Abstract
Central nervous system (CNS)‐relapsed mantle cell lymphoma (MCL) is a rare, aggressive non‐Hodgkin lymphoma without a standard treatment. Ibrutinib has shown promising results for inducing remission in other non‐Hodgkin lymphomas and may be considered as successful treatment for CNS‐relapsed MCL in the future as well.
Keywords: BTK inhibitor, central nervous system, ibrutinib, mantle cell lymphoma
1. CASE
Mantle cell lymphoma (MCL) is an uncommon malignancy that accounts for 3%‐6% of non‐Hodgkin lymphoma cases. While MCL is considered an aggressive and incurable type of lymphoma, patients receiving intensive therapy can achieve deep remissions lasting 10 years or more.1 Certain features have been identified that correlate with high‐risk disease, including a high‐risk mantle cell lymphoma international prognostic score (MIPI), a ki‐67 cell proliferation index greater than 30%, blastoid or pleomorphic morphology, and TP53 mutations.2, 3, 4, 5 For patients with any of these features, the survival is greatly diminished.
Central nervous system (CNS) involvement is exceedingly rare, accounting for only 4% of MCL cases, and is associated with a particularly poor prognosis. In one study, MCL patients with relapsed CNS involvement had a median survival of 3.7 months.6 Other studies have shown a similar dismal prognosis, ranging from 2 to 9 months.3, 7 MCL patients with a tumor ki‐67 proliferation index greater than 30% have a significantly increased risk of CNS relapse: up to 25.4% within the first 2 years.3 The blastoid variant of MCL also significantly increases the risk of CNS infiltration in comparison with nonblastoid variant, with 23.8% of patients relapsing within 5 years in one study.8 Due to the rarity of MCL with CNS relapse, no standard of care exists. We present a case of MCL with a third CNS relapse occurring 5 months after allogeneic stem cell transplant (alloSCT) that was effectively salvaged with ibrutinib monotherapy.
A 51‐year‐old Caucasian female initially presented to her primary care provider at an outside hospital with sinus congestion, fatigue, abdominal discomfort, and lymphadenopathy. Her peripheral white blood cell count was markedly elevated (70 100/mm3), and her lactate dehydrogenase (LDH) was elevated to 409 IU/L (upper limit of normal = 226 IU/L). A lymph node biopsy confirmed the diagnosis of MCL. The biopsy was notable for nonblastoid histology and a ki‐67 proliferation index of 60%. A MIPIb score of 8 was calculated, which correlates with high‐risk disease. Computed tomography (CT) scan revealed lymph node involvement above and below the diaphragm, and a bone marrow biopsy showed a low level of involvement, consistent with stage IV disease at initial presentation.
The patient was treated with six cycles of bendamustine and rituximab (BR) at the outside facility. Post‐treatment imaging was consistent with a complete response (CR). The patient was referred to our hospital for autologous stem cell transplant evaluation, but declined and continued treatment with her community oncologist. She was scheduled to begin maintenance rituximab in 2 months, but presented to her oncologist's office with abdominal fullness and right arm pain with numbness prior to initiation of maintenance therapy. A CT scan revealed relapsed disease in her omentum with mesenteric and right external iliac lymphadenopathy. An MRI of her spine showed an enhancing lesion at C2, confirming her first CNS relapse. She received 1 dose of bendamustine and 10 days of radiation to the cervical spine lesion with resolution of her symptoms. She received an opinion at UNC after this, and we recommended four cycles of alternating R‐CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) and R‐DHAP (rituximab, dexamethasone, high‐dose cytarabine, and cisplatin) followed by stem cell transplant evaluation. This regimen was given at an outside hospital, and she achieved a CR.
Two months after completion of R‐CHOP/R‐DHAP, the patient agreed to a transplant evaluation and presented to her transplant physician's office with 2‐3 weeks of increasing headaches, nausea, vomiting, and mild hearing changes. MRI of the brain revealed postcontrast enhancement of cranial nerves III through XII bilaterally, establishing her second CNS relapse. A lumbar puncture (LP) was performed and CSF flow cytometric analysis confirmed significant involvement by lymphoma (approximately 800 clonal cells/uL). Imaging with positron emission tomography (PET)‐CT was negative for systemic involvement (Deauville 1).
Extrapolating from the primary CNS lymphoma literature, the patient was treated with the regimen R‐MPV (rituximab, methotrexate, procarbazine, and vincristine). She completed five cycles and was found to have a partial response (PR); therefore, she continued for two more cycles and achieved a CR (seven cycles total). Additionally, she received intrathecal chemotherapy consisting of cytarabine, methotrexate, and hydrocortisone once each cycle, and her CSF cleared after two treatments. The patient tolerated the treatment well, with her only side effect being peripheral neuropathy that was controlled with gabapentin and reduction in vincristine from 2.4 to 2 mg starting with cycle 2. The patient then received a matched‐related donor, nonmyeloablative alloSCT. Her conditioning regimen was cyclophosphamide 50 mg/kg IV on Day −6, mesna 75 mg/kg infused over 36 hours starting on Day −6, fludarabine 25 mg/m2 IV on Days −6 to −2, and TIB 200 cGy on Day −1.9 She remained in complete remission 5 months post‐transplant and 9 months from her second CNS relapse.
Subsequently, the patient had a third CNS relapse manifesting as a headache and visual disturbance. A brain MRI showed relapse in her left occipital region and left optic nerve (Figure 1A,B,C). She was initiated on ibrutinib 560 mg daily and her neurologic symptoms improved within 1 week of therapy. A brain MRI after 5 weeks of therapy showed resolution of her lymphoma in the occipital region, and resolution of the left optic nerve enlargement and enhancement (Figure 1a,b,c). She has now been on ibrutinib therapy for 5 months and remains in CR with no neurologic deficits or side effects from therapy. She will remain on ibrutinib until progression or the development of unmanageable toxicity. Remarkably, it has been over 2 years since her initial MCL diagnosis, and over 1 year since her first CNS relapse.
Figure 1.
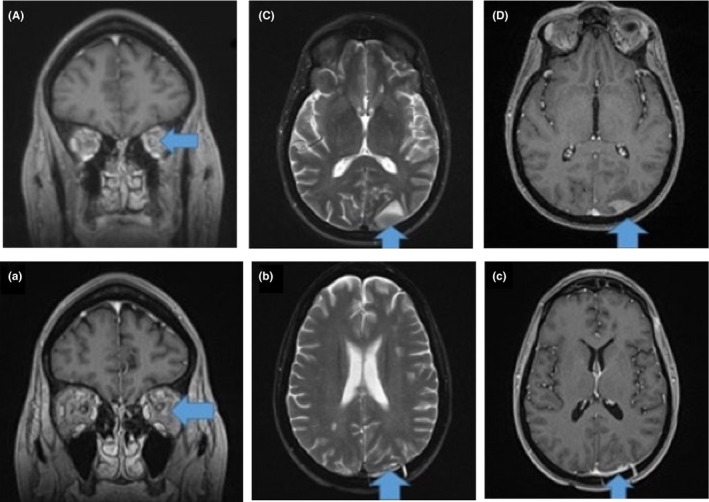
Brain MRI at second CNS relapse showing cranial nerve involvement. Axial postcontrast T1 images (left, A) reveal intense leptomeningeal bilateral internal auditory canal enhancement (arrows). An additional axial T1 postcontrast image (center, B) also demonstrates enhancement and thickening of the left CN V nerve roots as they exit the brainstem (arrows). A coronal postcontrast image (right C) demonstrates right‐sided CN V (inferior arrow) as well as right‐sided CN III (superior arrow) and left‐sided internal auditory canal CN VII and VIII leptomeningeal enhancement. Precontrast imaging appeared normal
We describe a rare case of MCL relapsed in the CNS for a third time that was effectively salvaged with ibrutinib monotherapy in the post‐alloSCT setting. Her primary risk factor for CNS involvement was an elevated ki‐67 proliferation index of 60%. To the best of our knowledge, this is the first description of ibrutinib leading to a CR in CNS MCL in the post‐alloSCT setting. The patient's symptoms improved within 1 week, and an MRI at 5 weeks showed a CR.
2. DISCUSSION
Central nervous system relapse of MCL is a rare event without any standard therapeutic approach and few reports in the literature (Table 1). The NCCN guidelines do not include this finding in their recommendations.10 A review of CNS MCL described the treatment approach for four patients.7 These patients received dexamethasone; dexamethasone, intrathecal methotrexate (IT MTX), radiation therapy, and hyperCVAD; dexamethasone, IT MTX and radiation; and dexamethasone with radiation therapy. Survival ranged from 2 to 9 months. Two other studies have reviewed the survival of MCL after CNS relapse.3, 6 In these retrospective studies, various combinations of high‐dose methotrexate, radiation therapy, and intrathecal chemotherapy were used, and the median overall survival was 3.7 and 8.3 months, respectively. Unfortunately, no statistical comparison of different regimens was reported. Most recently, four studies reported on the efficacy of ibrutinib in MCL patients with CNS relapse.11, 12, 13 Bernard et al11 reported a rapid response to full‐dose ibrutinib in three patients with intracerebral, symptomatic CNS relapse. One patient with concomitant systemic disease and another with an intraspinal lesion, achieved a CR. The third had CSF involvement and achieved a PR. None of these patients received an alloSCT prior to ibrutinib monotherapy. Mannina et al12 described a patient treated with dose‐reduced ibrutinib after CNS relapse isolated to the CSF who achieved a CR without a prior alloSCT. Vitagliano et al14 reported a CR to dose‐reduced ibrutinib in a patient with both CSF and systemic CNS relapse, ongoing at 17 months. A case series from the United Kingdom reviewed five patients who received full‐dose ibrutinib with response durations ranging from 6 days to 5 months at the time of publication.13 One patient received an alloSCT prior to ibrutinib with methylprednisolone and achieved a PR. While these studies support the efficacy of ibrutinib in CNS MCL, only one described a patient who received ibrutinib therapy after alloSCT, with a shorter duration and only partial response in comparison to our patient's complete response after alloSCT. Additionally, this patient also received methylprednisolone with ibrutinib therapy, which could confound the results for ibrutinib efficacy. Interestingly, ibrutinib has been described in the post‐alloSCT setting, but patients with CNS MCL were not evaluated.15
Table 1.
Comparison of CNS‐relapsed mantle cell lymphoma cases treated with ibrutinib in the literature
| Case 1 (Tucker) | Case 2 (Tucker | Case 3 (Tucker) | Case 4 (Tucker) | Case 5 (Tucker) | Case 6 (Vitagliano) | Case 7 (Bernard) | Case 8 (Bernard) | Case 9 (Bernard) | Case 10 (Mannina) | Ours | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 54 | 55 | 65 | 58 | 57 | 64 | 61 | 62 | 77 | 59 | 51 |
| Sex | M | M | M | M | M | F | M | M | F | M | F |
| ECOG | 3 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 4 | 0 | 0 |
| Ann Arbor | IV | III | IV | IV | Ie | IV | IV | IV | IV | IV | IV |
| MIPI | High | Int. | High | High | High | 8.7 | 5.5 (at CNS rel) | 5.8 (at CNS rel) | 7.7 (at CNS rel) | 5 (at dx) | 8 (5.5 at Dx) |
| CNS involvement at diagnosis? | No | No | No | No | No | No | No | ? | ? | ? | No |
| # Prior lines | 2 | 1 + SCT | 1 | 1 | 2 | 2 | 3 | 2 | 2 | 1 | |
| Previous treatments | R‐HiDAC, R‐CHOP‐V | R‐HiDAC‐D | R‐CHOP | R‐HiDAC | R‐HiDAC/R‐CHOP | R‐CEOP, R‐BAC + IT Cytarabine (HiDAC?) | R‐BEAM (high dose) | R‐BEAM (high dose) | ? | R‐CHOP | R‐CHOP/R‐DHAP, R‐MPV |
| Previous SCT | No | Allo | No | No | No | No | Auto | Auto | No | No | Allo |
| # CNS relapses prior to initiation | 2 | 2 | 1 | 1 | 1 (?) | 2 | 1 | 1 | ? | 1 | 3 |
| CNS therapy | Ibrutinib 560 mg/d | Ibrutinib 560 mg/d + methylprednisolone | Ibrutinib 560 mg/d + Dexamethasone + IT Cytarabine | Ibrutinib 560 mg/d | Ibruitinb 560 mg/d | Ibrutininb 560 mg initially + Depocyte, Ibrutinib reduced to 420 mg/d | Ibrutinib 560 mg/d | Ibrutinib 560 mg/d | Ibrutinib 560 mg/d | Ibrutinib 280 mg/d | Ibrutinib 560 mg/d |
| Clinical time to response | 1‐2 wk | 2 wk | 2 wk | 1 wk | 1 d | ? Follow up at 2 mo (−) | 1 wk | 3 d | 1 wk | ? | 1 wk |
| Response to ibrutinib | PR | PR | CR | PR | Transient PR | CR | CR | CR | PR | CR | CR |
?, Unknown.
Our patient had an unorthodox treatment course prior to initiation of her ibrutinib monotherapy. Our standard approach for young, fit patients is a regimen that includes high‐dose cytarabine followed by autoSCT. This patient would have been a candidate for this approach in the frontline setting, but we did not meet her until after she completed induction therapy. Additionally, when she relapsed in her CNS for the first time, she received a dose of bendamustine followed by radiation to her spinal lesion. While the radiation she received is a reasonable palliative approach until more intensive therapy can be pursued, we are not sure why she received one dose of bendamustine. This was not likely to be effective, since she already relapsed after six cycles of bendamustine‐rituximab, but was given at an outside facility. When we did see her, we recommended R‐CHOP/R‐DHAP, which is a standard high‐dose cytarabine‐based regimen. When she had her second CNS relapse, it was limited to the brain and our goal was to give induction therapy followed by consolidation with an alloSCT. We extrapolated from the primary CNS literature and proceeded with R‐MPV. Although not described in MCL previously, R‐MPV was a highly effective induction regimen that resulted in a CR and allowed the patient to receive an alloSCT.
In this report, we describe the safe and effective use of ibrutinib monotherapy for the treatment of a third CNS relapse of MCL after alloSCT. The patient had resolution of her CNS symptoms after 1 week of therapy and achieved a CR on MRI imaging after 5 weeks of ibrutinib therapy. Despite prior description of ibrutinib use in CNS MCL, to our knowledge, this is the first description of ibrutinib leading to a CR in CNS MCL in the post‐alloSCT setting.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
JDR: contributed to conception and design; drafting and editing manuscript. SMC: contributed to conception and design; editing manuscript. YF: provided pathology photomicrographs and interpretation; editing manuscript. VJ: provided radiographic images and interpretation; editing manuscript. WW: contributed to conception and design; editing manuscript. CD: contributed to conception and design; drafting and editing manuscript.
Rich JD, Clark SM, Fedoriw Y, Jewells V, Wood W, Dittus C. Complete remission with ibrutinib after allogeneic stem cell transplant for central nervous system relapse of mantle cell lymphoma: A case report and literature review. Clin Case Rep. 2019;7:1957–1961. 10.1002/ccr3.2257
REFERENCES
- 1. Cheah CY, Seymour JF, Wang ML. Mantle cell lymphoma. J Clin Onc. 2016;34(11):1256‐1269. [DOI] [PubMed] [Google Scholar]
- 2. Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced‐stage mantle cell lymphoma. Blood. 2008;111(2):558‐565. [DOI] [PubMed] [Google Scholar]
- 3. Chihara D, Asano N, Ohmachi K, et al. Ki‐67 is a strong predictor of central nervous system relapse in patients with mantle cell lymphoma (MCL). Ann Onc. 2015;26(5):966‐973. [DOI] [PubMed] [Google Scholar]
- 4. Bernard M, Gressin R, Lefrère F, et al. Blastic variant of mantle cell lymphoma: a rare but highly aggressive subtype. Leukemia. 2001;15(11):1785‐1791. [DOI] [PubMed] [Google Scholar]
- 5. Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130(17):1903‐1910. [DOI] [PubMed] [Google Scholar]
- 6. Cheah CY, George A, Giné E, et al. Central nervous system involvement in mantle cell lymphoma: clinical features, prognostic factors and outcomes from the European Mantle Cell Lymphoma Network. Ann Onc. 2013;24(8):2119‐2123. [DOI] [PubMed] [Google Scholar]
- 7. Gill S, Herbert KE, Prince HM, et al. Mantle cell lymphoma with central nervous system involvement: frequency and clinical features. Br J Haematol. 2009;147(1):83‐88. [DOI] [PubMed] [Google Scholar]
- 8. Ferrer A, Bosch F, Villamor N, et al. Central nervous system involvement in mantle cell lymphoma. Ann Oncol. 2007;19:135‐141. [DOI] [PubMed] [Google Scholar]
- 9. Mussetti A, Devlin SM, Castro‐Malaspina HR, et al. Non‐myeloablative allogeneic hematopoietic stem cell transplantation for adults with relapsed and refractory mantle cell lymphoma: a single center analysis in the rituximab era. Bone Marrow Transplant. 2015;50(10):1293‐1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Comprehensive Cancer Network . Mantle cell lymphoma, (Version 2.2016). https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed September 26, 2017.
- 11. Bernard S, Goldwirt L, Amorim S, et al. Activity of ibrutinib in mantle cell lymphoma patients with central nervous system relapse. Blood. 2015;126(14):1695‐1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mannina D, Loteta B. Ibrutinib treatment of mantle cell lymphoma relapsing at central nervous system: a case report and literature review. Case Rep Hematol. 2017;2017:9583257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tucker DL, Naylor G, Kruger A, Hamilton MS, Follows G, Rule SA. Ibrutinib is a safe and effective therapy for systemic mantle cell lymphoma with central nervous system involvement‐ a multi‐centre case series from the United Kingdom. Br J Haematol. 2017;178(2):327‐340. [DOI] [PubMed] [Google Scholar]
- 14. Vitagliano O, Trastulli F, Cacace F, et al. Ibrutinib as salvage therapy in mantle cell lymphoma with central nervous system involvement in a pretreated unfit patient. Leuk Lymphoma. 2017;59(7):1734‐1737. [DOI] [PubMed] [Google Scholar]
- 15. Michallet M, Dreger P, Sobh M, et al. Salvage use of ibrutinib after allogeneic hematopoietic stem cell transplantation (allo‐HSCT) for B cell malignancies: a study of the French cooperative group for CLL, the French society for blood and marrow transplantation (SFGM‐TC) and the European society for blood and marrow transplantation (EBMT) chronic malignancy and lymphoma working parties. Blood. 2016;128(22):4659. [Google Scholar]


