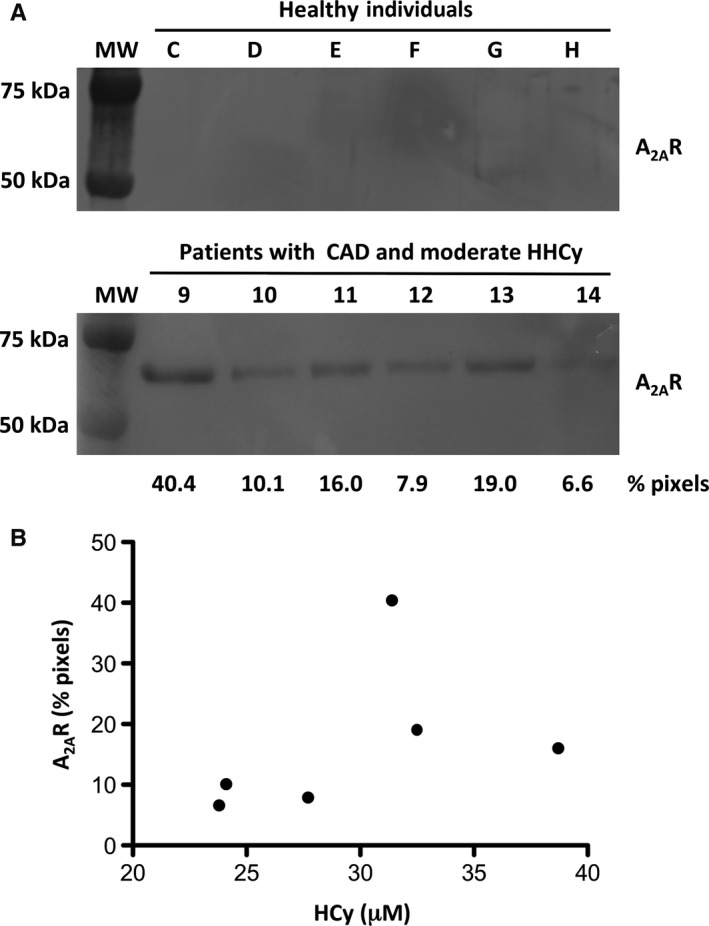
Relationship between A2AR export in EV and hyperhomocysteinemia (HHCy). Western blots of EV from six healthy individuals (upper) and six CAD patients with moderate hyperhomocysteinemia (lower). Blots were revealed using anti‐A2AR primary antibody. Molecular weight markers are on the left. Blots are representative of triplicates. Blotted bands were quantified and results are given in % of total pixels (A). Scatter plot to display the correspondence of A2AR levels in EV of CAD patients (% pixels) with their HCy levels (µmol/L) given in Table 1. Each point represents a patient (B)
4. DISCUSSION
We report here for the first time the presence of ubiquitinated A2AR in EV isolated from plasma of CAD patients. Ubiquitinated A2AR were also found in EV released in the culture supernatant of a stimulated human T cell line.
Plasma contains a mix of EV with different sizes derived from many different cells in varying proportions. Sorting of many membrane proteins into EV coincides with their association with tetraspanin membrane proteins.20 We found that A2AR expression in EV was related to a similar expression of EV assessed by CD9 and CD63 tetraspanins.
We also found here that A2AR in EV was ubiquitinated. Ubiquitin is a highly conserved 76 amino acid polypeptide that is covalently attached to substrate proteins via a lysine residue. Ubiquitination is a reversible modification and ubiquitin moieties can be removed from the substrate protein by a family of deubiquitinating enzymes.21 G protein‐coupled receptors that undergo agonist‐induced ubiquitination are usually internalized and targeted for degradation in lysosomes. However, after deubiquitination they can be redirected to the resensitization pathway and recycled back to the cell surface.22 The addition of a single ubiquitin to a substrate is defined as monoubiquitination and is implicated in various functions including endocytosis of plasma membrane proteins and sorting of proteins to the multivesicular body.23 Moreover, several lysine residues in the substrate can be tagged with single ubiquitin molecules, giving rise to multiple monoubiquitination.24 Here, the ubiquitinated A2AR migrated in SDS‐PAGE to approximately 61 kD suggesting that it contained at least two molecules of ubiquitin. Given the presence of two potential ubiquitination sites on lysine residues 315 and 391 in the intracellular part of A2AR according to an online calculator (Ubpred.org), A2AR could be monoubiquitinated at two sites.
EV with A2AR appeared strongly released in blood concomitantly to down‐regulation of cell surface A2AR expression in CAD patients with moderate hyperhomocysteinemia. This relationship was confirmed in cellulo. These results are in agreement with our previous observation that A2AR cell expression in hypoxia condition is down‐regulated by H2S produced from HCy via the transsulfuration pathway.14 In adenosinergic signalling, exoenzymes CD39 and CD73 are expressed in cancer exosomes where they produce adenosine, which inhibits T cell functions in tumour environment.25 In CAD, one could hypothesize that A2AR circulate from donor cells to target cells as a salvage pathway to be stimulated by adenosine when vasodilation is required for oxygen delivery in failing artery tissues. This mechanism may explain the low expression of A2AR with spare receptor characteristics (EC50 < KD) found in PBMC from CAD patients presenting a major cardiovascular risk 12, 13 by a release from the cells into the blood of EV carrying A2AR, constituting a circulating pool of receptors.
Here, moderate hyperhomocysteinemia in CAD patients was still associated with release of A2AR in EV, which questioned the molecular basis of this association. HCy can affect intracellular signalling by acting on the mitogen‐activated protein kinase pathways 26, 27 and cell signalling can be related to endocytosis and endosomal trafficking.28 We therefore assume that HCy could act on the ubiquitination process so that A2AR do not undergo proteosomal degradation but are exported into exosomes. In this case, hyperhomocysteinemia would favour the salvage pathway described above.
Exosomes contain ubiquitinated proteins that can serve as markers of exosomes 29 and ubiquitin plays a major role in the endosomal trafficking leading to exosomal release.30 Otherwise, ubiquitination controls cell surface expression of MHC class II molecules in dendritic cells 31 and the ubiquitin‐specific protease USP4 was reported to regulate the cell surface level of the A2AR in HEK293 transfected cells.32 Multiple monoubiquitins could confer resistance to the action of deubiquitinating enzymes that would otherwise result in rapid elimination of a single ubiquitin, thereby promoting receptor recycling.33 Given all these considerations, the putative presence here of bi‐monoubiquitinated A2AR in EV delivered in plasma from CAD patients with hyperhomocysteinemia and surpernatant from hypoxic CEM cells under HCy treatment suggests a pivotal role of the ubiquitination process in cell export of A2AR under pathological conditions.
In conclusion, our data show that plasma from CAD patients with hyperhomocysteinemia contains EV carrying ubiquitinated A2AR. Ubiquitin might function here as a tag for A2AR delivery into the blood. From a clinical point of view, EV with A2AR constitute a potential diagnostic tool in CAD and a promising treatment for ischemic tissue.
CONFLICT OF INTEREST
None.
AUTHORS' CONTRIBUTION
JR designed the study. FP provided the human samples. DV performed the experiments. JR, DV, FP and RG analysed the data. JR wrote the article with input from all authors.
Ruf J, Vairo D, Paganelli F, Guieu R. Extracellular vesicles with ubiquitinated adenosine A2A receptor in plasma of patients with coronary artery disease. J Cell Mol Med. 2019;23:6805–6811. 10.1111/jcmm.14564
Funding information
This work was supported by grants from the French National Institute of Health and Medical Research (INSERM), Aix‐Marseille University and Assistance Publique, Hôpitaux de Marseille (APHM), France.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat‐George F. Extracellular Vesicles in Angiogenesis. Circ Res. 2017;120:1658‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raposo G, Stoorvogel WJ. Extracellular vesicles: exosomes, microvesicles, and friends. Cell Biol. 2013;200:373‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corrado C, Raimondo S, Chiesi A, Ciccia F, De Leo G, Alessandro R. Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. Int J Mol. Sci. 2013;14:5338‐5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581‐593. [DOI] [PubMed] [Google Scholar]
- 5. EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347‐357. [DOI] [PubMed] [Google Scholar]
- 6. Kishore R, Srikanth VN, Gumpert A. Tiny shuttles for information transfer: exosomes in cardiac health and disease. J Cardiovasc Trans Res. 2016;9:169‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barile L, Moccetti T, Marbán E, Vassalli G. Roles of exosomes in cardioprotection. Eur. Heart J. 2017;38:1372‐1379. [DOI] [PubMed] [Google Scholar]
- 8. Hansson GK, Libby P. The immune response in atherosclerosis: a double‐ edged sword. Nat Rev Immunol. 2006;6:508‐519. [DOI] [PubMed] [Google Scholar]
- 9. Shryock JC, Snowdy S, Baraldi PG, et al. A2A‐adenosine receptor reserve for coronary vasodilation. Circulation. 1998;98:711‐718. [DOI] [PubMed] [Google Scholar]
- 10. Cushing DJ, Brown GL, Sabouni MH, Mustafa SJ. Adenosine receptor‐mediated coronary artery relaxation and cyclic nucleotide production. Am J Physiol. 1991;261:H343‐H348. [DOI] [PubMed] [Google Scholar]
- 11. Guieu R, Kipson N, Ruf J, et al. Low basal expression of A2A adenosine receptors and increase in adenosine plasma concentration are associated with positive exercise stress testing. Int J Cardiol. 2015;180:15‐17. [DOI] [PubMed] [Google Scholar]
- 12. Ruf J, Paganelli F, Bonello L, et al. Spare adenosine A2a receptors are associated with positive exercise stress test in coronary artery disease. Mol Med. 2016;22:530‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paganelli F, Resseguier N, Marlinge M, et al. Specific pharmacological profile of A2A adenosine receptor predicts reduced fractional flow reserve in patients with suspected coronary artery disease. J Am Heart Assoc. 2018;7:e008290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bruzzese L, Fenouillet E, Fromonot J, et al. High homocysteine levels prevent via H2S the CoCl2‐induced alteration of lymphocyte viability. J Cell Mol Med. 2016;20:1411‐1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gariboldi V, Vairo D, Guieu R, et al. Expressions of adenosine A2A receptors in coronary arteries and peripheral blood mononuclear cells are correlated in coronary artery disease patients. Int J Cardiol. 2017;230:427‐431. [DOI] [PubMed] [Google Scholar]
- 16. Pironti G, Strachan RT, Abraham D, et al. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation. 2015;131:2120‐2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gallart‐Palau X, Serra A, Wong AS, et al. Extracellular vesicles are rapidly purified from human plasma by protein organic solvent precipitation (PROSPR). Sci Rep. 2015;30(5):14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. By Y, Durand‐Gorde JM, Condo J, et al. Production of an agonist‐like monoclonal antibody to the human A2A receptor of adenosine for clinical use. Mol Immunol. 2009;46:400‐405. [DOI] [PubMed] [Google Scholar]
- 19. Foley GE, Lazarus H, Farber S, Uzman BG, Boone BA, McCarthy RE. Continuous culture of human lymphoblasts from peripheral blood of a child with acute leukemia. Cancer. 1965;18:522‐529. [DOI] [PubMed] [Google Scholar]
- 20. Andreu Z, Yáñez‐Mó M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503‐533. [DOI] [PubMed] [Google Scholar]
- 22. Skieterska K, Rondou P, Van Craenenbroeck K. Regulation of G protein‐coupled receptors by ubiquitination. Int J Mol Sci. 2017;18:E923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hicke L. Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol. 2001;2:195‐201. [DOI] [PubMed] [Google Scholar]
- 24. Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461‐466. [DOI] [PubMed] [Google Scholar]
- 25. Clayton A, Al‐Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187:676‐683. [DOI] [PubMed] [Google Scholar]
- 26. Kovalska M, Kovalska L, Tothova B, Mahmood S, Adamkov M, Lehotsky J. Combination of hyperhomocysteinemia and ischemic tolerance in experimental model of global ischemia in rats. J Physiol Pharmacol. 2015;66:887‐897. [PubMed] [Google Scholar]
- 27. Liu LB, Shen HF, Cha W, et al. SXBX pill suppresses homocysteine‐induced vascular smooth muscle cells dedifferentiation by inhibiting NLRP3 inflammasomes activation via ERK/p38 MAPK pathways. Am J Transl Res. 2019;11:806‐818. [PMC free article] [PubMed] [Google Scholar]
- 28. Sorkin A, von Zastrow M. Signalling by downstream kinases affects endocytosis and endosomal trafficking at many points. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buschow SI, Liefhebber JM, Wubbolts R, Stoorvogel W. Exosomes contain ubiquitinated proteins. Blood Cells Mol Dis. 2005;35:398‐403. [DOI] [PubMed] [Google Scholar]
- 30. Burke MC, Oei MS, Edwards NJ, Ostrand‐Rosenberg S, Fenselau CJ. Ubiquitinated proteins in exosomes secreted by myeloid‐derived suppressor cells. Proteome Res. 2014;13:5965‐5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shin JS, Ebersold M, Pypaert M, Delamarre L, Hartley A, Mellman I. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature. 2006;444:115‐118. [DOI] [PubMed] [Google Scholar]
- 32. Milojevic T, Reiterer V, Stefan E, et al. The ubiquitin‐specific protease Usp4 regulates the cell surface level of the A2A receptor. Mol Pharmacol. 2006;69:1083‐1094. [DOI] [PubMed] [Google Scholar]
- 33. Haglund K, Di Fiore PP, Dikic I. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem Sci. 2003;28:598‐603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


