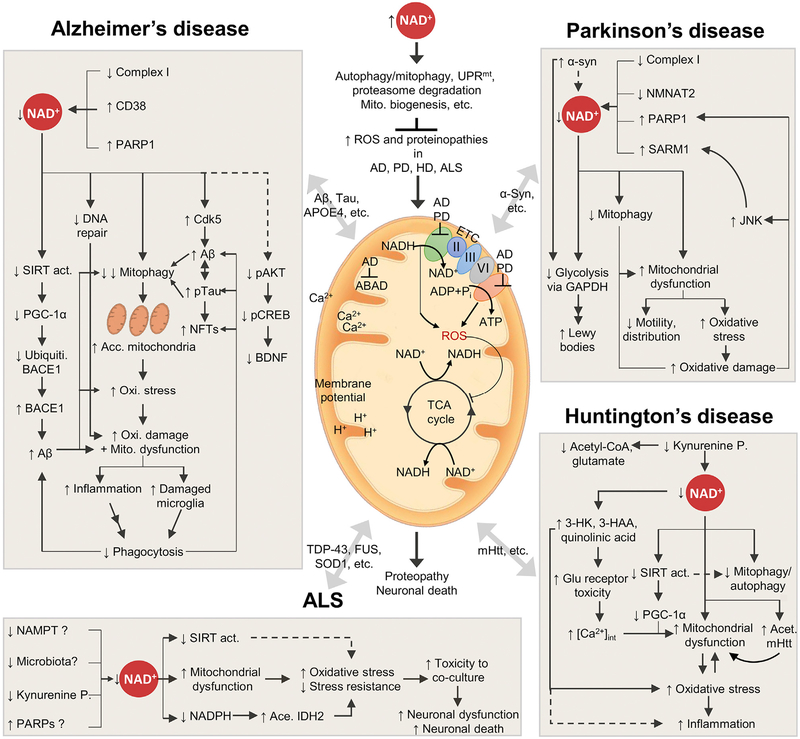
Figure 4. NAD+ Depletion and Impaired Mitophagy Are Pivotal Events in Common Neurodegenerative Diseases.
Based on the evidence summarized in the current review, we propose a hypothesis that may explain, in part, why age is the primary driver of the common neurodegenerative diseases. At a younger age, sufficient cellular NAD+ maintains mitochondrial quality to sustain normal neuronal function via the NAD+-dependent regulations of autophagy/mitophagy, UPRmt, proteasome degradation, and mitochondrial biogenesis. As we age, increased NAD+ consumption drives NAD+ depletion, leading to impaired mitochondrial homeostasis and neuronal function. Depending on the disease-related pathogenesis in the patient, age-dependent NAD+ depletion and impaired mitophagy may exacerbate the disease progression. This hypothesis explains why age makes people susceptible to neurodegenerative diseases, but it is not sufficient. Center: the mitochondrion in the center exhibits the various mitochondrial deficiencies observed in the four neurodegenerative diseases AD, PD, HD, and ALS. These include impairment of Complex I (CI) in the ETC utilizing NADH as an electron donor, creating NAD+. Impairments of both CI and ATP production create reactive oxygen species (ROS), which increase the level of oxidative stress, likely affecting both the ETC itself and the TCA cycle, and increasing the amount of oxidative damage. This can again affect the flux of Ca2+ and the membrane potential, resulting in dysfunctional mitochondria. Furthermore, compromised autophagy and/or mitophagy has been linked to several neurodegenerative diseases, resulting in accumulation of dysfunctional mitochondria. The four panels surrounding the mitochondrion illustrate the suggested explanations for NAD+ depleted and the downstream effects of NAD+ depletion in AD, PD, HD, and ALS. See the text for detailed explanations and references. Kynurenine P, kynurenine pathway.