Abstract
Background:
We conducted a meta-analysis to evaluate the efficacy and safety of upfront add-on immunotherapy for advanced non-small cell lung cancers (NSCLC).
Methods:
We performed a literature search on first-line chemotherapy ± immunotherapy in NSCLC. We utilized Revman version 5.3 to calculate the estimated pooled hazard ratio for overall survival (OS) and progression-free survival (PFS) and pooled risk ratio for objective response rate (ORR), all-grade and high-grade adverse events with 95% CI.
Results:
We analyzed 4322 patients. The pooled hazard ratios for OS, PFS and ORR were 0.74 (95% CI: 0.62–0.88; p = 0.0007), 0.62 (95% CI: 0.57–0.68; p = 0.00001) and 1.51 (95% CI: 1.3–1.74; p = 0.00001), respectively. The pooled risk ratios for all-grade and high-grade adverse events were 1.01 (95% CI: 0.99–1.03; p = 0.27) and 1.17 (95% CI: 1.07–1.28; p = 0.0006), respectively.
Conclusion:
Add-on immunotherapy significantly improves PFS, OS and ORR for the first-line treatment of advanced NSCLC with a reasonable safety profile.
Keywords: : advanced non-small-cell lung cancer, checkpoint inhibitors, chemotherapy, first-line therapy, immune-related adverse events, objective response rate, overall survival, progression-free survival, randomized controlled trials, systematic review and meta-analysis
Lay abstract
Lung cancer is the most frequent cancer and is the leading cause of cancer mortality worldwide – more than half of the patients presented at late-stage disease, which is associated with limited survival. To treat cancers, we use immune checkpoint inhibitors that release the brakes on the immune system; thus, the immune cells can kill cancer cells better. Multiple clinical trials have tested the role of immune checkpoint inhibitors combined with chemotherapy for lung cancer treatment. Based on these clinical trials, we conducted a systematic review that showed improvement in outcomes with combined chemotherapy and immunotherapy with acceptable adverse events.
Lung cancer is the most common cancer worldwide, with an estimated incidence of more than 2 million cases and approximately 1.8 million deaths, was also the leading cause of cancer mortality in 2018 [1]. In the USA, it is the second most frequently diagnosed cancer and is currently the leading cause of cancer death in both sexes [2]. There are estimated 228,150 cases of lung and bronchial carcinoma and approximately 142,670 deaths in 2019 in the USA [2]. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancers [3]. The metastatic disease represents approximately 55% of cases and long-term prognosis remains poor [3].
Subsets of patients with driver mutations and gene rearrangements gain significant benefits from molecularly targeted agents; however, the majority of patients without an identified molecular subtype rely mainly on traditional chemotherapy with modest improvement in survival and quality of life. An increasing in the understanding of the complex interactions between the immune system and cancer has led to the development of immune checkpoint inhibitors, namely monoclonal antibodies directed against programmed death receptor 1 (PD-1), programmed death ligand 1 (PD-L1) and cytotoxic T-cell lymphocyte antigen-4 (CTLA-4) monoclonal antibodies, which promote T-cell activation with subsequent formation of anti-tumor effect, resulting in durable response and improvement in outcome. Single-agent immunotherapy (nivolumab, pembrolizumab and atezolizumab) demonstrated superior overall survival (OS) and better safety profiles compared with chemotherapy in the subsequent management of both squamous and nonsquamous NSCLC [4–7]. However, single-agent immunotherapy in the first-line setting is limited to the small subset of NSCLC patients whose tumors have a PD-L1 tumor proportion score of 50% or more ‘without EGFR or ALK genomic tumor aberrations’, for which pembrolizumab is proven to be superior in terms of efficacy and safety profiles [8]. Increasing evidence suggests that combined chemoimmunotherapy can have synergistic anticancer activities through the immunomodulatory effect of checkpoint inhibitors and the immunogenic effect of chemotherapy, such as lowering regulatory T-cell activity and enhancing cross-presentation of tumor antigens [9,10].
Several randomized controlled trials (RCTs) have shown that the addition of immunotherapy to standard chemotherapy improves survival with manageable toxicity profiles. RCTs for the CTLA-4 inhibitor ipilimumab were not included in the analysis owing to separate mechanism of action, lack of OS benefit and different toxicity profiles [11,12]. Therefore, we conducted the meta-analysis of RCTs on PD-1 and PD-L1 inhibitors to evaluate the efficacy and safety of immune checkpoint inhibitors in combination with chemotherapy for the first-line treatment of advanced, metastatic NSCLC.
Methods
We conducted this systematic review according to the Cochrane Handbook for Systematic Reviews [13] and reported per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
Search methods
We conducted literature search in PubMed, EMBASE and SCOPUS databases using the terms ‘immune checkpoint inhibitors and NSCLC’, ‘nivolumab and NSCLC’, ‘pembrolizumab and NSCLC’, ‘atezolizumab and NSCLC’, ‘avelumab and NSCLC’ and ‘durvalumab and NSCLC’. A further search was performed on major oncology conferences throughout January 2019, including those of the American Society of Clinical Oncology, European Society of Medical Oncology and International Association for the Study of Lung Cancer. Clinical trials in English were retrieved and filtered, as mentioned in eligibility criteria.
Inclusion & exclusion criteria
The search results were narrowed to the following article types: clinical trial, Phase II; clinical trial, Phase III; checkpoint inhibitor plus chemotherapy versus chemotherapy plus placebo; studies that showed survival data and studies conducted for first-line treatment of advanced or metastatic NSCLC.
The exclusion criteria were as follows: review articles, systematic reviews, letter to editor and case reports; preclinical trials, Phase I trials, or nonrandomized trials; immune checkpoint inhibitors in second-line setting; immune checkpoint inhibitors in adjuvant or neoadjuvant settings; duplicates of previous publications on the same population and study of CTLA-4 inhibitor ipilimumab due to lack of OS benefit in combination with standard chemotherapy in the first-line setting [11,12].
Data extraction & quality assessment
AM Tun and WL Thein collected basic information of individual study and data were extracted independently. Discrepancies and disagreement were resolved through consensus with the third and the fourth reviewers (KZ Thein. and E Guevara). Extracted data include trial name, a surname of the first author, year of publication, study phase, treatment arms, participant characteristics and the number of patients evaluable for analysis. Analysis of hazard ratio (HR) for OS is the primary outcome of the study. Secondary outcomes were pooled progression-free survival (PFS), pooled overall response rate (ORR) and adverse events (AEs). The tool recommended by Cochrane Collaboration (London, UK) identified biases in each study. Biases were classified as selection bias, performance bias, detection bias, attrition bias, reporting bias and others. They are rated as low, high or unclear risk [14].
Statistical analysis
Review Manager, version 5.3 (Nordic Cochrane Centre; Copenhagen, Denmark) was used for data analyses; p < 0.05 were considered significant and I2 >50% is considered substantially heterogeneous [15]. The random-effect model was applied for all analyses due to heterogeneity among studies. We utilized the inverse variance method to analyze PFS and OS data and reported the outcomes as pooled HRs. Analysis of dichotomous outcomes, such as ORR and AEs, were done by the Mantel–Haenszel method and were reported as risk ratios (RRs) with 95% CIs. Subgroup analyses HRs for PFS and OS were conducted based on the degree of PD-L1 expressions (PD-L1 negative; low-PD-L1 expression: PD-L1 tumor proportion score of 1–49% for pembrolizumab trials or PD-L1 expression on 1–49% of tumor cells (TCs) or 1–9% of tumor-infiltrating immune cells (ICs) for atezolizumab trials; and high-PD-L1 expression: PD-L1 of 50% or greater for pembrolizumab trials or PD-L1 expression of 50% or greater on TCs or 10% or higher ICs in atezolizumab trials). We also performed subgroup analyses of PFS based on age (<65 vs ≥65 years), sex (male vs female) and Eastern Cooperative Oncology Group (ECOG) performance status (0 vs 1), and smoking status (current or former vs never). Publication bias was assessed by funnel plots. We did not perform sensitivity analysis since no study notably influences the results.
Results
Study selection
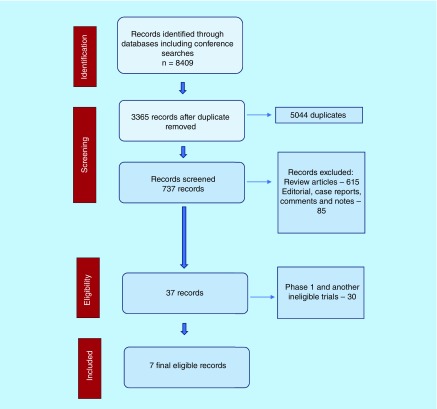
We retrieved 8409 potential references, and 5044 duplicates were removed. After application of exclusion criteria as mentioned above, seven RCTs were reviewed for the final analysis. We excluded Keynote 021 trial, which is a Phase II trial with expansion cohort from Phase I trial [16]. The data from IMpower-131, -132 and Checkmate-227 trials were extracted from conference abstracts and presentations. (Figure 1) We incorporated additional data from IMpower-130 trial in the analysis following its publication in July 2019 [17]. Figure 1 demonstrates study selection in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
Figure 1. . Study flow diagram in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
Study characteristics
The characteristics of the included studies are summarized in Table 1. A total of 4322 patients with advanced NSCLC were included in the meta-analysis. Overall, 2991 patients (69%) had nonsquamous NSCLC, among which 152 patients (5%) had EGFR or ALK alterations. A total of 1331 patients (31%) had squamous NSCLC. The median age of patients ranged from 63 to 65 years; all patients had ECOG performance status score of 0–1 with adequate organ function. All studies were done for the first-line treatment of advanced or metastatic NSCLC utilizing a platinum-based regimen with or without immunotherapy (nivolumab for Checkmate trial, atezolizumab for IMpower trials and pembrolizumab for Keynote trials). Data on the atezolizumab, carboplatin and paclitaxel study arms of IMpower-131 trial (three-arm trial) were not available and not included in the analysis. Another three-arm trial, IMpower-150, had two experimental arms: carboplatin, paclitaxel and atezolizumab (arm A) and carboplatin, paclitaxel, bevacizumab and atezolizumab (arm B) versus carboplatin, paclitaxel and bevacizumab (arm C). We did not include the data comparing between arm A and C because the HR may not reflect the actual effect of add-on immunotherapy (atezolizumab plus chemotherapy vs bevacizumab plus chemotherapy).
Table 1. . Characteristics of included studies (objective response rate; median progression-free survival; median overall survival; programmed death ligand 1; non-small-cell lung cancer).
| Study (year) [Ref.] | Phase | Participants | Patients (n) | Median age (years), study vs control | Intervention | PD-L1 expression | Median follow-up (months) | ORR (%) study arm vs control arm | mPFS (months), study vs control | mOS (months), study vs control |
|---|---|---|---|---|---|---|---|---|---|---|
| Gandhi et al. (2018) [18] Keynote-189 |
III | Metastatic nonsquamous NSCLC without EGFR or ALK mutations | 616 | 65 vs 63.5 | Pemetrexed + platin-based drug + pembrolizumab vs pemetrexed + platinum-based drug | ≥1% | 10.5 | 47.6 vs 18.9 | 8.8 vs 4.9 | Not reached vs 11.3 |
| Paz-Ares et al. (2018) [19] Keynote-407 |
III | Metastatic squamous NSCLC | 559 | 65 | Carboplatin + paclitaxel/nab-paclitaxel + pembrolizumab vs carboplatin + paclitaxel/nab-paclitaxel | Any | 7.8 | 57.9 vs 38.4 | 6.4 vs 4.8 | 15.9 vs 11.3 |
| Cappuzzo et al. (2018) [17,20] Impower-130 |
III | Advanced Nonsquamous NSCLC | 723 (44 patients had EGFR or ALK mutations) | 64 vs 65 | Carboplatin + nabpaclitaxel + atezolizumab vs carboplatin + nabpaclitaxel | Any | NA | 49.5 vs 31.9 | 7 vs 5.5 | 18.6 vs 13.9 |
| Jotte et al. (2018) [21] IMpower-131 |
III | Metastatic squamous NSCLC | 683 | 65 | Carboplatin + nabpaclitaxel + atezolizumab vs carboplatin + nabpaclitaxel | Any | 17.1 | 49 vs 41 | 6.3 vs 5.6 | 14 vs 13.9 |
| Papadimitrakopoulou et al. (2018) [22] IMpower-132 |
III | Advanced Nonsquamous NSCLC | 578 | 64 vs 63 | Pemetrexed + platin-based drug + atezolizumab vs pemetrexed + platinum-based drug | Any | 14.8 | 47 vs 32 | 7.6 vs 5.2 | 18.1 vs 13.6 |
| Socinski et al. (2018) [23,24] IMpower-150 |
III | Metastatic nonsquamous NSCLC | 800 (108 patients had EGFR or ALK alterations) | 63 | Carboplatin + paclitaxel + bevacizumab + atezolizumab vs Carboplatin + paclitaxel + bevacizumab | Any | 15.4 vs 15.5 | 63.5 vs 48 | 8.3 vs 6.8 | 19.2 vs 14.7 |
| Borghaei et al. (2018) [25] Checkmate-227 |
III | Metastatic NSCLC PDL1 <1% | 363 | 64 | Chemotherapy + nivolumab vs chemotherapy | <1% | Not available | 36.7 vs 23.1 | 5.6 vs 4.7 | pending |
NSCLC: Non-small-cell lung cancer; ORR: Objective response rate; PD-L1: Programmed death ligand 1.
Different PD-L1 assay methods were utilized in these studies: 22C3 pharmDx assay (Agilent, CA, USA) in nivolumab and pembrolizumab studies [18,19,26] and SP142 assay (Ventana, Roche, Basel, Switzerland) [23] in atezolizumab studies. Checkmate-227 study comparing platinum-based chemo with or without nivolumab was done on tumors with PD-L1 expression <1% [25].
Study quality, risk bias & publication bias
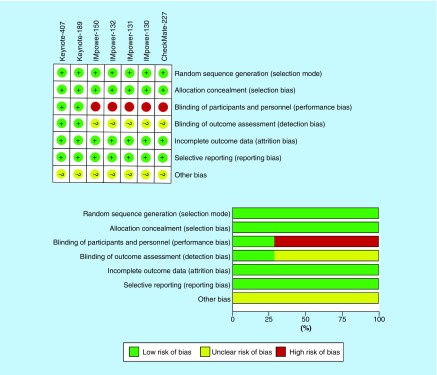
Risk of bias for each study was evaluated by RevMan 5.3 software (Cochrane) and is illustrated in Figure 2. IMpower-130, -131, -132, -150 and Checkmate-227 were open-label studies that lacked blinding between investigators and participants. Detection bias was unclear for IMpower-130,-131,-132,-150, and Checkmate-227 trials due to lack of blinding. Moreover, all the studies are sponsored by pharmaceutical companies so other biases remained uncertain. Publication bias was not identified in this study.
Figure 2. . Risk of bias for selected clinical trials.
Results
Primary outcome
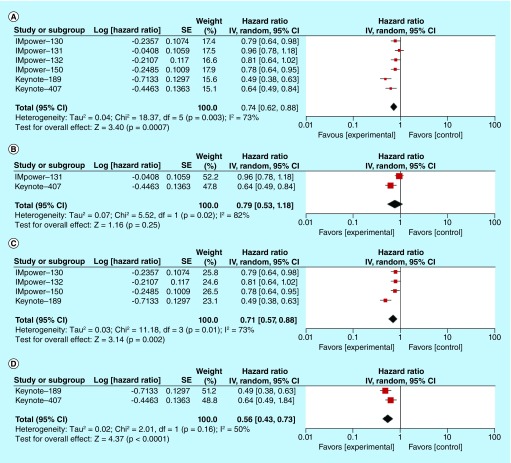
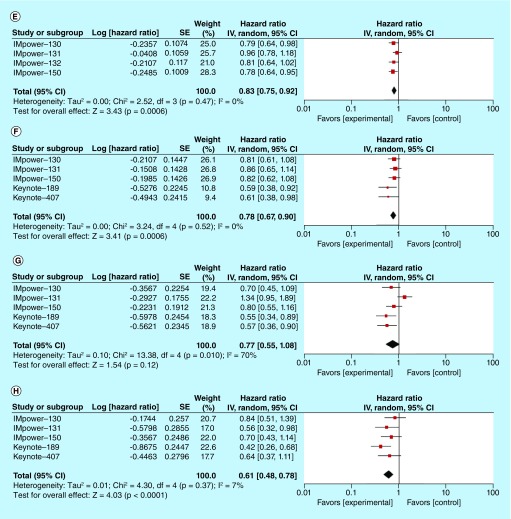
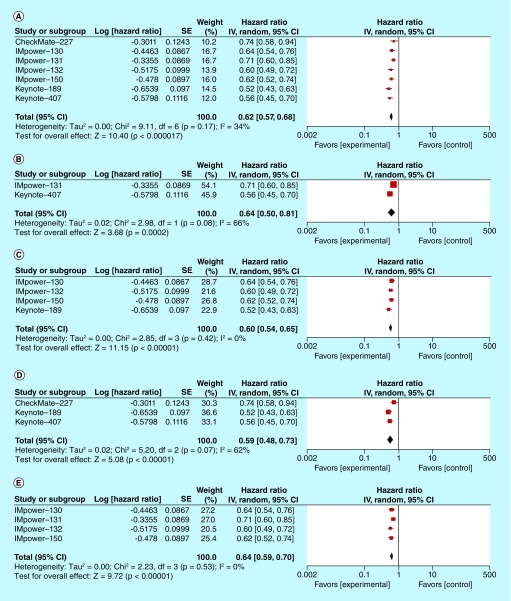
Median OS ranged from 14 months and is not reached in the Keynote-189 trial in the experimental arms, whereas median OS ranges from 11.3 to 14.7 months in the control arms. The pooled HR for OS was 0.74 (95% CI: 0.62–0.88; p = 0.0007, I2 = 73%), and is mentioned in Figure 3A. We performed pooled HR for OS based on histologic subtypes (squamous and nonsquamous) and the type of immunotherapy (PD-1 and PD-L1 monoclonal antibodies). Pooled HRs for OS were 0.79 (95% CI: 0.53–1.18; p = 0.25) for squamous NSCLC (Figure 3B) and 0.71 (95% CI: 0.57–0.88; p = 0.002) for nonsquamous NSCLC (Figure 3C). With regards to different treatment strategies, the pooled HR for PD-1 monoclonal antibody pembrolizumab was 0.56 (95% CI: 0.43–0.73; p = 0001) (Figure 3D) and pooled HR for PD-L1 monoclonal antibody atezolizumab was 0.83 (95% CI 0.75–0.92; p = 0.0006) (Figure 3E). The subgroup analysis was done for different levels of PD-L1 expressions (negative, low and high) and the forest plots for pooled HRs were shown in Figure 3F–H.
Figure 3. . Overall survival analysis in participants treated with first-line chemoimmunotherapy versus chemotherapy alone.
(A) Pooled HR for OS in patients with advanced NSCLC treated with first-line chemoimmunotherapy. (B) Pooled HR for OS in patients with advanced squamous NSCLC treated with first-line chemoimmunotherapy. (C) Pooled HR for OS in patients with advanced nonsquamous NSCLC treated with first-line chemoimmunotherapy. (D) Pooled HR for OS in patients with advanced NSCLC treated with PD-1 inhibitor (pembrolizumab) in combination with chemotherapy in the first-line setting. (E) Pooled HR for OS in patients with advanced NSCLC treated with PD-L1 inhibitor (atezolizumab) in combination with chemotherapy in the first-line setting. (F) Pooled HR for OS in PD-L1 negative patients with advanced NSCLC in the first-line setting. (G) Pooled HR for OS in PD-L1 low patients with advanced NSCLC in the first-line setting. (H) Pooled HR for OS in PD-L1 high patients with advanced NSCLC in the first-line setting.
HR: Hazard ratio; NSCLC: Non-small-cell lung cancer; OS: Overall survival; PD-1: Programmed death receptor 1; PD-L1: Programmed death ligand 1.
Secondary outcomes
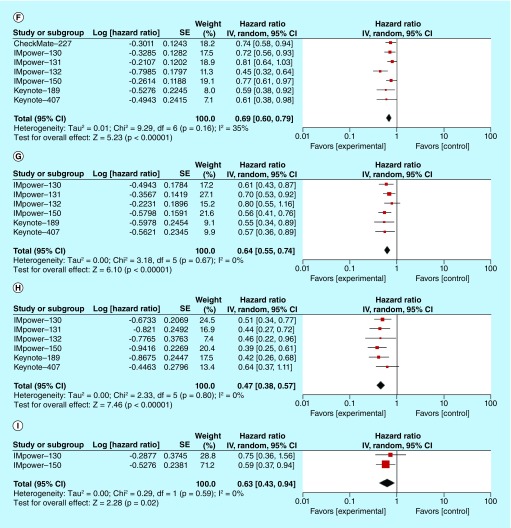
Median PFS ranged from 5.6 to 8.8 months in the study arms, while the control arms ranged from 4.7 to 6.8 months. The pooled HR for PFS was 0.62 (95% CI: 0.57–0.68; p = 0.00001) (Figure 4A). Heterogeneity was present with an I2 value of 34%. In addition, we performed pooled HR for PFS based on histologic subtypes (squamous and nonsquamous) and the type of immunotherapy (PD-1 and PD-L1 monoclonal antibodies). Pooled HR for squamous NSCLC and nonsquamous NSCLC were 0.64 (95% CI: 0.50–0.81; p = 0.0002) and 0.6 (95% CI: 0.54–0.65; p = 0.00001) (Figure 4B & C, respectively). The pooled HR for PD-1 monoclonal antibodies nivolumab and pembrolizumab was 0.59 (95% CI: 0.48–0.73; p = 00001) (Figure 4D) and the pooled HR for PD-L1 monoclonal antibody atezolizumab was 0.64 (95% CI: 0.59–0.70; p = 0.00001) (Figure 4E). Subgroup analyses of HRs for PFS based on the degree of PD-L1 expressions were performed and depicted in the Figure 4F–H. The addition of immune checkpoint inhibitor benefited across different levels of PD-L1 expressions. Moreover, subgroup analyses for PFS based on age, sex, ECOG performance status, smoking history and degree of PD-L1 expression (negative, low or high) were summarized in Table 2. IMpower-130 and -150 trials included patients with EGFR and ALK alterations [17,24]. The pooled HR for PFS in this patient population was 0.63 (95% CI: 0.43–0.94; p = 0.02, I2 = 0%), favoring patients treated with atezolizumab (Figure 4).
Figure 4. . Progression-free survival analysis in participants treated with first-line chemoimmunotherapy versus standard chemotherapy regimen.
(A) Pooled HR for PFS in patients with advanced NSCLC treated with first-line chemoimmunotherapy. (B) Pooled HR for PFS in patients with advanced squamous NSCLC treated with first-line chemoimmunotherapy. (C) Pooled HR for PFS in patients with advanced nonsquamous NSCLC treated with first-line chemoimmunotherapy. (D) Pooled HR for PFS in patients with advanced NSCLC treated with PD-1 inhibitor (nivolumab or pembrolizumab) in combination with chemotherapy in the first-line setting. (E) Pooled HR for PFS in patients with advanced NSCLC treated with PD-L1 inhibitor (atezolizumab) in combination with chemotherapy in the first-line setting. (F) Pooled HR for PFS in PD-L1 negative patients with advanced NSCLC in the first-line setting. (G) Pooled HR for PFS in PD-L1 low patients with advanced NSCLC in the first-line setting. (H) Pooled HR for PFS in PD-L1 high patients with advanced NSCLC in the first-line setting. (I) Pooled HR for PFS in patients with EGFR and ALK mutated advanced NSCLC treated with atezolizumab.
HR: Hazard ratio; NSCLC: Non-small-cell lung cancer; OS: Overall survival; PD-1: Programmed death receptor 1; PD-L1: Programmed death ligand 1; PFS: Progression-free survival.
Table 2. . Subgroup analyses of pooled hazard ratios for progression-free survival.
| Subgroups | Studies (n) | Pooled HR (95% CI) | I2 (%) | p-value |
|---|---|---|---|---|
| Age <65 years | 4 | 0.59 (0.38–0.84) | 66% | 0.0001 |
| Age ≥65 years | 4 | 0.65 (0.56–0.75) | 0% | 0.00001 |
| Male | 4 | 0.69 (0.62–0.78) | 0% | 0.00001 |
| Female | 4 | 0.48 (0.33–0.70) | 70% | 0.0001 |
| ECOG PS 0 | 4 | 0.59 (0.49–0.71) | 0% | 0.0001 |
| ECOG PS 1 | 4 | 0.66 (0.58–0.74) | 0% | 0.00001 |
| Smoking (current or former) | 3 | 0.64 (0.56–0.73) | 19% | 0.00001 |
| Smoking (never) | 3 | 0.55 (0.38–0.81) | 64% | 0.002 |
| PD-L1 <1% (negative) | 7 | 0.69 (0.60–0.79) | 35% | 0.00001 |
| PD-L1 ≥1–49% (low) | 6 | 0.64 (0.55–0.74) | 0% | 0.00001 |
| PD-L1 ≥50% (high) | 6 | 0.47 (0.38–0.57) | 0% | 0.00001 |
ECOG: Eastern Cooperative Oncology Group; HR: Hazard ratio; PD-L1: Programmed death ligand 1.
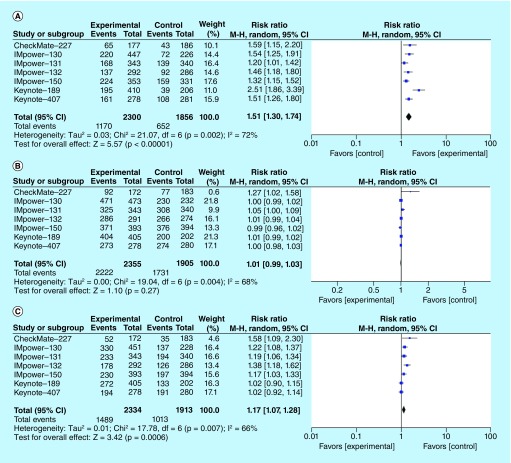
The RR for ORR by random-effect model was 1.51 (95% CI: 1.3–1.74; p = 0.00001, I2 = 72%), benefiting the chemoimmunotherapy group (Figure 5A). Higher rates of high-grade (grade 3 or higher) AEs were noted with the addition of immunotherapy in experimental arms, but no significant difference in rates of all-grade AEs was observed. The pooled RR for all-grade AEs (Figure 5B) and high-grade AEs (Figure 5C) were 1.01 (95% CI: 0.99–1.03; p = 0.27, I2 = 68%) and 1.17 (95% CI: 1.07–1.28; p = 0.0006, I2 = 66%), respectively. Specific immune-related AEs that are associated with statistically significant increased risk, with addition of immune checkpoint inhibitors, include hypothyroidism, hyperthyroidism, pneumonitis, colitis, hepatitis, hypophysitis, infusion reaction and rash. Pooled RRs for specific immune-related AEs are described in Table 3.
Figure 5. . Pooled risk ratio for objective response rate, all-grade adverse events, and high-grade adverse events in patients with NSCLC receiving chemoimmunotherapy versus chemotherapy.
(A) Pooled RR for ORR in patients with advanced NSCLC treated with first-line chemoimmunotherapy. (B) Pooled RR for all-grade AEs in patients with advanced NSCLC treated with first-line chemoimmunotherapy. (C) Pooled RR for high-grade AEs in patients with advanced NSCLC treated with first-line chemoimmunotherapy.
AE: Adverse event; NSCLC: Non-small-cell lung cancer; ORR: Objective response rate; RR: Risk ratio.
Table 3. . Pooled risk ratios for specific immune-related all-grade adverse events.
| Adverse events | Studies (n) | Chemoimmunotherapy | Chemotherapy | Pooled RR (95% CI) | I2 (%) | p-value | ||
|---|---|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||||
| Hypothyroidism | 6 | 226 | 2174 | 35 | 1716 | 5.18 (2.89–9.27) | 51 | 0.00001 |
| Hyperthyroidism | 6 | 92 | 2174 | 18 | 1716 | 3.73 (1.70–8.19) | 50 | 0.001 |
| Colitis | 6 | 41 | 2174 | 7 | 1716 | 3.46 (1.60–7.47) | 0 | 0.002 |
| Pneumonitis | 6 | 117 | 2174 | 30 | 1716 | 2.92 (1.95–4.37) | 0 | 0.00001 |
| Hepatitis | 6 | 135 | 2174 | 50 | 1716 | 2.41 (1.27–4.60) | 52 | 0.007 |
| Diabetes | 4 | 10 | 1605 | 3 | 1162 | 1.77 (0.56–5.59) | 0 | 0.33 |
| Pancreatitis | 4 | 14 | 1562 | 3 | 1102 | 2.35 (0.75–7.41) | 0 | 0.14 |
| Severe skin reaction | 4 | 21 | 1367 | 9 | 1150 | 1.63 (0.68–3.88) | 12 | 0.27 |
| Infusion reaction | 4 | 28 | 1308 | 10 | 1090 | 2.11 (1.02–4.37) | 0 | 0.04 |
| Adrenal insufficiency | 3 | 10 | 1271 | 4 | 828 | 1.12 (0.23–5.39) | 23 | 0.89 |
| Rash | 3 | 258 | 1018 | 149 | 1002 | 1.68 (1.13–2.50) | 78 | 0.01 |
| Hypophysitis | 3 | 9 | 1076 | 0 | 876 | 5.57 (1.01–30.76) | 0 | 0.05 |
| Nephritis | 3 | 12 | 1076 | 2 | 876 | 2.57 (0.62–10.59) | 0 | 0.19 |
| Meningoencephalitis | 2 | 5 | 866 | 0 | 626 | 4.13 (0.50–34.37) | 0 | 0.19 |
| Myositis | 2 | 3 | 798 | 1 | 596 | 1.81 (0.27–12.29) | 0 | 0.55 |
| Thyroiditis | 2 | 4 | 683 | 0 | 482 | 3.45 (0.39–30.27) | 0 | 0.26 |
| Ocular inflammatory toxicity | 1 | 3 | 393 | 0 | 394 | 7.02 (0.36–135.41) | NA | 0.2 |
| Encephalitis | 1 | 1 | 393 | 0 | 394 | 3.01 (0.12–73.60) | NA | 0.5 |
| Autoimmune hemolytic anemia | 1 | 1 | 393 | 1 | 394 | 1 (0.06–15.97) | NA | 1 |
| Vasculitis | 1 | 1 | 393 | 1 | 394 | 1 (0.06–15.97) | NA | 1 |
RR: Risk ratio.
Discussion
Multiple randomized clinical trials have been conducted to identify the optimal chemoimmunotherapy treatment strategy for advanced NSCLC patients. Chen et al. conducted pooled efficacy and safety analyses of immune checkpoint inhibitors in NSCLC patients as the first-line treatment option [27]. The study showed statistically improved PFS, OS but not ORR. It is important to note that the analysis also included studies that compare immune checkpoint inhibitors against chemotherapy. We excluded these studies in our review [27]. The meta-analysis done by Xu et al. showed improvement in PFS but not OS in first-line treatment of NSCLC [28]. The study mainly includes Phase I trials [28]. Analysis by Shen et al. showed improvement in PFS, OS and ORR [29]. A recent meta-analysis by Addeo et al. [30] incorporated additional Phase III studies (IMpower-130, -131, -132 and Checkmate-227 studies) that showed significantly prolonged PFS and OS with the addition of immune checkpoint inhibitor [30]. In addition to PFS and OS analyses of EGFR and ALK wild-type patient population, we analyzed pooled HR for PFS in patients with EGFR and ALK alterations (Figure 4I) that showed statistically significant PFS benefit. However, this benefit is mainly driven by the IMpower-150 trial, which utilized bevacizumab plus chemotherapy as a backbone regimen. We also performed a comprehensive review of safety profiles. Our study met the primary end point of significantly improved OS. The study also revealed statistically significant improvement in PFS and ORR.
Pooled analysis for OS based on histologic subtypes favored both histologic subtypes (both squamous NSCLC and nonsquamous NSCLC) but was not statistically significant for the squamous NSCLC subtype. A longer follow-up of the IMpower-131 trial may change this result. Besides, we identified substantial heterogeneity with I2 of 82% among squamous NSCLC studies with the favorable outcome being driven by the pembrolizumab study (Figure 3B). The pooled HRs for PFS showed statistically significant benefits for both squamous and nonsquamous NSCLC. Moreover, statistically significant OS and PFS benefits were seen on separate analyses of PD-1 and PD-L1 monoclonal antibodies.
Subgroup analyses of both OS and PFS based on the degree of PD-L1 expressions yielded the statistically significant OS and PFS benefits across different levels of PD-L1 expressions, except pooled HR for OS in patients with low PD-L1 expression (HR: 0.77; 95% CI: 0.55–1.08; p = 0.12). We noted substantial heterogeneity in this analysis. The HRs from the Keynote trials contributed more to the survival benefit in low PD-L1 subgroup. In addition, studies utilized different PD-L1 assay methods, further complicating the picture. Nonetheless, subgroup analyses should be interpreted with caution since they are observational by nature and are not based on randomized comparisons. There are significant false positive and false negative findings which could be misleading [13].
Immune checkpoint inhibitors showed relatively tolerable safety profiles. There was no statistically significant increase in rates of all-grade AEs, but a slight increase in high-grade (grade 3 or higher) AEs with the addition of immunotherapy.
There are several limitations to our review. The PD-L1 assay methods are not consistent across different studies; in the atezolizumab studies, PD-L1 immunohistochemistry is read on both TCs and tumor-infiltrating ICs [20,21,23]. However, trials of nivolumab and pembrolizumab applied PD-L1 expression only on TCs [18,19,25]. Moreover, we require longer follow-up and more mature data from the trials, which may change the overall efficacy and safety in the future. At last, our analysis is not designed to identify the optimal combination strategy, but to prove the impact of add-on immunotherapy to chemotherapy in patients with advanced NSCLC.
Conclusion
Overall, our meta-analysis suggests that the addition of immune checkpoint inhibitor to standard chemotherapy benefited OS and PFS, including tumors with EGFR and ALK alterations in first-line, advanced, metastatic NSCLC. The analysis also showed statistically significant improvement in ORR in the overall patient population with the addition of immune checkpoint inhibitor. The combined regimen had a slight increase in high-grade AEs without a statistically significant increase in rates of all-grade AEs.
Future perspective
It is clear that checkpoint blockade, along with cytotoxic chemotherapy, provides additional therapeutic benefit. Despite successful incorporation of PD-1 and PD-L1 inhibitors into the management of metastatic lung cancer, achieving durable remission remains a challenge. Understanding of complex tumor microenvironments and tumor-IC interaction plays a crucial role in the development of novel therapeutic strategies. Combination strategies utilizing multiple immune-checkpoint blockades is an attractive option and have been implemented in the Checkmate-227 trial [26]. Further strategies may include PD-1 blockade in combination with concurrent activation of the immune system, such as vaccination and use of stimulating antibodies. A greater understanding of molecular medicine along with molecular subclassification of this heterogeneous disease and identification of reliable biomarkers to tailor an optimal treatment strategy is crucial in the era of precision oncology. Additional basic science and clinical research will likely help us to understand more about molecular biology, develop newer biomarkers and evolve new therapeutic approaches which will ultimately improve long-term outcome.
Summary points.
Immune checkpoint blockade plus chemotherapy is proven to be effective in randomized controlled trials (RCTs) for the treatment of lung cancer.
We conducted a literature search for RCTs in advanced non-small-cell lung cancer (NSCLC); seven RCTs with a total of 4322 patients were included in the study.
The study population comprises nonsquamous NSCLC, 69% (5% had EGFR or ALK alterations) and squamous NSCLC, 31%.
The analysis showed that the addition of an immune checkpoint inhibitor lead to improvement in overall survival, progression-free survival and objective response rate.
Addition of atezolizumab to standard therapeutic regimen improved progression-free survival in cohorts of patients with EGFR and ALK altered nonsquamous NSCLC.
Subgroup analysis failed to show OS benefit from add-on immunotherapy in patients with squamous NSCLC that expresses low PD-L1.
The study showed slightly higher rates of high-grade (grade 3 or higher) AEs, but no significant difference in rates of all-grade AEs with addition of immune checkpoint inhibitor.
We identified substantial heterogeneity among studies. The programmed death ligand 1 assay methods are not consistent across different studies.
Long-term follow-up is required for mature data.
Increasing understanding of molecular biology and the development of newer biomarkers and novel therapeutic approaches will improve outcome in this patient population.
Footnotes
Author contributions
All authors have full access to the data in the study and take responsibility for the accuracy of the analysis and the integrity of the data. AM Tun and E Guevara did study concept and design. All authors participated in acquisition, analysis, and interpretation of the data.
Note
This meta-analysis was presented as a poster presentation at the Annual meeting of the American Society of Clinical Oncology Immuno-Oncology Symposium in San Francisco in February 2019 (Abstract#117).
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Creative Commons Attribution 4.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68(6), 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 69(1), 7–34 (2019). [DOI] [PubMed] [Google Scholar]
- 3.SEER Cancer Statistics Review, 1975–2010. National Cancer Institute, MD, USA: (2018). https://seer.cancer.gov/csr/1975_2014/ [Google Scholar]
- 4.Rittmeyer A, Barlesi F, Waterkamp D. et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a Phase III, open-label, multicentre randomised controlled trial. Lancet 389(10066), 255–265 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst RS, Baas P, Kim D-W. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027), 1540–1550 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Borghaei H, Paz-Ares L, Horn L. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373(17), 1627–1639 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer J, Reckamp KL, Baas P. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373(2), 123–135 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fülöp A, Lubiniecki GM, Tafreshi A. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375(19), 1823–1833 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Spila A, Roselli M, Guadagni F. et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology 2(10), e27025 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 21(1), 15–25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch TJ, Bondarenko I, Luft A. et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non–small-cell lung cancer: results from a randomized, double-blind, multicenter Phase II study. J. Clin. Oncol. 30(17), 2046–2054 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Govindan R, Szczesna A, Ahn M-J. et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J. Clin. Oncol. 35(30), 3449–3457 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. 0 [updated March 2011]. The Cochrane Collaboration; (2011). http://www.cochrane-handbook.org [Google Scholar]
- 14.Higgins JPT, Altman DG. Assessing risk of bias in included studies. In: Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Higgins JPT, Green S (Eds). Wiley, NJ, USA, 187–241 (2008). [Google Scholar]
- 15.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 327(7414), 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langer CJ, Gadgeel SM, Borghaei H. et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, Phase II cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 17(11), 1497–1508 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West H, McCleod M, Hussein M. et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, Phase III trial. Lancet Oncol. 20(7), 924–937 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Gandhi L, Rodríguez-Abreu D, Gadgeel S. et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N. Engl. J. Med. 378(22), 2078–2092 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Paz-Ares L, Luft A, Vicente D. et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N. Engl. J. Med. 379(21), 2040–2051 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Cappuzzo F, McCleod M, Hussein M. et al. IMpower130: progression-free survival (PFS) and safety analysis from a randomised Phase III study of carboplatin+nab-paclitaxel (CnP) with or withoutatezolizumab (atezo) as first-line (1L) therapy in advanced non-squamous NSCLC. Ann. Oncol. 29(Suppl. 8), mdy424–065 (2018). [Google Scholar]
- 21.Jotte RM, Cappuzzo F, Vynnychenko I. et al. IMpower131: primary PFS and safety analysis of a randomized Phase III study of atezolizumab+carboplatin+paclitaxel or nab-paclitaxel vs carboplatin+nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J. Clin. Oncol. 36(Suppl.18), LBA9000 (2018). [Google Scholar]
- 22.Papadimitrakopoulou V, Cobo M, Bordoni R. et al. IMpower132: PFS and safety results with 1L atezolizumab+ carboplatin/cisplatin+ pemetrexed in stage IV non-squamous NSCLC. J. Thorac. Oncol. 13(Suppl. 10), S332–S333 (2018). [Google Scholar]
- 23.Socinski MA, Jotte RM, Cappuzzo F. et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 378(24), 2288–2301 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Socinski MA, Jotte RM, Cappuzzo F. et al. Overall survival (OS) analysis of IMpower150, a randomized Phase III study of atezolizumab (atezo)+ chemotherapy (chemo) ± bevacizumab (bev) vs chemo+ bev in 1L nonsquamous (NSQ) NSCLC. J. Clin. Oncol. 36(Suppl. 15), 9002 (2018). [Google Scholar]
- 25.Borghaei H, Hellmann MD, Paz-Ares LG. et al. Nivolumab (Nivo) + platinum-doublet chemotherapy (Chemo) vs chemo as first-line (1L) treatment (Tx) for advanced non-small cell lung cancer (NSCLC) with <1% tumor PD-L1 expression: results from CheckMate 227. J. Clin. Oncol. 36(Suppl. 15), 9001 (2018). [Google Scholar]
- 26.Reck M, Geese WJ, Chang H. et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 378(22), 2093–2104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen R, Hou X, Yang L, Zhao D. Comparative efficacy and safety of first-line treatments for advanced non-small cell lung cancer with immune checkpoint inhibitors: a systematic review and meta-analysis. Thorac. Cancer. 10(4), 607–623 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, Huang Z, Zheng L, Fan Y. The efficacy and safety of anti-PD-1/PD-L1 antibodies combined with chemotherapy or CTLA4 antibody as a first-line treatment for advanced lung cancer. Int. J. Cancer 142(11), 2344–2354 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Shen K, Cui J, Wei Y. et al. Effectiveness and safety of PD-1/PD-L1 or CTLA4 inhibitors combined with chemotherapy as a first-line treatment for lung cancer: a meta-analysis. J. Thorac. Dis. 10(12), 6636–6652 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Addeo A, Banna GL, Metro G, Di Maio M. Chemotherapy in combination with immune checkpoint inhibitors for the first-line treatment of patients with advanced non-small cell lung cancer: a systematic review and literature-based meta-analysis. Front. Oncol. 9, 264 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]