Abstract
NAD+ is a pivotal metabolite involved in cellular bioenergetics, genomic stability, mitochondrial homeostasis, adaptive stress responses, and cell survival. Multiple NAD+-dependent enzymes are involved in synaptic plasticity and neuronal stress resistance. Here, we review emerging findings that reveal key roles for NAD+ and related metabolites in the adaptation of neurons to a wide range of physiological stressors and in counteracting processes in neurodegenerative diseases, such as those occurring in Alzheimer’s, Parkinson’s, and Huntington diseases, and amyotrophic lateral sclerosis. Advances in understanding the molecular and cellular mechanisms of NAD+-based neuronal resilience will lead to novel approaches for facilitating healthy brain aging and for the treatment of a range of neurological disorders.
NAD+ Synthesis and Metabolism
NAD+ Synthesis in Cells
Nicotinamide adenine dinucleotide (NAD+) is a fundamental molecule in health and disease, as it is central to several cellular bioenergetic functions. NAD+ is synthesized via three major pathways, including de novo biosynthesis, the Preiss-Handler pathway, and the salvage pathway (Figure 1). While the aspartate pathway is the de novo NAD+ pathway in most photosynthetic eukaryotes, the kynurenine pathway is the only de novo NAD+ synthetic pathway in mammals. The kynurenine pathway starts with the catabolism of the amino acid tryptophan that is converted via two steps to the intermediate kynurenine, which can generate NAD+, kynurenic acid, or xanthurenic acid (Vécsei et al., 2013). The kynurenine pathway modulates neuronal functions as it is involved in the synthesis of two fundamental neuro-transmitters (glutamate and acetylcholine) as well as regulates N-methyl-D-aspartate (NMDA) receptor activity and free radical production (Vécsei et al., 2013). The kynurenine pathway exhibits “double-edged sword” effects on neurons with both neuroprotective metabolites (tryptophan, kynurenic acid, and picolinic acid) and neurotoxic intermediates, including 3-hydroxykynure-nine (3-HK) that generates free radicals, 3-hydroxyanthranilic acid (3-HAA), and quinolinic acid (that induces glutamate receptor excitotoxicity) (Figure 1). While kynurenic acid is an NMDA receptor antagonist, quinolinic acid is an NMDA receptor agonist (Vécsei et al., 2013). The ambient levels of these metabolites are determined by different enzymes, which in the brain are preferentially localized in microglia and astrocytes, suggesting necessary glial cell-neuron communication (Schwarcz and Pellicciari, 2002).
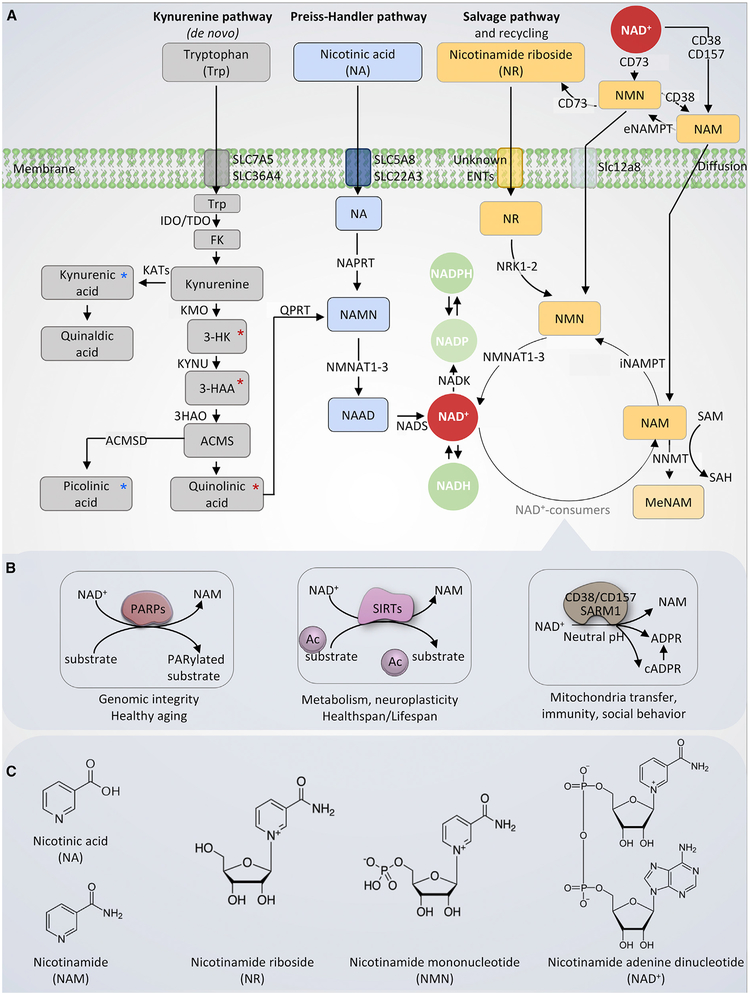
Figure 1. Nicotinamide Adenine Dinucleotide (NAD+) Production and Catabolism in Mammalian Cells.
(A) NAD+ is produced via three major pathways in mammals. The first is the de novo biosynthesis from tryptophan (Trp), also called the kynurenine pathway. Trp enters the cell via the transporters SLC7A5 and SLC36A4. Within the cell, Trp is converted to formylkynurenine (FK), which is further converted to kynurenine. Kynurenine can be converted to kynurenic acid (via kynurenine aminotransferases/KATs) and finally quinaldic acid. Additionally, kynurenine can be converted to 3-hydroxykynurenine (3-HK) by kynurenine 3-monooxygenase (KMO), and further to 3-hydroxyanthranilic acid (3-HAA) by tryptophan 2,3-dioxygenase (KYNU). The next step is performed by 3-hydroxyanthranilic acid oxygenase (3HAO) to produce α-amino-β-carboxymuconate-ϵ-semialdehyde (ACMS). Via a spontaneous reaction, ACMS converts to quinolinic acid, which further formulates to NAMN by quinolinate phosphoribosyltransferase (QPRT), and to nicotinic acid adenine dinucleotide (NAAD), and finally to NAD+. 3-HK, 3-HAA, and quinolinic acid are neurotoxic (denoted with red asterisk), whereas kynurenic acid and picolinic acid are neuroprotective (marked with green asterisk). The second pathway is the Preiss-Handler pathway during which nicotinic acid (NA) is used as an NAD+ precursor. NA enters the cell via SLC5A8 or SLC22A3 transporters. The Preiss-Handler pathway is initiated by the conversion of NA to NAMN by NA phosphoribosyl-transferase (NAPRT). NAMN, an intermediate in both kynurenine pathway and the Preiss-Handler pathway, is converted to form NAAD by NAM mononucleotide transferases (NMNATs). Lastly, NAAD is converted to NAD+ by NAD+ synthase (NADS). The third pathway is the salvage pathway with the cells generating NAD+ from nicotinamide riboside (NR) and recycling nicotinamide (NAM) back to NAD+ via nicotinamide mononucleotide (NMN). Extracellularly, NAD+ or NAM can be converted to NMN, which is in turn dephosphorylated to NR, possibly by CD73. NR is transported into the cell via an unknown nucleoside transport. Intracellularly, NR forms NMN via NRK1 or NRK2 in a tissue-specific manner. NMN is then converted to NAD+ by NMNATs. The enzyme NAM N-methyltransferase (NNMT) methylates NAM, using S-adenosyl methionine (SAM) as a methyl donor. This removes NAM from recycling and indirectly affects NAD+ levels.
(B) The four major NAD+-consuming enzymes. From left: poly(ADP-ribose) polymerases (PARPs), especially PARP1 and PARP2, use NAD+ as a co-substrate to PARylate target proteins, generating NAM as a by-product. The deacetylation activity of sirtuin (SIRT)1, SIRT3, and SIRT6 depends on NAD+, generating NAM as a by-product, with NAM at high cellular levels inhibiting the activity of SIRTs. The NADases or cyclic ADP-ribose synthases (cADPRSs) CD38 and CD157 hydrolyze NAD+ to NAM, generating ADPR and cADPR; in addition, CD38 can degrade NMN to NAM, removing NMN from NAD+ synthesis. Sterile alpha and TIR motif-containing 1 (SARM1) was recently identified as a NADase, which cleaves NAD+ to NAM, cADPR, and ADPR.
(C) The chemical structures of NAD+ and the NAD+ precursors. From left: nicotinic acid (NA), nicotinamide (NAM), nicotinamide riboside (NR), nicotinamide mononucleotide (NMN), and oxidized form of nicotinamide adenine dinucleotide (NAD+).
Note that CD38, CD73, and CD157 are membrane proteins. For simplicity, we did not attach them to the membrane in this schematic model.
The Preiss-Handler pathway and the salvage pathway synthesize NAD+ from pyridine bases. The Preiss-Handler pathway synthesizes NAD+ from nicotinic acid (NA) in three steps via the intermediate nicotinic acid adenine dinucleotide (NAAD). One important step in the Preiss-Handler pathway constitutes the nicotinamide mononucleotide adenylyltransferases (NMNATs), which are also involved in the kynurenine and salvage pathways. Three mammalian NMNATs exist, NMNAT1–3, showing neuroprotective effects in both mice and D. melanogaster models (Ali et al., 2013). While NMNAT1 and NMNAT3 are ubiquitously expressed, NMNAT2 is enriched in the brain, and adequate levels of NMNAT2 seem to be essential for axon development and survival (Gilley et al., 2019). The NAD+ salvage pathway starts from the recycling of nicotinamide (NAM) to nicotinamide mononucleotide (NMN) by intracellular nicotinamide phosphoribosyltransferase (iNAMPT), followed by the conversion of NMN into NAD+ via the NMNATs (Bogan and Brenner, 2008; Verdin, 2015). Additionally, nicotinamide riboside (NR) integrates in this pathway via the conversion of NR into NMN by nicotinamide riboside kinase 1 (NRK1) or NRK2 (Bieganowski and Brenner, 2004; Ratajczak et al., 2016). Despite NAMPT being relatively highly expressed in brown adipocyte, liver, and kidney tissues compared to brain tissue in mice, several studies have supported an essential role of iNAMPT in neuronal NAD+ metabolism (Stein and Imai, 2014; Stein et al., 2014; Zhang et al., 2010). Experimental evidence suggests that blood NA and NAM are able to cross the plasma membrane, while blood NAD+ cannot be taken up by cells directly but needs to be converted to smaller uncharged molecules to enter the cells (Hara et al., 2007; Ratajczak et al., 2016). Extracellularly, NAD+ can be digested to NAM by the membrane-bound CD38 and CD157, with NAM further metabolized into NMN by extracellular NAMPT (eNAMPT); however, NAD+ can also be converted directly into NMN by CD73 (Bogan and Brenner, 2008; Verdin, 2015). Three ways for extracellular NMN to enter the cells have been proposed. First, extracellular NMN converts into NR by CD73, followed by NR being taken up by the cells via a presumptive nucleoside transporter (Fletcher et al., 2017; Grozio et al., 2013; Nikiforov et al., 2011; Ratajczak et al., 2016; Sociali et al., 2016). Second, CD38 may metabolize NMN, but not NR, into NAM, which is able to cross the plasma membrane (Camacho-Pereira et al., 2016; Grozio et al., 2013; Sauve et al., 1998). Third, NMN has been reported to enter cells directly (Grozio et al., 2019; Yoshino et al., 2011). A newly reported NMN transporter, the Slc12a8, is highly expressed and regulated by NAD+, in the murine small intestine, and Slc12a8 deficiency abrogates the uptake of NMN in vitro and in vivo (Grozio et al., 2019). These pathways are detailed in Figure 1. Studies in mice and humans indicate that NR supplementation dramatically upregulates intracellular NAAD, suggesting unknown NAD+ metabolic pathways, including possibilities of NAD+ conversion to NAAD and/or NMN to nicotinic acid mononucleotide (NAMN) (Trammell et al., 2016a). Thus, although the NAD+ metabolic pathways have been intensively characterized for a long time, there are steps remaining to be determined.
NAD+ Has Numerous Functions in Cells
NAD+ is a vital redox cofactor for metabolism and ATP production, and a key substrate for at least four families of enzymes involved in healthspan and longevity (Fang et al., 2017; Gomes et al., 2013; Verdin, 2015). NAD+ plays an essential role in glycolysis and the citric acid (TCA) cycle, by its ability to accept hydride equivalents, forming NADH during ATP production (Krebs, 1970; Wallace, 2012). NADH is one of the central electron donors in oxidative phosphorylation (OXPHOS) in the mitochondria, providing electrons to the electron transport chain (ETC) to generate ATP (Krebs, 1970; Wallace, 2012). The ratio of NAD+/NADH is important in various bioenergetic reactions in different subcellular compartments, and increased activity of one of these reactions can influence the metabolic homeostasis via changes in the NAD+/NADH ratio (Ying, 2008). Additionally, the conversion of NAD+ to NADP+/NADPH is important in the involvement of different cellular functions, including antioxidation and generation of oxidative stress, calcium homeostasis, and cell survival or death (Ying, 2008).
In addition to cellular bioenergetics, NAD+ is also a substrate for different NAD+-consuming proteins, which catabolize NAD+ to NAM. They are class III histone deacetylases sirtuins (SIRTs), poly (ADP-ribose) polymerases (PARPs), ADP ribosyl-cyclases (CD38/CD157), and NADase sterile alpha and TIR motif-containing 1 (SARM1) (Figure 1). In mammals, there are seven SIRTs, which regulate a large number of cellular pathways, including neuronal survival, stem cell rejuvenation, cancer, and healthy longevity (Chalkiadaki and Guarente, 2015). The SIRTs are NAD+-dependent enzymes that regulate a wide spectrum of cellular pathways involved in health and disease (Chalkiadaki and Guarente, 2015; Imai et al., 2000). For example, SIRT1 consumes NAD+ to regulate glycolysis, gluconeogenesis, and mitochondrial homeostasis via the balance between mitochondrial biogenesis and mitophagy and adaptive responses of neurons to exercise and metabolic/excitatory challenges (Bonkowski and Sinclair, 2016; Cheng et al., 2016; Fang, 2019). Furthermore, SIRT1 has been shown to promote neurite outgrowth and axon development, in addition to regulating dendritic arborization, long-term potentiation and learning, and memory (Gao et al., 2010). Among the 17 PARPs, only four of them are capable of adding multiple ADP-ribose units (poly[ADP-ribosyl]ation) or PARylation; they are PARP1, PARP2, PARP5a (tankyrase 1), and PARP5b (tankyrase 2) (Leung, 2017; Rouleau et al., 2010). Experimental evidence supports that PARP1 transfers the first ADP-ribose moiety from NAD+ to lysine, arginine, glutamate, aspartate, and serine residues on an acceptor protein, followed by addition of multiple ADP-ribose units to the preceding ones, thereby forming poly(ADP-ribose) (PAR) chains (Bonfiglio et al., 2017; Daniels et al., 2014). The majority of PARylation is executed by PARP1, which participates in a number of necessary cellular processes, such as DNA repair, DNA/RNA metabolism, and cellular stress response. Additionally, PAR is serving as a signaling molecule and scaffolding element (Fang et al., 2016b; Leung, 2017), e.g., PARP1 is critical in the stabilization of DNA repair forks, with its catalytic activity necessary for multiple DNA repair pathways, such as the repair of single-strand breaks, bulky lesions, and double-strand breaks (DSBs) (Ray Chaudhuri and Nussenzweig, 2017). However, excessive PARP1 activation can trigger cell death, termed parthanatos, through a mechanism in which PAR formation triggers the release of mitochondrial apoptosis-inducing factor (AIF) from the cytosolic side of the mitochondrial outer membrane. AIF is then translocated to the nucleus to activate macrophage migration inhibitory factor (MIF, a nuclease), which finally results in MIF-dependent chromatinolysis and cell death (Wang et al., 2011, 2016; Yu et al., 2002). Notably, excessive PARP1 activation induces ATP depletion through PAR-dependent inhibition of hexokinase activity, resulting in dysfunctional glycolysis, a mechanism likely independent of NAD+ depletion (Andrabi et al., 2014; Fouquerel et al., 2014). In addition to ATP loss, hyperactivation of PARP1-induced NAD+ depletion can induce neuronal loss and accelerated aging (Fang et al., 2016a). In view of such detrimental roles of PARP1 in endogenous and exogenous stress, e.g., glutamate excitotoxicity, ischemia-reperfusion injury, and inflammation-induced (Yu et al., 2002) neuronal death, targeting the regulation of PARP1 activity may provide therapeutic strategies for neurodegenerative diseases.
CD38 catalyzes the synthesis of the Ca2+-responsive messenger cyclic ADP-ribose (cADPR) by use of NAD+ and plays a key role in multiple physiological processes such as immunity, metabolism, inflammation, and even social behaviors (Jin et al., 2007). While CD38 molecules are expressed in both a type II form(i.e., large extracellular C-terminal) and a type III form (with its catalytic domain facing the cytosol) (Liu et al., 2017, 2008), there is an age-dependent increase of CD38, which may contribute to cellular NAD+ depletion and impaired mitochondrial function (Camacho-Pereira et al., 2016). Despite CD38 being a lymphocyte differentiation antigen, it is also expressed in the brain (Mizuguchi et al., 1995), and CD38 knockout mice show significant protection against ischemic brain damage (Long et al., 2017). SARM1 is a newly recognized class of NADase that cleaves NAD+ into NAM, ADPR, and cADPR via its TIR domain. It is expressed in both brain and non-brain tissues, including the liver (Essuman et al., 2017; Pan and An, 2018). SARM1 exhibits both cyclase and glycohydrolase activities, and the estimated Michaelis constant (Km) is 24 μM, which is similar to that of the other known NAD+-consumers (PARP1, 50–97 μM; SIRT1, 94–96 μM; CD38, 15–25 μM) (Cantó et al., 2015). The NADase activity of SARM1 may contribute to its role in axonal degeneration (Essuman et al., 2017) and is therefore a potential target for therapeutic intervention in neurodegenerative diseases. SARM1 also holds a mitochondrial localization signal, but its role in mitochondrial function is not clear.
SIRTS, PARPs, CD38/CD157, and SARM1 compete with each other to consume cellular NAD+; thus, the hyperactivation of one enzyme can impair the activities of other NAD+-dependent enzymes. The interrelationships of these enzymes have been reviewed recently (Bonkowski and Sinclair, 2016; Fang et al., 2017; Verdin, 2015).
NAD+ Subcellular Equilibrium
NAD+ is in constant equilibrium between synthesis, consumption, and recycling in various subcellular compartments, including the cytoplasm, nucleus, mitochondria, and Golgi apparatus. Two major mechanisms are involved in the regulation of subcellular balance of NAD+, including the expression of subcellular-specific NAD+-synthetic enzymes and subcellular transporters for NAD+ and related metabolites. NMNATs convert NMN to NAD+. The three mammalian NMNATs include the nuclear NMNAT1, the cytoplasmic NMNAT2, and the mitochondrial NMNAT3, which is also present in the cytosol (Berger et al., 2005). In neurons, NMNAT2 localizes to Golgi and Rab7-containing late endosomes as well as to both synaptic terminals and axons (Gilley and Coleman, 2010; Mayer et al., 2010). Thus, one can imagine that cells can regulate subcellular NAD+ levels through the control of these subcellular-specific NAD+ synthetic enzymes. Similarly, NAMPT (Figure 1) is primarily localized in the nucleus and cytoplasm, where it regulates subcellular NAD+ levels. The existence of mitochondrial NAMPT is still debated. While a small fraction of NAMPT has been shown to co-purify with mitochondria in liver extracts (Yang et al., 2007), results from mitochondrial purification and immunofluorescence in cell lines suggested an exclusion of NAMPT from the mitochondrial matrix, suggesting the lack of NAM recycling to NAD+ in the mitochondria (Pittelli et al., 2010).
It is still not fully understood whether or how NAD+ and its precursors enter the cell and even less clear how or if they enter the mitochondria. While the existence of mitochondrial NAD+ transporters is well documented in yeast and plants, a mammalian counterpart has yet to be identified. Recent studies have demonstrated that both NMN and NAD+/NADH can be transported into the mitochondria by unknown systems, and that NMN is converted to NAD+ by NMNAT3 within the mitochondrial matrix, whereas NR is likely converted to NMN or NAD+ in the cytoplasm before transport into mitochondria (Davila et al., 2018; Nikiforov et al., 2011). Furthermore, some NAD+ transporters (SLC25A17) localize in peroxisomes, where NAD+ participates in b-oxidation like in mitochondria (Agrimi et al., 2012). Peroxisomes have recently been shown to have a shared mitochondrial and endoplasmic reticulum (ER) origin, further linking the function of mitochondria and peroxisomes (Sugiura et al., 2017). In addition to a role in mitophagy, USP30 was recently demonstrated to be involved in the peroxisome-specific degradation process, pexophagy. Peroxisomes have a half-life of 1.5–2.5 days, though pexophagy can be regulated by the metabolic state of the cells. This suggests a role for NAD+ in pexophagy regulation (Marcassa et al., 2018). Since the NAD+/NADH ratio is important in the regulation of redox and metabolism, cytoplasmic and mitochondrial NAD+/NADH ratios are tightly regulated by the malate-aspartate and the glyceraldehyde 3-phosphate (G3P) shuttles (Verdin, 2015). Furthermore, in the pentose phosphate pathway (PPP), glucose-6-phosphate (G6P) dehydrogenase (G6PD) converts G6P to 6-p-gluconate, when the NAD+-dependent SIRT2 deacetylates and activates G6PD. The PPP pathway converts NADP+ to NADPH and regenerates the antioxidant glutathione (GSH), thus promoting cell survival during oxidative stress (Wu and Sinclair, 2014). A schematic representation of the sub-cellular NAD+ balance is shown in Figure 2. Advances in tracing strategies have allowed for the detection and tracing of NAD+ and NAD+ intermediates in different subcellular compartments of the cell (Cambronne et al., 2016; Cameron et al., 2016; Dölle et al., 2010) and will facilitate further mechanistic explorations of subcellular NAD+ control.
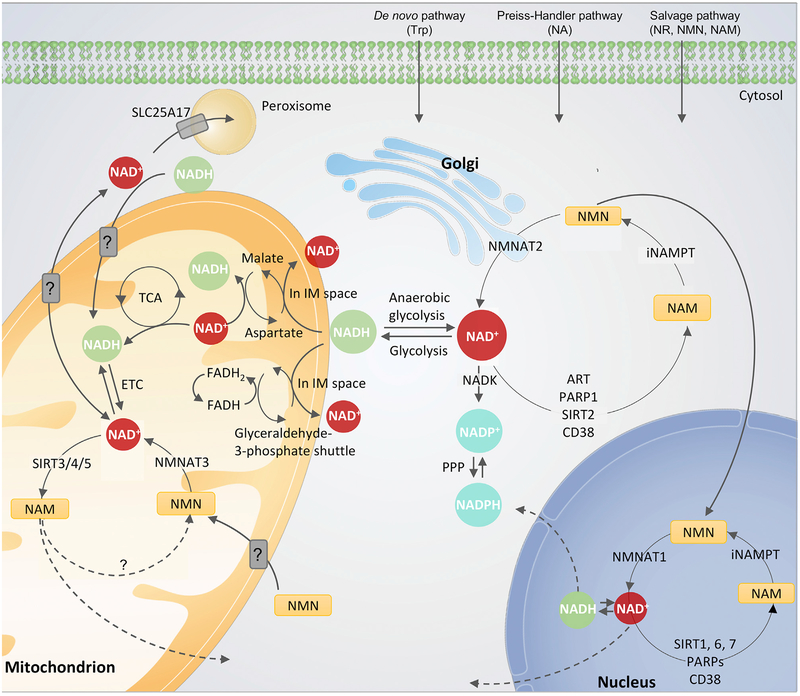
Figure 2. Subcellular Homeostasis of NAD+.
The equilibrium of NAD+ is a balance of synthesis, consumption, and recycling in various subcellular compartments including the cytosol and Golgi, the nucleus, and the mitochondria. The expression of subcellular-specific NAD+-consuming enzymes in addition to the subcellular transporters and redox reactions of NAD+ affect the equilibrium. After cell entrance, NAD+ precursors are metabolized via three major pathways (Figure 1) to NAD+. In the cytosol nicotinamide (NAM) is converted to nicotinamide mononucleotide (NMN) by the intracellular form of NAM phosphoribosyltransferase (iNAMPT). NMN is then converted to NAD+ by NMN transferase 2 (NMNAT2), associated to the outer Golgi membrane in the cytosol. NAD+/NADH is utilized during glycolysis, and NADH is also used by the malateaspartate and the glyceraldehyde-3-phosphate shuttles located in the inner mitochondrial membrane. In the mitochondrial matrix, the malate-aspartate shuttle oxidizes NAD+ to NADH, whereas the glyceraldehyde-3-phosphate shuttle converts flavin adenine dinucleotide (FADH) to FADH2, providing electron donors for the ETC. In the mitochondrion, NMN is converted to NAD+ by NMNAT3. NAD+ is utilized by TCA cycle in the mitochondrion to generate ATP, and additionally used by the NAD+-dependent mitochondrial sirtuin 3–5 (SIRT3–5) generating NAM. It is still not known whether NAM can be converted back to NMN within the mitochondrion or whether it is transported/diffusing out of the mitochondrion to the cytosol before conversion. Additionally, studies have indicated transporters of NAD+, NADH, and NMN in the mitochondrial membrane, but no specific transporters have been identified yet. An NAD+ transporter has been found in peroxisomes, SLC25A17, where NAD+ likely participates in b-oxidation. Within the nucleus, NMN is converted to NAD+ by NMNAT1, and NAD+ is here consumed predominantly by SIRT1, 6, 7, and poly (ADP-ribose) polymerase 1–3 (PARP1–3). Like in the cytosol, NAM is recycled back to NMN by iNAMPT.
NAD+ in Brain Aging
NAD+ in Normal Brain Aging
During normal aging, lower NAD+ levels are observed in tissues of various organisms including humans, mice, and C. elegans. Using a non-invasive 31P magnetic resonance (MR)-based in vivo NAD assay, Zhu et al. demonstrated an age-dependent reduction of NAD+ levels, NAD+/NADH, and total NAD(H) contents in intact human brain from healthy volunteers (Zhu et al., 2015). In mice, there was a nearly 40% decrease of NAD+ levels in the hippocampus in 10- to 12-month-old mice compared with 1-month-old mice (Stein and Imai, 2014). Similarly, an age-dependent reduction of organismal NAD+ in wild-type (WT) worms has been shown (Fang et al., 2014). Comprehensive summaries of reduced tissue NAD+ levels in aging and diseases are available (Fang et al., 2017; Yoshino et al., 2018). In line with the role of NAD+ in maintaining healthy brain function, genetic approaches to reduce brain NAD+ production via CA1-region-specific knockdown of Nampt recapitulate hippocampal cognitive phenotypes of old mice (Johnson et al., 2018). NMN treatment improved cognition in this Nampt-knockdown (CA1) mouse model, partially through upregulation of calcium/calmodulin-dependent serine protein kinase (Cask), a potential downstream effector, in a SIRT1-dependent manner (Johnson et al., 2018). Furthermore, adding extracellular vesicles containing eNAMPT improved cognitive/neuronal function and extended lifespan in WT mice (Yoshida et al., 2019). Possible explanations for reduced cellular NAD+ during aging include increased NAD+ consumption by PARPs and CD38 (Camacho-Pereira et al., 2016; Fang et al., 2014) and reduced NAD+ production, such as that evidenced by reduced eNAMPT in the aged mice and humans (Yoshida et al., 2019). While mounting evidence in animals shows age-dependent NAD+ depletion, similar data on NAD+ changes through normal aging in humans are sparse, and data from large, well-controlled population studies will be required.
NAD+ Depletion in Premature Aging Diseases with Neurodegeneration
NAD+ depletion is observed not only during normal aging, but also in accelerated aging. Several accelerated aging diseases exhibit neurodegeneration, including ataxia telangiectasia (AT), xeroderma pigmentosum group A (XPA), and Cockayne syndrome (CS). NAD+ depletion has been shown in both C. elegans and brain tissues from mouse models of AT, XPA, and CS. In line with the importance of NAD+ depletion in neurodegeneration, multiple NAD+ augmentation strategies (including treatment with the NAD+ precursors NR or NMN, or treatment with a PARP1 inhibitor or a SIRT1 activator) improved healthspan and lifespan in the accelerated aging animal models (Fang et al., 2014, 2016a; Scheibye-Knudsen et al., 2014). Moreover, NAD+ augmentation restores mitochondrial function and bioenergetics, leading to enhanced neuronal survival and improved cognitive function in premature aging animal models. The mechanism underlying the benefits of NAD+ augmentation likely involves reduced levels of DNA damage, leading to decreased PARP1 activity. Additionally, increased SIRT1 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) activities were observed after NAD+ augmentation, which, when combined with enhanced DNA repair, suggest improved nuclear-to-mitochondrial communication (Fang et al., 2016b). These effects of bolstering cellular NAD+ levels suggest a central role for mitochondrial dysfunction and impaired mitophagy in the neurodegenerative features of these premature aging disorders.
NAD+ and the Hallmarks of Brain Aging
During aging, the cellular milieu of the brain exhibits mitochondrial dysfunction, intracellular accumulation of oxidatively damaged macromolecules (DNA, lipids, and proteins), dysregulated energy metabolism, impaired cellular “waste disposal” mechanisms, impaired adaptive stress response signaling, compromised DNA repair, aberrant neuronal network activity, dysregulated neuronal Ca2+ handling, stem cell exhaustion, and inflammation. We refer to these as the 10 hallmarks of brain aging (Mattson and Arumugam, 2018). Two hallmarks of aging in proliferative tissues, including cellular senescence and telomere damage, may also apply in some types of glial cells and neural progenitor cells in the brain, but this remains elusive (Mattson and Arumugam, 2018). Emerging findings are revealing linkages by which age-related NAD+ depletion is positively related to the 10 hallmarks of brain aging. Based on the established linkages between NAD+ depletion and the hallmarks of aging (Fang et al., 2017), the linkage of NAD+ and autophagy/mitophagy, and the emerging evidence of an important role of defective mitophagy in neurodegenerative disorders (Lou et al., 2019), we propose a model of the relationships between NAD+ and the 10 hallmarks of brain aging (Figure 3) (Mattson and Arumugam, 2018).
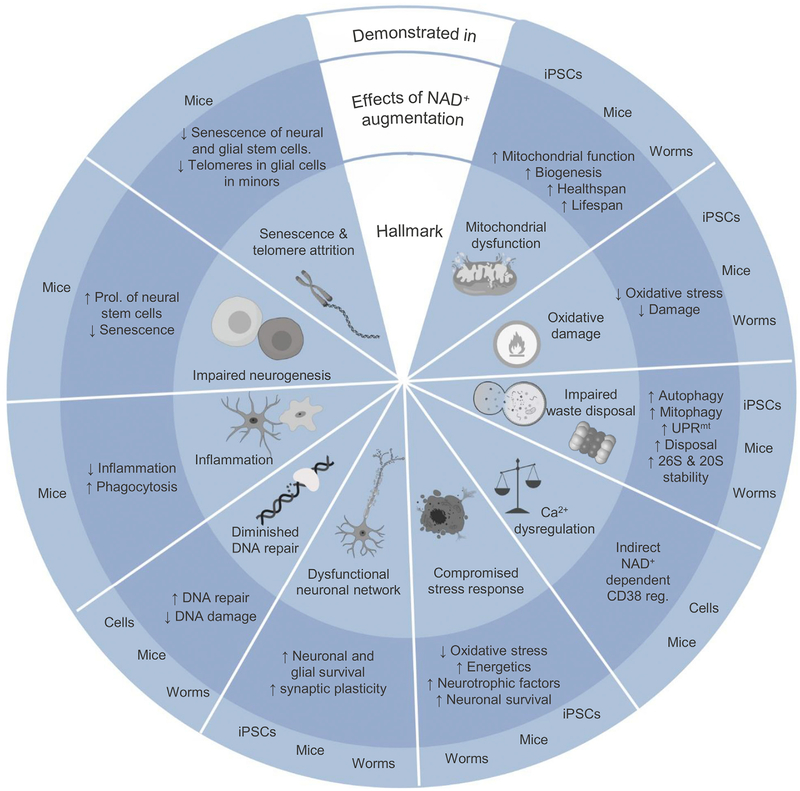
Figure 3. Relationships between NAD+ and the Ten Hallmarks of Brain Aging.
The 10 hallmarks of brain aging include mitochondrial dysfunction; accumulation of oxidative damage; impaired waste disposal including autophagy, mitophagy, and proteostasis; Ca2+ deregulation; compromised adaptive stress responses; dysfunctional neuronal network; impaired DNA repair; inflammation; impaired neurogenesis; and senescence and telomere attrition. Evidence from cell culture, C. elegans, and mouse studies shows that NAD+ augmentation counteracts the adversities of the hallmarks of brain aging. See the text for details. iPSCs, induced pluripotent stem cells; NAD+, nicotinamide adenine dinucleotide; UPRmt, mitochondrial unfolded protein response.
NAD+ Depletion in Mitochondrial Dysfunction.
Mitochondria isolated from animal and post-mortem human brain tissues show an age-dependent rise in mitochondrial functional heterogeneity, increased oxidative damage, reduced function of the ETC, disrupted membrane potential, impaired Ca2+ handling, and/or an accumulation of dysfunctional mitochondria (Lin and Beal, 2006; Mattson et al., 2008; Sorrentino et al., 2017). Mitochondria generate ATP to support neuronal activities including neurotransmission and Ca2+ homeostasis and are a source of signals that regulate nuclear-mitochondrial communication and even the arbitration of neuronal survival and death (Mattson et al., 2008).
NAD+ is central to mitochondrial homeostasis, including in mitochondrial biogenesis, mitophagy, the mitochondrial unfolded protein response (UPRmt), and nuclear-mitochondrial communication (Fang et al., 2014; Gomes et al., 2013; Mouchiroud et al., 2013). In brain tissues from old normal mice and accelerated aging mice, a decline in NAD+ levels contributes to the age-associated decline of mitochondrial biogenesis via impaired SIRT1-PGC-1a signaling (Fang et al., 2014; Mouchiroud et al., 2013; Stein and Imai, 2014). Furthermore, an age-dependent reduction in the cellular ability to clear damaged mitochondria via mitophagy/macro-autophagy has been shown (Fang et al., 2019; Hansen et al., 2018; Palikaras et al., 2015). Reduced NAD+ levels play a pivotal role in brain aging and neurodegenerative disorders because NAD+ replenishment improves mitochondrial function and mitochondrial biogenesis and reduces accumulation of damaged mitochondria in both premature aging models and Alzheimer’s disease (AD) models (Fang et al., 2014, 2016a; Scheibye-Knudsen et al., 2014). Furthermore, NAD+ depletion induces a pseudohypoxic state that disrupts PGC-1α/β-independent nuclear-mitochondrial communication, which contributes to the decline in mitochondrial function with age (Gomes et al., 2013). Thus, NAD+ is critical for the coupling of mitochondrial biogenesis and mitophagy to maintain mitochondrial homeostasis in neurons. As described in the following sections, reduced NAD+ levels contribute to most, if not all, of the hallmarks of brain aging.
NAD+ Depletion and Accumulation of Oxidatively Damaged Molecules.
During aging, neurons accumulate dysfunctional proteins and subcellular organelles as a result of the actions of reactive oxygen species (ROS). This has been exemplified by the observed increase in oxidative damage to DNA, proteins, and lipids in the aging brain (Mattson and Arumugam, 2018). Accordingly, long-lived animals, including the naked mole-rat, rodents maintained on calorie restricted (CR) and intermittent fasting (IF) diets, and long-lived daf-2 mutant worms exhibit decreased levels of oxidative damage and are more resistant to oxidative stress (Fontana and Partridge, 2015; Guarente and Kenyon, 2000; Pérez et al., 2009). Exogenous stress (e.g., UV exposure and γ-radiation and the use of the mitochondrial toxicant paraquat), endogenous DNA damage, and impaired ROS detoxifying pathways are common causes of cellular oxidative damage, while exceeding a threshold may lead to neuronal death. Superoxide produced during mitochondrial electron transport and via the activity of NADPH oxidase are the two major routes of cellular ROS production, and both NAD+/NADH and NADP+/NADPH play key roles in cellular antioxidant systems (Ying, 2008). The aging of rats has been associated with a decrease in NAD+ levels that is correlated with increased lipid and protein oxidation as well as increased DNA damage in the heart, lung, kidney, and liver (Braidy et al., 2011). Moreover, NAD+ augmentation decreases the elevated levels of ROS in cell and animal models of premature aging diseases through reduced ROS production, improved mitochondrial function, and possibly also increased ROS detoxification (Fang et al., 2016a, 2019; Scheibye-Knudsen et al., 2014). However, relationships between NAD+ levels and oxidatively damaged molecules in the human brain remain to be determined.
NAD+ Depletion and Impaired Lysosome and Proteasome Function.
Compared to the dominant replicative cells in the human body, post-mitotic neurons are likely more fragile and susceptible to age-dependent accumulation of damaged or dysfunctional molecules and subcellular organelles due to impaired autophagy or proteasomes. As we age, levels of autophagy and proteasomal degradation gradually decline, leading to proteinopathies, a result of aberrant protein misfolding and aggregation (Menzies et al., 2015; Nixon, 2013). The autophagy-lysosome pathway plays important roles in life and health, such as in longevity, germ-cell lineage, and the rejuvenation of hematopoietic stem cells (HSCs) and neural stem cells (NSCs). The lysosome pathway plays an important role in longevity of C. elegans, where one of the pathways is involved in the lyso-some-to-nucleus signaling pathway. A major protein involved is the lysosomal acid lipase LIPL-4 (orthologue of the human LIPA), which induces nuclear translocation of the lysosomal lipid chaperone LBP-8 and extends worm lifespan; however, whether LIPL-4 has a role in neural function remains to be determined (Folick et al., 2015). In both C. elegans and Xenopus, sperm-secreted hormones re-establish oocyte proteostasis via activation of the vacuolar H+-ATPase (V-ATPase), which acidifies lysosomes (Bohnert and Kenyon, 2017). Furthermore, the autophagy-lysosome pathway maintained stemness of HSCs in mice through elimination of active and healthy mitochondria to maintain quiescence and low metabolism (Ho et al., 2017), an outcome that can be achieved by NAD+ augmentation (Vannini et al., 2019).
In the adult brain, the autophagy-lysosome pathway keeps healthy brain function via the maintenance of the survival and function of mature neurons and keeping a “youthful state” of NSC pool. NSC pools comprise activated and quiescent populations of stem cells, with the former containing active proteasomes and the latter having large lysosomes (Leeman et al., 2018). In quiescent NSCs from old mice, an age-dependent reduction of lysosome activity with accumulated protein aggregates was observed. Genetic (expression of the transcription factor EB/TFEB, a master regulator of autophagy and lysosome biogenesis) or pharmacological (feeding with the mTOR inhibitor rapamycin) enhancement of the lysosome activity in old quiescent NSCs cleared protein aggregates and inhibited the NSCs’ activation to maintain the “youthful state” (Leeman et al., 2018). Impaired autophagy with accumulated proteinopathies are common pathological features in many age-predisposed neurodegenerative diseases, including AD, Parkinson’s disease (PD), Huntington disease (HD), and tauopathies. Importantly, impaired lysosome function and autophagy/mitophagy are common features among them (Kerr et al., 2017; Lou et al., 2019; Menzies et al., 2015; Nixon, 2013). Genetic and pharmacological restorations of autophagic/mitophagic clearance in animal models of these diseases ameliorate disease-defining pathologies and behavioral deficits (Fang et al., 2019; Ravikumar et al., 2004; Sliter et al., 2018). NAD+ levels are reduced in many neurodegenerative diseases, and NAD+ augmentation forestalls pathologies in animal and cell culture models of AD, PD, and HD, at least partially through induction of autophagy/mitophagy (detailed in the next section). Recently, it was shown that knockdown of any of three mitophagy-related genes (pink1, pdr-1, and dct-1) in a C. elegans AD model eliminated benefits of NAD+ augmentation on behavioral deficits, and a similar pivotal role for mitophagy was demonstrated in AD mice and AD patient-derived induced pluripotent stem cells (iPSCs) (Fang et al., 2019). NAD+-dependent proteins involved in stimulation of autophagy/mitophagy include SIRT1, SIRT3, SIRT6, SIRT7, CD38, SARM1, and IL10-dependent upregulation of the expression of autophagic/mitophagic proteins (Fang, 2019).
In addition to the autophagy-lysosome pathway, experimental evidence suggests an important role for the ubiquitin-proteasome system (UPS)-mediated protein degradation in brain function. Some key proteins involved in UPS and memory are 19S/20S/26S proteasomes, small ubiquitin-related modifier I (SUMO-I), ubiquitin-specific protein 14 (USP14), and the proteasome phosphorylation-regulating protein CaMKII (Jarome and Devulapalli, 2018; Jarome et al., 2013, 2016). Similar to age-dependent reduction of autophagy, evidence of impaired UPS in the aging brain arises from studies showing age-dependent accumulation of polyubiquitinated proteins in neurons and a brain region-specific reduction of age-dependent proteasome activity in the hippocampus and cerebral cortex (Keller et al., 2002; Mattson and Arumugam, 2018). Impaired UPS can be caused by the global aging process and by intracellular proteinopathies, such as Ab and tau aggregates (Saez and Vilchez, 2014). Accordingly, enhancement of UPS can improve healthy aging as well as longevity in disease and aged animals, although the specific benefits to neurons and brain function are still elusive (Saez and Vilchez, 2014; Walther et al., 2015). Interestingly, the reduced form of NAD+, NADH, binds and stabilizes 26S proteasomes, and may also regulate the function of 20S proteasomes, the two main proteolytic complexes (Asher et al., 2005; Tsvetkov et al., 2014). Collectively, there is a strong link between impaired autophagy-lysosome and UPS pathways in the aging brain, while detailed linkages between NAD+ levels and such pathways in the aging brain remain to be further studied.
NAD+ Depletion and Dysregulation of Neuronal Calcium Homeostasis.
The calcium ion (Ca2+) regulates neuronal function and structural adaptations of neuronal networks to various environmental challenges. Perturbed Ca2+ signaling is implicated in the aging brain and age-related neurodegenerative diseases. During synaptic activity, the transient Ca2+ influx affects glutamate receptor trafficking, cytoskeletal remodeling, and local protein synthesis through activation of cytosolic kinases, phosphatases, and the major neuronal transcription factors, including PGC-1α and cyclic AMP response element-binding protein (CREB) (Mattson and Arumugam, 2018; Tsien et al., 1988). During aging, the neurons suffer impaired proteostasis and energy production as well as increased oxidative damage, resulting in compromised subcellular Ca2+ handling, which may lead to caspase-regulated apoptosis and PARP1-mediated neuronal death (Bezprozvanny and Mattson, 2008; Mairet-Coello et al., 2013). NAD(P) is a precursor of nicotinic acid adenine dinucleotide phosphate (NAADP), cADPR, and ADPR, which are key regulators of Ca2+ signaling and are synthesized by CD38 and CD157 by the use of NAD+ (Figure 1) (Guse, 2015). NAADP and cADPR stimulate the release of Ca2+ from ER stores, whereas cADPR regulates the entry of Ca2+ from the extracellular space. The NAD+-dependent CD38 plays a fundamental role in cellular Ca2+ homeostasis, social memory, and transfer of mitochondria from astrocytes to neurons after stroke and may protect against amnesia and autism (Adebanjo et al., 1999; Hayakawa et al., 2016; Higashida et al., 2012). Thus, with advancing age, a depletion of NAD+ levels can compromise these neuronal protective functions of CD38.
Additionally, studies in aged mice show that calcium/calmodulin-dependent serine protein kinase (CASK) is a potential downstream effector in response to age-related NAD+ depletion in the hippocampus (Johnson et al., 2018). However, it remains to be determined whether a decline of NAD+ levels is responsible for dysregulation of Ca2+ signaling in neurons during aging.
NAD+ Depletion and Compromised Adaptive Cellular Stress Responses.
Three major initiators of adaptive cellular stress responses in neurons are ATP consumption, ROS production, and Ca2+ signaling. With advancing age, these stress responses might become impaired, which will render the neurons more vulnerable to various forms of stress of either endogenous or exogenous origin (Mattson et al., 2018). This will in turn compromise synaptic function due to reduced expression and activity of neurotrophic factors and decreased mitochondrial function. As previously mentioned, the strong links between NAD+ and mitochondrial function (ATP production), ROS, and Ca2+ signaling suggest that bolstering cellular NAD+ levels can improve adaptive cellular stress responses in neurons. Multiple molecular mechanisms are involved in enhancement of adaptive cellular stress responses by NAD+. Among the different subcellular pools of NAD+, in vitro cell culture and in vivo rodent studies suggest that reduced mitochondrial NAD+ might be more detrimental to cell death than the other subcellular NAD+ pools (Yang et al., 2007). For example, mitochondrial NAD+ levels remain unchanged following genotoxic stress and can maintain cell viability in the conditions of depleted nuclear and cytoplasmic NAD+. In Sprague-Dawley rats, short-term fasting (48 h) can increase tissue mitochondrial NAD+ levels, at least partially through upregulation of the NAD+ biosynthetic enzyme NAMPT, which provides protection against cell death and requires an intact mitochondrial NAD+ salvage pathway as well as the mitochondrial NAD+-dependent proteins SIRT3 and SIRT4 (Yang et al., 2007). In neurons, SIRT3 mediates the adaptive responses of neurons to exercise, intermittent fasting, and excitatory challenges and helps prevent noise-induced hearing loss and spiral ganglia neurite degeneration (Brown et al., 2014; Cheng et al., 2016; Liu et al., 2019). NAD+ also improves adaptive cellular stress responses in neurodegenerative diseases. The neurotrophin brain-derived neurotrophic factor (BDNF) is important in the development, maintenance, and plasticity of the central and peripheral nervous system; it also protects the aging brain against injury and disease through stimulation of glucose transport and mitochondrial biogenesis (Marosi and Mattson, 2014). In different neurodegenerative mouse models including AD, HD, and the premature aging disease A-T, NAD+ augmentation increases the expression and activity of BDNF, leading to enhanced synaptic plasticity and function (Fang et al., 2016a; Hathorn et al., 2011; Liu et al., 2013).
NAD+ Depletion and Impaired Neuronal Network Plasticity.
Human neuronal circuits consist primarily of excitatory glutamatergic neurons that form synapses with other glutamatergic neurons and with inhibitory GABAergic inhibitory interneurons. Structural integrity and proper integration of the synaptic activity of different neurotransmitter systems are both required for normal brain function. During aging, neuronal network activity within and between brain regions, including impaired GABAergic signaling, occurs to various degrees and can be pathological (McQuail et al., 2015). Several of the hallmarks of brain aging, including persistent oxidative stress, accrual of damaged and misfolded proteins and subcellular organelles, and inflammation, can lead to increased vulnerability of neural circuits to hyperexcitability and excitotoxicity. Supplementation with NAD+ precursors has been shown to improve synaptic plasticity and neuronal morphology in AD mouse models (Fang et al., 2019; Hou et al., 2018). Moreover, NAD+ biosynthesis from tryptophan is also related to neurotransmitter synthesis since tryptophan, in addition to being incorporated into proteins, is also converted into the neurotransmitter serotonin. Additionally, a number of the intermediates in the kynurenine pathway are neuroactive and have been related to neurodegenerative diseases, including AD, PD, and HD (Maddison and Giorgini, 2015). In summary, NAD+ plays an important role in the maintenance and structural and functional plasticity of neuronal circuits. While the data from animal studies are encouraging, it remains to be determined whether bolstering cellular NAD+ levels will improve cognition during aging and/or in neurodegenerative disorders in humans.
NAD+ Depletion and Impaired DNA Repair.
Highly replicative NSCs and the lifelong survival and function of differentiated mature neurons require an efficient DNA repair system. Multiple DNA repair pathways exist to ensure genetic fidelity in neurons. While homologous recombination (HR), non-homologous end joining (NHEJ), and mismatch repair (MMR) are active during neurogenesis, NHEJ, base excision repair (BER)/single-strand break repair (SSBR), and nucleotide excision repair (NER) are necessary for DNA repair in differentiated neurons and glial cells (Fang et al., 2016b; McKinnon, 2013). In neurons, BER, the primary pathway for the repair of oxidative DNA damage, is reduced in elderly humans and in AD patients (Hou et al., 2018; McKinnon, 2013). Furthermore, mutations of the genes encoding DNA repair proteins can cause numerous human neurological syndromes. These diseases include ocular motor apraxia (mutation of XRCC1, a gene involved in SSBR), spinocerebellar ataxia with axonal neuropathy-1 (SCAN1, mutation in tyrosyl phosphodiesterase 1/TDP1, a gene involved in SSBR), CS (mutations in CSA and CSB, which are involved in NER), XPA (mutation in XPA, which is involved in NER), and A-T (mutation in ATM, mainly participating in HR and BER) (Fang et al., 2014, 2016a; Hou et al., 2017, 2018; Scheibye-Knudsen et al., 2014). NAD+ plays a critical role in DNA repair through multiple pathways, including the regulation of protein-protein interactions, the activation of the DNA repair signaling PARylation via PARPs, and via the NAD+- SIRT1/SIRT6 pathways (Fang et al., 2016b; Li et al., 2017). NAD+ can regulate protein-protein interactions through binding to the Nudix homology domains (NHDs) of target proteins, including binding to deleted in breast cancer 1 (DBC1). In normal mice, there is an age-dependent increase of tissue DBC1, which binds to and inhibits PARP1, leading to accumulation of DNA damage, a process rapidly reversed by restoring cellular NAD+ levels (Li et al., 2017). Moreover, NAD+ depletion is evident as a phenotypic driver in many of these DNA damage-induced diseases, including at least CS, XPA, and A-T, since restoration of tissue NAD+ through PARPs inhibition ameliorates disease pathologies (Fang et al., 2014). At the molecular level, NAD+ rescues neurodegeneration in CS, XPA, and A-T through mitophagic maintenance of mitochondrial homeostasis and enhanced DNA repair (Fang et al., 2014, 2016b). Impaired autophagy and DNA repair have been suggested as causal and intertwined mechanisms in the pathogenesis of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) (Walker and El-Khamisy, 2018). NAD+ augmentation ameliorates ALS phenotypes, which is a testament to the importance of NAD+ in the protection of DNA-damage-induced neuronal dysfunction (de la Rubia et al., 2019). Collectively, impaired DNA repair byproducts and intermediates accumulate in cells of the aging brain and may contribute to numerous human neurological diseases, at least partially through NAD+ depletion driven by hyperactivation of PARP.
NAD+ Depletion in Inflammation and Glial Cell Activation.
Inflammation is commonly seen in brain aging and involves activated microglia (M2 phenotype), which produce pro-inflammatory cytokines and ROS, including nitric oxide and superoxide (Latta et al., 2015). Complement is one part of the immune system participating in host defense, including the initiating protein of the classical complement cascade, C1q, and the central component of complement C3 (a key inflammatory protein activated in AD), among others (Hong et al., 2016; Morgan, 2018). The complement cascade, a series of protein-protein interactions that attack and damage the cell membrane, can also be activated in brain aging. Inhibition of the complement cascade (pharmacologically or genetically) can ameliorate synapse loss and neuronal death seen during normal aging, in AD and stroke mouse models (Arumugam et al., 2007; Hong et al., 2016; Morgan, 2018; Shi et al., 2015). In AD mouse models, supplementation with the NAD+ precursor NR inhibited the disease-associated neuroinflammation, decreased the numbers of activated microglia and astrocytes, and reduced pro-inflammatory cytokines (Fang et al., 2019; Hou et al., 2018). The effect of NAD+ augmentation on inflammation has also been demonstrated in relation with diabetes, a disease-state predisposed to developing AD (Lee et al., 2015; Trammell et al., 2016b; Yoshino et al., 2011). The underlying molecular mechanism by which NAD+ suppresses neuroinflammation involves the elimination of damaged pro-inflammatory mitochondria and inhibition of the major inflammasome NLRP3 (Fang et al., 2019; Lautrup et al., 2019).
NAD+ and Impaired Neurogenesis.
Adult neurogenesis is the process by which neurons are generated from NSCs in the hippocampus and subventricular zone of the brain. Neurogenesis is critical for the function and plasticity of the hippocampus and olfactory system. Animal studies have shown that neurogenesis declines during aging and this may also occur in humans, although there is evidence that neurogenesis may occur in healthy people up to the ninth decade of life (Kuhn et al., 2018). In contrast, there is a sharp decline in the size and maturation in hippocampal neurogenesis in AD (Moreno-Jiménez et al., 2019). Mounting evidence supports a critical role for NAD+ in stem cell rejuvenation, including in HSCs, endothelial stem cells, muscle stem cells, and melanocyte stem cells (Das et al., 2018; Vannini et al., 2019; Zhang et al., 2016). Similarly, NR treatment of aged mice resulted in increased proliferation and increased neurogenesis in both the subventricular zone and the dentate gyrus of the hippocampus in aged mice (Zhang et al., 2016). NR treatment also improved neurogenesis and proliferation of neural progenitor cells in a BER-defective 3xTg AD mouse model (Hou et al., 2018). Molecular mechanisms underlying NAD+-dependent stem cell rejuvenation may include autophagic/mitophagic elimination of superfluous mitochondria, regulation of UPRmt, inhibition of the senescence phenotype, and the maintenance of the NAD+ and H2S signaling (Das et al., 2018; Fang et al., 2014; Vannini et al., 2019; Zhang et al., 2016)
NAD+ and Cell Senescence and Telomere Shortening.
During aging, most, if not all, tissues exhibit accumulation of senescent cells that exhibit distinct features, including permanent cell-cycle arrest, resistance to apoptosis, and the acquisition of an inflammatory senescence-associated secretory phenotype (SASP). Cellular senescence is a double-edged sword: while on one hand it is necessary for disabling potentially cancerous cells and for wound healing, senescent cells accumulate with age and contribute to aging phenotypes and pathologies, including inflammation and cognitive impairment (Wiley et al., 2016). Emerging evidence suggests a detrimental impact of senescent cells, including p16INK4A-positive senescent astrocytes and microglia, and Aβ plaque-associated Olig2- and NG2-expressing p16INK4A-positive oligodendrocyte progenitor cells (OPCs) in neuronal loss and cognitive impairment in mouse models of AD (Bussian et al., 2018; Zhang et al., 2019). NAD+ augmentation exhibits anti-senescence capabilities. In primary cell culture, supplementation with NR delays cellular senescence (Wiley et al., 2016), and NR treatment also reduces the senescent state in both NSCs and other stem cell types (Zhang et al., 2016). Molecular mechanisms between NAD+ depletion and senescence may also involve CD38 and the AMPK pathway. Studies in endothelial cells and bone marrow-derived macrophages show that the SASP factors released by senescent cells induce both high expression and NADase activity of CD38, leading to further NAD+ depletion, thereby creating a vicious cycle (Chini et al., 2019). Furthermore, the activity of AMPK is low in senescent cells, correlating with low NAD+ levels. By activating AMPK with metformin or berberine, NAD+ levels were restored and oxidative stress-induced senescence was prevented (Han et al., 2016). Collectively, the knowledge so far suggests an important role of reduced NAD+ levels in senescence, though further research is needed to understand the mechanisms upstream and downstream of NAD+ depletion in cell senescence in the aging brain.
In addition to senescence, another hallmark of aging in proliferative tissues is telomere shortening, which may occur in some types of glial cells and neural progenitor cells in the brain (Mattson and Arumugam, 2018). Experimental evidence from human neurons, gray matter glial cells, and white matter glial cells suggests telomeres of neurons remain stable throughout life, and there is no significant correlation between telomere length and adult age (from young adult to elderly) in the three cell types; however, there is likely an age-dependent telomere shortening in white matter glial cells in the adolescents (Tomita et al., 2018). A recent study showed that NMN treatment ameliorated telomere shortening in hepatocytes from successive generation 4 (G4) TERT knockout mice (Amano et al., 2019). Therefore, it would be interesting to explore NAD+ augmentation in telomere length in neuronal stem cells and glial cells in the future.
The hallmarks of brain aging are mechanistically interrelated and converge on impaired cellular energy metabolism (Mattson and Arumugam, 2018). It remains to be determined whether reduced NAD+ level is a pivotal factor in brain aging. Despite the strong links between NAD+ depletion and hallmarks of brain aging, it is very likely that there are NAD+-independent pathways as well. Considerable further studies on animal models will be required to more clearly understand the roles for NAD+ and the various proteins and cellular processes it supports in normal brain aging.
NAD+ Depletion in Different Neurological Disorders with NAD+ Replenishment in Neural Resilience
NAD+ and AD
AD, the most common type of dementia, is characterized by the progressive impairment of memory. There are currently no treatments that affect the progression of AD. In 2015, there were 47 million people with dementia worldwide, a number estimated to triple by 2050, bringing formidable healthcare and socioeconomic challenges (Livingston et al., 2017). The defining neuropathological hallmarks of AD are amyloid β-peptide (Aβ) plaques and neurofibrillary tangles (NFTs). In most cases, AD occurs late in life (7th–9th decades) and does not have an obvious family history of AD. However, people with the epsilon 4 isoform of apolipoprotein E (ApoE4) are at an increased risk for late-onset AD. Rare cases of early-onset dominantly inherited familial AD are caused by mutations in either the Aβ precursor protein (APP) or presenilin 1, the enzymatic component of the γ-secretase protein complex that cleaves APP at the C terminus, which releases the Aβ that self-aggregates and can be neurotoxic. Neurons affected by Aβ and p-Tau neurofibrillary pathology in AD exhibit oxidative damage, impaired Ca2+ handling, defective lysosome function and mitophagy, and reduced DNA repair (Canter et al., 2016; Fang et al., 2019; Mattson et al., 2018; Polanco et al., 2018).
Emerging evidence points to a key role for NAD+ depletion and impairment of NAD+-dependent pathways in AD pathophysiology (Figure 4; Table 1). In rodent models of early-onset familial AD, brain NAD+ depletion and metabolic dysfunction have been demonstrated (Dong and Brewer, 2019; Hou et al., 2018). Exposure of cultured rat cortical neurons to aggregating Aβ results in reduced NAD+ levels (Liu et al., 2013). Accordingly, NAD+ augmentation inhibited AD-related pathology and cognitive decline in different AD animal models, including 3xTgAD mice treated with NAM (Liu et al., 2013), 3xTgAD/Polβ+/− mice treated with NR (Hou et al., 2018), and in neuronal Aβ1–42 and the neuronal Tau (pro-aggregant F3ΔK280 tau fragment) transgenic C. elegans models (Fang et al., 2019) (summarized in Table 1). This provides strong support for the contribution of NAD+ depletion to AD progression. While Aβ, p-Tau, and inflammation are major contributors to AD pathogenesis, NAD+ augmentation can ameliorate cognitive deficits in AD mouse models by suppressing these pathologies. NAD+ augmentation may or may not lessen Aβ pathology depending upon the animal model (Gong et al., 2013; Liu et al., 2013). Reduced Aβ1–42 accumulation in NAM- or NR-treated AD mice could result from reduced Aβ1–42 production through the activation of PGC-1α-mediated BACE1 (β-secretase) degradation and/or increased phagocytosis of Aβ plaques by microglia (Fang et al., 2019; Gong et al., 2013).
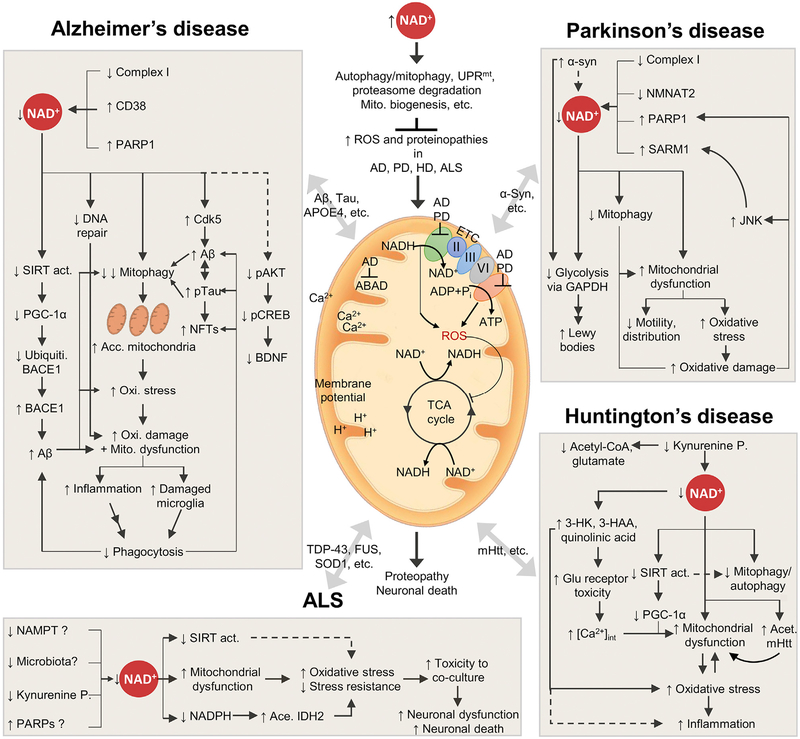
Figure 4. NAD+ Depletion and Impaired Mitophagy Are Pivotal Events in Common Neurodegenerative Diseases.
Based on the evidence summarized in the current review, we propose a hypothesis that may explain, in part, why age is the primary driver of the common neurodegenerative diseases. At a younger age, sufficient cellular NAD+ maintains mitochondrial quality to sustain normal neuronal function via the NAD+-dependent regulations of autophagy/mitophagy, UPRmt, proteasome degradation, and mitochondrial biogenesis. As we age, increased NAD+ consumption drives NAD+ depletion, leading to impaired mitochondrial homeostasis and neuronal function. Depending on the disease-related pathogenesis in the patient, age-dependent NAD+ depletion and impaired mitophagy may exacerbate the disease progression. This hypothesis explains why age makes people susceptible to neurodegenerative diseases, but it is not sufficient. Center: the mitochondrion in the center exhibits the various mitochondrial deficiencies observed in the four neurodegenerative diseases AD, PD, HD, and ALS. These include impairment of Complex I (CI) in the ETC utilizing NADH as an electron donor, creating NAD+. Impairments of both CI and ATP production create reactive oxygen species (ROS), which increase the level of oxidative stress, likely affecting both the ETC itself and the TCA cycle, and increasing the amount of oxidative damage. This can again affect the flux of Ca2+ and the membrane potential, resulting in dysfunctional mitochondria. Furthermore, compromised autophagy and/or mitophagy has been linked to several neurodegenerative diseases, resulting in accumulation of dysfunctional mitochondria. The four panels surrounding the mitochondrion illustrate the suggested explanations for NAD+ depleted and the downstream effects of NAD+ depletion in AD, PD, HD, and ALS. See the text for detailed explanations and references. Kynurenine P, kynurenine pathway.
Table 1.
A Summary of Laboratory Evidence of Linkages between NAD+ Depletion/Supplementation and Neurological Disorders
| Condition | NAD+ Precursor | Effects of Supplementation in Disease Models | Demonstrated in | References |
|---|---|---|---|---|
| Brain Aging | NR | ↑ UPRmt and prohibitins → ↑ proliferation of neural stem cells (NSCs) and muscle stem cells → ↑ neurogenesis → ↑ lifespan | 22- to 24-month-old C57BL/6J (400 mg/kg/day for 6 weeks) | Zhang et al., 2016 |
| NMN | ↑ NAD+ → ↑ SIRT1/SIRT2 → ↑ NSCs/progenitor cells likely via ↑ neurogenesis | 18-month-old C57BL/6 (300 mg/kg/day for 12 months), neurospheres (100 μM) | Stein and Imai, 2014 | |
| AD | NAM | ↑ SIRT1 expression, antioxidant levels and autophagy-lysosome clearance → ↑ oxidative stress resistance → ↑ mitochondrial function/integrity → ↓ Aβ and p-Tau → ↑ neuronal plasticity/cognitive function | 3xTgAD cultured cortical neurons (40 mg/kg/day for 8 months) | Liu et al., 2013 |
| ↑ p25/Cdk5 → ↓ p-Tau231 via degradation of p-Tau231 | 3xTgAD (200 mg/kg/day for 4 months) | Green et al., 2008 | ||
| ↑ acet. A-Tubulin, perhaps via SIRT2 inhibition → ↑ microtubule stability | ||||
| NR | ↑ NAD+ → ↑ SIRT3, SIRT6 and ↓ PARP1 + PAR due to ↓ DNA damage (γH2AX) → ↓ p-Tau, no effect on Aβ → ↑ neurogenesis, ↑ LTP and cognitive function, ↓ neuroinflammation (NLRP3, Caspase 3) | 3xTgAD and 3xTgAD/Polβ+/− (12 mM for 6 months) | Hou et al., 2018 | |
| ↑ NAD+ → ↑ PGC-1α (likely via SIRT1) → ↑ BACE1 degradation → ↓ Aβ → ↑ cognition | Tg2576 (250 mg/kg/day for 3 months), hippocampal slices from Tg2576 (10 μM) | Gong et al., 2013 | ||
| ↑ mitophagy and ↑ UPRmt → ↑ mitochondrial function → ↓ Aβ aggregation → ↑ fitness, memory, and lifespan | Aβ model of C. elegans, 3xTgAD mice, APP/PS1 mice, APP-SH-SY5Y cells (1 or 3 mM for worms and cells, 400 mg/kg/day for 10 weeks) | Sorrentino et al., 2017 | ||
| NMN | ↑ mitophagy → ↑ mitochondrial integrity → ↑ microglial function incl. phagocytosis → ↑ neuronal function | APP/PS1, 3xTgAD, Aβ1–42 and Tau models of C. elegans (5 mM) | Fang et al., 2019 | |
| CD38 KD | ↑ NAD+ → ↓ Aβ → ↑ cognition | APP/PS1 mice | Blacher et al., 2015 | |
| PD | NAM | ↑ mitochondrial function, ↓ oxidative stress → ↓ DNA damage and protein oxidation → ↑ motor function (Drosophila) | MPP+ induced cellular model (101 mg/L), α-syn transgenic Drosophila model (15 or 30 mg/100 g diet). | Jia et al., 2008 |
| ↑ NAD+ → ↑ mitochondrial function → ↑ neuroprotection → ↓ loss of dopaminergic neurons | parkin or pinkl mutant Drosophila (5 mM) | Lehmann et al., 2016, 2017 | ||
| NAD+ | ↑ NAD+ → ↑ sensitivity toward MeHg → ↓ MeHg-induced neuronal damage → ↑ DAergic neuron morophology and behavior | MeHg-treated C. elegans (1 mM) | Caito and Aschner, 2016 | |
| ALS | NR or NMN | in astrocytes: ↑ NAD+ → ↑ NADPH /↑ SIRT1, 3 and/or 6 → ↓ acet. of IDH2 / activation of Nrf2 → ↑ oxidative stress resistance → ↑ mitochondrial function → ↓ toxicity toward cocultured neurons | primary astrocytes from SOD1 mutant mice, astrocytes isolated post-mortem from spinal cord of ALS patients (5 mM) | Harlan etal., 2016, 2019 |
| HD | NAM | NAM was used as a HDAC inhibitor → ↑ acet. of mHtt → ↑ autophagic clearance of mHtt → reversed toxic effects of mHtt | HD C. elegans model (5 mM) | Jeong et al., 2009 |
| NAM was used as an HDAC/SIRT2 (Sir2) inhibitor → inhibition of Rpd3 and Sir2 → ↑ neuroprotection | Htt transgenic Drosophila (2 to 20 mM) | Pallos et al., 2008 | ||
| ↑ PGC-1α and ↑ BDNF → no effect on aggregation phenotyp, ↑ of motor functions | B6.HD6/1 mice (250 mg/kg for 12 weeks) | Hathorn et al., 2011 | ||
| NR | ↑ NAD+ → ↑ act. Of SIRT1/PGC-1α and SIRT3 → ↑ oxidative metabolism → ↑ neuroprotection → ↓ HD-related motor dysfunction and related pathways | STHdhQ111 cells, R6/2 and BACHD mouse models (unpublished data) | Unpublished data; Lloret and Beal, 2019 |
3xTgAD, triple transgenic Alzheimer’s disease mouse model; 3xTgAD/Polβ+/−, 3xTgAD heterozygous in DNA polymerase beta; Aβ, amyloid beta; acet, acetylated; act, activity; AD, Alzheimer’s disease; ALS, amyotrophic lateral sclerosis; APP/PS1, mice containing human transgenes for both APP bearing the Swedish mutation and PSEN1 containing an L166P mutation; BACE1, beta-secretase; BDNF, brain-derived neurotrophic factor; Cdk5, cyclin-dependent kinase 5; Daergic, dopaminergic; HD, Huntington disease; HDAC, histone deacetylase; IDH2, isocitrate dehydrogenase 2; LTP, long-term potentiation; MeHg, methyl mercury; mHtt, mutant huntingtin; NAD+, nicotinamide adenine dinucleotide; NADPH, NAD phosphate; NAM, nicotinamide; NLRP3, NLR family pyrin domain-containing 3; NMN, nicotinamide mononucleotide; NR, nicotinamide riboside; Nrf2, nuclear factor erythroid 2-related factor 2; NSCs, neural progenitor cells; PAR, poly(ADP) ribosylation; PARP1, PAR polymerase 1; PD, Parkinson’s disease; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; p-Tau, phosphorylated Tau; Rpd3, histone deacetylase Rpd3; SIRT, Sirtuin; UPRmt, mitochondrial unfolded protein response; Tg2576, mice expressing the human APP (amyloid precursor protein) gene carrying the Swedish mutation.
p-Tau is a prerequisite for the formation of NFTs, and inhibition of p-Tau by NAD+ augmentation has been demonstrated in different settings. NAD+ augmentation inhibited Tau phosphorylation at different sites (Thr181, Ser202, Thr205, and Thr231) possibly through suppression of cyclin-dependent kinase 5 (Cdk5)-p25 complex activity (Fang et al., 2019; Green et al., 2008). While glycogen synthase 3 (GSK-3) and Cdk5 are thought to be the main tau kinases, p25 is a calcium-dependent degradation product of p35, the principal Cdk5 activator. p25 is important in synaptic plasticity and hippocampus-dependent memory (Fischer et al., 2005). The p25 protein level is reduced by more than 60% in vulnerable brain regions of AD patients at an early symptomatic stage (Engmann et al., 2011). Four months of NAM treatment increased the level of p25 2-fold, reduced p-TauThr231 levels, and improved cognitive function in 3xTgAD mice (Green et al., 2008). Note that p25 upregulation is normally associated with increased Cdk5 activity and hence higher p-Tau levels; however, there was reduced p-Tau in NAM-treated 3xTgAD mice (Green et al., 2008). This paradoxical phenomenon can be explained by the opposing roles of p25: while prolonged p25 production causes severe cognitive deficits, transiently increased p25 expression enhances long-term potentiation (LTP) and memory (Fischer et al., 2005).
The NAD hydrolase CD38 is an ectoenzyme highly expressed in endothelial and inflammatory cells as well as brain cells, including neurons, astrocytes, and microglia (Blacher et al., 2015). In mice, the expression and activity of CD38 increase during aging, and CD38 contributes significantly to age-related NAD+ depletion and mitochondrial dysfunction at least in part via inhibition of NAD+/SIRT3 activity (Camacho-Pereira et al., 2016). Knockdown of CD38 in APPswePS1ΔE9 (APP.PS) mice reduced Aβ plaques and soluble Aβ levels, and increased spatial learning, possibly through upregulation of brain NAD+ levels (Blacher et al., 2015). LTP is a classic measure of synaptic plasticity essential for learning and memory. While LTP is impaired in the 3xTgAD mice, NR restores LTP, correlating with improvements in learning and memory (Hou et al., 2017). In summary, NAD+ augmentation enhances synaptic plasticity and improves memory in different AD mouse models possibly through the reduction of Aβ and p-Tau pathologies and suppression of inflammation (see below).
NAD+ supports several major cellular processes that can protect neurons against Aβ and p-Tau pathologies. First, NAD+ augmentation improves DNA repair in AD mice. It has been proposed that DNA damage is a cause of neuronal loss in AD, with oxidative damage likely being the most significant type of DNA damage (Hou et al., 2017). In a BER-defective 3xTgAD/Polβ+/− mouse model, NR supplementation reduced DNA damage as evidenced by reduced γH2AX staining (marking DNA DSBs) in the hippocampus (Hou et al., 2018). DSBs are lethal to neurons and predominantly repaired via NHEJ (Fang et al., 2016b). Consistent with NHEJ being bolstered by NAD+, NR treatment of ATM-deficient neurons improved neuronal NHEJ activity through deacetylation and activation of the key NHEJ protein Ku70 in addition to facilitating the chromatin binding of Ku70 and DNA-PKcs, an additional NHEJ repair protein (Fang et al., 2016a). Second, NAD+ augmentation inhibits neuroinflammation in AD mice (Hou et al., 2018). The underlying molecular mechanisms could involve NAD+-dependent mitophagy and inhibition of the NLRP3 inflammasome pathway (Fang et al., 2019; Lautrup et al., 2019). As such, a NAD+-dependent programing of immune responses could lead to improved resolution of inflammation and phagocytic activity (Minhas et al., 2019) as well as inhibition of mitochondrial dysfunction-associated senescence (MiDAS) (Wiley et al., 2016). Third, NAD+ augmentation restores neurogenesis in AD mice by promoting the proliferation of neural progenitor cells (Hou et al., 2018). In line with this, studies in large numbers of human brain samples indicate that hippocampal neurogenesis progressively declines as AD advances (Moreno-Jiménez et al., 2019). The stem cell rejuvenating activity of NR/NMN may therefore contribute to amelioration of hippocampus-dependent learning and memory deficits. While the underlying molecular mechanisms of NAD+-dependent neurogenesis in AD are still elusive, mechanistic studies of other tissues show the involvement of the mTORC1/SIRT1 pathway (Igarashi and Guarente, 2016), the regulation of the UPRmt (Zhang et al., 2016), and the stimulation of hematopoiesis through mitophagy (Vannini et al., 2019). Additionally, accumulation of senescent brain cells is an etiopathological feature of the aging brain and the neuropathological lesions in AD brain. Senolytic elimination of Aβ-associated senescent OPCs or Tau pathology-associated senescent glial cells ameliorates cognitive impairment in different AD mouse models (Bussian et al., 2018; Zhang et al., 2019). NR supplementation delayed senescence of adult NSCs in 2-year-old mice (Zhang et al., 2016), suggesting a role for a deficit in NAD+ or one or more of the enzymes it supports in neural-cell senescence.
Mitophagy plays a major role in specific recognition and degradation of damaged mitochondria, with the central participating proteins PTEN-induced kinase 1 (PINK1), Parkin, NIX (BNIP3L), BCL2/adenovirus E1B 19-kDa protein-interacting protein 3 (BNIP3), FUNDC1, and cardiolipin (Kerr et al., 2017; Palikaras et al., 2018). On the basis of evidence from post-mortem brain tissues from AD patients as well as different AD mouse models, damaged mitochondria and increased oxidative stress are common features of AD (Lin and Beal, 2006; Mattson et al., 2008). Additionally, recent studies suggest that there is an accumulation of damaged mitochondria in AD caused by defective mitophagy (Cummins et al., 2019; Fang et al., 2019). It was recently reported that treatment with the NAD+ precursor NMN and mitophagy-inducing chemicals can reduce the accumulation of dysfunctional mitochondria in human AD patient iPSC-derived neurons and in AD mouse and C. elegans models (Fang et al., 2019). This in turn reduces neuroinflammation, lessens the burden of Tau and Ab proteinopathies, and improves learning and memory. Combined with data from previous studies, the mechanisms behind NAD+ augmentation likely include the prevention of Tau and Ab-induced impairment of the initiation of the mitophagic machinery (reduced p-TBK1 and p-ULK1) (Fang et al., 2019) and Tau-based binding and segregation of cytoplasmic Parkin, preventing its translocation to damaged mitochondria (Cummins et al., 2019). Genetic strategies (upregulation of PINK1) and pharmacological approaches (use of robust neuronal mitophagy inducers including NR, NMN, urolithin A, and actinonin) reduce damaged mitochondria, forestall synaptic dysfunction, and bolster learning and memory in several AD animal models (Du et al., 2017; Fang et al., 2019; Sorrentino et al., 2017). NAD+-induced mitophagy likely plays a major role in the observed benefits, as mutation of key mitophagy genes (pink1, parkin/pdr-1, and nix/dct-1) abolishes NAD+- dependent memory improvement in Aβ and p-Tau C. elegans models (Fang et al., 2019).
In addition, NAD+ may alleviate AD pathologies by regulating the functions of lysosomes and UPS. NAD+ augmentation via NR supplementation induced UPS in both the hippocampus and cortex of Tg2576 AD mice (Gong et al., 2013) and restored UPRmt in APPswe/PSEN1dE9 AD mice. Further evidence to support lysosome and UPS functions in maintaining a healthy brain comes from studies showing that the AD toxic proteins Aβ and/or p-Tau inhibit the proteasome and autophagy/mitophagy and dominantly accumulate in the hippocampus and prefrontal cortex, while NAD+ augmentation ameliorates both Ab and p-Tau pathologies (Fang et al., 2019; Tseng et al., 2008).
Despite the recent studies filling in some of the gaps in our understanding of the relations between NAD+, AD, and mitochondrial function, we still lack knowledge of how NAD+ activates the mitophagic machinery, whether the mitophagic machinery is functional but deregulated due to unknown upstream defects, and what side effects mitophagy induction might have. Furthermore, the interconnection between NAD+ and inflammation in AD remains elusive and requires further investigation.
NAD+ and PD
PD is a progressive fatal neurological disorder of aging that profoundly impairs one’s ability to control body movements as the result of loss of dopaminergic neurons in the substantia nigra, which exhibit abnormal accumulation of α-synuclein fibrils in their cell body and neurites (Kam et al., 2018; Spillantini et al., 1997). Alphα-synuclein spreads in a prion-like manner in cultured neurons and in mice in vivo (Li et al., 2008). Interestingly, emerging evidence suggests a gut-to-brain spread of α-synucleinopathy and related loss of dopaminergic neurons and motor and non-motor symptoms. Studies from mice suggest that the neurons initially affected by α-synuclein pathology are those that innervate the gut (enteric neurons) and the pathology then spreads retrogradely via the vagus nerve to the brainstem and hence to the midbrain (Kim et al., 2019; Kishimoto et al., 2019). Despite Lewy bodies with α-synuclein being a defining pathological characteristic of PD, the driver of the production of α-synuclein fibrils and how abnormally aggregated α-synuclein induces the loss of dopaminergic neurons are not fully understood. In addition to aging and environmental factors, early-onset inherited cases of PD are caused by mutations of SNCA, LRRK2, PINK1, and PARK2 (which encode the proteins α-synuclein, leucine rich repeat kinase 2, PINK1, and Parkin, respectively; Kitada et al., 1998; Sliter et al., 2018; Valente et al., 2004), PARK15 (encoding F-box only protein 7, Fbxo7; Cookson, 2010), and GBA-PD (encoding the lysosomal enzyme β-glucocerebrosidase; Sidransky et al., 2009).
Emerging evidence suggests that neurons affected in PD experience a deficit of NAD+ (Table 1; Figure 4). As evidenced, neurons expressing the LRRK2 G2019S mutation exhibit mitochondrial dysfunction and a 30% reduction of NAD+ levels. Interestingly, this mutation seems to specifically affect dopaminergic neurons, since no mitochondrial dysfunction or NAD+ reduction was observed in glutamatergic or sensory neurons expressing the mutation (Schwab et al., 2017; Sison and Ebert, 2018). Mitochondrial impairments in dopaminergic neurons expressing mutant LRRK2 include decreased mitochondrial mass, aberrant subcellular distribution of mitochondria, altered mitochondrial respiration and ATP levels, and impaired mitochondrial motility (Schwab et al., 2017; Sison and Ebert, 2018). Dopaminergic neurons derived from iPSCs expressing mutant GBA exhibit a reduced cytosolic NAD+/NADH ratio, although no significant change of total cellular NAD+ was seen (Schöndorf et al., 2018). The reduction of cytoplasmic NAD+/NADH ratio in the GBA-mutant neurons was possibly due to a reduction of the NAD+ synthetic enzyme NMNAT2, which localizes to the cytosol (Schöndorf et al., 2018). Knockdown of PINK1 expression in primary neurons and neuroblastoma cells causes increased oxidative stress and 50% reduction of NADH levels (Gandhi et al., 2009). Mutant PINK1 also dramatically impairs NAD+ metabolism in flies, including reduced levels of NAD+ (69% reduction), NMN (81% reduction), and NR (11% reduction) (Lehmann et al., 2017). A similar phenomenon was shown in parkin mutant flies, including a 24% reduction of NR, 92% reduction of NMN, and 78% reduction of NAD+, compared with controls (Lehmann et al., 2016). Of note, PINK1 depletion may not be overtly detrimental (e.g., no dramatic inhibition of basal mitophagy), possibly due to the compensatory responses by other PINK1-independent pathways (Lee et al., 2018; McWilliams et al., 2018). Supporting a role of PINK1 in the aging brain, PINK1 deletion in rhesus monkeys resulted in neurodegeneration, but this phenotype was not associated with altered mitochondrial morphology in the neurons, suggesting the existence of a mitophagy-independent role of PINK1 (Yang et al., 2019). However, NAD+ levels were not measured in these studies, and the effect of aging or age-related dysfunctions was not examined. Moreover, Fbxo7 directly binds to PINK1 and Parkin, and participates in Parkinmediated mitophagy (Burchell et al., 2013). Total cellular NAD+ levels were approximately 50% lower in both Fbxo7-deficient SH-SY5Y cells and patient fibroblasts expressing the Fbxo7 R378G mutation (Delgado-Camprubi et al., 2017).
Further evidence supporting that a cellular NAD+ deficit is common to most, if not all, cases of PD comes from a study showing that hyperactivation of the NADase SARM1 may contribute to NAD+ depletion and axonal degeneration. The TIR domain of SARM1 induces axonal degeneration depleting axonal NAD+ (Essuman et al., 2017; Gerdts et al., 2015). In oxidative stress conditions, the c-jun N-terminal kinase (JNK) phosphorylates SARM1 at serine 548, which enhances its NADase activity. Moreover, SARM1 activity, measured as its ability to cleave NAD+, was constitutively increased in neuronal cells from a PD patient, providing a potential explanation of NAD+ depletion in PD (Murata et al., 2018). However, experimental evidence including p-SARM1 (serine 548) in human PD-patient brain or at least in mouse models of PD is required to support a role for this JNK-SARM1 pathway in PD. In addition to hyper-activation of SARM1, the activity of the NAD+-consuming PARPs as well as PAR levels was increased in brains and the cerebrospinal fluid of PD patients (Kam et al., 2018). Mechanistically, pathologic α-synuclein activates PARP-1 and increases PARylation, leading to the formation of more toxic pathologic α-synuclein in a feedforward loop, which finally results in neuronal death via parthanatos (Kam et al., 2018). Supporting an important role of PARP1 in PD, PARP inhibition or NAD+ augmentation forestalled PD pathology in PD animal models (Kam et al., 2018; Lehmann et al., 2016). There is also evidence showing that α-synuclein competes with NAD+ for binding to the partially oxidized glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which co-localizes with α-synuclein in Lewy bodies in PD (Barinova et al., 2018). The immediate consequence of increased α-synuclein binding on GAPDH is the inhibition of glycolysis. The mitochondrial matrix protein TRAP1 (tumor necrosis factor type 1 receptor-associated protein, also known as HSP75) participates in mitophagy when phosphorylated by PINK1. Genetic screenings of PD patients and controls have identified a TRAP1 mutation that leads to complete loss of functional protein in fibroblasts, from a 71-year-old patient with late-onset PD (Fitzgerald et al., 2017). Surprisingly, compared with fibroblasts from a healthy control, this PD-patient’s fibroblasts exhibit increased complex I activity, OCR, and NAD+ levels (Fitzgerald et al., 2017). The involvement of complex I deficiency in PD is well established although it is unclear whether it is an early causal factor in PD or a prominent alteration downstream of primary triggers of mitochondrial dysfunction (Palin et al., 2013). Nevertheless, NAM treatment restored mitochondrial function and reduced oxidative stress in a 1-methyl-4-phenylpyridinium (MPP+; a complex I inhibitor) PD model, and NAM treatment significantly improved climbing ability in a fly model of PD (Jia et al., 2008). Furthermore, NAM supplementation ameliorated PD phenotypes, including loss of dopaminergic neurons, in both pink1 mutant and parkin mutant flies (Lehmann et al., 2016, 2017).
Combined, the data suggest the involvement of NAD+ depletion and mitochondrial/mitophagic defects in PD, though the underlying mechanisms are complex (Poewe et al., 2017) and more studies are needed to understand the involvement of NAD+ insufficiency.
NAD+ and HD
HD is an autosomal-dominant neurodegenerative disease with a clinical presentation of involuntary choreiform movements that result from degeneration of medium spiny neurons in the striatum. As the disease progresses and affects neuronal circuits in the cerebral cortex, HD patients exhibit cognitive impairment and psychiatric symptoms. HD is caused by trinucleotide (CAG) expansions in the Htt gene resulting in polyglutamine repeats in the huntingtin protein (Lloret and Beal, 2019). Mutant huntingtin (mHtt) self-aggregates and forms inclusions in vulnerable neurons. While mitochondrial dysfunction and escalated oxidative stress are the well-defined cellular features of HD, impairment of the kynurenine pathway and consequent reduction of NAD+ production may occur in HD. The under-recognized kynurenine pathway plays important roles in neuronal function and resilience, as it is the primary pathway metabolizing 95% of tryptophan in the entire body. Impairment of the kynurenine pathway occurs in dementias and schizophrenia (Stone and Darlington, 2013). Compared with normal humans, HD patients show reduced level of kynurenic acid and increased levels of 3-HK and quinolinic acid (Beal et al., 1990; Zwilling et al., 2011). The impairment of the kynurenine pathway might contribute to elevated glutamatergic neurotransmission, increased intracellular Ca2+ levels, and impaired mitochondrial function, finally leading to neuronal dysfunction and cell death (Zwilling et al., 2011). Thus, correcting the metabolic imbalance in the kynurenine pathway is a viable therapeutic approach. One possibility is to inhibit kynurenine 3-monooxygenase (KMO) to inhibit the production of the neurotoxic 3-HK, 3-HAA, and quinolinic acid and perhaps shift the metabolic flow toward enhanced production of kynurenic acid and NAD+. Indeed, pharmacological inhibition of KMO prevented synaptic loss and decreased CNS inflammation in both fly and mouse models of HD (Campesan et al., 2011; Zwilling et al., 2011). Likely, there is a similar impaired metabolic profile of the kynurenine pathway in multiple brain regions as suggested by prevention of spatial memory deficits, anxiety-related behavior, and synapse loss when KMO is inhibited (Zwilling et al., 2011).
In addition to the modulation of the kynurenine pathway, direct supplementation with NAD+ precursors as well as an agonistic augmentation of the NAD+/SIRT1-PGC-1α axis and SIRT3 activity show therapeutic potential for HD (Lloret and Beal, 2019). NAM administration (with a histone deacetylase inhibitor trichostatin A) reversed the toxic effects of mHtt in primary striatal and cortical neurons through autophagic clearance of acetylated mHtt (Jeong et al., 2009). In an Httex1p Q93-expressing HD fly model, both NAM and NA inhibited mHtt-induced loss of photo-receptor neurons (Pallos et al., 2008). Although these two studies concluded that the effects of NAM were the result of inhibiting HDACs by over-activating SIRTs, data from these studies did not consider the role of increased NAD+ levels after NAM treatment. Studies from the Beal group showed that NR increases NAD+ levels in both cell cultures and mouse brain tissues, including a 2-fold increase of NAD+ in brain mitochondria (Lloret and Beal, 2019). Intriguingly, NR supplementation ameliorated motor deficits and molecular phenotypes in two HD models (R6/2 and BACHD), possibly through activation of SIRT1-PGC-1a- and SIRT3-related pathways (Lloret and Beal, 2019). Thus, strong evidence for linkage between NAD+ metabolism/catabolism and HD exists, but further detailed mechanistic studies in HD mice as well as in HD patient-derived iPSCs are needed.
NAD+ and ALS
ALS is a group of rare neurological diseases with impairment of voluntary muscle movement caused by the progressive degeneration of motor neurons in the spinal cord, brain stem, and motor cortex. The death of motor neurons leads to muscle weakness and paralysis, causing death of the patients within 1–5 years from the time of symptom onset, typically due to respiratory failure (Tang, 2017). Though the pathology of ALS is complex, mitochondrial dysfunction and increased oxidative stress are common and prominent features of motor neurons (Carrì et al., 2017). Most of the ALS cases are sporadic, whereas 5%–10% are familial. Mutations in superoxide dismutase 1 (SOD1) account for up to 20% of familial cases and 1%–2% of sporadic cases of ALS, whereas other genes mutated include Fused in Sarcoma (FUS) and the C9orf72 non-coding hexanucleotide repeat expansion, which is the most common cause of familial ALS (Tang, 2017). Additionally, transcriptomic profile analyses have reported changes in multiple neurodegeneration-associated genes encoding APP, Tau, PINK1, and Cdk5 in ALS. Familial and sporadic cases are indistinguishable at the clinical and histopathological levels. The function of SOD1 clearly relates to constraint of oxidative stress, although the underlying mechanism of the toxicity of the SOD1 mutations remains unknown. Interestingly, the ALS-related toxicity to motor neurons is believed to arise from an adverse effect on astrocytes resulting in impaired glutamate transport which promotes neuronal excitotoxicity.
ALS has been linked to NAD+ metabolism via impairment of the kynurenine pathway and the potential involvement of altered SIRT1 and SIRT3 activities. Similar to that in HD patients, the kynurenine pathway is also impaired in ALS. In ALS patients, there were higher levels of CSF and serum tryptophan, kynurenine, and quinolinic acid, and decreased serum picolinic acid; furthermore, there was higher microglial inflammation and increased neuronal and microglial expression of indoleamine 2,3-dioxygenase and quinolinic acid in the motor cortex and spinal cord tissues from ALS patients (Chen et al., 2010). Though NAD+ levels were not reported, all the data suggest an inflammation-related excitotoxic-chelation defective mechanism in ALS patients. SIRT1 activity is altered in mouse models of ALS and in postmortem tissue samples from ALS patients; however, the direction of change has been debated (Kim et al., 2007; Körner et al., 2013; Lee et al., 2012; Valle et al., 2014). The discrepancies between the findings on SIRT1 levels in ALS might be due to different cell lines used or altered expression of SIRT1 during disease progression. In addition, SIRT3 expression has been linked to ALS in a PGC-1α-dependent pathway, though the mechanism remains unknown (Buck et al., 2017; Harlan et al., 2016). Interestingly, treatment with SIRT1 activators, including resveratrol, ameliorates the disease progress in ALS animal models (reviewed in Tang, 2017). ALS has also been directly linked to NAD+ metabolism in a study showing that enhancement of the NAD+ salvage pathway protects astrocytes expressing an ALS-linked mutant SOD1 and thereby promotes motor neuron survival (Harlan et al., 2016). Interestingly, overexpression of NAMPT, the protein converting NAM to NMN, or overexpression of a mitochondria-targeted NAMPT results in increased levels of NAD+, including the mitochondrial NAD+ fraction. NAD+ precursor treatment (NMN or NR) or NAMPT overexpression results in increased oxidative stress resistance in motor neurons and prevents the toxicity of co-cultured SOD1-mutant astrocytes. SIRT1, SIRT3, and/or SIRT6 are likely activated as a result of improved NAD+ salvage, leading to decreased acetylation of IDH2 and activation of Nrf2, thereby improving the resistance of motor neurons to oxidative stress and the mitochondrial function (Harlan et al., 2016, 2019). Consistent with a protective role of NAD+ and its metabolites in ALS, a recent study reported lower NAM levels in the ALS-prone SOD1-Tg mice due to impairment of the gut microbiome via diminution of the protective bacterium Akkermansia muciniphila (Blacher et al., 2019). Importantly, the NAM level was decreased in both serum and CSF samples from ALS patients (n = 60 for serum and 14 for CSF) compared with healthy controls (n = 33 for serum and 17 for CSF) (Blacher et al., 2019). Accordingly, NAM supplementation dramatically improved both behavioral and pathological phenotypes in the ALS-prone SOD1-Tg mice (Blacher et al., 2019). The importance of NAD+ metabolism and SIRT1 activation has been supported by a recent preliminary clinical trial with EH301, consisting of an NAD+ precursor and a SIRT1 activator (de la Rubia et al., 2019).
The involvement of cellular NAD+ depletion and the potential therapeutic benefits of treatments that bolster NAD+ levels include, but are not limited to, AD, PD, HD, and ALS. Alterations in the kynurenine system have been expanded to cerebral ischemia, multiple sclerosis, epilepsy, neuropathic pain, and migraine and have been reviewed elsewhere (Vécsei et al., 2013).
NAD+ Restoration as a Therapeutic Strategy
Clinical Trials
Multiple clinical trials of NAD+ precursors, including NAM, NR, and NMN, in neurological disorders are in progress (Table 2). NR is orally bioavailable, and it is well tolerated and elevates NAD+ in healthy middle-aged and older adults as well as in old diabetic males. The Brenner group reported oral bioavailability of NR and up to 2.7-fold increased NAD+ levels and 45-fold increase in the intermediate NAAD in human blood after oral NR supplementation (1,000 mg/day for 7 days) (Trammell et al., 2016a). A late clinical trial of NR in combination with the polyphenol pterostilbene (PT) (NRPT or EH301: 500 mg NR + 100 mg PT for 8 weeks, n = 40 between the ages of 60 and 80 years) led by the Guarente lab showed that NRPT treatment increased whole blood NAD+ levels by 90% with no serious adverse events (Dellinger et al., 2017). Martens and colleagues performed a clinical trial of 1,000 mg NR per day for 6 weeks in 24 healthy adults (average age 65 ± 7), and the data confirmed that NR was bioavailable and safe, with potential cardiovascular benefits (Martens et al., 2018). A recent study with NR on diabetic men also showed that NR was safe even at 2,000 mg/day (twice a day) for 12 weeks (Dollerup et al., 2018). Currently, studies on NR in relation to mitochondrial function, bioenergetics, aging (and premature aging), and obesity are conducted or recruiting participants and results from these clinical trials will likely reveal the potential of NR in humans. Thus, at least four independent clinical trials indicate NR supplementation (1–2 g/day for up to 3 months) is orally bioavailable and relatively safe.
Table 2.
A Summary of Clinical Trials with NAD+ Precursors: Focusing on Cognitive Function and Neurodegenerative Diseases
| Disease | NAD+ Precursor | Dose and Treatment Duration | Main Endpoints | Status and Results | References/NCT |
|---|---|---|---|---|---|
| AD and MCI | NAM | 1,500 mg twice daily, orally for 48 weeks | effect on P-tau231 and total tau in CSF | recruiting, no results | |
| 1,500 mg twice daily for 6 months | Alzheimer’s Disease Assessment Scale-Cognitive Subscale (cognitive function) | completed, no effect reported | ; Phelan et al., 2017 | ||
| NR | dose escalation, 250–1,000 mg for 10 weeks | change in cognitive accessments, cerebral blood flow, plasma NAD levels, and physical performances in MCI patients | recruiting, no results | ||
| 500 mg twice daily for 12 weeks | memory, brian blood flow, and cognitive function | recruiting, no results | |||
| NADH | 10 mg/day for 8–12 weeks (i.v.) | cognitive score (MMSE) | completed, no effect on cognitive score | Rainer et al., 2000 | |
| cognitive score (MMSE) | completed, improved cognitive score | Birkmayer, 1996 | |||
| PD | NR | 1,000 mg/day for 52 weeks | MDS-UPDRS, levels of NAD metabolites in blood | not yet recruiting, no results | |
| 500 mg/day for 30 days | changes in PD related pattern, neurometabolic profile, and motor function | recruiting, no results | |||
| NA or NAM | 100 mg twice daily for 18 months | unified PD rating scale changes, cognitive function (MMSE), sleep, niacin changes, and inflammatory markers in CSF | not yet recruiting, no results | ||
| NADH | 25–50 mg/day, i.v. or i.m. | changes in PD-related disabilities, including movements | completed, benefits on PD-related disabilities like movement | Birkmayer et al., 1989, 1993 | |
| 25 mg/day/4 days i.v., after 2–4 weeks 25 mg/day/4 days i.m. | unified PD rating scale changes including disabilities and movements | completed, no effects of treatment | Dizdar et al., 1994 | ||
| ALS | EH301 | not published | ALS-FRSr Functional Rating Scale, MRC grading scale index, FVC, muscle activity, fat, and muscle weights | completed, improvements of at least 1 out of 3 clinical measures in all patients compared to placebo | ; de la Rubia etal., 2019 |
| A-T | NR | 25 mg/kg/day for 4 months | ataxia, dysarthria, quality of life, laboratory parameters, intelligibility, and fatigue status | enrolling by invitation, no results | |
| F-A | NAM | 4 g/day or highest tolerated dose (min. 2 g/day) for 1 year | ataxia, quality of life, progression of cerebellar severity, and safety issues | not yet recruiting, no results | |
| dose-escalation, 2–8 g for 9–12 months | level of frataxin, ataxia, and other clinical characteristics; identification of novel biomarkers; and determine safety and tolerability | unknown, no results | |||
| Axon denervation in healthy individuals | NR | 900 mg twice daily for 3 months, orally | denervation of skin and reinnervation of skin after experimental denervation with capsaicin | not yet recruiting, no results | |
| Cognitive function, mood, and sleep in healthy elderly | NR | 300 mg/day or 1,000 mg/day for 8 weeks | differences between low-dose treatment and placebo/baseline in executive function (CNS vital signs tests) | active, no results |
AD, Alzheimer’s disease; ALS, amytrophic lateral sclerosis; ALS-FRSr, ALS functional rating scale; A-T, ataxia telangiectasia; CNS, central nervous system; CSF, cerebrospinal fluid; EH301, drug consisting of a combination of 1-(b-D-Ribofuranosyl) nicotinamide chloride and 3,5-dimethoxy-40-hydroxy-transstilbene; F-A, Friedreich ataxia; FVC, functional respiratory capacity; i.m., intramuscularly; i.v., intravenously; MCI, mild cognitive impairment; MMSE, minimental state examination; MDS-UPDRS, Movement Disorder Society Unified Parkinson disease rating scale; MRC, Medical Research Council; NAM, nicotinamide; NMN, nicotinamide mononucleotide; NR, nicotinamide riboside; PD, Parkinson’s disease; P-Tau, phosphorylated Tau.
Clinical trials with NAD+ precursors in relation to cognitive decline with age are underway (Table 2). One trial is focused on the effects of NR on memory and brain blood flow in adults with mild cognitive impairment (n = 26, multiple doses from 250 mg/day to 1 g/day for a total of 10 weeks). A similar trial is also in progress (500 mg twice daily, n = 58 for 12 weeks).
NADH, NR, and NAM have been and are in clinical trials for AD. While an open-label study in 17 AD patients (33–84 years, mean age 67.7) reported that NADH ingestion (twice a day with a total 10 mg/day) for 8–12 weeks improved cognitive effects, a follow-up clinical study with similar design did not result in any cognitive improvements (Rainer et al., 2000). Several decades of clinical studies of NAM for a variety of diseases have been performed, with the clinical data showing NAM is relatively safe (1,000–2,000 mg/day) and is readily absorbed from the gastrointestinal tract with peak serum concentrations in 1 h after oral ingestion (Chen et al., 2015; Petley et al., 1995; Phelan et al., 2017). Based on the promising data from AD mice (Green et al., 2008), a phase II clinical trial of NAM on 15 AD patients with mild to moderate dementia (1,500 mg twice daily) for 24 weeks was performed () (Phelan et al., 2017). However, NAM did not show any significant effects, though the lack of efficacy may have been due to several contributing factors, such as small sample size and a relatively short treatment period (Phelan et al., 2017). A new clinical trial with NAM on AD is now recruiting with the aim of studying the effect on p-Tau (p-Tau181) and total Tau levels in the cerebrospinal fluid (Table 2).
Several clinical trials of NADH and NR in PD patients have been performed. With relation to PD, NADH has shown beneficial effects on movement. An early study using NADH (intravenous administration, 25 mg/day) in 34 PD patients for 4 days, reported that 21 patients (61.7%) showed dramatic benefits, while the remaining 13 PD patients showed moderate improvement of motor deficits (Birkmayer et al., 1989). The benefits were confirmed in a following study with 885 PD patients, also showing similar benefits between parenteral and oral applications (Birkmayer et al., 1993). However, benefits were not seen in a double-blinded study on 5 clinically moderate PD patients treated with NADH (25 mg) given intravenously once a day for 4 days followed by NADH (25 mg) given intramuscularly after 2 and 4 weeks (Dizdar et al., 1994). Niacin/nicotinic acid (NA) has also been tested in one 78-year-old male PD patient. This report indicated that 500 mg NA twice a day improved rigidity and bradykinesia, while a higher dose at 1,000 mg twice a day produced severe nightmares and skin rash (Alisky, 2005). The side effects were not surprising since painful flushing is a common side effect of high NA doses (DiPalma and Thayer, 1991). A new clinical trial with NA is currently recruiting PD patients, with the aim of examining the effects on inflammation and motor function/symptoms in PD (Table 2). Since NR is orally bioavailable and has shown no detectable side effects as described above (Trammell et al., 2016a), it would be desirable for clinical trial. Currently, two clinical trials with NR in PD are starting, with the aims of investigating the neuro-metabolic profile and motor function after treatment (Table 2).
There are fewer clinical trials of treatments that bolster NAD+ levels in ALS patients. Following the clinical trial of NRPT/EH301 in healthy elderly people (Dellinger et al., 2017), a trial in ALS patients was completed (de la Rubia et al., 2019). The trial included 10 ALS patients receiving placebo and 10 patients receiving EH301 for 4 months. The study was a randomized double-blind design and included both males and females. NRPT/EH301 treatment improved ALS functional rating scale revised (ALSFRS-R), increased the MRC grading scale index (grades muscle power), and forced vital capacity (FVC, functional respiratory capacity test), as well as increased electrical activity in muscles. All patients in the EH301 group choose to continue the treatment after 4 months, and thus the treatment was finalized after 1-year treatment, where no deterioration, except of FVC, was observed (de la Rubia et al., 2019). Despite the low level of participants in this study, it sets the stage for the potential for NAD+ augmentation/SIRT1 activation as a treatment for ALS, and large-scale phase trials are warranted.
NAD+ precursors are also being tested for other neurodegenerative conditions including a clinical trial of NR in premature aging diseases including CS and A-T (Table 2) based on its significant benefit in cross-species preclinical studies (Fang et al., 2014, 2016a). Additionally, NAM is being tested in two clinical trials for Friedreich’s ataxia, an early-onset autosomal recessive hereditary ataxia caused by a pathological expansion of a GAA repeat in the first intron of the frataxin gene (FXN).
A Cautionary Note on Interventions that Bolster NAD+ Levels
While NAD+ replenishment appears safe in the healthy elderly, there are concerns with administering NAD+ precursors to patients with cancer. Major NAD+ metabolic enzymes, e.g., iNAMPT, eNAMPT, and/or NMNAT2, are overexpressed in a broad variety of cancers including breast, pancreatic, prostate, and colorectal cancers (Demarest et al., 2019). Evidence from laboratory studies show that increased NAD+, through either overexpressed NAD+ synthetic enzymes or supplementation with NAD+ precursors, promotes cancer cell proliferation, migration, and resistance to anticancer chemo-/radiotherapy (Demarest et al., 2019). Thus, strategies to deplete cellular NAD+ and the inhibition of NAD+ synthetic enzymes can trigger cancer cell death (Buonvicino et al., 2018). It is not known whether NAD+ replenishment would increase cancer risk in the aged general population. A recent study shows that high NAD+ levels may lead to cancer promotion (at least in pancreatic cancer) through changes in the secretory activity of senescent cells to release pro-inflammatory cytokines (Nacarelli et al., 2019). Other studies indicate that NAD+ replenishment delays cellular senescence (including mitochondrial dysfunction-associated senescence) (Wiley et al., 2016). Mature neurons are post-mitotic cells, while cancer cells are highly replicative, and so both cell types have a high energy demand. Despite both being nutrition demanding, cancer cells and post-mitotic cells exhibit a completely different metabolism, which may explain why the neuroprotective conditions of NAD+ augmentation and autophagy (Sousa et al., 2016) can also promote the growth of specific cancers (e.g., pancreatic cancer).
However, in some cases NAD+/NADH and its metabolites can inhibit cancer growth and metastasis. For example, a clinical case reports suggest that NADH inhibits the metastasis of invasive duct carcinoma, liver cancer, and breast cancer (Birkmayer, 1995). A phase-III randomized large clinical trial reported that oral NAM was safe and effective in reducing the rates of new nonmelanoma skin cancers and actinic keratosis in high-risk patients (Chen et al., 2015). Because of limited studies in this field, it is still an open question for future studies.
Outstanding Questions and Future Perspectives
Recent studies on animals and human clinical trials have expanded our understanding of NAD+ and NAD+-dependent enzymes in neuroplasticity, brain aging, and neurodegenerative disorders. Emerging evidence suggests NAD+ depletion in brain cells is common during aging and is accentuated in many neurodegenerative disorders including AD, PD, HD, and ALS, and that NAD+ augmentation inhibits pathological features in animal models of these disorders (Table 1). Emerging findings are revealing a critical role for NAD+ in neural resilience and in the molecular mechanisms of the hallmarks of brain aging. Although NAD+ depletion occurs in vulnerable neuronal populations in several major neurodegenerative diseases, it remains to be established whether impaired NAD+-dependent processes occur upstream and/or downstream of the specific proteopathic lesions that define each of the neurodegenerative diseases (Figure 4).
Different NAD+ precursors, NR, NMN, NA, and NAM, have been and are in clinical trials of different diseases, and it is necessary to compare them on safety, therapeutic effects, and side effects. Increasing NAD+ levels in the brain using NAD+, NADH, or NAD+ precursors can be limited by their cell impermeability, the different routes of administration, stability, and side effects. While mouse studies show that NR exhibits a unique oral bioavailability compared to NA and NAM (Trammell et al., 2016a), direct comparisons in humans are not available. While modern, sedentary, and overindulgent lifestyles contribute to brain aging and age-related neurodegenerative diseases, intermittent bioenergetic challenges, as exemplified by exercise, fasting, and intellectual challenges may protect the human brain against age-related dysfunction and disease (Mattson and Arumugam, 2018; Mattson et al., 2018). Studies on the effects of intermittent metabolic challenges on NAD+ levels, its metabolism, and the activities of the NAD+-dependent enzymes in humans are required. In addition, it will be of considerable interest to compare and contrast the efficacy and safety profiles of NAD+ precursors with the ketone β-hydroxybutyrate, which elevates NAD+ levels in neurons and has been shown to suppress Aβ and p-Tau pathologies and ameliorate behavioral deficits in a mouse model of AD (Kashiwaya et al., 2013; Marosi et al., 2016; Pawlosky et al., 2017) and also protects dopaminergic neurons in an animal model of PD (Tieu et al., 2003). The focus of this review is on brain aging, but the importance of NAD+- related biogenetics also involves neurons and related pathologies outside the brain. One such example is the group of neurodegenerative diseases termed glaucoma, which is characterized by progressive dysfunction and loss of the retinal ganglion cells. Also here, NAD+ depletion and mitochondrial abnormalities have been shown, and beneficial effects of NAM treatment of mouse models have been reported (Williams et al., 2017). Furthermore, the gut-to-brain axis may also show effects of NAD+ augmentation and, hereby, provide a new target of interest in neurodegenerative diseases (Blacher et al., 2019).
There are many unanswered questions surrounding NAD+ that need to be further addressed. First, a direct role for impaired NAD+-dependent processes and neurodegenerative diseases in humans remains to be established. Large longitudinal studies that include the quantification of NAD+ and related metabolites in using magnetic resonance spectroscopy or novel imaging methods could clarify whether brain regional NAD+ levels decline early in the disease process (Figure 4). Second, the complex relationships between NAD+ and cancer should be further studied to provide the necessary information to guide the use of NAD+ precursors as diet supplements for the general population. Third, it should be kept in mind that NAD+ precursors may not be beneficial in specific conditions. For example, NMN may exacerbate axon degeneration in vitro induced by the chemotherapeutic agent vincristine (Liu et al., 2018). Fourth, studies on the roles of NAD+ precursors in neuronal Ca2+ homeostasis and the interactions between NAD+ and different hallmarks of brain aging are necessary. Additionally, encouraged by the positive roles of NAD+ augmentation in neuroprotection in A-T, it would be interesting to explore the effects of NAD+ on other neurodegenerative diseases in which neuronal DNA repair is impaired including XRCC1 mutation-related neurodegeneration, FTD, and SCAN1.
ACKNOWLEDGMENTS
E.F.F. thanks Professor Vilhelm Bohr at the National Institute on Ageing for his former postdoc mentorship and his expertise on this project. The authors acknowledge the valuable work of the many investigators whose published articles they were unable to cite owing to space limitations. The authors thank Brian Christopher Gilmour and Dominic Wiesmann for reading of the manuscript, and Dr. Guofeng Lou for some illustrations. E.F.F. is supported by the HELSE SøR-ØST (#2017056), the Research Council of Norway (#262175 and #277813), NSFC (#81971327), and an Akershus University Hospital Strategic grant (#269901), and was a recipient of the 2019 Rosa Sløyfe Award project (#207819). D.A.S. was supported by the Glenn Foundation for Medical Research and grants from the NIH (R37 AG028730, R01 AG019719, and R01 DK100263).
Footnotes
DECLARATION OF INTERESTS
E.F.F. is a visiting professor at the First Affiliated Hospital, Sun Yat-Sen University (Guangzhou, China), a Guest Professor at School of Medicine, Jinan University (Guangzhou, China), and a Visiting Professor at the Department of Clinical Gerontology, the First Affiliated Hospital, Zhengzhou University (Zhengzhou, China). E.F.F. has CRADA arrangements with ChromaDex and is a consultant to Aladdin Healthcare Technologies and the Vancouver Dementia Prevention Centre. D.A.S. is a founder, equity owner, board member, advisor to, director of, consultant to, investor in, and/or inventor on patents licensed to Vium, Jupiter Orphan Therapeutics, Cohbar, Galilei Biosciences, GlaxoSmithKline, OvaScience, EMD Millipore, Wellomics, Inside Tracker, Caudalie, Bayer Crop Science, Longwood Fund, Zymo Research, EdenRoc Sciences (and affiliates Arc-Bio, Dovetail Genomics, Claret Bioscience, Revere Biosensors, UpRNA and MetroBiotech [an NAD booster company], and Liberty Biosecurity), and Life Biosciences (and affiliates Selphagy, Senolytic Therapeutics, Spotlight Biosciences, Animal Biosciences, Iduna, Immetas, Prana, Continuum Biosciences, Jumpstart Fertility [an NAD booster company], and Lua Communications). D.A.S. sits on the board of directors of both companies. D.A.S. is an inventor on a patent application filed by Mayo Clinic and Harvard Medical School that has been licensed to Elysium Health; his personal royalty share is directed to the Sinclair lab. For more information, see https://genetics.med.harvard.edu/sinclair-test/people/sinclair-other.php.
REFERENCES
- Adebanjo OA, Anandatheerthavarada HK, Koval AP, Moonga BS, Biswas G, Sun L, Sodam BR, Bevis PJ, Huang CL, Epstein S, et al. (1999). A new function for CD38/ADP-ribosyl cyclase in nuclear Ca2+ homeostasis. Nat. Cell Biol. 1, 409–414. [DOI] [PubMed] [Google Scholar]
- Agrimi G, Russo A, Scarcia P, and Palmieri F (2012). The human gene SLC25A17 encodes a peroxisomal transporter of coenzyme A, FAD and NAD+. Biochem. J 443, 241–247. [DOI] [PubMed] [Google Scholar]
- Ali YO, Li-Kroeger D, Bellen HJ, Zhai RG, and Lu HC (2013). NMNATs, evolutionarily conserved neuronal maintenance factors. Trends Neurosci. 36, 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alisky JM (2005). Niacin improved rigidity and bradykinesia in a Parkinson’s disease patient but also caused unacceptable nightmares and skin rash–a case report. Nutr. Neurosci 8, 327–329. [DOI] [PubMed] [Google Scholar]
- Amano H, Chaudhury A, Rodriguez-Aguayo C, Lu L, Akhanov V, Catic A, Popov YV, Verdin E, Johnson H, Stossi F, et al. (2019). Telomere dysfunction induces sirtuin repression that drives telomere-dependent disease. Cell Metab. 29, 1274–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrabi SA, Umanah GK, Chang C, Stevens DA, Karuppagounder SS, Gagné JP, Poirier GG, Dawson VL, and Dawson TM (2014). Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc. Natl. Acad. Sci. USA 111, 10209–10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Tang SC, Lathia JD, Cheng A, Mughal MR, Chigurupati S, Magnus T, Chan SL, Jo DG, Ouyang X, et al. (2007). Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc. Natl. Acad. Sci. USA 104, 14104–14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher G, Tsvetkov P, Kahana C, and Shaul Y (2005). A mechanism of ubiquitin-independent proteasomal degradation of the tumor suppressors p53 and p73. Genes Dev. 19, 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barinova K, Khomyakova E, Semenyuk P, Schmalhausen E, and Muronetz V (2018). Binding of alphα-synuclein to partially oxidized glyceralde-hyde-3-phosphate dehydrogenase induces subsequent inactivation of the enzyme. Arch. Biochem. Biophys 642, 10–22. [DOI] [PubMed] [Google Scholar]
- Beal MF, Matson WR, Swartz KJ, Gamache PH, and Bird ED (1990). Kynurenine pathway measurements in Huntington’s disease striatum: evidence for reduced formation of kynurenic acid. J. Neurochem 55, 1327–1339. [DOI] [PubMed] [Google Scholar]
- Berger F, Lau C, Dahlmann M, and Ziegler M (2005). Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J. Biol. Chem 280, 36334–36341. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, and Mattson MP (2008). Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 31, 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieganowski P, and Brenner C (2004). Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell 117, 495–502. [DOI] [PubMed] [Google Scholar]
- Birkmayer JGD (1995). Nicotinamide adenine dinucleotide (NADH)- a new therapeutic approach: preliminary results with cancer patients and patients with dementia of the Alzheimer type. J. Tumor Marker Oncol. 10, 1–10. [Google Scholar]
- Birkmayer JG (1996). Coenzyme nicotinamide adenine dinucleotide: new therapeutic approach for improving dementia of the Alzheimer type. Ann. Clin. Lab. Sci 26, 1–9. [PubMed] [Google Scholar]
- Birkmayer W, Birkmayer GJ, Vrecko K, Mlekusch W, Paletta B, and Ott E (1989). The coenzyme nicotinamide adenine dinucleotide (NADH) improves the disability of parkinsonian patients. J. Neural Transm. Park. Dis. Dement. Sect 1, 297–302. [DOI] [PubMed] [Google Scholar]
- Birkmayer JG, Vrecko C, Volc D, and Birkmayer W (1993). Nicotinamide adenine dinucleotide (NADH)–a new therapeutic approach to Parkinson’s disease. Comparison of oral and parenteral application. Acta Neurol. Scand. Suppl 146, 32–35. [PubMed] [Google Scholar]
- Blacher E, Dadali T, Bespalko A, Haupenthal VJ, Grimm MO, Hartmann T, Lund FE, Stein R, and Levy A (2015). Alzheimer’s disease pathology is attenuated in a CD38-deficient mouse model. Ann. Neurol 78, 88–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bach-ash M, Kleimeyer C, Moresi C, Harnik Y, Zur M, et al. (2019). Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572, 474–480. [DOI] [PubMed] [Google Scholar]
- Bogan KL, and Brenner C (2008). Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr 28, 115–130. [DOI] [PubMed] [Google Scholar]
- Bohnert KA, and Kenyon C (2017). A lysosomal switch triggers proteostasis renewal in the immortal C. elegans germ lineage. Nature 551, 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfiglio JJ, Fontana P, Zhang Q, Colby T, Gibbs-Seymour I, Atanassov I, Bartlett E, Zaja R, Ahel I, and Matic I (2017). Serine ADP-ribosylation depends on HPF1. Mol. Cell 940, 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski MS, and Sinclair DA (2016). Slowing ageing by design: the rise of NAD(+) and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol 17, 679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, and Grant R (2011). Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in Wistar rats. PLoS One 6, e19194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Brown KD, Maqsood S, Huang JY, Pan Y, Harkcom W, Li W, Sauve A, Verdin E, and Jaffrey SR (2014). Activation of SIRT3 by the NAD(+) precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 20, 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck E, Bayer H, Lindenberg KS, Hanselmann J, Pasquarelli N, Ludolph AC, Weydt P, and Witting A (2017). Comparison of sirtuin 3 levels in ALS and Huntington’s disease-differential effects in human tissue samples vs. transgenic mouse models. Front. Mol. Neurosci 10, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonvicino D, Mazzola F, Zamporlini F, Resta F, Ranieri G, Camaioni E, Muzzi M, Zecchi R, Pieraccini G, Dölle C, et al. (2018). Identification of the nicotinamide salvage pathway as a new toxification route for antimetabolites. Cell Chem. Biol 25, 471–482. [DOI] [PubMed] [Google Scholar]
- Burchell VS, Nelson DE, Sanchez-Martinez A, Delgado-Camprubi M, Ivatt RM, Pogson JH, Randle SJ, Wray S, Lewis PA, Houlden H, et al. (2013). The Parkinson’s disease-linked proteins Fbxo7 and Parkin interact to mediate mitophagy. Nat. Neurosci 16, 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, and Baker DJ (2018). Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caito SW, and Aschner M (2016). NAD+ supplementation attenuates methylmercury dopaminergic and mitochondrial toxicity in Caenorhabditis elegans. Toxicol. Sci 151, 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Pereira J, Tarragó MG, Chini CCS, Nin V, Escande C, Warner GM, Puranik AS, Schoon RA, Reid JM, Galina A, et al. (2016). CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 23, 1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambronne XA, Stewart ML, Kim D, Jones-Brunette AM, Morgan RK, Farrens DL, Cohen MS, and Goodman RH (2016). Biosensor reveals multiple sources for mitochondrial NAD(+). Science 352, 1474–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron WD, Bui CV, Hutchinson A, Loppnau P, Gräslund S, and Rocheleau JV (2016). Apollo-NADP(+): a spectrally tunable family of genetically encoded sensors for NADP(+). Nat. Methods 13, 352–358. [DOI] [PubMed] [Google Scholar]
- Campesan S, Green EW, Breda C, Sathyasaikumar KV, Muchowski PJ, Schwarcz R, Kyriacou CP, and Giorgini F (2011). The kynurenine pathway modulates neurodegeneration in a Drosophila model of Huntington’s disease. Curr. Biol 21, 961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canter RG, Penney J, and Tsai LH (2016). The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 539, 187–196. [DOI] [PubMed] [Google Scholar]
- Cantó C, Menzies KJ, and Auwerx J (2015). NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 22, 31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrì MT, D’Ambrosi N, and Cozzolino M (2017). Pathways to mitochondrial dysfunction in ALS pathogenesis. Biochem. Biophys. Res. Commun 483, 1187–1193. [DOI] [PubMed] [Google Scholar]
- Chalkiadaki A, and Guarente L (2015). The multifaceted functions of sirtuins in cancer. Nat. Rev. Cancer 15, 608–624. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stankovic R, Cullen KM, Meininger V, Garner B, Coggan S, Grant R, Brew BJ, and Guillemin GJ (2010). The kynurenine pathway and inflammation in amyotrophic lateral sclerosis. Neurotox. Res 18, 132–142. [DOI] [PubMed] [Google Scholar]
- Chen AC, Martin AJ, Choy B, Fernández-Peñas P, Dalziell RA, McKenzie CA, Scolyer RA, Dhillon HM, Vardy JL, Kricker A, et al. (2015). A Phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N. Engl. J. Med 373, 1618–1626. [DOI] [PubMed] [Google Scholar]
- Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, Liu Y, Wan R, Marosi K, Misiak M, et al. (2016). Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab. 23, 128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini C, Hogan KA, Warner GM, Tarragó MG, Peclat TR, Tchkonia T, Kirkland JL, and Chini E (2019). The NADase CD38 is induced by factors secreted from senescent cells providing a potential link between senescence and age-related cellular NAD(+) decline. Biochem. Biophys. Res. Commun 513, 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR (2010). The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat. Rev. Neurosci 11, 791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins N, Tweedie A, Zuryn S, Bertran-Gonzalez J, and Götz J (2019). Disease-associated tau impairs mitophagy by inhibiting Parkin translocation to mitochondria. EMBO J. 38, e99360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels CM, Ong SE, and Leung AK (2014). Phosphoproteomic approach to characterize protein mono- and poly(ADP-ribosyl)ation sites from cells. J. Proteome Res. 13, 3510–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, et al. (2018). Impairment of an endothelial NAD(+)-H2S signaling network is a reversible cause of vascular aging. Cell 173, 74–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila A, Liu L, Chellappa K, Redpath P, Nakamaru-Ogiso E, Paolella LM, Zhang Z, Migaud ME, Rabinowitz JD, and Baur JA (2018). Nicotinamide adenine dinucleotide is transported into mammalian mitochondria. Elife 7, e33246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rubia JE, Drehmer E, Platero JL, Benlloch M, Caplliure-Llopis J, Villaron-Casales C, de Bernardo N, AlarcÓn J, Fuente C, Carrera S, et al. (2019). Efficacy and tolerability of EH301 for amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled human pilot study. Amyotroph. Lateral Scler. Frontotemporal Degener. 20, 115–122. [DOI] [PubMed] [Google Scholar]
- Delgado-Camprubi M, Esteras N, Soutar MP, Plun-Favreau H, and Abramov AY (2017). Deficiency of Parkinson’s disease-related gene Fbxo7 is associated with impaired mitochondrial metabolism by PARP activation. Cell Death Differ. 24, 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger RW, Santos SR, Morris M, Evans M, Alminana D, Guarente L, and Marcotulli E (2017). Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD(+) levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study. NPJ Aging Mech. Dis 3, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarest TG, Truong GTD, Lovett J, Mohanty JG, Mattison JA, Mattson MP, Ferrucci L, Bohr VA, and Moaddel R (2019). Assessment of NAD(+)metabolism in human cell cultures, erythrocytes, cerebrospinal fluid and primate skeletal muscle. Anal. Biochem 572, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPalma JR, and Thayer WS (1991). Use of niacin as a drug. Annu. Rev. Nutr 11, 169–187. [DOI] [PubMed] [Google Scholar]
- Dizdar N, Kågedal B, and Lindvall B (1994). Treatment of Parkinson’s disease with NADH. Acta Neurol. Scand 90, 345–347. [DOI] [PubMed] [Google Scholar]
- Dölle C, Niere M, Lohndal E, and Ziegler M (2010). Visualization of subcellular NAD pools and intra-organellar protein localization by poly-ADP-ribose formation. Cell. Mol. Life Sci. 67, 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollerup OL, Christensen B, Svart M, Schmidt MS, Sulek K, Ringgaard S, Stødkilde-Jørgensen H, Møller N, Brenner C, Treebak JT, et al. (2018). A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am. J. Clin. Nutr 108, 343–353. [DOI] [PubMed] [Google Scholar]
- Dong Y, and Brewer GJ (2019). Global metabolic shifts in age and Alzheimer’s disease mouse brains pivot at NAD +/NADH redox sites.J. Alzheimers Dis. 71, 119–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Yu Q, Yan S, Hu G, Lue LF, Walker DG, Wu L, Yan SF, Tieu K, and Yan SS (2017). PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer’s disease. Brain 140, 3233–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engmann O, Hortobágyi T, Thompson AJ, Guadagno J, Troakes C, Soriano S, Al-Sarraj S, Kim Y, and Giese KP (2011). Cyclin-dependent kinase 5 activator p25 is generated during memory formation and is reduced at an early stage in Alzheimer’s disease. Biol. Psychiatry 70, 159–168. [DOI] [PubMed] [Google Scholar]
- Essuman K, Summers DW, Sasaki Y, Mao X, DiAntonio A, and Mil-brandt J (2017). The SARM1 toll/interleukin-1 receptor domain possesses intrinsic NAD(+) cleavage activity that promotes pathological axonal degeneration. Neuron 93, 1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF (2019). Mitophagy and NAD(+) inhibit Alzheimer disease. Autophagy 15, 1112–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Scheibye-Knudsen M, Brace LE, Kassahun H, SenGupta T, Nilsen H, Mitchell JR, Croteau DL, and Bohr VA (2014). Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell 157, 882–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Kassahun H, Croteau DL, Scheibye-Knudsen M, Marosi K, Lu H, Shamanna RA, Kalyanasundaram S, Bollineni RC, Wilson MA, et al. (2016a). NAD(+) replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metab. 24, 566–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Scheibye-Knudsen M, Chua KF, Mattson MP, Croteau DL, and Bohr VA (2016b). Nuclear DNA damage signalling to mitochondria in ageing. Nat. Rev. Mol. Cell Biol. 17, 308–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Lautrup S, Hou YJ, Demarest TG, Croteau DL, Mattson MP, and Bohr VA (2017). NAD(+) in aging: molecular mechanisms and translational implications. Trends Mol. Med 23, 899–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, Lautrup S, Hasan-Olive MM, Caponio D, Dan X, et al. (2019). Mitophagy inhibits amyloid-b and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci 22, 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Pang PT, Lu B, and Tsai LH (2005). Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron 48, 825–838. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JC, Zimprich A, Carvajal Berrio DA, Schindler KM, Maurer B, Schulte C, Bus C, Hauser AK, Kübler M, Lewin R, et al. (2017). Metformin reverses TRAP1 mutation-associated alterations in mitochondrial function in Parkinson’s disease. Brain 140, 2444–2459. [DOI] [PubMed] [Google Scholar]
- Fletcher RS, Ratajczak J, Doig CL, Oakey LA, Callingham R, Da Silva Xavier G, Garten A, Elhassan YS, Redpath P, Migaud ME, et al. (2017). Nicotinamide riboside kinases display redundancy in mediating nicotinamide mononucleotide and nicotinamide riboside metabolism in skeletal muscle cells. Mol. Metab 6, 819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folick A, Oakley HD, Yu Y, Armstrong EH, Kumari M, Sanor L, Moore DD, Ortlund EA, Zechner R, and Wang MC (2015). Aging. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans. Science 347, 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, and Partridge L (2015). Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquerel E, Goellner EM, Yu Z, Gagné JP, Barbi de Moura M, Feinstein T, Wheeler D, Redpath P, Li J, Romero G, et al. (2014). ARTD1/PARP1 negatively regulates glycolysis by inhibiting hexokinase 1 independent of NAD+ depletion. Cell Rep. 8, 1819–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, et al. (2009). PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol. Cell 33, 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang WY, Mao YW, Gräff J, Guan JS, Pan L, Mak G, Kim D, Su SC, and Tsai LH (2010). A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466, 1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, and Milbrandt J (2015). SARM1 activation triggers axon degeneration locally via NAD(+) destruction. Science 348, 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley J, and Coleman MP (2010). Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 8, e1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley J, Mayer PR, Yu G, and Coleman MP (2019). Low levels of NMNAT2 compromise axon development and survival. Hum. Mol. Genet 28, 448–458. [DOI] [PubMed] [Google Scholar]
- Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, et al. (2013). Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155, 1624–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, et al. (2013). Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol. Aging 34, 1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KN, Steffan JS, Martinez-Coria H, Sun X, Schreiber SS, Thompson LM, and LaFerla FM (2008). Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J. Neurosci 28, 11500–11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozio A, Sociali G, Sturla L, Caffa I, Soncini D, Salis A, Raffaelli N, De Flora A, Nencioni A, and Bruzzone S (2013). CD73 protein as a source of extracellular precursors for sustained NAD+ biosynthesis in FK866-treated tumor cells. J. Biol. Chem 288, 25938–25949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozio A, Mills KF, Yoshino J, Bruzzone S, Sociali G, Tokizane K, Lei HC, Cunningham R, Sasaki Y, Migaud ME, et al. (2019). Slc12a8 is a nicotinamide mononucleotide transporter. Nat. Metab 1, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L, and Kenyon C (2000). Genetic pathways that regulate ageing in model organisms. Nature 408, 255–262. [DOI] [PubMed] [Google Scholar]
- Guse AH (2015). Calcium mobilizing second messengers derived from NAD. Biochim. biophys. Acta 1854, 1132–1137. [DOI] [PubMed] [Google Scholar]
- Han X, Tai H, Wang X, Wang Z, Zhou J, Wei X, Ding Y, Gong H, Mo C, Zhang J, et al. (2016). AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD(+) elevation. Aging Cell 15, 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Rubinsztein DC, and Walker DW (2018). Autophagy as a promoter of longevity: insights from model organisms. Nat. Rev. Mol. Cell Biol. 19, 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara N, Yamada K, Shibata T, Osago H, Hashimoto T, and Tsuchiya M (2007). Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J. Biol. Chem 282, 24574–24582. [DOI] [PubMed] [Google Scholar]
- Harlan BA, Pehar M, Sharma DR, Beeson G, Beeson CC, and Vargas MR (2016). Enhancing NAD+ salvage pathway reverts the toxicity of primary astrocytes expressing amyotrophic lateral sclerosis-linked mutant superoxide dismutase 1 (SOD1). J. Biol. Chem 291, 10836–10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan BA, Pehar M, Killoy KM, and Vargas MR (2019). Enhanced SIRT6 activity abrogates the neurotoxic phenotype of astrocytes expressing ALS-linked mutant SOD1. FASEB J. 33, 7084–7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathorn T, Snyder-Keller A, and Messer A (2011). Nicotinamide improves motor deficits and upregulates PGC-1alpha and BDNF gene expression in a mouse model of Huntington’s disease. Neurobiol. Dis 41, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, and Lo EH (2016). Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida H, Yokoyama S, Huang JJ, Liu L, Ma WJ, Akther S, Higashida C, Kikuchi M, Minabe Y, and Munesue T (2012). Social memory, amnesia, and autism: brain oxytocin secretion is regulated by NAD+ metabolites and single nucleotide polymorphisms of CD38. Neurochem. Int 61, 828–838. [DOI] [PubMed] [Google Scholar]
- Ho TT, Warr MR, Adelman ER, Lansinger OM, Flach J, Verovskaya EV, Figueroa ME, and Passegué E (2017). Autophagy maintains the metabolism and function of young and old stem cells. Nature 543, 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, et al. (2016). Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Song H, Croteau DL, Akbari M, and Bohr VA (2017). Genome instability in Alzheimer disease. Mech. Ageing Dev. 161, 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, Zhang Y, Moritoh K, O’Connell JF, Baptiste BA, et al. (2018). NAD(+) supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc. Natl. Acad. Sci. USA 115, E1876–E1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, and Guarente L (2016). mTORC1 and SIRT1 cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell 166, 436–450. [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, and Guarente L (2000). Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800. [DOI] [PubMed] [Google Scholar]
- Jarome TJ, and Devulapalli RK (2018). The ubiquitin-proteasome system and memory: moving Beyond protein degradation. Neuroscientist 24, 639–651. [DOI] [PubMed] [Google Scholar]
- Jarome TJ, Kwapis JL, Hallengren JJ, Wilson SM, and Helmstetter FJ (2013). The ubiquitin-specific protease 14 (USP14) is a critical regulator of long-term memory formation. Learn. Mem 21, 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Ferrara NC, Kwapis JL, and Helmstetter FJ (2016). CaMKII regulates proteasome phosphorylation and activity and promotes memory destabilization following retrieval. Neurobiol. Learn. Mem 128, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H, Then F, Melia TJ Jr., Mazzulli JR, Cui L, Savas JN, Voisine C, Paganetti P, Tanese N, Hart AC, et al. (2009). Acetylation targets mutant huntingtin to autophagosomes for degradation. Cell 137, 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Li X, Gao H, Feng Z, Li X, Zhao L, Jia X, Zhang H, and Liu J (2008). High doses of nicotinamide prevent oxidative mitochondrial dysfunction in a cellular model and improve motor deficit in a Drosophila model of Parkinson’s disease. J. Neurosci. Res 86, 2083–2090. [DOI] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, et al. (2007). CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446, 41–45. [DOI] [PubMed] [Google Scholar]
- Johnson S, Wozniak DF, and Imai S (2018). CA1 Nampt knockdown recapitulates hippocampal cognitive phenotypes in old mice which nicotinamide mononucleotide improves. NPJ Aging Mech. Dis 4, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam TI, Mao X, Park H, Chou SC, Karuppagounder SS, Umanah GE, Yun SP, Brahmachari S, Panicker N, Chen R, et al. (2018). Poly(ADP-ribose) drives pathologic alphα-synuclein neurodegeneration in Parkinson’s disease. Science 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, Okun E, Clarke K, Mattson MP, and Veech RL (2013). A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging 34, 1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Gee J, and Ding Q (2002). The proteasome in brain aging. Ageing Res. Rev 1, 279–293. [DOI] [PubMed] [Google Scholar]
- Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, and Fang EF (2017). Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci. 40, 151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, et al. (2007). SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 26, 3169–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA, et al. (2019). Transneuronal propagation of pathologic alphα-synuclein from the gut to the brain models Parkinson’s disease. Neuron 103, 627–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Zhu W, Hosoda W, Sen JM, and Mattson MP (2019). Chronic mild gut inflammation accelerates brain neuropathology and motor dysfunction in alphα-synuclein mutant mice. Neuromolecular Med. 21, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, and Shimizu N (1998). Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608. [DOI] [PubMed] [Google Scholar]
- Körner S, Böselt S, Thau N, Rath KJ, Dengler R, and Petri S (2013). Differential sirtuin expression patterns in amyotrophic lateral sclerosis (ALS) postmortem tissue: neuroprotective or neurotoxic properties of sirtuins in ALS? Neurodegener. Dis 11, 141–152. [DOI] [PubMed] [Google Scholar]
- Krebs HA (1970). Rate control of the tricarboxylic acid cycle. Adv. Enzyme Regul. 8, 335–353. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Toda T, and Gage FH (2018). Adult hippocampal neurogenesis: a coming-of-age story. J. Neurosci 38, 10401–10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latta CH, Sudduth TL, Weekman EM, Brothers HM, Abner EL, Popa GJ, Mendenhall MD, Gonzalez-Oregon F, Braun K, and Wilcock DM (2015). Determining the role of IL-4 induced neuroinflammation in microglial activity and amyloid-beta using BV2 microglial cells and APP/PS1 transgenic mice. J. Neuroinflamm 12, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautrup S, Lou G, Aman Y, Nilsen H, Tao J, and Fang EF (2019). Micro-glial mitophagy mitigates neuroinflammation in Alzheimer’s disease. Neurochem. Int 129, 104469. [DOI] [PubMed] [Google Scholar]
- Lee JC, Shin JH, Park BW, Kim GS, Kim JC, Kang KS, and Cha CI (2012). Region-specific changes in the immunoreactivity of SIRT1 expression in the central nervous system of SOD1(G93A) transgenic mice as an in vivo model of amyotrophic lateral sclerosis. Brain Res. 1433, 20–28. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Hong YS, Jun W, and Yang SJ (2015). Nicotinamide riboside ameliorates hepatic metaflammation by modulating NLRP3 inflammasome in a rodent model of type 2 diabetes. J. Med. Food 18, 1207–1213. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Sanchez-Martinez A, Zarate AM, Benincá C, Mayor U, Clague MJ, and Whitworth AJ (2018). Basal mitophagy is widespread in Drosophila but minimally affected by loss of Pink1 or parkin. J. Cell Biol. 217, 1613–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman DS, Hebestreit K, Ruetz T, Webb AE, McKay A, Pollina EA, Dulken BW, Zhao X, Yeo RW, Ho TT, et al. (2018). Lysosome activation clears aggregates and enhances quiescent neural stem cell activation during aging. Science 359, 1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann S, Costa AC, Celardo I, Loh SH, and Martins LM (2016). Parp mutations protect against mitochondrial dysfunction and neurodegeneration in a Parkin model of Parkinson’s disease. Cell Death Dis. 7, e2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann S, Loh SH, and Martins LM (2017). Enhancing NAD(+) salvage metabolism is neuroprotective in a PINK1 model of Parkinson’s disease. Biol. Open 6, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AKL (2017). PARPs. Curr. Biol 27, R1256–R1258. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Björklund A, et al. (2008). Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med 14, 501–503. [DOI] [PubMed] [Google Scholar]
- Li J, Bonkowski MS, Moniot S, Zhang D, Hubbard BP, Ling AJ, Rajman LA, Qin B, Lou Z, Gorbunova V, et al. (2017). A conserved NAD(+) binding pocket that regulates protein-protein interactions during aging. Science 355, 1312–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, and Beal MF (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795. [DOI] [PubMed] [Google Scholar]
- Liu Q, Graeff R, Kriksunov IA, Lam CM, Lee HC, and Hao Q (2008). Conformational closure of the catalytic site of human CD38 induced by calcium (dagger) (double dagger). Biochemistry 47, 13966–13973. [DOI] [PubMed] [Google Scholar]
- Liu D, Pitta M, Jiang H, Lee JH, Zhang G, Chen X, Kawamoto EM, and Mattson MP (2013). Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: evidence for improved neuronal bioenergetics and autophagy procession. Neurobiol. Aging 34, 1564–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhao YJ, Li WH, Hou YN, Li T, Zhao ZY, Fang C, Li SL, and Lee HC (2017). Cytosolic interaction of type III human CD38 with CIB1 modulates cellular cyclic ADP-ribose levels. Proc. Natl. Acad. Sci. USA [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HW, Smith CB, Schmidt MS, Cambronne XA, Cohen MS, Migaud ME, Brenner C, and Goodman RH (2018). Pharmacological bypass of NAD(+) salvage pathway protects neurons from chemotherapy-induced degeneration. Proc. Natl. Acad. Sci. USA 115, 10654–10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cheng A, Li YJ, Yang Y, Kishimoto Y, Zhang S, Wang Y, Wan R, Raefsky SM, Lu D, et al. (2019). SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat. Commun 10, 1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, et al. (2017). Dementia prevention, intervention, and care. Lancet 390, 2673–2734. [DOI] [PubMed] [Google Scholar]
- Lloret A, and Beal MF (2019). PGC-1alpha, sirtuins and PARPs in Huntington’s disease and other neurodegenerative conditions: NAD+ to rule them all. Neurochem. Res. Published online May 7, 2019 10.1007/s11064-019-02809-1. [DOI] [PubMed] [Google Scholar]
- Long A, Park JH, Klimova N, Fowler C, Loane DJ, and Kristian T (2017). CD38 knockout mice show significant protection against ischemic brain damage despite high level poly-ADP-ribosylation. Neurochem. Res 42, 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou G, Palikaras K, Lautrup S, Scheibye-Knudsen M, Tavernarakis N, and Fang EF (2019). Mitophagy and neuroprotection. Trends Mol. Med [DOI] [PubMed] [Google Scholar]
- Maddison DC, and Giorgini F (2015). The kynurenine pathway and neurodegenerative disease. Semin. Cell Dev. Biol 40, 134–141. [DOI] [PubMed] [Google Scholar]
- Mairet-Coello G, Courchet J, Pieraut S, Courchet V, Maximov A, and Polleux F (2013). The CAMKK2-AMPK kinase pathway mediates the synaptotoxic effects of Abeta oligomers through Tau phosphorylation. Neuron 78, 94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcassa E, Kallinos A, Jardine J, Rusilowicz-Jones EV, Martinez A, Kuehl S, Islinger M, Clague MJ, and Urbé S (2018). Dual role of USP30 in controlling basal pexophagy and mitophagy. EMBO Rep. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marosi K, Kim SW, Moehl K, Scheibye-Knudsen M, Cheng A, Cutler R, Camandola S, and Mattson MP (2016). 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons.J. Neurochem 139, 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marosi K, and Mattson MP (2014). BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab 25, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, Chonchol M, and Seals DR (2018). Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD(+) in healthy middle-aged and older adults. Nat. Commun 9, 1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, and Arumugam TV (2018). Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab. 27, 1176–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Gleichmann M, and Cheng A (2008). Mitochondria in neuro-plasticity and neurological disorders. Neuron 60, 748–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Moehl K, Ghena N, Schmaedick M, and Cheng A (2018). Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci 19, 63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer PR, Huang N, Dewey CM, Dries DR, Zhang H, and Yu G (2010). Expression, localization, and biochemical characterization of nicotinamide mononucleotide adenylyltransferase 2. J. Biol. Chem 285, 40387–40396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon PJ (2013). Maintaining genome stability in the nervous system. Nat. Neurosci 16, 1523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Frazier CJ, and Bizon JL (2015). Molecular aspects of age-related cognitive decline: the role of GABA signaling. Trends Mol. Med 21, 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams TG, Prescott AR, Montava-Garriga L, Ball G, Singh F, Barini E, Muqit MMK, Brooks SP, and Ganley IG (2018). Basal mitophagy occurs independently of PINK1 in mouse tissues of high metabolic demand. Cell Metab. 27, 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies FM, Fleming A, and Rubinsztein DC (2015). Compromised auto-phagy and neurodegenerative diseases. Nat. Rev. Neurosci 16, 345–357. [DOI] [PubMed] [Google Scholar]
- Minhas PS, Liu L, Moon PK, Joshi AU, Dove C, Mhatre S, Contrepois K, Wang Q, Lee BA, Coronado M, et al. (2019). Macrophage de novo NAD(+) synthesis specifies immune function in aging and inflammation. Nat. Immunol 20, 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi M, Otsuka N, Sato M, Ishii Y, Kon S, Yamada M, Nishina H, Katada T, and Ikeda K (1995). Neuronal localization of CD38 antigen in the human brain. Brain Res. 697, 235–240. [DOI] [PubMed] [Google Scholar]
- Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N,Ávila J, and Llorens-Martín M (2019). Adult hippo-campal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med 25, 554–560. [DOI] [PubMed] [Google Scholar]
- Morgan BP (2018). Complement in the pathogenesis of Alzheimer’s disease. Semin. Immunopathol 40, 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Cantó C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, et al. (2013). The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154, 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata H, Khine CC, Nishikawa A, Yamamoto KI, Kinoshita R, and Sakaguchi M (2018). C-Jun N-terminal kinase (JNK)-mediated phosphorylation of SARM1 regulates NAD(+) cleavage activity to inhibit mitochondrial respiration. J. Biol. Chem 293, 18933–18943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacarelli T, Lau L, Fukumoto T, Zundell J, Fatkhutdinov N, Wu S, Aird KM, Iwasaki O, Kossenkov AV, Schultz D, et al. (2019). NAD(+) metabolism governs the proinflammatory senescence-associated secretome. Nat. Cell Biol. 21, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforov A, Dölle C, Niere M, and Ziegler M (2011). Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J. Biol. Chem 286, 21767–21778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA (2013). The role of autophagy in neurodegenerative disease. Nat. Med 19, 983–997. [DOI] [PubMed] [Google Scholar]
- Palikaras K, Lionaki E, and Tavernarakis N (2015). Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 521, 525–528. [DOI] [PubMed] [Google Scholar]
- Palikaras K, Lionaki E, and Tavernarakis N (2018). Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 20, 1013–1022. [DOI] [PubMed] [Google Scholar]
- Palin EJ, Paetau A, and Suomalainen A (2013). Mesencephalic complex I deficiency does not correlate with parkinsonism in mitochondrial DNA maintenance disorders. Brain 136, 2379–2392. [DOI] [PubMed] [Google Scholar]
- Pallos J, Bodai L, Lukacsovich T, Purcell JM, Steffan JS, Thompson LM, and Marsh JL (2008). Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a Drosophila model of Huntington’s disease. Hum. Mol. Genet 17, 3767–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZG, and An XS (2018). SARM1 deletion restrains NAFLD induced by high fat diet (HFD) through reducing inflammation, oxidative stress and lipid accumulation. Biochem. Biophys. Res. Commun 498, 416–423. [DOI] [PubMed] [Google Scholar]
- Pawlosky RJ, Kemper MF, Kashiwaya Y, King MT, Mattson MP, and Veech RL (2017). Effects of a dietary ketone ester on hippocampal glycolytic and tricarboxylic acid cycle intermediates and amino acids in a 3xTgAD mouse model of Alzheimer’s disease. J. Neurochem 141, 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez VI, Buffenstein R, Masamsetti V, Leonard S, Salmon AB, Mele J, Andziak B, Yang T, Edrey Y, Friguet B, et al. (2009). Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc. Natl. Acad. Sci. USA 106, 3059–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petley A, Macklin B, Renwick AG, and Wilkin TJ (1995). The pharmacokinetics of nicotinamide in humans and rodents. Diabetes 44, 152–155. [DOI] [PubMed] [Google Scholar]
- Phelan MJ, Mulnard RA, Gillen DL, and Schreiber SS (2017). Phase II clinical trial of nicotinamide for the treatment of mild to moderate Alzheimer’s disease. J. Geriatr. Med. Gerontol 3, 1–7. [Google Scholar]
- Pittelli M, Formentini L, Faraco G, Lapucci A, Rapizzi E, Cialdai F, Romano G, Moneti G, Moroni F, and Chiarugi A (2010). Inhibition of nicotinamide phosphoribosyltransferase: cellular bioenergetics reveals a mitochondrial insensitive NAD pool. J. Biol. Chem 285, 34106–34114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, and Lang AE (2017). Parkinson disease. Nat. Rev. Dis. Primers 3, 17013. [DOI] [PubMed] [Google Scholar]
- Polanco JC, Li C, Bodea LG, Martinez-Marmol R, Meunier FA, and Götz J (2018). Amyloid-beta and tau complexity - towards improved bio-markers and targeted therapies. Nat. Rev. Neurol 14, 22–39. [DOI] [PubMed] [Google Scholar]
- Rainer M, Kraxberger E, Haushofer M, Mucke HA, and Jellinger KA (2000). No evidence for cognitive improvement from oral nicotinamide adenine dinucleotide (NADH) in dementia. J. Neural Transm. (Vienna) 107, 1475–1481. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Joffraud M, Trammell SA, Ras R, Canela N, Boutant M, Kulkarni SS, Rodrigues M, Redpath P, Migaud ME, et al. (2016). NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat. Commun 7, 13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scar-avilli F, Easton DF, Duden R, O’Kane CJ, et al. (2004). Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet 36, 585–595. [DOI] [PubMed] [Google Scholar]
- Ray Chaudhuri A, and Nussenzweig A (2017). The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 18, 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, and Poirier GG (2010). PARP inhibition: PARP1 and beyond. Nat. Rev. Cancer 10, 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez I, and Vilchez D (2014). The mechanistic links between proteasome activity, aging and age-related diseases. Curr. Genomics 15, 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve AA, Munshi C, Lee HC, and Schramm VL (1998). The reaction mechanism for CD38. A single intermediate is responsible for cyclization, hydrolysis, and base-exchange chemistries. Biochemistry 37, 13239–13249. [DOI] [PubMed] [Google Scholar]
- Scheibye-Knudsen M, Mitchell SJ, Fang EF, Iyama T, Ward T, Wang J, Dunn CA, Singh N, Veith S, Hasan-Olive MM, et al. (2014). A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in Cockayne syndrome. Cell Metab. 20, 840–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöndorf DC, Ivanyuk D, Baden P, Sanchez-Martinez A, De Cicco S, Yu C, Giunta I, Schwarz LK, Di Napoli G, Panagiotakopoulou V, et al. (2018). The NAD+ precursor nicotinamide riboside rescues mitochondrial defects and neuronal loss in iPSC and fly models of Parkinson’s disease. Cell Rep. 23, 2976–2988. [DOI] [PubMed] [Google Scholar]
- Schwab AJ, Sison SL, Meade MR, Broniowska KA, Corbett JA, and Ebert AD (2017). Decreased sirtuin deacetylase activity in LRRK2 G2019S iPSC-derived dopaminergic neurons. Stem Cell Reports 9, 1839–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, and Pellicciari R (2002). Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J. Pharmacol. Exp. Ther 303, 1–10. [DOI] [PubMed] [Google Scholar]
- Shi Q, Colodner KJ, Matousek SB, Merry K, Hong S, Kenison JE, Frost JL, Le KX, Li S, Dodart JC, et al. (2015). Complement C3-deficient mice fail to display age-related hippocampal decline. J. Neurosci 35, 13029–13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, et al. (2009). Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med 361, 1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sison SL, and Ebert AD (2018). Decreased NAD+ in dopaminergic neurons. Aging (Albany NY) 10, 526–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliter DA, Martinez J, Hao L, Chen X, Sun N, Fischer TD, Burman JL, Li Y, Zhang Z, Narendra DP, et al. (2018). Parkin and PINK1 mitigate STING-induced inflammation. Nature 561, 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sociali G, Raffaghello L, Magnone M, Zamporlini F, Emionite L, Sturla L, Bianchi G, Vigliarolo T, Nahimana A, Nencioni A, et al. (2016). Anti-tumor effect of combined NAMPT and CD73 inhibition in an ovarian cancer model. Oncotarget 7, 2968–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino V, Romani M, Mouchiroud L, Beck JS, Zhang H, D’Amico D, Moullan N, Potenza F, Schmid AW, Rietsch S, et al. (2017). Enhancing mitochondrial proteostasis reduces amyloid-beta proteotoxicity. Nature 552, 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, et al. (2016). Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536, 479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, and Goedert M (1997). Alphα-synuclein in Lewy bodies. Nature 388, 839–840. [DOI] [PubMed] [Google Scholar]
- Stein LR, and Imai S (2014). Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 33, 1321–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LR, Wozniak DF, Dearborn JT, Kubota S, Apte RS, Izumi Y, Zorumski CF, and Imai S (2014). Expression of Nampt in hippocampal and cortical excitatory neurons is critical for cognitive function. J. Neurosci 34, 5800–5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW, and Darlington LG (2013). The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br. J. Pharmacol 169, 1211–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A, Mattie S, Prudent J, and McBride HM (2017). Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature 542, 251–254. [DOI] [PubMed] [Google Scholar]
- Tang BL (2017). Could sirtuin activities modify ALS onset and progression? Cell. Mol. Neurobiol 37, 1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, et al. (2003). D-beta-hydroxybuty-rate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Invest 112, 892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita KI, Aida J, Izumiyama-Shimomura N, Nakamura KI, Ishikawa N, Matsuda Y, Arai T, Ishiwata T, Kumasaka T, Takahashi-Fujigasaki J, et al. (2018). Changes in telomere length with aging in human neurons and glial cells revealed by quantitative fluorescence in situ hybridization analysis. Geriatr. Gerontol. Int 18, 1507–1512. [DOI] [PubMed] [Google Scholar]
- Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Del-linger RW, Li Z, Abel ED, Migaud ME, and Brenner C (2016a). Nicotin-amide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun 7, 12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trammell SA, Weidemann BJ, Chadda A, Yorek MS, Holmes A, Coppey LJ, Obrosov A, Kardon RH, Yorek MA, and Brenner C (2016b). Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci. Rep 6, 26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BP, Green KN, Chan JL, Blurton-Jones M, and LaFerla FM (2008). Abeta inhibits the proteasome and enhances amyloid and tau accumulation. Neurobiol. Aging 29, 1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RW, Lipscombe D, Madison DV, Bley KR, and Fox AP (1988). Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 11, 431–438. [DOI] [PubMed] [Google Scholar]
- Tsvetkov P, Myers N, Eliav R, Adamovich Y, Hagai T, Adler J, Navon A, and Shaul Y (2014). NADH binds and stabilizes the 26S proteasomes independent of ATP. J. Biol. Chem 289, 11272–11281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, et al. (2004). Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304, 1158–1160. [DOI] [PubMed] [Google Scholar]
- Valle C, Salvatori I, Gerbino V, Rossi S, Palamiuc L, René F, and Carrì MT (2014). Tissue-specific deregulation of selected HDACs characterizes ALS progression in mouse models: pharmacological characterization of SIRT1 and SIRT2 pathways. Cell Death Dis. 5, e1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini N, Campos V, Girotra M, Trachsel V, Rojas-Sutterlin S, Tratwal J, Ragusa S, Stefanidis E, Ryu D, Rainer PY, et al. (2019). The NAD-booster nicotinamide riboside potently stimulates hematopoiesis through increased mitochondrial clearance. Cell Stem Cell 24, 405–418. [DOI] [PubMed] [Google Scholar]
- Vécsei L, Szalárdy L, Fülöp F, and Toldi J (2013). Kynurenines in the CNS: recent advances and new questions. Nat. Rev. Drug Discov. 12, 64–82. [DOI] [PubMed] [Google Scholar]
- Verdin E (2015). NAD(+) in aging, metabolism, and neurodegeneration. Science 350, 1208–1213. [DOI] [PubMed] [Google Scholar]
- Walker C, and El-Khamisy SF (2018). Perturbed autophagy and DNA repair converge to promote neurodegeneration in amyotrophic lateral sclerosis and dementia. Brain 141, 1247–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC (2012). Mitochondria and cancer. Nat. Rev. Cancer 12, 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DM, Kasturi P, Zheng M, Pinkert S, Vecchi G, Ciryam P, Morimoto RI, Dobson CM, Vendruscolo M, Mann M, et al. (2015). Widespread proteome remodeling and aggregation in aging C. elegans. Cell 161, 919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kim NS, Haince JF, Kang HC, David KK, Andrabi SA, Poirier GG, Dawson VL, and Dawson TM (2011). Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase-1-dependent cell death (parthanatos). Sci. Signal 4, ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, An R, Umanah GK, Park H, Nambiar K, Eacker SM, Kim B, Bao L, Harraz MM, Chang C, et al. (2016). A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, et al. (2016). Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 23, 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Harder JM, Foxworth NE, Cochran KE, Philip VM, Porciatti V, Smithies O, and John SW (2017). Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 355, 756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LE, and Sinclair DA (2014). SIRT2 controls the pentose phosphate switch. EMBO J. 33, 1287–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, et al. (2007). Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130, 1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Liu Y, Tu Z, Xiao C, Yan S, Ma X, Guo X, Chen X, Yin P, Yang Z, et al. (2019). CRISPR/Cas9-mediated PINK1 deletion leads to neurodegeneration in rhesus monkeys. Cell Res. 29, 334–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W (2008). NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid. Redox Signal. 10, 179–206. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Satoh A, Lin JB, Mills KF, Sasaki Y, Rensing N, Wong M, Apte RS, and Imai SI (2019). Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metab. 30, 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Mills KF, Yoon MJ, and Imai S (2011). Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 14, 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Baur JA, and Imai SI (2018). NAD(+) intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. 27, 513–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, and Dawson VL (2002). Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297, 259–263. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xie Y, Wang T, Bi J, Li H, Zhang LQ, Ye SQ, and Ding S (2010). Neuronal protective role of PBEF in a mouse model of cerebral ischemia. J. Cereb. Blood Flow Metab. 30, 1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, et al. (2016). NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352, 1436–1443. [DOI] [PubMed] [Google Scholar]
- Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, Abdelmohsen K, Bohr VA, Misra Sen J, Gorospe M, et al. (2019). Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci 22, 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XH, Lu M, Lee BY, Ugurbil K, and Chen W (2015). In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc. Natl. Acad. Sci. USA 112, 2876–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwilling D, Huang SY, Sathyasaikumar KV, Notarangelo FM, Guidetti P, Wu HQ, Lee J, Truong J, Andrews-Zwilling Y, Hsieh EW, et al. (2011). Kynurenine 3-monooxygenase inhibition in blood ameliorates neurode-generation. Cell 145, 863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]