Abstract
Background:
Acute kidney injury (AKI) is a complex and heterogeneous clinical syndrome with limited effective treatment options. Therefore, a coherent research structure considering AKI pathophysiology, treatment, translation, and implementation is critical to advancing patient care in this area.
Purpose of review:
In this narrative review, we discuss novel therapies for AKI from their journey from bench to bedside to population and focus on roadblocks and opportunities to their successful implementation.
Sources of information:
Peer-reviewed articles, opinion pieces from research leaders and research funding agencies, and clinical and research expertise.
Methods:
This narrative review details the challenges of translation of preclinical studies in AKI and highlights trending research areas and innovative designs in the field. Key developments in preclinical research, clinical trials, and knowledge translation are discussed. Furthermore, this article discusses the current need to involve patients in clinical research and the barriers and opportunities for effective knowledge translation.
Key findings:
Preclinical studies have largely been unsuccessful in generating novel therapies for AKI, due both to the complexity and heterogeneity of the disease, as well as the limitations of commonly available preclinical models of AKI. The emergence of kidney organoid technology may be an opportunity to reverse this trend. However, the roadblocks encountered at the bench have not precluded researchers from running well-designed and impactful clinical trials, and the field of renal replacement therapy in AKI is highlighted as an area that has been particularly active. Meanwhile, knowledge translation initiatives are bolstered by the presence of large administrative databases to permit ongoing monitoring of clinical practices and outcomes, with research output from such evaluations having the potential to directly impact patient care and inform the generation of meaningful clinical practice guidelines.
Limitations:
There are limited objective data examining the process of knowledge creation and translation in AKI, and as such the opinions and research areas of the authors are significantly drawn upon in the discussion.
Implications:
The use of an organized knowledge-to-action framework involving multiple stakeholders, especially patient partners, is critical to translating basic research findings to improvements in patient care in AKI, an area where effective treatment options are lacking.
Keywords: translational medical research, acute kidney injury, kidney organoids, randomized controlled trials as topic, patient-centered care, community-based participatory research
Abrégé
Contexte:
L’insuffisance rénale aigüe (IRA) est un syndrome clinique complexe et hétérogène pour lequel les options de traitement efficaces sont limitées. Ainsi, une structure de recherche cohérente, tenant compte de la physiopathologie et du traitement de l’IRA, de même que de la transposition et de l’application des résultats de recherche, est essentielle à l’avancement des soins aux patients.
Justification:
Dans cette revue narrative, nous discutons des nouveaux traitements de l’IRA, et du parcours que ces derniers empruntent du laboratoire au chevet des patients, et jusqu’à la population générale, en se concentrant sur les obstacles et les facilitateurs qui influencent la réussite de leur application.
Sources:
Des articles révisés par les pairs, des avis d’éminents chercheurs et d’organismes de financement de la recherche, de même que l’expertise clinique et de recherche des auteurs.
Méthodologie:
Cette revue narrative expose les défis de la transposition des études précliniques en IRA, et met en lumière les nouveaux axes de recherche et les modèles novateurs dans le domaine. La discussion porte également sur les principaux développements en recherche préclinique, en essais cliniques et en transfert des connaissances. Enfin, cet article aborde la nécessité d’impliquer les patients en recherche clinique, de même que les obstacles et possibilités pour une transfert efficace de connaissances.
Principaux résultats:
Les études précliniques ont en grande partie échoué à proposer de nouveaux traitements à l’IRA en raison de la complexité et de l’hétérogénéité de la maladie, mais également des limites inhérentes aux modèles précliniques communément utilisés. Une tendance qui pourrait s’inverser grâce à l’émergence de la technologie des organoïdes rénaux. Les difficultés rencontrées au laboratoire n’ont toutefois pas empêché les chercheurs de mener des essais cliniques significatifs et bien conçus; la recherche sur les thérapies de remplacement rénal est d’ailleurs un domaine de recherche particulièrement actif. Parallèlement, les initiatives visant le transfert des connaissances sont appuyées par d’importantes bases de données administratives qui permettent un suivi constant des pratiques et des résultats cliniques; les résultats des recherches issues de ces évaluations pourraient avoir une incidence directe sur les soins aux patients et l’élaboration de lignes directrices pertinentes en matière de pratique clinique.
Limites:
Il existe peu de données objectives examinant la création et le transfert de connaissances en IRA. À ce titre, les opinions et domaines de recherche des auteurs sont largement pris en compte dans la discussion.
Conclusion:
Un cadre de recherche « du savoir à l’action » impliquant plusieurs intervenants, surtout des patients partenaires, est essentiel à l’application des découvertes de la recherche fondamentale et à l’amélioration des soins aux patients atteints d’IRA; un domaine où les options de traitement efficaces font défaut.
Why is this review important?
Acute Kidney Injury (AKI) is a common clinical syndrome for which few effective therapies are available. Basic research has an important role in the discovery of novel treatments for AKI, but the translation of those discoveries into clinical practice has been challenging. An understanding of barriers impeding the process of clinical translation is essential to reducing research waste and optimizing outcomes for patients with AKI.
What are the key messages?
The use of a comprehensive research framework to translate research findings from the bench to the bedside could enhance the generation of novel therapies for AKI. Such a strategy must bridge the two “Death Valleys” of AKI research: from basic biomedical research to clinical trials, and from synthesized and aggregated research findings to implementation in clinical practice.
Introduction
Acute kidney injury (AKI) is a rapid decline in glomerular filtration rate that is common in hospitalized adults, with an overall incidence of 22%1 that varies depending on the setting, population, and definition of AKI.2-4 AKI is associated with an increased risk of morbidity, including cardiovascular (CV) events,5 the development of chronic kidney disease (CKD)6 and end-stage renal disease (ESRD),7 reduced health-related quality of life,8,9 and mortality,5,10 but whether or not this is causal is uncertain. Regardless, AKI is important from a patient, clinician, and health care system perspective given its associations with adverse outcomes and health care costs.11
Acute kidney injury is a heterogeneous clinical syndrome, the classification of which does not provide any insight into its complex pathophysiology. Pre-renal causes and acute tubular necrosis (ATN) are responsible for 65% to 75% of AKI, but even within these episodes, there is interindividual variability in responses to injury and outcomes. There are many different causes of ATN including hypotension, surgery, sepsis, and nephrotoxins that often occur with other comorbidities and conditions such as diabetes, CKD, and heart failure which may modify the underlying pathophysiology of AKI. The pathogenesis of ATN involves an impairment of renal perfusion leading to ischemia, injury to endothelial and epithelial cells leading to intratubular obstruction, immune activation, and inflammation12 followed by repair and recovery. Currently, therapies for the prevention or treatment of ATN are limited. Despite a multitude of treatments evaluated, including diuretics, dopamine, fenoldopam, atrial natriuretic peptide, N-acetylcysteine, statins, corticosteroids, off-pump CV surgery, and remote ischemic preconditioning, these strategies have failed to improve outcomes and management is largely supportive with dialysis if necessary. Novel approaches for the detection, prevention, and treatment of AKI are needed to improve patient outcomes and reduce the global burden of disease.13
In this narrative review, we discuss novel therapies for AKI from their journey from bench to bedside to population and focus on roadblocks and opportunities to their implementation. We propose potential solutions to the 2 “death valleys” of research including the first from basic biomedical research in the laboratory to the bedside and the second that involves synthesizing, disseminating, and integrating research into clinical practice and policy. We focus on stem cell research and regenerative medicine as an example for basic science and randomized clinical trials (RCTs) for the timing of dialysis initiation as an example of clinical research.
Challenges of Preclinical Studies
The road toward novel therapies for AKI begins in the preclinical arena, which involves research on mammalian kidney cells cultured in vitro and live studies on nonhuman animals. These cell cultures and live animal models are essential for expanding our knowledge of the pathophysiology of disease processes like AKI, as such studies usually cannot be performed on humans due to ethical concerns. These models can also be used to rapidly screen novel therapies for toxicity and efficacy, to identify the safest and most promising target candidates for human translation. Among animal models, which better recapitulate multiorgan physiology than isolated cells, rodents have risen to prominence, as they are relatively inexpensive, easy to house, and amenable to precise genetic and environmental manipulation. Unlike the case with humans, a cohort of genetically identical (inbred) rodents can be studied to minimize heterogeneity and strengthen the association between the disease condition studied and the phenotype observed. Still, important limitations to the use of animal models must be kept in mind. For instance, genetic homogeneity can be a confounder when an unidentified genetic variant interacts with the intervention being studied, which may mislead investigators into concluding that the resulting phenotype is directly attributable to the intervention administered. In addition, animal physiology differs from that of humans in relevant ways. For example, mice have an elevated metabolic rate of roughly 7 times that of humans, associated with increased cellular mitochondrial content and increased mass of metabolically active tissues such as the liver, kidney, and brown adipose tissue.14 Consequently, they have evolved a higher capillary density and lower hemoglobin affinity to permit the effective delivery of oxygen to peripheral tissues, in turn resulting in the increased generation of reactive oxygen species and oxidative damage.14 These and other rodent-human differences are especially relevant to models of renal disease, as the kidney is a highly vascularized and metabolically active organ. For example, rodents exhibit significant resistance to the development of diabetic and hypertensive nephropathies and often require much higher weight-adjusted doses of nephrotoxins such as gentamicin to induce ATN.15 As a result, models that have been developed to induce phenotypes of human renal diseases in rodents may be driven by different disease mechanisms and may respond very differently to therapies than would be observed with human disease.
In addition to properly modeling the disease itself, preclinical studies must also recreate relevant disease contexts. First, patient-specific contexts, including comorbid medical conditions such as diabetes and CV disease, are known to significantly modulate susceptibility to AKI and may thus influence response to therapy.16,17 It is becoming increasingly apparent that genetic heterogeneity within patient cohorts adds another element of complexity that may affect the response to promising therapies, and research initiatives in this area on relevant patient populations will hopefully inform the design of better preclinical models.18-21 In contrast, preclinical studies are typically performed on pure disease processes with minimal consideration given to the genetic background of the model or cell line chosen.22 The context of treatment must also be considered—many preclinical studies attempt to prevent renal injury in response to a specific insult, whereas, in clinical practice, AKI is usually established at the time therapy is being considered.22 These factors are likely at play when promising preclinical therapies have disappointing outcomes in human studies. Meanwhile, careful attention to disease contexts in promising preclinical studies may also improve clinical translation by informing the design of subsequent human studies.
Finally, and perhaps of most concern, is the endemic problem of limited reproducibility of preclinical studies.23 This issue is rooted in several separate but related issues, such as a publishing culture strongly favoring the publication of positive results and lower methodological rigor in the design of interventional studies than in human clinical trials (eg, infrequent use of power calculations, not adjusting for multiplicity testing, lack of blinding and randomization).23 Standardization of experimental design could facilitate greater reproducibility of published works while also increasing relevance to human disease. For example, the Animal Models of Diabetic Complications Consortium (sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK]) mandates specific criteria before a model can be considered relevant to human diabetic nephropathy and also suggests protocols for specific techniques, such as the induction of diabetes in mice.24 Such a consortium may also improve the clinical transition of therapies for AKI.
Opportunities for Developing Novel Therapies for AKI: Kidney Organoids
Given the major limitations of preclinical models, it is not surprising that disappointingly few candidate therapies have overcome the challenges of clinical translation. A promising development in preclinical kidney research is the field of kidney organoids. Organoids are defined as in vitro cell culture systems that recapitulate both structural and functional properties of an organ of interest and are usually stem cell derived.25 Current in vitro studies rely on mature cell lines, such as the proximal tubular cell line HK-2 (human kidney 2).26,27 Such cell lines are typically genetically modified to facilitate in vitro culture and thus may have limited relevance to human physiology due to genetic or epigenetic abnormalities acquired during immortalization or prolonged culture. Kidney organoids, meanwhile, are derived from stem cells that have the broad potential to differentiate into every tissue of an adult organism, which is a developmental state known as “pluripotency.” Human pluripotent stem cells (hPSCs) have emerged as powerful tools in the study of cellular mechanisms that govern fetal kidney development, and, following several major advances, hPSCs have been successfully differentiated into kidney organoids containing a multitude of different epithelial kidney cell types.28 An important feature of kidney organoids is the recapitulation of 3-dimensional (3D) renal architecture which may enhance their ability to replicate normal kidney physiology, as compared with more conventional culture methods of growing cells on plastic as layers 1 cell thick (also known as monolayer culture).29
Despite significant advances in the ability to generate nephron and interstitial cell types in vitro, a major hurdle in kidney organoid research has been the generation of mature and functional nephron components. For instance, established protocols allow the generation of podocytes (identified by the expression of the slit diaphragm protein nephrin and the transcription factor WT1), but these podocytes are morphologically immature, as evidenced by their ill-defined basement membranes and the primitive structure of their foot processes.28,30 Recent reports have described culture conditions that improve the maturation of organoid-derived glomeruli, including the use of soft culture surfaces to enhance the formation of slit diaphragms between adjacent podocytes,31 and exposure to fluidic sheer stress to promote the formation of a vascular network within the organoid.32 There has been less focus on the generation of mature tubular epithelial cells, and assessment of maturity has been limited to characterization of ultrastructural morphology, transcriptional profiles, and expression of solute transporters. Assessed in this way, recent protocols have led to progress in tubular maturation.32,33 Demonstration of functional properties of nephrons, such as the ability to generate a selective filtrate for glomeruli, and the regulation of solute content for tubular cells, would be the ultimate test for maturity of hPSC-derived kidney organoids, but such assays remain essentially nonexistent.
Given the limited functional status of kidney organoids, the clinical potential for hPSC-derived kidney organoids is currently restricted to modeling of monogenic or congenital diseases.34-36 Moreover, their therapeutic potential remains uncertain, as organoids cannot currently be grown large enough to restore meaningful function in humans. Although limited integration of hPSC-derived renal progenitors into injured mouse kidneys has been reported, the resulting improvement in kidney function appears modest at best.37 However, kidney organoids, in their current state, do offer opportunities for use in AKI research. Kidney tubular organoids exhibit sensitivity to the known nephrotoxins cisplatin and gentamicin,38 and expression and release of the AKI biomarker (kidney injury molecule 1 [KIM-1]) in response to such injury have been demonstrated.39 Although the effect of nephrotoxins may only be present at very high drug concentrations, this injurious response supports the potential of kidney organoids for use in drug toxicity screens and drug discovery. Of course, in the absence of a functional vasculature, it is unlikely that ischemic ATN can be recapitulated in kidney organoids. In addition, the lack of multisystemic physiology (which is a major advantage of animal models) hinders the study of more complex AKI causes, particularly those involving the immune system such as tubulointerstitial nephritis and glomerulonephritis. The outlook for such in vitro disease modeling, however, is promising, particularly following recent advances in multiorgan-on-a-chip technology.40 As organoid technology continues to evolve, it may soon become a valuable preclinical tool in the arsenal of the AKI researcher.
Randomized Controlled Trials for AKI
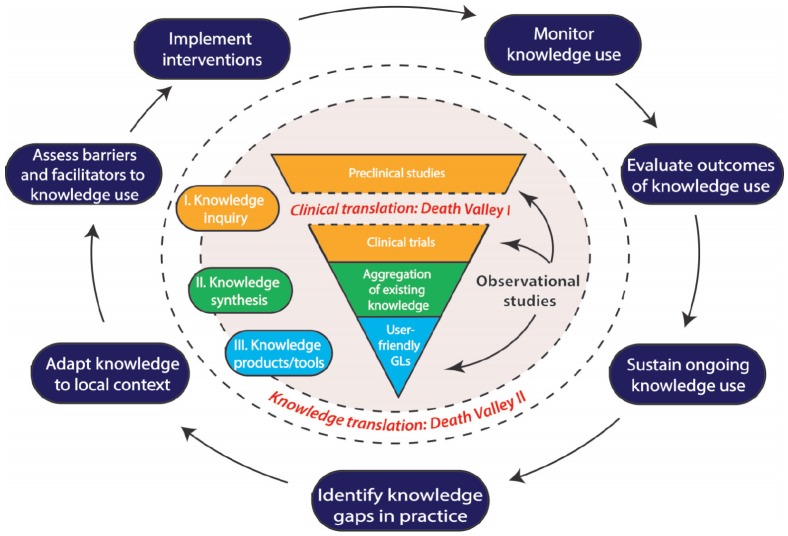
Although preclinical studies are essential to the discovery of novel therapies, trials involving humans must be performed to translate preclinical findings into treatments for patients. Identifying bench-to-bedside translation strategies for novel AKI preventative therapies and treatments can be challenging, particularly given the AKI-specific limitations of preclinical studies described above, which make it difficult to directly apply findings from preclinical studies to patients with AKI. Therefore, creative strategies are needed, to translate the knowledge both from basic science to clinical models and then from clinical models to clinical practice. Silver et al41 described those translation needs as the “death valleys of biomedical research to clinical practice.” The transfer of basic science discoveries into clinical models, “death valley 1” (see Figure 1), is known as translational research.43 Specifically for translational research, validated therapeutic targets with high probabilities of success in clinical settings are necessary prior to attempting to bridge the journey from bench to bedside. Again, the limitations of preclinical models often lead to uncertainties in intervention mechanisms, delivery, dosing, efficacy, and toxicity, and this can result in delays in the appropriate evaluation of any novel therapy. However, for those therapies that appear most promising, many of these parameters can be directly assessed by well-designed, early-phase clinical trials.
Figure 1.
Schematic illustrating the movement of research findings from preclinical models to implementation in clinical practice, based on the knowledge-to-action framework elaborated by Graham et al,70 and incorporating the concept of the “death valleys of biomedical research,” as described by Silver et al.41
Note. This pathway begins with knowledge creation, depicted within the inner gray circle. The first “death valley” occurs here, in the movement from preclinical studies to clinical trials, also known as “translational research.” Human observational studies are an important part of this process, informing preclinical studies, clinical trials, and often even guidelines (GLs) directly. The second process refers to the “Action Cycle,” or “knowledge translation,” whereby aggregated research findings are incorporated into patient care. The second “death valley” begins at the end of knowledge creation, in the generation of user-friendly tools such as guidelines, and continues into knowledge translation with attempts to implement these tools into specific health care contexts.
Randomized clinical trials are the highest quality of evidence used to inform treatment decisions given that, if appropriately performed, they minimize bias and provide accurate estimates of treatment benefits and risks. Nephrology lags behind most other specialties in the conduct of clinical trials44 because of both systemic factors (lack of experts, infrastructure, and funding) and patient factors (elderly, frail, comorbidities, and competing risks). In 2017, it was shown that although the number of clinical kidney-related trials doubled between 2004 and 2014, deficits in reporting quality including design, randomization, and intention-to-treat analysis still exist.45
Contemporary Trials for the Timing of Dialysis Initiation
Although there are many barriers in the conduct of high-quality RCTs in nephrology, the timing of dialysis initiation is a success story. Given the lack of novel therapies elaborated by preclinical studies, current RCTs in the realm of AKI have focused on optimizing outcomes with existing therapies, such as dialysis. There is uncertainty regarding the optimal timing of dialysis initiation in individuals with AKI due to ATN requiring renal replacement therapy (RRT). Initiating dialysis prior to the development of life-threatening complications may improve outcomes such as mortality, length of stay, and renal recovery. This has led to several RCTs in this area including the AKIKI (Artificial Kidney Initiation in Kidney Injury),46 ELAIN (Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury),47 IDEAL-ICU (Initiation of Dialysis Early Versus Delayed in Intensive Care Unit), 48 and STARRT-AKI49 (Standard versus Accelerated Initiation of Renal Replacement Therapy in Acute Kidney Injury) trials that compared early to delayed RRT therapy with conflicting results. This is in the setting of a replication crisis that has spilled over into the realm of clinical research50,51 with the credibility of results questioned regarding internal validity and generalizability always remaining an issue. AKIKI was a multicenter RCT with 620 subjects with AKI KDIGO (Kidney Disease Improving Global Outcomes) stage 3 requiring mechanical ventilation or vasopressors that found no difference in mortality. ELAIN, on the contrary, was a single-center RCT with 231 subjects with AKI KDIGO stage 2 and plasma neutrophil gelatinase–associated lipocalin (NGAL) > 150 ng/mL that was “positive” showing that early RRT reduced 90-day mortality with a hazard ratio of 0.66 (95% confidence interval: 0.45-0.97). IDEAL-ICU was a multicenter RCT in individuals with the failure stage of the AKI RIFLE (Risk/Injury/Failure/Loss/End-Stage) classification system and septic shock that was stopped early for futility after no difference in mortality at 90 days after 488 subjects were randomized. STARRT-AKI is an international multicenter trial designed to definitively answer the question of early versus late RRT that is larger than all previous trials in the area combined. These 4 RCTs are the result of an evolving line of scientific inquiry that will span almost a decade from which much will be learned with knowledge translation (KT) remaining a critical step as STARRT-AKI nears completion.
Trial Design
In 2010, a NIDDK workshop was held to discuss optimal AKI trial methodology including recommendations for patient selection, outcomes, sample size, in prevention trials and other clinical settings.52,53 In 2013, clinician-trialists in nephrology through KDIGO met to provide guidance for clinical trials in nephrology54 with similar recommendations.
All trials require carefully formulating the research question, selecting a population that is aligned with the overall objective of the trial (explanatory vs pragmatic), calculating a sample size with reasonable assumptions and performing the proper statistical analyses. Eligibility criteria should be balanced to enrich the trial population to include subjects likely to respond to therapy while maintaining feasibility with recruitment and generalizability. Classifying AKI using the RIFLE or Acute Kidney Injury Network (AKIN) criteria in addition to novel biomarkers can assist with risk stratification for enrollment and enrichment. The selection of outcomes for assessing treatment effects should include kidney-specific outcomes (eg, dialysis), non-kidney-specific outcomes (eg, death), and patient-reported outcome measures (PROMs) if appropriate. Endpoints must be able to be measured accurately and reliably as well as be meaningful to patients, and include death, the need for dialysis, and the sustained loss of kidney function. Trial design elements, including implementation, are relevant beyond the trial because the trial’s applicability to real-world settings will be questioned by clinicians and its benefits/risks used in shared decision making with patients. Trial conduct including recruitment, data collection, monitoring, adherence, and follow-up is critical but their intensity and complexity must be balanced with resource availability and subject expectations. They should also reasonably reflect clinical practice while balancing efficacy versus effectiveness.
The timing of dialysis initiation trials have incorporated many of the elements discussed above which are responsible for their success. Their research questions and trial populations were well defined and they capitalized on clinical equipoise in the nephrology and intensive care communities to meet their recruitment goals. STARRT-AKI specifically did not include any biomarkers as eligibility criteria given their infrequent use in most clinical settings but allowed the use of a furosemide stress test to facilitate eligibility decisions from a clinician perspective. Their outcomes were clinically meaningful to knowledge users and in some cases were assessed using administrative data without significantly added complexity or costs. AKIKI and IDEAL-ICU included only centers in France and ELAIN only a single center in Germany, but STARRT-AKI required international collaboration to meet its recruitment goals and ensure global generalizability.
Other challenges in AKI trial design and conduct include the role of patient engagement, the use of surrogate outcomes for CKD and ESRD such as percent decline in glomerular filtration rate,55 the development of valid and reliable AKI-specific PROMs56 to be used along with generic PROMs as secondary outcomes to capture patient-important outcomes and accounting for the competing risk of death57 when interventions do not modify nonrenal illness trajectory in trials.
Innovative Design: Opportunities for Trials in AKI
The Kidney Research National Dialogue58 (KRND) identified several themes that are promising catalysts for the advancement of basic and clinical kidney research. It acknowledged the need for interventional studies and specifically pragmatic RCTs that leverage the preexisting infrastructure of health care systems to evaluate interventions using data obtained from electronic health records. This can facilitate recruitment and outcome ascertainment and control costs. For example, AKI trials could use electronic alerts59 to identify patients that meet eligibility criteria and use clinical records and laboratory systems to assess dialysis dependency or renal function at follow-up. Pragmatic trials are an efficient design to test the effectiveness of therapies and provide highly generalizable findings. The KRND also recommended establishing clinical trial networks which can facilitate shared learning, prioritization of trials, and coordinated recruitment. For example, the STARRT-AKI trial was reviewed and is supported by the Canadian Nephrology Trials Network60 and the Canadian Critical Care Trials Group61 which includes clinicians, trialists, and multidisciplinary members from across Canada. Cluster RCTs using administrative data to compare interventions can also facilitate recruitment as well as generalizability, especially if an intervention is compared to a standard of care and informed consent is waived.62 Other novel designs that include enrichment, basket, or umbrella designs add efficiency and flexibility and the comparison of multiple treatment arms in an adaptive manner, but rely on biomarkers with excellent analytic performance and good preclinical models for treatment mechanisms.63
Patient Engagement
Another innovation in clinical research is the involvement of patient partners. Cancer, heart disease, hemophilia, and a variety of orphan diseases have organized and effective patient advocacy groups that have been crucial in advancing their agenda and directing research in their respective areas. Patient engagement64 is defined as a “meaningful and active collaboration in governance, priority setting, conducting research and knowledge translation” that results in a mutually beneficial relationship between patients and researchers. Involving patients in health research allows those affected by a disease to have a say in what and how research is performed. The collaboration between patients and researchers increases the quality, efficiency, and impact of research. However, it requires time and resources to successfully perform.65 Patient engagement can occur at all stages of research, but there its value across stages by stakeholders from industry, academia, and patients is still controversial.66
The Canadian Institutes of Health Research (CIHR) has launched a Strategy for Patient-Oriented Research (SPOR) with dedicated funding of SPOR networks across a variety of chronic diseases. Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (Can-SOLVE CKD)67 is a SPOR network for CKD that consists of patients, caregivers, researchers, health care providers, policymakers, industry, and renal agencies. In the United States, the Patient-Centered Outcomes Research Institute68 (PCORI) has mandated patient and stakeholder engagement and has funded projects related to kidney disease with successful partnerships dependent on defining roles and processes for the incorporation of input, identification of patients and stakeholders, and engagement and personal investment in the research process.68 Patient partners can be involved in RCTs as members of steering committees, in ethics applications or review, and in the development of patient-centered trial protocols, informed consent forms, and subject information sheets. Finally, patient and public involvement in clinical trials has been shown to improve enrollment with uncertain effects on retention.69
Research priority exercises for AKI have largely not involved patients and caregivers, in contrast to those recently completed for CKD, dialysis, and transplantation. The following factors may contribute to the lack of patient engagement in this area: the heterogeneity of AKI and its operationalization; AKI being a syndrome and not a disease; the degree of acuity and high mortality rate with AKI; the lack of patient advocacy groups (which are common in chronic disease settings); our lack of knowledge about the perceived value of patient engagement to current stakeholders; and our lack of knowledge about the willingness of current stakeholders to engage patients and caregivers (which is known to be challenging). We believe that there is a need for patient engagement, not only to see if current research interests align with those previously identified by other stakeholders, but also to inform trial design, outcome selection, and KT. Challenges are faced in KT.70 Even though KT steps are clear, it is a complex process that depends on high-quality data and adequate implementation. Regarding the detection, prevention, and treatment of AKI, KT advances are moving slowly (“death valley 2”; see Figure 1). In 2012, guidelines regarding the prevention and management of AKI have been released by KDIGO that have variable levels of evidence due to a lack of high-quality clinical studies leading to differing opinions from national nephrology societies (eg, CSN [Canadian Society of Nephrology], KDQOI [Kidney Disease Outcomes Quality Initiative], ERBP [European Renal Best Practice]).71-74 Even though some of the suggestions in the guideline were contested by researchers, e.g. limitations in the diagnosis of AKI as assessed by creatinine- and urine-based criteria, requiring additional diagnostic tools (new AKI biomarkers, renal ultrasound, measurement of intraabdominal pressure, autoimmunity serologies, renal biopsy, etc),75 interestingly, no updates have yet been made to these guidelines. In addition to that, KDIGO AKI guidelines were developed by a working group formed with physicians and medical scholars, and did not include in its development important stakeholders such as allied health team members and patient partners. 5 years after the publication of these KDIGO AKI guidelines, there is a lot of uncertainty regarding optimal management of patient outcomes. However, the consensus definition of AKI led to an increase in publications in AKI improving the understanding on its incidence, management, risk, and prognosis.76
In addition, there is a lack of PROMs specific to AKI as compared with CKD, dialysis, and transplantation settings.56 Outcomes typically include mortality, the need for RRT, renal recovery (RRT independence and estimated glomerular filtration rate in follow-up), health care utilization (length of stay and costs), and adverse events. The degree to which these are patient-important outcomes and how they can be complemented by generic and disease-specific PROMs require further evaluation.
KT Examples and Opportunities
Considering the array of research questions and methods being employed, one would expect fast and effective advances in clinical treatments. However, another barrier to effective KT implementation is research waste. Throughout the knowledge generation and other stages of research, there is an estimated 50% waste of research efforts.77,78 Therefore, research priority setting is a critical exercise to identify the most clinically relevant questions to pursue in a disease area given funding limitations.79 Effective research priority setting also needs the involvement of different stakeholders, including patients, to be successful.80 However, patient involvement in the process of research priority setting for kidney disease was uncommon42 until recently,81 despite readily apparent differing perspectives between health care providers, patients, and their caregivers. In 2008, a clinical research agenda for AKI was developed by the AKIN using a modified Delphi process that included 43 subjects representing a variety of stakeholders without patient representation.82 Priority was placed on studying the optimal timing and dose of RRT, AKI outcomes, and biomarkers for the prediction of AKI and renal recovery. A concerted effort by AKI researchers to focus on these priority areas, along with implementing effective KT steps to disseminate findings, will no doubt accelerate translation across both “death valleys” of research (see Figure 1).
In Canada, the CIHR adopts and encourages the use of a knowledge-to-action (KTA) framework for promoting the application of research into clinical practice. Knowledge-to-action framework is an iterative and dynamic framework that seeks to bridge the gap from knowledge creation to knowledge utilization (see Figure 1).70 The knowledge creation cycle comprises 3 phases: knowledge inquiry (primary research), synthesis (aggregation of existing knowledge), and products/tools (user-friendly evidence-based practice guidelines).70,83,84 Importantly, the KTA identifies multiple stakeholders such as researchers, patients, health care professionals, and policymakers as being critical to this process and emphasizes the involvement and collaboration of all stakeholders at all levels, aligning with the recent trends described above.84
An important feature of KTA is its cyclical nature. Critical to this cyclical process is bridging the promotion of established practices (“sustain ongoing knowledge” in Figure 1) and the identification of areas of weakness (“identify gaps in practice” in Figure 1). In areas where randomized controlled trials are not readily available, observational studies play an important role. It is known that observational studies have limited generalizability, but still they are able to provide valuable insights and direction to the development of research inquires and disease pathway discoveries. An example of one such study generating important knowledge is the retrospective cohort study by Brar et al (2018). They investigated if the use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) after hospital discharge was associated with better outcomes in patients with AKI; their results demonstrated a potential benefit of ACEI or ARB use after AKI, bringing attention to the need for a trial to further evaluate those positive results before the actual application in clinical practice.85
Once new research findings are disseminated, evidence is tested, and clinical practice guidelines are implemented, the resulting practices should be evaluated on an ongoing basis. That evaluation might take various forms, but the use of administrative data seems well suited for this purpose, because it is simple to use and diagnostic codes are available to identify AKI, dialysis outcomes, and changes in medications. Currently, in Canada, opportunities to use such administrative data exist in Ontario at the Institute for Clinical Evaluative Sciences (IC/ES) and Alberta at the Alberta Kidney Disease Network (AKDN). One example of such administrative data use is the work by Karsanji et al,86 which investigated knowledge users’ opinions on the KDIGO clinical practice guideline for patient follow-up after AKI hospitalization. They analyzed the information provided by Canadian nephrologists surveyed to identify their likelihood of recommending follow-up for patients hospitalized with severe AKI after clinical discharge and compared it with administrative health data.86 They found that nephrologists’ response indicated that follow-up should occur for most hospitalized severe AKI patients, yet analysis of real practice data shows that the opposite occurs.86 Leung et al87 also used administrative data in their research but to analyze AKI patient outcomes with the use of cardiac medications after coronary angiography. They used information from 2 different Alberta databases (the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease [APPROACH] database and the AKDN repository) to identify the cohort to be studied and the creatinine measures for those patients.87 They found that the use of cardiac medications was associated with lower mortality rates, but strategies to optimize the use of such medications should be further investigated.87 The results of both studies bring to attention the need to efficiently use the KT cycle to ensure effective implementation of evidence into practice. There are also clear opportunities for future use of administrative databases to measure specific AKI interventions on a population-based level. One such opportunity would be the evaluation of the actual use of early versus late dialysis in the AKI setting to address the impact of published (AKIKI,46 ELAIN,47 IDEAL-ICU48) and upcoming (STARRT-AKI)49 trials on this topic. Such an effort, which will certainly require further investment of resources and expertise, will maximize the chances that findings from these promising studies will positively impact the care of patients with AKI.
Conclusion
Although the paradigm for caring for patients with AKI has remained largely unchanged over the past decades, an acknowledgment of the unique challenges in AKI research has not prevented researchers and clinicians from pursuing novel ways to care for affected patients. Optimization of available therapies such as dialysis, elaboration of research priorities to reduce waste, and support for promising preclinical therapies are all efforts from which the effects have yet to be fully witnessed. However, an understanding of the components that create the chasms between the bench and the patient bedside is critical for all stakeholders to continually move forward in improving outcomes for patients with AKI. Given the recent advances in elaborating novel technologies and implementing frameworks for generating and translating knowledge in nephrology, we can be hopeful that a quantum leap in the care of patients with AKI awaits in the near future.
Acknowledgments
The authors would like to thank Drs Todd Alexander, Adeera Levin, and Sunny Hartwig for reviewing our manuscript.
Footnotes
Ethics Approval and Consent to Participate: Ethics approval and Consent to Participate was not required for this publication.
Consent for Publication: All authors have read and approved the final version of this manuscript.
Availability of Data and Materials: No primary data was generated for this publication.
Author Contributions: P.S.M., V.S.e.S., and D.C. contributed equally to the conception, drafting, and revision of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: P.S.M. was supported by a Canada Graduate Studies scholarship from the Canadian Institute of Health Research, has received salary support from the University of Toronto Department of Medicine’s Eliot Phillipson Clinician-Scientist Training Program, and a postdoctoral fellowship award from the KRESCENT Program, a national kidney research training partnership of the Kidney Foundation of Canada, the Canadian Society of Nephrology, and the Canadian Institutes of Health Research. V.S.e.S. was supported by an allied health doctoral fellowship award from the KRESCENT Program. D.C. was supported by a postdoctoral fellowship award from the KRESCENT Program.
ORCID iDs: Paraish S. Misra  https://orcid.org/0000-0002-0514-8560
https://orcid.org/0000-0002-0514-8560
David Collister  https://orcid.org/0000-0002-2323-6521
https://orcid.org/0000-0002-2323-6521
References
- 1. Susantitaphong P, Cruz DN, Cerda J, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482-1493. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kidney Disease: Improving Global Outcomes and Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1-138. [Google Scholar]
- 3. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care (London, England). 2004;8:R204-R112. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961-973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442-448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179-1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 8. Ahlstrom A, Tallgren M, Peltonen S, Rasanen P, Pettila V. Survival and quality of life of patients requiring acute renal replacement therapy. Intensive Care Med. 2005;31:1222-1228. doi: 10.1007/s00134-005-2681-6. [DOI] [PubMed] [Google Scholar]
- 9. Johansen KL, Smith MW, Unruh ML, Siroka AM, O’Connor TZ, Palevsky PM. Predictors of health utility among 60-day survivors of acute kidney injury in the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study. Clin J Am Soc Nephrol. 2010;5:1366-1372. doi: 10.2215/CJN.02570310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coca SG, Peixoto AJ, Garg AX, Krumholz HM, Parikh CR. The prognostic importance of a small acute decrement in kidney function in hospitalized patients: a systematic review and meta-analysis. Am J Kidney Dis. 2007;50:712-720. doi: 10.1053/j.ajkd.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 11. Collister D, Pannu N, Ye F, et al. Health care costs associated with AKI. Clin J Am Soc Nephrol. 2017;12:1733-1743. doi: 10.2215/CJN.00950117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agarwal A, Dong Z, Harris R, et al. Cellular and molecular mechanisms of AKI. J Am Soc Nephrol. 2016;27:1288-1299. doi: 10.1681/ASN.2015070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehta RL, Cerda J, Burdmann EA, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616-2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 14. Perlman RL. Mouse models of human disease: an evolutionary perspective. Evol Med Public Health. 2016;2016:170-176. doi: 10.1093/emph/eow014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Susztak K, Bitzer M, Meyer TW, Hostetter TH. Animal models of renal disease. Kidney Int. 2008;73:526-528. doi: 10.1038/sj.ki.5002724. [DOI] [PubMed] [Google Scholar]
- 16. Mittalhenkle A, Stehman-Breen CO, Shlipak MG, et al. Cardiovascular risk factors and incident acute renal failure in older adults: the cardiovascular health study. Clin J Am Soc Nephrol. 2008;3:450-456. doi: 10.2215/CJN.02610607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patschan D, Muller GA. Acute kidney injury in diabetes mellitus. Int J Nephrol. 2016;2016:6232909. doi: 10.1155/2016/6232909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Awdishu L, Nievergelt CM, Davenport A, et al. Rationale and design of the genetic contribution to Drug Induced Renal Injury (DIRECT) study. Kidney Int Rep. 2016;1:288-298. doi: 10.1016/j.ekir.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee-Son K, Jetton JG. AKI and genetics: evolving concepts in the genetics of acute kidney injury: implications for pediatric AKI. J Pediatr Genet. 2016;5:61-68. doi: 10.1055/s-0035-1557112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Larach DB, Engoren MC, Schmidt EM, Heung M. Genetic variants and acute kidney injury: a review of the literature. J Crit Care. 2018;44:203-211. doi: 10.1016/j.jcrc.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 21. Zhao B, Lu Q, Cheng Y, et al. A genome-wide association study to identify single-nucleotide polymorphisms for acute kidney injury. Am J Respir Crit Care Med. 2017;195:482-490. doi: 10.1164/rccm.201603-0518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fiorentino M, Kellum JA. Improving translation from preclinical studies to clinical trials in acute kidney injury. Nephron. 2018;140:81-85. doi: 10.1159/000489576. [DOI] [PubMed] [Google Scholar]
- 23. de Caestecker M, Humphreys BD, Liu KD, et al. Bridging translation by improving preclinical study design in AKI. J Am Soc Nephrol. 2015;26:2905-2916. doi: 10.1681/ASN.2015070832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brosius FC, III, Alpers CE, Bottinger EP, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2009;20:2503-2512. doi: 10.1681/ASN.2009070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586-1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 26. Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45:48-57. [DOI] [PubMed] [Google Scholar]
- 27. Xu X, Wang J, Yang R, Dong Z, Zhang D. Genetic or pharmacologic inhibition of EGFR ameliorates sepsis-induced AKI. Oncotarget. 2017;8:91577-91592. doi: 10.18632/oncotarget.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Little MH, Combes AN, Takasato M. Understanding kidney morphogenesis to guide renal tissue regeneration. Nat Rev Nephrol. 2016;12:624-635. doi: 10.1038/nrneph.2016.126. [DOI] [PubMed] [Google Scholar]
- 29. Hale LJ, Howden SE, Phipson B, et al. 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat Commun. 2018;9:5167. doi: 10.1038/s41467-018-07594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim YK, Refaeli I, Brooks CR, et al. Gene-edited human kidney organoids reveal mechanisms of disease in podocyte development. Stem Cells. 2017;35:2366-2378. doi: 10.1002/stem.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garreta E, Prado P, Tarantino C, et al. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat Mater. 2019;18:397-405. doi: 10.1038/s41563-019-0287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Homan KA, Gupta N, Kroll KT, et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods. 2019;16:255-262. doi: 10.1038/s41592-019-0325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boreström C, Jonebring A, Guo J, et al. A CRISP(e)R view on kidney organoids allows generation of an induced pluripotent stem cell-derived kidney model for drug discovery. Kidney Int. 2018;94:1099-1110. doi: 10.1016/j.kint.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 34. Forbes TA, Howden SE, Lawlor K, et al. Patient-iPSC-derived kidney organoids show functional validation of a ciliopathic renal phenotype and reveal underlying pathogenetic mechanisms. Am J Hum Genet. 2018;102:816-831. doi: 10.1016/j.ajhg.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cruz NM, Song X, Czerniecki SM, et al. Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nat Mater. 2017;16:1112-1119. doi: 10.1038/nmat4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Freedman BS, Brooks CR, Lam AQ, et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun. 2015;6:8715. doi: 10.1038/ncomms9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Toyohara T, Mae S, Sueta S, et al. Cell therapy using human induced pluripotent stem cell-derived renal progenitors ameliorates acute kidney injury in mice. Stem Cells Transl Med. 2015;4:980-992. doi: 10.5966/sctm.2014-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Little MH, Hale LJ, Howden SE, Kumar SV. Generating kidney from stem cells. Annu Rev Physiol. 2019;81:335-357. doi: 10.1146/annurev-physiol. [DOI] [PubMed] [Google Scholar]
- 39. Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol. 2015;33:1193-1200. doi: 10.1038/nbt.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Skardal A, Murphy SV, Devarasetty M, et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep. 2017;7:8837. doi: 10.1038/s41598-017-08879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Silver SA, Cardinal H, Colwell K, Burger D, Dickhout JG. Acute kidney injury: preclinical innovations, challenges, and opportunities for translation. Can J Kidney Health Dis. 2015;2:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tong A, Chando S, Crowe S, et al. Research priority setting in kidney disease: a systematic review. Am J Kidney Dis. 2015;65:674-683. doi: 10.1053/j.ajkd.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 43. Proudfoot A, McAuley D, Hind M, Griffiths MJ. Translational research: what does it mean, what has it delivered and what might it deliver. Curr Opin Crit Care. 2011;17:495-503. doi: 10.1097/MCC.0b013e32834a4b19. [DOI] [PubMed] [Google Scholar]
- 44. Strippoli GF, Craig JC, Schena FP. The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol. 2004;15:411-419. [DOI] [PubMed] [Google Scholar]
- 45. Chatzimanouil MKT, Wilkens L, Anders HJ. Quantity and reporting quality of kidney research. J Am Soc Nephrol. 2019;30:13-22. doi: 10.1681/ASN.2018050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375:122-133. doi: 10.1056/NEJMoa1603017. [DOI] [PubMed] [Google Scholar]
- 47. Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315:2190-2199. doi: 10.1001/jama.2016.5828. [DOI] [PubMed] [Google Scholar]
- 48. Barbar SD, Clere-Jehl R, Bourredjem A, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. New Engl J Med. 2018;379:1431-1442. doi: 10.1056/NEJMoa1803213. [DOI] [PubMed] [Google Scholar]
- 49. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier: NCT02568722, Standard vs. Accelerated Initiation of RRT in Acute Kidney Injury (STARRT-AKI: Principal Trial); 2015 Oct 6 [cited 2019 Sept 30]; [about 4 screens]. Available from: https://clinicaltrials.gov/ct2/show/NCT02568722?term=starrt+aki&rank=2
- 50. Baker M. 1,500 scientists lift the lid on reproducibility. Nature. 2016;533:452-454. [DOI] [PubMed] [Google Scholar]
- 51. Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. JAMA. 2005;294:218-228. doi: 10.1001/jama.294.2.218. [DOI] [PubMed] [Google Scholar]
- 52. Molitoris BA, Okusa MD, Palevsky PM, et al. Design of clinical trials in AKI: a report from an NIDDK workshop. Trials of patients with sepsis and in selected hospital settings. Clin J Am Soc Nephrol. 2012;7:856-860. [DOI] [PubMed] [Google Scholar]
- 53. Palevsky PM, Molitoris BA, Okusa MD, et al. Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol. 2012;7:844-850. [DOI] [PubMed] [Google Scholar]
- 54. Baigent C, Herrington WG, Coresh J, et al. Challenges in conducting clinical trials in nephrology: conclusions from a Kidney Disease-Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2017;92:297-305. doi: 10.1016/j.kint.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Grams ME, Sang Y, Coresh J, et al. Candidate surrogate end points for ESRD after AKI. J Am Soc Nephrol. 2016;27:2851-2859. doi: 10.1681/ASN.2015070829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aiyegbusi OL, Kyte D, Cockwell P, et al. Measurement properties of patient-reported outcome measures (PROMs) used in adult patients with chronic kidney disease: a systematic review. PLoS ONE. 2017;12:e0179733. doi: 10.1371/journal.pone.0179733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Austin PC, Fine JP. Accounting for competing risks in randomized controlled trials: a review and recommendations for improvement. Stat Med. 2017;36:1203-1209. doi: 10.1002/sim.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bonventre JV, Boulware LE, Dember LM, et al. The Kidney Research National Dialogue: gearing up to move forward. Clin J Am Soc Nephrol. 2014;9:1806-1811. doi: 10.2215/CJN.07310714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lachance P, Villeneuve PM, Rewa OG, et al. Association between e-alert implementation for detection of acute kidney injury and outcomes: a systematic review. Nephrol Dial Transplant. 2017;32:265-272. doi: 10.1093/ndt/gfw424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rigatto C, Walsh M, Zalunardo N, et al. Establishing a Canadian national clinical trials network for kidney disease: proceedings of a planning workshop. Can J Kidney Health Dis. 2015;2:46. doi: 10.1186/s40697-015-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Marshall JC, Cook DJ. Investigator-led clinical research consortia: the Canadian Critical Care Trials Group. Crit Care Med. 2009;37:S165-S172. doi: 10.1097/CCM.0b013e3181921079. [DOI] [PubMed] [Google Scholar]
- 62. Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. New Engl J Med. 2018;378:829-839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Simon R. Genomic alteration-driven clinical trial designs in oncology. Ann Intern Med. 2016;165:270-278. doi: 10.7326/M15-2413. [DOI] [PubMed] [Google Scholar]
- 64. Canadian Institutes of Health Research. Strategy for Patient-Oriented Research: patient engagement framework. http://www.cihr-irsc.gc.ca/e/48413.html#a4. Published 2014. Accessed September 20, 2019.
- 65. Domecq JP, Prutsky G, Elraiyah T, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14:89. doi: 10.1186/1472-6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smith SK, Selig W, Harker M, et al. Patient engagement practices in clinical research among patient groups, industry, and academia in the United States: a survey. PLoS ONE. 2015;10:e0140232. doi: 10.1371/journal.pone.0140232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Levin A, Adams E, Barrett BJ, et al. Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (Can-SOLVE CKD): form and function. Can J Kidney Health Dis. 2018; 5: 2054358117749530. 2018/01/27. doi: 10.1177/2054358117749530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cukor D, Cohen LM, Cope EL, et al. Patient and other stakeholder engagement in Patient-Centered Outcomes Research Institute funded studies of patients with kidney diseases. Clin J Am Soc Nephrol. 2016;11:1703-1712. doi: 10.2215/CJN.09780915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Crocker JC, Ricci-Cabello I, Parker A, et al. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis. BMJ. 2018;363:k4738. doi: 10.1136/bmj.k4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Graham ID, Logan J, Harrison MB, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof. 2006;26:13-24. doi: 10.1002/chp.47. [DOI] [PubMed] [Google Scholar]
- 71. James M, Bouchard J, Ho J, et al. Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61:673-685. doi: 10.1053/j.ajkd.2013.02.350. [DOI] [PubMed] [Google Scholar]
- 72. Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61:649-672. doi: 10.1053/j.ajkd.2013.02.349. [DOI] [PubMed] [Google Scholar]
- 73. Jorres A, John S, Lewington A, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 2: renal replacement therapy. Nephrol Dial Transplant. 2013;28:2940-2945. doi: 10.1093/ndt/gft297. [DOI] [PubMed] [Google Scholar]
- 74. The Ad-hoc Working Group of ERBP, Fliser D, Juillard L, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27:4263-4272. doi: 10.1093/ndt/gfs375%. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ostermann M, Joannidis M. Acute kidney injury 2016: diagnosis and diagnostic workup. Crit Care. 2016;20:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lameire N, Vanmassenhove J, Lewington A. Did KDIGO guidelines on acute kidney injury improve patient outcome. Intensive Care Med. 2017;43:921-923. doi: 10.1007/s00134-017-4740-1. [DOI] [PubMed] [Google Scholar]
- 77. Macleod MR, Michie S, Roberts I, et al. Biomedical research: increasing value, reducing waste. Lancet (London, England). 2014;383:101-104. doi: 10.1016/s0140-6736(13)62329. [DOI] [PubMed] [Google Scholar]
- 78. Moher D, Glasziou P, Chalmers I, et al. Increasing value and reducing waste in biomedical research: who’s listening. Lancet. 2016;387:1573-1586. doi: 10.1016/S0140-6736(15)00307-4. [DOI] [PubMed] [Google Scholar]
- 79. Chalmers I, Bracken MB, Djulbegovic B, et al. How to increase value and reduce waste when research priorities are set. Lancet. 2014;383:156-165. doi: 10.1016/S0140-6736(13)62229-1. [DOI] [PubMed] [Google Scholar]
- 80. Minogue V, Cooke M, Donskoy A-L, Vicary P, Wells B. Patient and public involvement in reducing health and care research waste. Res Involv Engagem. 2018;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Manns B, Hemmelgarn B, Lillie E, et al. Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol. 2014;9:1813-1821. doi: 10.2215/CJN.01610214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kellum JA, Mehta RL, Levin A, et al. Development of a clinical research agenda for acute kidney injury using an international, interdisciplinary, three-step modified Delphi process. Clin J Am Soc Nephrol. 2008;3:887-894. doi: 10.2215/CJN.04891107. [DOI] [PubMed] [Google Scholar]
- 83. Straus S, Tetroe J, Graham I. Defining knowledge translation. CMAJ. 2009;181:165-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Straus S, Tetroe J, Graham ID, Zwarenstein M, Bhattacharyya O, Shepperd S. Knowledge Translation in Health Care: Moving From Evidence to Practice. Hoboken, NJ: John Wiley; 2011. [Google Scholar]
- 85. Brar S, Ye F, James MT, et al. Association of Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use With Outcomes After Acute Kidney Injury. JAMA Intern Med. 2018;178:1681-1690. doi: 10.1001/jamainternmed.2018.4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Karsanji DJ, Pannu N, Manns BJ, et al. Disparity between nephrologists’ opinions and contemporary practices for community follow-up after AKI hospitalization. Clin J Am Soc Nephrol. 2017;12:1753-1761. doi: 10.2215/CJN.01450217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Leung KCW, Pannu N, Tan Z, et al. Contrast-associated AKI and use of cardiovascular medications after acute coronary syndrome. Clin J Am Soc Nephrol. 2014;9:1840-1848. doi: 10.2215/CJN.03460414. [DOI] [PMC free article] [PubMed] [Google Scholar]