Influenza viruses, particularly influenza A viruses, cause a substantial degree of morbidity and mortality worldwide and are a persistent threat to global health. Influenza viruses have 2 distinct mechanisms of antigenic diversity, termed “antigenic drift” and “antigenic shift,” that manifest in differing epidemiological forms, referred to as seasonal and pandemic influenza, respectively [1]. Antigenic drift is a continuous process that results from the accumulation of point mutations in the viral hemagglutinin (HA) and neuraminidase (NA) genes. This process occurs in both influenza A and B viruses and is responsible for seasonal influenza epidemics, as these mutations allow the virus to escape immune protection induced by prior natural exposures and/or vaccinations. Seasonal influenza outbreaks consistently occur each year, yet they typically garner less public attention than an influenza pandemic. However, they arguably have caused as much or more cumulative morbidity and mortality over time than have pandemics. It is estimated that between 291 243 and 645 832 deaths due to seasonal influenza–associated respiratory disease occur worldwide each year [2], with approximately 12 000–56 000 deaths in the United States alone [3]. To illustrate this, the 2017–2018 influenza season in the United States was remarkably severe, with influenza-like illness activity akin to that of the 2009 influenza A(H1N1) pandemic, coupled with the highest rates of seasonal influenza–related hospitalizations seen in recent history [4].
Antigenic shift, on the other hand, is an unpredictable event that occurs when novel influenza A viruses, to which the vast majority of the human population do not have immunity, arise either de novo from an animal source or via recombination between animal and human viruses. When these novel viruses also have the capacity to spread efficiently among humans, an influenza pandemic results. Four influenza pandemics have occurred in the past 100 years, in 1918, 1957, 1968, and 2009 [1]. The most severe of these was the 1918 influenza pandemic, which caused an estimated 50 million–100 million deaths worldwide.
The mainstay of influenza prevention is vaccination. Current influenza vaccines are designed to protect specifically against a single influenza strain resulting in “strain-specific” immunity. Given the strong tendency for influenza virus strains to drift, influenza vaccines must be developed each year against viruses predicted to circulate in the upcoming season, to provide maximal protection for each seasonal outbreak. Historically, seasonal influenza vaccination clearly decreases the number of hospitalizations and deaths due to influenza. However, its effectiveness against medically attended laboratory-confirmed illness in the United States during 2004–2018 has ranged from 10% to 60% [5]. This degree of effectiveness is considerably lower than that of many other licensed vaccines for common infectious diseases, such as measles vaccine, which has an effectiveness of 97% [6]. To illustrate this point, the 2017–2018 seasonal influenza vaccine used in the United States offered mixed degrees of protection, with very low interim effectiveness reported against the predominant viral strain (influenza A[H3N2] virus) in most people, except young children [5]. This degree of seasonal influenza vaccine effectiveness may be due in part to a vaccine manufacturing timeline that requires vaccine virus strains to be selected at least 6 months before the vaccine becomes available to the public. Since the vast majority of influenza vaccine doses are produced by growing the virus in eggs, this amount of time is required to produce enough vaccine doses for widespread deployment. In some years, the circulating influenza virus strains drift significantly within that 6-month time frame from strain selection to completion of production. Once the production process is initiated, it is impractical to begin anew with a different strain. The end result is an antigenic mismatch between the vaccine virus and the circulating virus strain, resulting in low vaccine effectiveness.
Other factors may also influence vaccine effectiveness. In the 2017–2018 influenza season, for example, circulating influenza A(H3N2) viruses appeared to be antigenically well matched to the virus used in the vaccine production process, yet interim reports suggest that the overall vaccine efficacy was only 25% against circulating influenza A(H3N2) viruses [5]. One factor that may have contributed to this low level of effectiveness is the influenza vaccine manufacturing process itself, namely the requirement for growth of the vaccine viruses in eggs [7]. When influenza viruses are grown in eggs, mutations occur that favor the growth of the virus in eggs. Mutations as such may have no substantial impact on the match between circulating virus and vaccine virus. However, if the mutations occur on a part of the influenza virus critical to the induction of a protective immune response, such as key residues on the head of the HA molecule, this may result in an accidental mismatch with circulating strains, again lowering vaccine effectiveness. However, vaccine virus mismatch is unlikely to be the only factor at play. For example, the 2017–2018 influenza vaccine worked much better in young children than in other populations, suggesting that there are key differences in the immune response to influenza vaccines related to age or pre-existing immunity that need to be better characterized.
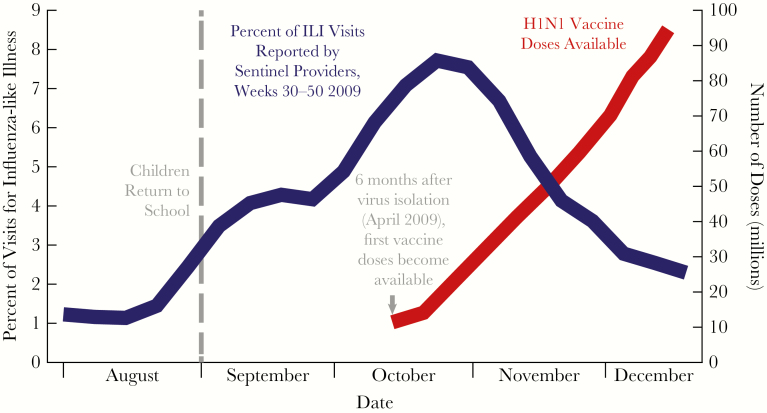
Apart from issues discussed above related to protection against seasonal influenza viruses, strain-specific influenza vaccines are unlikely to provide much, if any, protection in the event of an influenza pandemic. Pandemics are usually completely unexpected, and when they do occur a novel vaccine would need to be created, usually after the pandemic is already underway. Clearly, this is not an effective strategy for pandemic preparedness. To underscore this point, during the 2009 influenza A(H1N1) influenza pandemic, despite awareness in March and April 2009 of the high likelihood that a pandemic would occur in the winter of 2009–2010, which allowed several months for vaccine development and production, an influenza A(H1N1) vaccine was not available to the public until well after the peak of the pandemic [8] (Figure 1).
Figure 1.
Vaccine availability lags behind 2009 H1N1 influenza pandemic
Source: Adapted from CDC, combined data from 1) https://www.cdc.gov/flu/weekly/pastreports.htm, 2) https://www.cdc.gov/h1n1flu/vaccination/vaccinesupply.htm
One approach to mitigate this delay in pandemic response is to develop and stockpile vaccines against animal viruses that have jumped species to infect humans. We call these viruses “prepandemic viruses” since they can infect humans; however, they have not yet evolved into a pandemic virus capable of spreading efficiently from human to human. This process of anticipating a pandemic and preemptively making a vaccine against the prepandemic strain is costly and limited by the same factors that influence seasonal influenza vaccination. For example, the US government previously developed and stockpiled vaccines against prepandemic avian influenza A(H7N9) virus that began causing isolated human infections in China in 2013 with a high mortality of 30%–40%. Since 2013, influenza A(H7N9) viruses have continued to cause isolated human infections without developing the capability of efficiently spreading from human to human. However, the recently (2017) circulating influenza A(H7N9) viruses have mutated to create a mismatch with the vaccine that had been developed against the original 2013 strain [9]. Thus, new vaccines are being developed against the 2017 version of influenza A(H7N9) virus. This has created an untenable situation of having to continually chase these prepandemic viruses at a substantial economic cost. Given the potentially catastrophic nature of such a pandemic, it is essential that we pay attention to and respond to the possibility that these prepandemic viruses might evolve into true pandemic strains. Yet, it is noteworthy that every pandemic that we have experienced over the past 100 years has come as a surprise and was not antedated for years by a recognizable animal prepandemic strain. Thus, it is essential that we develop a better strategy to address the dual threats of seasonal and pandemic influenza.
The ultimate influenza vaccination strategy involves a universal influenza vaccine that would protect against both seasonal and pandemic viruses. We are faced with 2 main challenges when designing such a vaccine: improving production strategies for influenza vaccines of all types and advancing from strain-specific vaccines to universal influenza strain coverage. As discussed above, current influenza vaccines are, for the most part, produced in eggs. This is a time-honored but antiquated and time-consuming process that may allow the virus in the meantime to mutate away from the vaccine virus. Furthermore, the egg-adaptation process undergone by the vaccine virus may in itself lead to mutations that make the vaccine less effective. The vicissitudes of growing influenza vaccine viruses in eggs may be mitigated by using newer manufacturing techniques, such as growing viruses in cells. This may somewhat improve the manufacturing process; however, state of the art vaccine platform technologies such as DNA, messenger RNA, virus-like particles, vector-based vaccines, and self-assembling nanoparticles, will ultimately reflect the future of vaccinology, including that of influenza vaccines.
In addition to improving how we manufacture influenza vaccines, it is critical to pursue innovative strategies to advance from strain-specific vaccines to universal influenza virus strain coverage. Strain-specific vaccines primarily generate an immune response to the head of the HA protein, which mutates easily and differs between influenza virus strains. In this regard, researchers are designing vaccines that generate an immune response to parts of the influenza virus that are less likely to change. They also are working on new ways of displaying these parts of the virus to the immune system to induce a stronger immune response.
Recent advances in the areas of influenza virology, structural biology, immunology, bioinformatics, and vaccinology make addressing these 2 challenges and achieving the goal of a universal influenza vaccine more feasible than ever before. For example, knowledge of the atomic-level structure of HA has led to the design of novel vaccine antigens, such as chimeric HA and stabilized HA stem proteins [10]. This more detailed knowledge of atomic structure has also led to the design of probes that can be used for selection, characterization, and sequencing of single B cells responding to influenza vaccination or infection so that selected antibody lineages can be targeted by future vaccines. A wealth of human monoclonal antibodies, including broadly neutralizing antibodies to conserved epitopes found on the head and stem of HA, have been developed using these approaches [11]. Identification of these conserved epitopes can inform the targeted design of immunogens that can be coupled with new vaccine manufacturing platforms. In this regard, self-assembling nanoparticles can display HA or other viral proteins in immunogenically potent conformations that are more likely to generate a robust immune response [12]. Gene-based approaches, such as those involving messenger RNA [13], may increase the immunogenicity of the vaccine by inducing both antibodies and CD8 T cells, and can be synthetically produced rapidly and deployed, eliminating the slow and costly egg-based production process. Additionally, the development of well-characterized human challenge models provides a potential forum for the rapid testing and comparison of novel vaccine candidates. Cutting across the advancements in influenza virology, immunology, and vaccinology is an increase in publicly available research data and the improved ability to use such data, which lends itself to innovative universal influenza vaccine design in a way that was not possible in the past. Finally, there is a strong desire within the influenza field to better coordinate research efforts toward this shared goal, thus providing a unique opportunity and unprecedented momentum for universal influenza vaccine development.
One or more of these scientific advances may lead, in the short-term, to a much better seasonal influenza vaccine that would protect against the drift of influenza viruses from season to season or allow us to better prepare for an influenza pandemic by creating prepandemic vaccines that are less impacted by point mutations in the prepandemic viruses. However, a truly universal influenza vaccine that would protect against all seasonal and pandemic viruses is likely many years in the future. To achieve that longer-term, more auspicious goal, many fundamental questions about the influenza virus and our immune responses to it will need to be answered. Some of these questions and the ongoing research in these areas will be highlighted in this supplement. In addition, developing a truly universal influenza vaccine is the focus of a new strategic plan for National Institute of Allergy and Infectious Diseases influenza vaccine research [14]. The process almost certainly will be iterative and progressive, with vaccines first against all versions of a single subtype such as influenza A(H3N2) virus, then by vaccines against all subtypes within an entire group (eg, either group 1 or group 2 influenza viruses), and finally by a truly universal vaccine that would protect against all influenza A viruses. In the short term, to borrow a baseball analogy, we aim to have some base hits—singles or doubles—that improve our seasonal influenza vaccines; however, in the long-term, we are committed to the game-changing home run of influenza vaccines—a truly universal vaccine that would provide long-lasting protection against multiple seasonal and pandemic influenza viruses.
Notes
Supplement sponsorship. This work is part of a supplement sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH).
Potential conflicts of interest. Both authors: No reported conflicts of interest. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Paules C, Subbarao K. Influenza. Lancet 2017; 390:697–708. [DOI] [PubMed] [Google Scholar]
- 2. Iuliano AD, Roguski KM, Chang HH, et al. ; Global Seasonal Influenza-associated Mortality Collaborator Network Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018; 391:1285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Disease burden of influenza 2017. https://www.cdc.gov/flu/about/disease/burden.htm Accessed 18 September 2017.
- 4. Weekly U.S. Influenza Surveillance Report; 2017–2018 Influenza Season Week 20 ending May 19, 2018 2018. https://www.cdc.gov/flu/weekly/ Accessed 31 May 2018.
- 5. Seasonal influenza vaccine effectiveness, 2005–2018 2018. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm Accessed 25 May 2018.
- 6. Measles, mumps, and rubella (MMR) vaccination: what everyone should know 2018. https://www.cdc.gov/vaccines/vpd/mmr/public/index.html Accessed 7 June 2018.
- 7. Zost SJ, Parkhouse K, Gumina ME, et al. . Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A 2017; 114:12578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pandemic Influenza-Past Pandemics 2017. https://www.cdc.gov/flu/pandemic-resources/basics/past-pandemics.html Accessed 31 May 2018.
- 9. Iuliano AD, Jang Y, Jones J, et al. . Increase in human infections with avian influenza A(H7N9) virus during the fifth epidemic - China, October 2016-February 2017. MMWR Morb Mortal Wkly Rep 2017; 66:254–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berlanda Scorza F, Tsvetnitsky V, Donnelly JJ. Universal influenza vaccines: Shifting to better vaccines. Vaccine 2016; 34:2926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sano K, Ainai A, Suzuki T, Hasegawa H. The road to a more effective influenza vaccine: Up to date studies and future prospects. Vaccine 2017; 35:5388–95. [DOI] [PubMed] [Google Scholar]
- 12. Kanekiyo M, Wei CJ, Yassine HM, et al. . Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 2013; 499:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bahl K, Senn JJ, Yuzhakov O, et al. . Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol Ther 2017; 25:1316–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erbelding EJ, Post DJ, Stemmy EJ, et al. . A universal influenza vaccine: the strategic plan for the national institute of allergy and infectious diseases. J Infect Dis 2018; 218:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]