Abstract
PDAC bone metastases represent a clinical challenge characterized by multifaceted biological entity.
Keywords: bone metastases, pancreatic ductal adenocarcinoma, PDAC, zoledronic acid
1. INTRODUCTION
Bone metastases are more frequent in prostate, breast, and lung cancers.1 Their occurrence represents a clinical issue leading to hypercalcemia, severe pain, and risk of spinal cord injury contributing to an unfavorable prognosis and a much worse quality of life.2 Gastrointestinal tumors metastasize less common to the skeleton with a particular low incidence in PDAC patients whose disease is characterized by a more frequent metastatization to liver, peritoneal cavity, and lungs.3, 4, 5, 6, 7 The most common site of bone metastases is the spine which is associated with back pain that could be confused with symptoms correlated with the primary tumor.8 In a few cases, the skeleton may represent its sole metastatic site.6, 9
In this study, we describe a rare case of bone vertebral metastasis from PDAC which appeared at the onset of the disease.
2. CASE PRESENTATION
A 64‐year‐old man presented severe back pain. He did not report trauma or comorbidities for osteoarticular pathologies in his past medical history. Drugs prescribed for pain control did not relieve symptoms. X‐ray examination showed mild lumbar right‐convex scoliosis and signs of osteophytic spondylosis. Vertebral discomfort and aggravating pain at the level of D11 and D12 vertebrae prompted a neurological assessment which revealed slight inferior limb numbness and weakness, mainly in the left leg in combination with hypoesthesia and dysesthesia in the same region; perineal reflexes were present. Computerized tomography images of vertebral column revealed bone rarefaction with D11 vertebral fracture and spinal cord compression (Figure 1). Laboratory tests showed an increase of carcinoembryonic antigen serum levels (61 ng/mL), while prostate‐specific antigen, beta 2 microglobulin, and CA 19.9 were within the normal ranges.
Figure 1.
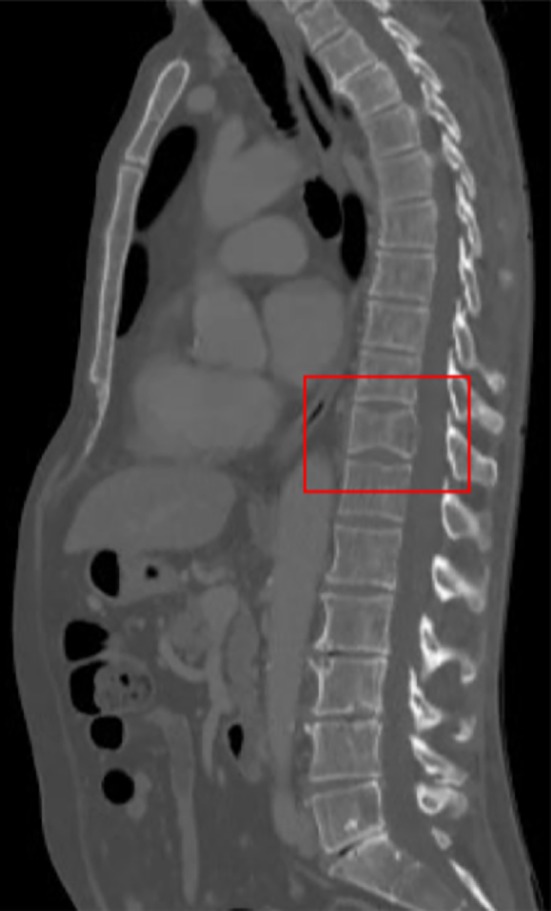
Computerized tomography images of vertebral column revealed bone rarefaction with D11 vertebral fracture and spinal cord compression
Total‐body computerized tomography revealed only a 23 × 14 mm ill‐defined mass at the pancreatic tail with loss of acinar structure. Percutaneous vertebroplasty in emergency with vertebral biopsy was performed. Bone scan showed a hypocaptation area at D11 vertebral, and abnormal uptake in the right iliac wing, iliac crest, left clavicle, bilateral ribs, and D10 vertebra (Figure 2). Histopathological examination of vertebra soft tissue biopsy led to the diagnosis of metastatic adenocarcinoma with features resembling pancreatic cancer (Figure 3A‐B).
Figure 2.
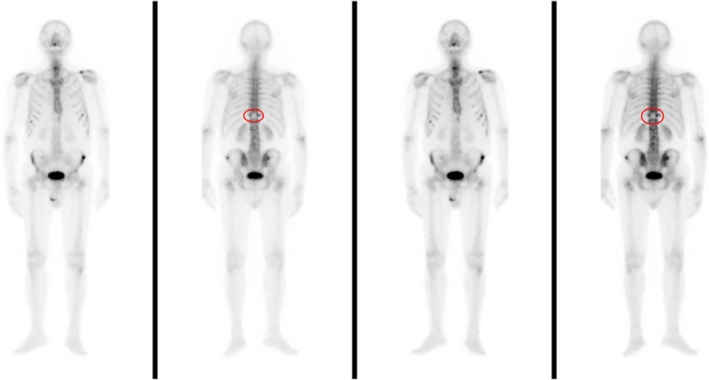
Bone scan showed a hypocaptation area at D11 vertebral, and abnormal uptaking in the right iliac wing, iliac crest, left clavicle, bilateral ribs, and D10 vertebra. In the red rounds the result of percutaneous vertebroplasty
Figure 3.
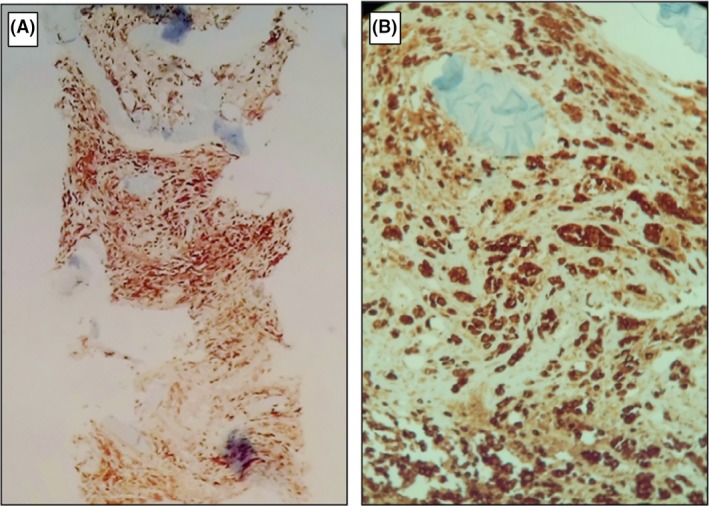
A, Vertebral biopsy speciem 2.5×, CKAE1/AE3 diffusely positive. B, 10×, CKAE1/AE3 diffusely positive in metastasis of adenocarcinoma with acinar pattern
A first‐line chemotherapy regimen was administered with gemcitabine plus nab‐paclitaxel in combination with zoledronic acid. Unfortunately, an early disease bone and liver progression was observed with an overall survival of 6 months.
3. DISCUSSION
PDAC is thought to become the second leading cause of cancer death by 2030 with a 5‐year overall survival rate around 7%.10, 11 The metastatic process is strictly connected to tumor intrinsic and extrinsic characteristics.12 As in other several solid and hematologic cancer,13, 14, 15 the tumor microenvironment emerged as a pivotal driver of metastatic niche 16 being correlated at the same time to cell‐adhesion dependent and independent drug‐resistance development.17, 18, 19, 20 Thus, several approaches have been envisioned in order to molecularly overcome this malignant phenotype, in order to target both the cancer cells and the tumoral milieu.21, 22, 23 Variable incidence of bone metastases from PDAC reported in literature (from 5% to 20%) should be conditioned by either the possible overlapping between symptoms related to the primary tumor and bone localization or the longer survival obtained in the last few years due the availability of new and more active chemotherapy regimens in both adjuvant and advanced settings.6, 24, 25, 26, 27 The predominance of osteolytic or osteoblastic bone metastasis is controversial in these patients.8, 9, 28
We reported a case of a metastatic PDAC with an osteoblastic/osteolytic bone involvement of both the right iliac wing and the body of the D11 and L3 vertebrae, the main sites of bone colonization in PDAC. Diagnostic biopsy was performed during the vertebroplasty, while the diagnosis of a primitive pancreatic cancer was performed through radiological images.
Our choice of the gemcitabine plus nab‐paclitaxel systemic regimen was supported by an intriguing preclinical study demonstrating that association between nitrogen‐containing bisphosphonates (ie, zoledronic acid) and nab‐paclitaxel reduced fibrosis, peritoneal dissemination, angiogenesis, and cell proliferation.26, 29 Furthermore, in vitro studies showed an antitumor activity of zoledronic acid against PDAC cells.30, 31 In addition, zoledronic acid has been reported to stimulate lymphocytes antitumor response through γδ‐type T cell receptors and impact on the median time to the first skeletal‐related event and on overall survival.32, 33, 34, 35, 36 Remarkably, also innate immunity seems to be related to zoledronic acid efficacy, via tumor‐associated macrophages (TAM).37 Nonetheless, we observed a very poor survival in our patient.
Scanty data are available in the literature concerning the clinical outcome of PDAC patients with bone metastases. Borad et al reported a series of seven patients who developed skeletal metastases between 2 and 17.3 months (median: 5.5 months) after the onset of the disease. Survival from the evidence of skeletal metastases ranged between 0.3 and 9 months. Most of these patients (71.4%) received bisphosphonates in combination with systemic chemotherapy.8 Rades et al collected data of 15 PDAC with metastatic epidural spinal cord compression from PDAC who underwent radiotherapy. Three of them showed an improvement of motor function, while the others demonstrated no further progression or deterioration. This improvement was significantly associated with the absence of visceral involvement at the time of radiotherapy (P = .025), with a 6‐month survival rate of 33%. Moreover, the same authors demonstrated that radiotherapy of 1 × 8 Gy appeared to be not inferior to multi‐fraction radiation schedules considering the post‐treatment motor function in patients with vertebral involvement in PDAC.38 Habermehl et al described data of 33 patients with PDAC affected by bone metastases. The investigators showed a median overall survival of 3.1 months (95% CI 1.9‐4.3) and presented survival rates of 75.3%, 46.5%, and 19.9% after 1, 3, and 6 months, respectively. Treatment protocols were different, with most patients treated with 3000 cGy in 10 fractions and a median treatment duration of 15 days.39 Few reports described the role of surgery in the management of bone involvement in PDAC. Chih et al reported a patient with L2 vertebra involvement treated with percutaneous vertebroplasty with an improvement of performance status and pain control.40
Survival of our patient was lower than the median overall survival of a metastatic PDAC patient treated with first‐line gemcitabine and nab‐paclitaxel. In previous reports, we described a relationship between bone metastases and poorer prognosis in patients affected by gastric cancer and hepatocellular carcinoma.34, 36 Recently, several data have suggested a potential prognostic and predictive role of molecular characterization in PDAC patients.41 As emerged for invasive PDAC the genomic landscape can represent the new frontier to envision therapeutic solutions for aggressive disease.42, 43
Conclusively, we hypothesize that bone metastatization could represent the phenotypic expression of the underlying molecular PDAC subgroups characterized by unfavorable outcome. This patient's subgroup might be characterized by peculiar prognostic feature, being potentially candidate to specific targeted therapies.44
CONFLICT OF INTEREST
The authors have no conflict of interests.
AUTHOR CONTRIBUTION
AA, OB, AGS: Conceptualization, AC, AA, AN and AC: methodology, MMGP, AA, AN, AC and AGS: formal analysis, AA, OB and AGS: investigation, AA, MMGP and AN: data curation, AA, OB and AGS: writing—original draft preparation, AA and OB: writing—review and editing, AA and OB: resources, OB and AGS: supervision, AGS: funding acquisition.
ACKNOWLEDGMENTS
This research project was supported in part by the Apulian Regional Project “Medicina di Precisione”.
Argentiero A, Calabrese A, Solimando AG, Notaristefano A, Panarelli MMG, Brunetti O. Bone metastasis as primary presentation of pancreatic ductal adenocarcinoma: A case report and literature review. Clin Case Rep. 2019;7:1972–1976. 10.1002/ccr3.2412
REFERENCES
- 1. Yin JJ, Pollock CB, Kelly K. Mechanisms of cancer metastasis to the bone. Cell Res. 2005;15:57‐62. [DOI] [PubMed] [Google Scholar]
- 2. Santoni M, Conti A, Procopio G, et al. Bone metastases in patients with metastatic renal cell carcinoma: are they always associated with poor prognosis? J Exp Clin Cancer Res. 2015;34:10 10.1186/s13046-015-0122-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oweira H, Petrausch U, Helbling D, et al. Prognostic value of site‐specific metastases in pancreatic adenocarcinoma: a surveillance epidemiology and end results database analysis. World J Gastroenterol. 2017;23(10):1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Portales F, Thézenas S, Samalin E, et al. Bone metastases in gastrointestinal cancer. Clin Exp Metas. 2014;32:7‐14. 10.1007/s10585-014-9686-x [DOI] [PubMed] [Google Scholar]
- 5. Longo V, Brunetti O, D'Oronzo S, et al. Bone metastases in hepatocellular carcinoma: an emerging issue. Cancer Metastasis Rev. 2014;33:333‐342. 10.1007/s10555-013-9454-4 [DOI] [PubMed] [Google Scholar]
- 6. Saif MW, Galanina N, Ravage‐Mass LB, et al. Bone metastasis as the only metastatic site in a patient with pancreatic cancer following distal pancreatectomy. Case Reports in Medicine. 2010;2010:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iguchi H, Yasuda M, Matsuo T, et al. Clinical features and management of pancreatic cancer with bone metastases. Nippon Shokakibyo Gakkai Zasshi. 2004;101:872‐878. [PubMed] [Google Scholar]
- 8. Borad MJ, Saadati H, Lakshmipathy A, et al. Skeletal metastases in pancreatic cancer: retrospective study and review of the literature. Yale Journal of Biology and Medicine. 2009;82:1‐6. [PMC free article] [PubMed] [Google Scholar]
- 9. Pneumaticos SG, Savidou C, Korres DS, et al. Pancreatic cancer's initial presentation: back pain due to osteoblastic bone metastasis. Eur J Cancer Care (Engl). 2010;19:137‐140. [DOI] [PubMed] [Google Scholar]
- 10. Siegel R, Miller KD, Jemal A. Cancer Statistics. CA Cancer J Clin. 2017;67:7‐30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 11. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913‐2921. 10.1158/0008-5472.CAN-14-0155 [DOI] [PubMed] [Google Scholar]
- 12. Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang M, Zhao J, Zhang L, et al. Role of tumor microenvironment in tumorigenesis. Journal of Cancer. 2017;8(5):761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 15. Di Marzo L, Desantis V, Solimando AG, et al. Microenvironment drug resistance in multiple myeloma: emerging new players. Oncotarget. 2016;7(37):60698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fu Y, Liu S, Zeng S, et al. The critical roles of activated stellate cells‐mediated paracrine signaling, metabolism and onco‐immunology in pancreatic ductal adenocarcinoma. Mol Cancer. 2018;17(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shain KH, Dalton WS. Cell adhesion is a key determinant in de novo multidrug resistance (MDR): new targets for the prevention of acquired MDR. Mol Cancer Ther. 2001;1(1):69‐78. [PubMed] [Google Scholar]
- 18. Solimando AG, Brandl A, Mattenheimer KM, et al. JAM‐A as a prognostic factor and new therapeutic target in multiple myeloma. Leukemia. 2018;32(3):736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi Y, Gao W, Lytle NK, et al. Targeting LIF‐mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature. 2019;569(7754):131‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Porcelli L, Iacobazzi RM, Di Fonte R, et al. CAFs and TGF‐β Signaling activation by mast cells contribute to resistance to gemcitabine/nabpaclitaxel in pancreatic cancer. Cancers. 2019;11(3):330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chand S, O'Hayer K, Blanco FF, et al. The landscape of pancreatic cancer therapeutic resistance mechanisms. Int J Biol Sci. 2016;12(3):273‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rudelius M, Rosenfeldt MT, Leich E, et al. Inhibition of focal adhesion kinase overcomes resistance of mantle cell lymphoma to ibrutinib in the bone marrow microenvironment. Haematologica. 2018;103(1):116‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Silvestris N, Brunetti O, Vasile E, et al. Multimodal treatment of resectable pancreatic ductal adenocarcinoma. Crit Rev Oncol Hematol. 2017;111:152‐165. [DOI] [PubMed] [Google Scholar]
- 25. Silvestris N, Longo V, Cellini F, et al. Neoadjuvant multimodal treatment of pancreatic ductal adenocarcinoma. Crit Rev Oncol Hematol. 2016;98:309‐324. [DOI] [PubMed] [Google Scholar]
- 26. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817‐1825. [DOI] [PubMed] [Google Scholar]
- 28. Joffe N, Antonioli DA. Osteoblastic bone metastases secondary to adenocarcinoma of the pancreas. Clin Radiol. 1978;29:41‐46. [DOI] [PubMed] [Google Scholar]
- 29. Gonzalez‐Villasana V, Rodriguez‐Aguayo C, Arumugam T, et al. Bisphosphonates inhibit stellate cell activity and enhance antitumor effects of nanoparticle albumin‐bound paclitaxel in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2014;13:2583‐2594. 10.1158/1535-7163.MCT-14-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Elsayed M, Kobayashi D, Kubota T, et al. Synergistic antiproliferative effects of zoledronic acid and fluvastatin on human pancreatic cancer cell lines: an in vitro study. Biol Pharm Bull. 2016;39(1238–1246):1. [DOI] [PubMed] [Google Scholar]
- 31. Yamaguchi Y, Katata Y, Okawaki M, et al. A prospective observational study of adoptive immunotherapy for cancer using zoledronate‐activated killer (ZAK) cells ‐ an analysis for patients with incurable pancreatic cancer. Anticancer Res. 2016;36:2307‐2313. [PubMed] [Google Scholar]
- 32. Miyagawa F, Tanaka Y, Yamashita S, et al. Essential requirement of antigen presentation by monocyte lineage cells for the activation of primary human gamma delta T cells by amino bisphosphonate antigen. J Immunol. 2001;166:5508‐5514. [DOI] [PubMed] [Google Scholar]
- 33. Santini D, Procopio G, Porta C, et al. Natural history of malignant bone disease in renal cancer: final results of an Italian bone metastasis survey. PLoS One. 2013, 8(12):e83026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Santini D, Pantano F, Riccardi F, et al. Natural history of malignant bone disease in hepatocellular carcinoma: final results of a multicenter bone metastasis survey. PLoS One. 2014;9(8):e105268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santini D, Tampellini M, Debus J, et al. Natural history of bone metastasis in colorectal cancer: final results of a large Italian bone metastase study. Ann Oncol. 2012;23:2072‐2077. 10.1093/annonc/mdr572 [DOI] [PubMed] [Google Scholar]
- 36. Silvestris N, Pantano F, Ibrahim T, et al. Natural history of malignant bone disease in gastric cancer: final results of a multicenter bone metastasis survey. PLoS One. 2013;8(10):e74402 10.1371/journal.pone.0074402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valilou SF, Keshavarz‐Fathi M, Silvestris N, et al. The role of inflammatory cytokines and tumor associated macrophages (TAMs) in microenvironment of pancreatic cancer. Cytokine Growth Factor Rev. 2018;39:46‐61. [DOI] [PubMed] [Google Scholar]
- 38. Rades D, Huttenlocher S, Schild SE, et al. Metastatic spinal cord compression from pancreatic cancer. Anticancer Res. 2014;34:3727‐3730. [PubMed] [Google Scholar]
- 39. Habermehl D, Brecht IC, Debus J, et al. Palliative radiation therapy in patients with metastasized pancreatic cancer ‐ description of a rare patient group. Eur J Med Res. 2014;19:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chih YP, Wu WT, Lin CL, et al. Vertebral compression fracture related to pancreatic cancer with osteoblastic metastasis: a case report and literature review. Medicine (Baltimore). 2016;95(5):e2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47. [DOI] [PubMed] [Google Scholar]
- 43. Argentiero A, De Summa S, Di Fonte R, et al. Gene expression comparison between the lymph node‐positive and negative reveals a peculiar immune‐microenvironment signature and a theranostic role for wnt targeting in pancreatic ductal adenocarcinoma. A Pilot Study. Cancers. 2019;11(7):942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Santini D, Galluzzo S, Zoccoli A, et al. New molecular targets in bone metastases. Cancer Treat Rev. 2010;36(Suppl 3):S6‐S10. [DOI] [PubMed] [Google Scholar]


