Abstract
Background and Purpose:
After large-vessel intracranial occlusion (LVO), the fate of the ischemic penumbra, and ultimately final infarct volume, largely depends on tissue perfusion. In this study, we evaluated whether blood pressure reduction and sustained relative hypotension during endovascular thrombectomy (EVT) are associated with infarct progression and functional outcome.
Methods:
We identified consecutive patients with LVO ischemic stroke who underwent mechanical thrombectomy at two comprehensive stroke centers. Intra-procedural mean arterial pressure (MAP) was monitored throughout the procedure. ∆MAP was calculated as the difference between admission MAP and lowest MAP during EVT until recanalization. Sustained hypotension was measured as the area between admission MAP and continuous measurements of intra-procedural MAP (aMAP). Final infarct volume was measured using MRI at 24 hours, and functional outcome was assessed using the modified Rankin Scale (mRS) at discharge and 90 days. Associations with outcome were analyzed using linear and ordinal multivariable logistic regression.
Results:
390 patients (mean age 71±14 years, mean NIHSS 17) were included in the study; of these, 280 (72%) achieved TICI 2B/3 reperfusion. 87% of patients experienced MAP reductions during EVT (mean 31±20 mmHg). ∆MAP was associated with greater infarct growth (p=0.036) and final infarct volume (p=0.035). Mean ∆MAP among patients with favorable outcomes (mRS 0–2) was 20±21 mmHg compared to 30±24 mmHg among patients with poor outcome (p=0.002). In the multivariable analysis, ∆MAP was independently associated with higher (worse) mRS scores at discharge (aOR per 10-mmHg 1.17; 95%CI 1.04–1.32; P=0.009) and at 90 days (aOR per 10-mmHg 1.22; 95%CI 1.07–1.38; P=0.003). The association between aMAP and outcome was also significant at discharge (p=0.002) and 90 days (p=0.001).
Conclusions:
Blood pressure reduction prior to recanalization is associated with larger infarct volumes and worse functional outcomes for patients affected by LVO stroke. These results underscore the importance of BP management during EVT, and highlight the need for further investigation of blood pressure management after LVO stroke.
Keywords: Stroke, Blood Pressure, Thrombectomy, Brain Ischemia
Subject terms: Cerebrovascular Disease/Stroke, Ischemic Stroke, Revascularization, Blood Pressure
Introduction
Endovascular thrombectomy (EVT) has profoundly changed the landscape of acute stroke therapy.1-4 However, there remains a major unmet medical need for adjunctive treatment strategies to improve the outcomes of stroke victims treated with EVT. A recent meta-analysis with 1287 participants found that overall only 46% of subjects receiving EVT were functionally independent at 90 days and only 10% were neurologically normal.5 Blood pressure is an important modifiable parameter to ensure proper cerebral perfusion during EVT and a focus of clinical care in every stroke patient. Importantly, following large-vessel occlusion, the fate of the ischemic penumbra and final infarct volume largely depends on the ability to maintain perfusion above the threshold for infarction.6,7 Yet currently, there are only limited data to guide optimal hemodynamic management peri-procedurally.
Hypotension prior to reperfusion may compromise collateral flow. Exhaustion of compensatory vasodilatory capacity distal to the occluded vessel and the loss of intrinsic autoregulatory function in the ischemic tissue lead to pressure passivity rendering the patient vulnerable to blood pressure changes.8,9 In this situation, reductions in systemic blood pressure may result in corresponding decreases in cerebral blood flow to the ischemic tissue, potentially causing infarct progression and worse outcome.10
In this study, we examined the effects of blood pressure reductions and sustained relative hypotension during EVT on infarct progression and functional outcome.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files.
Study design
We conducted a retrospective, observational study of consecutive acute ischemic stroke patients with LVO undergoing EVT. The study was conducted at two US academic comprehensive stroke centers: Yale-New Haven Hospital, CT and University of Iowa Hospitals and Clinics, IA. The study was approved by the Institutional Review Board of each participating center. Individual patient consent was waived because of the study’s retrospective design without any patient intervention. The analysis was planned prior to data collection.
Subjects
We retrospectively reviewed the prospectively collected stroke databases at each institution and included a consecutive sample of patients ≥18 years of age with anterior circulation large-vessel occlusion acute ischemic stroke involving the intracranial internal carotid artery or middle cerebral artery (M1 or M2). All patients were treated with EVT between 2014 and 2018. Patient medical history, demographic information, baseline characteristics, initial imaging and angiographic results were obtained from the electronic medical records.
All stroke management decisions including delivery of thrombolytic drugs, intraprocedural blood pressure targets and choice of anesthesia were made by the patients’ attending providers and clinical care team in accordance with current American Heart Association (AHA) guidelines.11 All patients were managed by an anesthesia care team, however, general anesthesia was reserved for patients who could not cooperate with the procedure despite conscious sedation, had respiratory failure, or were unable to protect their airway. Baseline admission MAP was defined as the earliest MAP in the patient’s chart, recorded either by emergency medical services, an outside facility prior to transfer, or upon presentation to the EVT-capable Comprehensive Stroke Center.
Blood Pressure Data
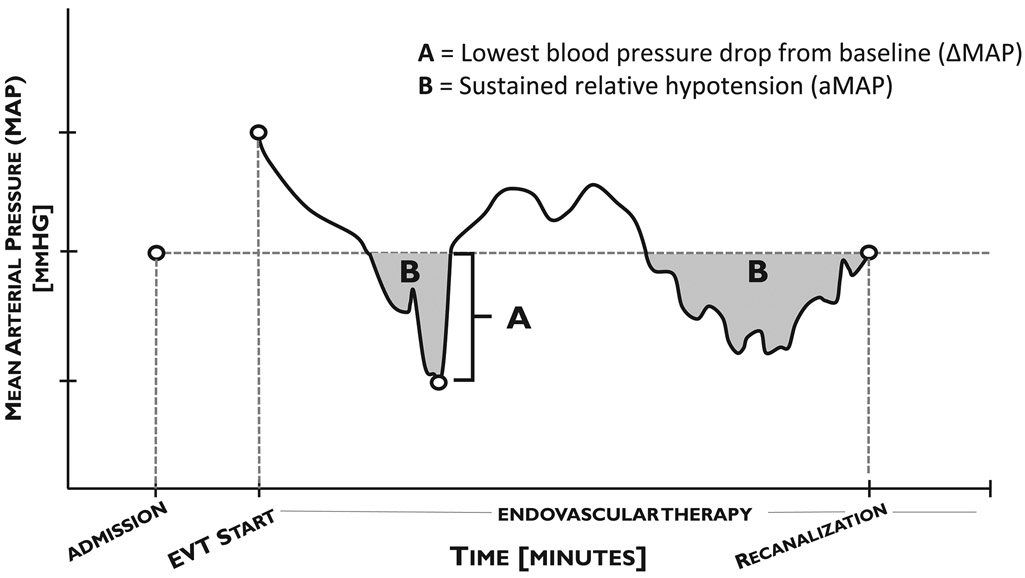
Blood pressure during EVT was measured throughout the procedure and recorded either every minute using an intra-arterial catheter, or every three minutes using a non-invasive blood pressure cuff. Blood pressure data was obtained electronically from the patient’s anesthesia report following intervention, and analyzed with a custom computer algorithm using MATLAB (Release 2018b, The Mathworks, Inc., Natick, Massachusetts, USA). Decreases in blood pressure were calculated as the difference between admission MAP and the single lowest MAP prior to recanalization during EVT (∆MAP). Additionally, to distinguish between one-time blood pressure reductions and sustained relative hypotension, the area between baseline admission MAP and continuous measurements of intraprocedural MAP from procedure start to vessel recanalization was measured (aMAP) and expressed in mmHg*min [Figure 1].
Figure 1: Schematic of data analysis and blood pressure reduction parameters.
MAP was measured continuously during EVT (black line). ∆MAP was measured as the single greatest drop in blood pressure during EVT from baseline admission levels (A). aMAP was calculated as the total area between admission MAP and intraprocedural MAP (B). Measurements were calculated until the time of vessel recanalization.
Outcome measurements
We evaluated functional outcome using the modified Rankin scale (mRS) including mortality at discharge and 90 days.12 The mRS scale assigns an ordinal score from 0 (no symptoms) to 6 (deceased) to patients after stroke. Outcomes were assessed either in person or by telephone by certified raters blinded to the measurement results. As part of their routine stroke evaluation for EVT, all patients underwent a non-contrast head CT as well as a CT angiogram of the head and neck. Since 2016, the clinical protocol has also included CT perfusion imaging, which was processed with the use of fully automated software (RAPID, iSchemaView, Menlo Park, CA, USA). Irreversibly injured brain (“ischemic core”) was diagnosed if the relative cerebral blood flow was less than 30% of that in normal tissue.13 In addition, all patients underwent magnetic resonance imaging (MRI) as part of their routine clinical work-up at 24 hours post-intervention. Final infarct volumes were measured using RAPID software and defined as pixels with apparent diffusion coefficient (ADC) values below 680 × 10−6 mm2/sec. Since automated volume computation based on ADC threshold values can become inaccurate in the setting of significant hemorrhagic transformation, manual tracings were employed in these instances to calculate the final infarct volume (Analyze 11.0, AnalyzeDirect, Overland Park, KS, USA). Infarct growth was calculated as the difference between the initial, pre-EVT infarct core and final infarct volume on follow-up imaging. The degree of reperfusion was determined by the Thrombolysis in Cerebral Infarction (TICI) score, where a score of 2B (reperfusion of more than half of the previously occluded target artery ischemic territory) or 3 represented successful reperfusion.14
Statistical Analyses
Baseline characteristics of included subjects were summarized by means and standard deviations (SD) for normally distributed continuous variables, by medians and interquartile ranges (IQR) for skewed continuous variables, and by numbers (%) for categorical variables. We dichotomized mRS scores into favorable (mRS 0–2) and unfavorable (mRS ≥3) outcomes. We used χ2-, t- or Mann-Whitney U tests as appropriate for unadjusted comparisons and performed adjusted analyses via ordinal logistic or linear regression modeling, as appropriate. Given the large number of possible confounders with p<0.01 in the univariable analysis, variable selection for adjusted analyses was performed based on knowledge from prior studies and theoretical considerations. An odds ratio (OR) above 1 constituted a decrease in blood pressure associated with a shift on the mRS towards a worse outcome. Since reperfusion is a major predictor of final infarct size and functional outcome, we performed sensitivity analyses in the subgroup of patients with successful reperfusion. An additional subgroup analysis was performed for patients treated with general anesthesia. All statistics were computed using SPSS (Version 24, IBM Corp). Statistical significance was set at P<0.05 (two tailed) for the primary hypothesis that ∆MAP/aMAP is associated with functional outcome.
Results
Subject characteristics
Three hundred-ninety patients undergoing endovascular thrombectomy were included for data analysis (mean age 71 ± 14 years, mean admission NIHSS 17 ± 6), of which two hundred ninety-four were assessed for functional outcome at 90 days. Baseline characteristics for all patients are displayed in Table 1. The median procedure duration was 117 minutes (range 25 – 379). For patients who were successfully recanalized (TICI = 2B, 3), the median time from procedure start to recanalization was 66 minutes (range 9 – 323).
Table 1: Demographics and baseline characteristics.
Data are mean (standard deviation) and n (%). ICA, internal carotid artery; tICA, terminal internal carotid artery; ACA, anterior cerebral artery; MCA, middle cerebral artery; NIHSS, National Institutes of Health Stroke Scale; MAP, mean arterial pressure; TICI, thrombolysis in cerebral infarctions score.
| Total patients | 390 |
| Outcomes assessed at 90 days, n(%) | 294 (75) |
| Age, mean±SD | 71±14 |
| Gender, female(%) | 219 (56) |
| Race, n(%) | |
| White | 326 (84) |
| Black or African American | 37 (9) |
| Asian | 4 (1) |
| Other | 16 (4) |
| Medical History*, n(%) | |
| Hypertension | 238 (61) |
| Coronary artery disease/myocardial infarction | 73 (19) |
| Hyperlipidemia | 143 (37) |
| Chronic heart failure | 45 (12) |
| Atrial fibrillation | 137 (35) |
| Diabetes mellitus | 83 (21) |
| Past ischemic stroke | 41 (11) |
| Current/past smoker | 93 (24) |
| Occlusion on CTA*, n(%) | |
| ICA/tICA | 81 (18) |
| ACA | 7 (2) |
| M1 MCA | 282 (63) |
| M2 MCA | 79 (18) |
| Admission NIHSS, mean±SD | 17±6 |
| Admission MAP, mean±SD | 105±18 |
| ASPECTS, median (IQR) | 9 (7–10) |
| Core infarct volume, median (IQR) | 8 (0–25) |
| Treated with tPA, n(%) | 264 (68) |
| General anesthesia, n(%) | 140 (35) |
| Mean onset to bolus time, minutes±SD | 138±74 |
| Mean onset to admission, minutes±SD | 304±291 |
| Mean onset to EVT, minutes±SD | 404±345 |
| Mean onset to reperfusion, minutes±SD | 463±266 |
| Mean baseline BP measurement to EVT, median (IQR) | 78 (48–114) |
| TICI, n (%) | |
| 0 | 60 (15) |
| 1 | 8 (2) |
| 2A | 34 (9) |
| 2B | 160 (41) |
| 3 | 120 (31) |
| In-hospital mortality, n(%) | 46 (12) |
| 90-day mortality, n(%) | 84 (29) |
Percentages may add up to more than 100% due to comorbidity.
Blood pressure reductions and functional outcome
The mean MAP at admission was 105 ± 18 mmHg, which increased to 114 ± 21 mmHg upon arrival in the angiosuite. Compared to their own admission blood pressure, 87% of patients experienced reductions in MAP during endovascular thrombectomy. The average maximum MAP reduction (∆MAP) was 31 ± 20 mmHg. ∆MAP did not significantly vary by institution or by site of occlusion (Supplemental Table I and II).
Ninety-six (33%) of patients achieved functional independence (mRS 0–2) at 3 months. Patients with good outcome were overall younger (65 vs. 74 years, P<0.001), more likely to be male (56% vs. 38%, P=0.004), and had fewer comorbidities and lower NIHSS scores at presentation (14 vs. 18, P=0.001). They were more frequently treated with tPA prior to endovascular thrombectomy (70% vs. 55%, P=0.0016). Differences were also observed in the distribution of ASPECT scores (P=0.005) and degree of reperfusion (P=0.001). The mean ∆MAP among patients with favorable outcomes was 20 ± 21 mmHg compared to 30 ± 24 mmHg among patients with unfavorable outcomes (P=0.002). Results were similar for SBP reductions and functional outcome at 90 days (Table 2).
Table 2: Hemodynamic variables.
Data are mean (SD) and n (%). SD, standard deviation; mRS, modified Rankin scale; MAP, mean arterial pressure; SBP, systolic blood pressure; ΔMAP, difference between baseline and lowest MAP during endovascular thrombectomy; aMAP, area between baseline and intraprocedural MAP; ΔSBP, difference between baseline and lowest SBP during endovascular thrombectomy; aSBP, area between baseline and intraprocedural SBP.
| Favorable outcomes (mRS 0–2) |
Unfavorable outcomes (mRS 3–6) |
p-value | ||
|---|---|---|---|---|
| Total patients | 390 | 96 | 198 | |
| Baseline SBP, mmHg±SD | 149±26 | 142±24 | 152±25 | 0.001 |
| Baseline MAP, mmHg±SD | 106±19 | 102±17 | 107±19 | 0.055 |
| Mean minimum SBP, mmHg±SD | 113±26 | 114±25 | 110±27 | 0.182 |
| Mean minimum MAP, mmHg±SD | 80±18 | 82±18 | 77±19 | 0.043 |
| Mean maximum SBP, mmHg±SD | 186±31 | 183±33 | 189±31 | 0.164 |
| Mean maximum MAP, mmHg±SD | 132±23 | 130±23 | 134±22 | 0.211 |
| Mean procedural SBP, mmHg±SD | 144±19 | 143±22 | 144±18 | 0.494 |
| Mean procedural MAP, mmHg±SD | 100±13 | 99±15 | 100±12 | 0.394 |
| Patients with intra-procedural blood pressure reduction below admission, n(%) | 339 (87) | 80 (83) | 176 (89) | 0.183 |
| Mean ΔSBP, mmHg±SD | 37±34 | 28±29 | 43±35 | <0.001 |
| Mean ΔMAP, mmHg±SD | 26±23 | 20±21 | 30±24 | 0.002 |
| Mean percent ΔSBP,% | 23±20 | 18±19 | 26±21 | 0.002 |
| Mean percent ΔMAP,% | 22±20 | 19±19 | 26±21 | 0.005 |
| SBP hypotensive area, mmHg*min | 592 (118–1968) | 370 (43–1120) | 786 (190–2714) | <0.001 |
| MAP hypotensive area, mmHg*min | 409 (58–1431) | 225 (20–990) | 603 (94–1943) | <0.001 |
Associations of mRS at discharge and 90 days for all patients and their respective values for ∆MAP and aMAP are summarized in Table 3. ∆MAP was independently associated with higher (worse) mRS scores at discharge (adjusted OR per 10-mmHg 1.17; 95% CI 1.04–1.32; P=0.009) and at 90 days (adjusted OR per 10-mmHg 1.22; 95% CI 1.07–1.38; P=0.003) after adjusting for age, ASPECT score, baseline blood pressure, admission NIHSS, TICI score, and onset-to-reperfusion time (Figure 2). For every 10-mmHg reduction in MAP, there was a 22% increased likelihood of shifting towards worse outcomes on the mRS at 90 days. The association between aMAP and outcome was also significant at discharge (P=0.002) and 90 days (P=0.001). A similar effect size and significance was found for the relationship using systolic blood pressure (SBP) measurements to substitute for MAP (Table 3) and for the subpopulation of reperfused patients (Supplemental Table III).
Table 3: Association of hemodynamic variables with functional outcome.
aOR, adjusted odds ratio (adjusted for age, ASPECT score, baseline blood pressure, admission NIHSS, TICI score, and time-to-reperfusion); OR, odds ratio; MAP, mean arterial pressure; SBP, systolic blood pressure; mRS, modified Rankin scale; ΔMAP, difference between baseline and lowest MAP during endovascular thrombectomy; aMAP, area between baseline and intraprocedural MAP; ΔSBP, difference between baseline and lowest SBP during endovascular thrombectomy; aSBP, area between baseline and intraprocedural SBP.
| Likelihood of a shift towards worse outcome on discharge mRS | ||||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p Value | aOR | 95% CI | p Value |
| ΔMAP, per 10-mmHg | 1.19 | 1.1–1.28 | <0.001 | 1.17 | 1.04–1.32 | 0.009 |
| ΔSBP, per 10-mmHg | 1.16 | 1.09–1.22 | <0.001 | 1.12 | 1.04–1.22 | 0.005 |
| aMAP, per 300-mmHg*min | 1.1 | 1.06–1.15 | <0.001 | 1.1 | 1.03–1.16 | 0.002 |
| aSBP, per 300-mmHg*min | 1.08 | 1.05–1.11 | <0.001 | 1.07 | 1.01–1.1 | 0.014 |
| Likelihood of a shift towards worse outcome on 90-day mRS | ||||||
| Variables | OR | 95% CI | p Value | aOR | 95% CI | p Value |
| ΔMAP, per 10-mmHg | 1.22 | 1.12–1.34 | <0.001 | 1.22 | 1.07–1.38 | 0.003 |
| ΔSBP, per 10-mmHg | 1.17 | 1.1–1.25 | <0.001 | 1.25 | 1.06–1.26 | 0.002 |
| aMAP, per 300-mmHg*min | 1.15 | 1.09–1.21 | <0.001 | 1.15 | 1.06–1.24 | 0.001 |
| aSBP, per 300-mmHg*min | 1.1 | 1.06–1.14 | <0.001 | 1.09 | 1.03–1.14 | 0.002 |
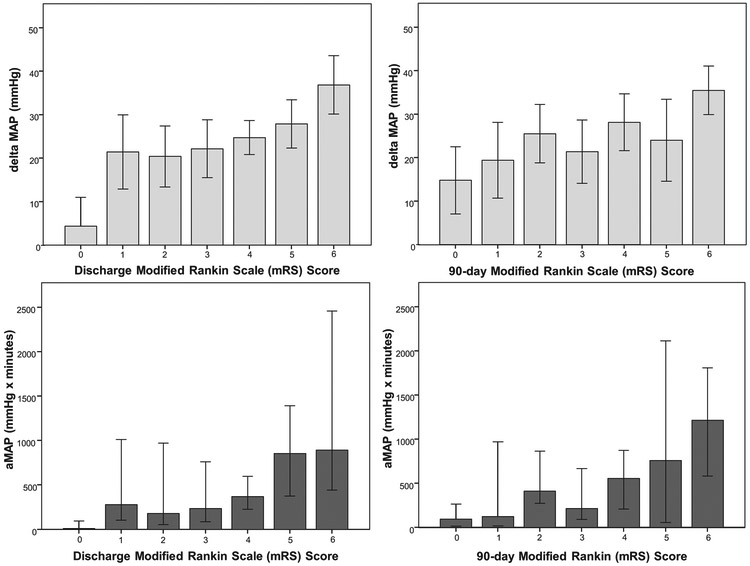
Figure 2: Association of blood pressure reduction with functional outcomes at discharge and 90-days.
The difference between baseline mean arterial pressure (MAP) and lowest MAP during endovascular thrombectomy (∆MAP, light blue) and area between admission MAP and intraprocedural MAP (aMAP, dark blue) were plotted per each mRS score at discharge and 90-days. Bar graphs represent mean (∆MAP) and median (aMAP) for each mRS score category; error bars indicate the 95% confidence interval.
Blood pressure reductions and infarct progression
We calculated infarct growth in 154 patients who underwent both CT perfusion imaging with sufficient quality to estimate initial core infarct (CBF<30%) and follow-up MRI at 24 hours for determination of final infarct size.
In this subgroup, intraprocedural blood pressure reductions (∆MAP) and sustained relative hypotension (aMAP) were significantly associated with infarct growth (P=0.003 and P=0.005, Figure 3, Supplemental Table IV). After adjusting for age, admission NIHSS, TICI score and core infarct volume, ∆MAP remained independently associated with infarct growth (P=0.036) and final infarct volume (P=0.035). On average, there was a 4.1 ml increase in infarct volume for every 10-mmHg decrease in MAP during endovascular thrombectomy.
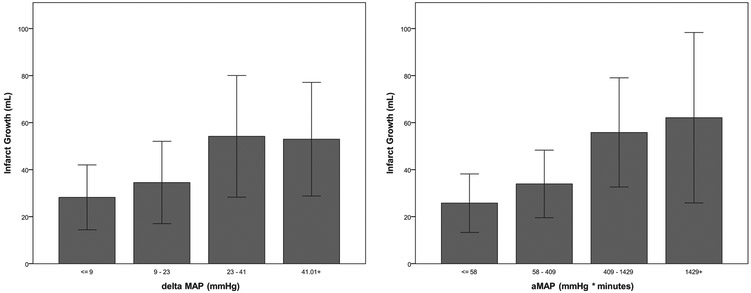
Figure 3: Association of blood pressure reduction with infarct growth.
∆MAP (left) and aMAP (right) were divided into equal quartiles and plotted against infarct growth. Bar graphs represent mean for each quartile of blood pressure reduction and relative hypotension; error bars indicate the 95% confidence interval.
Discussion
In this cohort of LVO patients from two large academic stroke centers, we demonstrated that blood pressure reductions and sustained relative hypotension during endovascular thrombectomy are independent predictors of worse functional outcome at discharge and 90 days.
Our results are in agreement with several other retrospective analyses that suggest that hypotension may contribute to worse outcomes related to the use of general anesthesia in endovascular stroke therapy.10,15-17 In a post-hoc analysis of the MR CLEAN trial, Treurniet et al. demonstrated similar “dose-dependent” association between blood pressure reductions and unfavorable outcome among patients undergoing general anesthesia for mechanical thrombectomy.10 Two additional retrospective studies found that SBP below 140 mmHg and MAP below 70 mmHg were predictors of poor neurological outcome15,18 Our study provides evidence for a potential mechanism leading to worse outcomes associated with blood pressure reductions. Using short-term imaging endpoints, we found greater infarct progression and larger final infarct volumes associated with blood pressure reductions. This finding supports the construct that the ischemic penumbra is dependent on maintenance of cerebral perfusion pressure for preservation of flow to ischemic areas. 6,7,19 Because blood vessels in these areas are not only maximally dilated but also have lost their intrinsic autoregulatory capacity, reductions in systemic blood pressure may be detrimental to this pressure-passive system and result in infarct progression. A strength of our study is the large sample size and inclusion of patients from two large academic stroke centers. Baseline characteristics by institution are summarized in Supplemental Table V. The significant number of patients included in this study allowed for adjustment for important covariates and known predictors of poor outcome. Blood pressure was managed according to AHA guidelines without specific protocol, reflecting current clinical practice.11 While existing guidelines recognize that hypotension and hypovolemia should be corrected to maintain perfusion, parameters for patient selection and specific blood pressure targets remain elusive. A single blood pressure target below 180/105 mmHg for all patients, as currently recommended, is likely inadequate for the management of this highly complex and heterogeneous patient population.
Although the majority of thrombectomies in our cohort (65%) were performed under conscious sedation, which has been associated with greater hemodynamic stability compared to general anesthesia, we observed frequent and substantial reductions in blood pressure. Hemodyanmic parameters and outcomes by anesthesia type are summarized in Supplemental Table VI. Whalin et al. reported similar reductions in blood pressure among patients treated with conscious sedation and found that a greater than 10% decrease in blood pressure from pre-procedural baseline was a predictor of poor outcome.20 Instead of calculating blood pressure reductions below currently recommended thresholds21 or pre-procedural baseline, we used earliest recorded blood pressure as the reference as it is unaffected by commonly-performed interventions such as hemodynamic augmentation with intravenous fluids or blood pressure lowering for administration of tPA. This earlier blood pressure reference better reflects a patient’s true baseline after acute LVO stroke. A sensitivity analysis using pre-procedural blood pressure as the baseline to compute ∆MAP and aMAP showed similar results, but a smaller effect size (Supplemental Table VII). Thirty-six patients had intraprocedural blood pressure decreases compared to their pre-procedural baseline, but never dropped below their admission blood pressure. These patients had smaller final infarct volumes (median 20 vs. 34 ml) and increases in MAP from admission to arrival in the angiosuite may have provided some protection against blood pressure reductions. Hemodynamic parameters based on admission and pre-procedural blood pressure baselines are also compared in Supplemental Table VIII.
Blood pressure is commonly elevated after large-vessel occlusion stroke and may represent a response to maintain cerebral perfusion.22,23 The rate of infarct growth after LVO stroke is highly variable and depends not only on the duration of arterial occlusion but also the ability to maintain collateral blood flow above the threshold of infarction.24 Patients with unfavorable outcome had similar average intraprocedural blood pressure compared to those with good outcomes, but presented with significantly higher baseline MAP and SBP. Several authors have demonstrated a strong association between higher admission blood pressure and poorer collaterals, suggesting the need for a greater hypertensive response to maintain at least partial perfusion to the ischemic tissue.25,26 These patients may be more vulnerable to even small blood pressure reductions and simply maintaining blood pressure above 140 mmHg may be inadequate. In contrast, patients presenting with lower NIHSS scores despite intracranial large-vessel occlusion and presumably better collateral circulation have been shown to tolerate even substantial intraprocedural hypotension.20 In addition to sustained relative hypotension below individual admission blood pressure, we found that even one-time reductions pose a significant risk to patients perhaps due to vascular collapse downstream of the occlusion.
Our findings stand in contrast with a secondary analysis of the GOLIATH trial in which patients undergoing endovascular stroke therapy were randomized to general anesthesia or conscious sedation. In a post-hoc analysis, the authors found no statistically relevant association between hemodynamic parameters and outcome.27 However, the trial included a blood pressure management protocol that aimed at simultaneously maintaining mean arterial pressure and systolic blood pressure above 70 and 140 mmHg, respectively.28 While the authors report significant differences in hemodynamic variables among treatment groups, the rigorous blood pressure protocol and frequent use of vasopressors (98% in the general anesthesia group, 57% in the conscious sedation group) may have protected patients from more severe blood pressure reductions and sustained relative hypotension. In addition, the decrease in metabolic demand during general anesthesia may provide some protection against blood pressure reductions.
Our study has several limitations. First, although larger than prior studies, it remains a retrospective analysis. As with all such studies, missing data may bias the results in unpredictable ways. For example, we did not collect data on anesthetic medications administered during EVT. Three-month functional outcomes were available for only 75% (294/390) of patients. However, both groups (patients with and without 3-months outcomes) were comparable in terms of important baseline characteristics (Supplemental Table IX). Furthermore, a similar association and effect size between blood pressure reductions and outcome were found when discharge mRS was assessed for the entire cohort and the subgroup of patient with 90-day outcomes. Second, while intraprocedural blood pressure was managed according to current guidelines, a lower blood pressure target has not been clearly defined. As a result, blood pressure interventions such as use of vasopressor therapy were inconsistently applied in this observational cohort. This may have prevented or mitigated blood pressure declines in some patients, but is unlikely to have affected the observed relationship in which greater reductions in blood pressure were associated with increased risk for larger infarct volumes and higher likelihood for unfavorable outcome. Third, the growth rate of early DWI lesions and clinical outcomes are strongly associated with the degree of reperfusion achieved during EVT. In addition to adjusting for the degree of reperfusion, we performed a sensitivity analysis selecting only those patients who achieved successful reperfusion (TICI 2B or 3). The odd ratios for worse outcome were similar in models that were either unadjusted or adjusted for degree of reperfusion as well as in the subgroup of reperfused patients, supporting the notion that the effect of blood pressure declines on outcome was independent of reperfusion status (Supplemental Table III). Nevertheless, we cannot exclude the possibility that the blood pressure effect was at least partly due to differences in reperfusion. Fourth, blood pressure was measured using either non-invasive cuff and invasive arterial catheter. Despite this lack of uniform measurement, our main findings held across blood pressure measurement type (Supplemental Table X). Lastly, since our study was limited to patients with anterior circulation large-vessel occlusion, our results may not be generalizable to patients with posterior circulation strokes.
The question of optimal blood pressure management in the initial stages of large-vessel occlusion stroke is of particular importance in the current era of endovascular thrombectomy where patients are commonly transferred to a comprehensive stroke center. A recent meta-analysis reported a median emergency department door-to-reperfusion time of 148 minutes (IQR 112, 197).29 Patients who initially present to an outside facility on average face an additional delay of 129 minutes during which they are vulnerable to hemodynamic instability and blood pressure reductions, which may contribute to the increase in mortality associated with interfacility transfer.30,31 However, it remains unknown if an intervention to maintain or augment blood pressure can actually improve outcomes. Additional studies are needed to approach this question through a prospective, randomized trial evaluating blood pressure management in the acute phase of large-vessel occlusion stroke. Such a trial should take into consideration individual patient factors such as baseline blood pressure, site of vascular occlusion and collateral blood vessel status. Until the results of such a trial are available, it seems reasonable to minimize blood pressure reductions during endovascular stroke therapy by maintaining blood pressure close to an individual patient’s baseline.
Conclusions
Blood pressure reductions during endovascular stroke therapy are common and may lead to larger infarct volumes and worse functional outcomes. Every 10-mmHg reduction in mean arterial pressure prior to reperfusion increased the risk of worse outcome by 22%. These results underline the importance of blood pressure management during EVT and highlight the need for further investigation of active blood pressure management in LVO stroke.
Supplementary Material
Acknowledgments
Funding
This work was supported by American Heart Association Grant # 17MCPRP33460188/Nils Petersen/2017 and by CTSA Grant # KL2 TR001862 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of AHA or NIH.
KNS receives NIH support from U24NS107215, U24NS107136, U01NS106513, R01NR018335, AHA support from 17CSA33550004, grants to Yale from Novartis, Biogen, Bard, Hyperfine for his role as PI in several multicenter stroke trials. Dr. Sheth reports equity interests in Alva Health.
Footnotes
Disclosures
NHP declares no competing interests.
References
- 1.Saver JL, Goyal M, Bonafe A, Diener H-C, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015;372:2285–2295. [DOI] [PubMed] [Google Scholar]
- 2.Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. N. Engl. J. Med. 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015;372:1009–1018. [DOI] [PubMed] [Google Scholar]
- 4.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. The Lancet. 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 6.Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12:723–725. [DOI] [PubMed] [Google Scholar]
- 7.Heiss W-D, Sobesky J, Hesselmann V. Identifying thresholds for penumbra and irreversible tissue damage. Stroke. 2004;35:2671–2674. [DOI] [PubMed] [Google Scholar]
- 8.Olsen TS, Larsen B, Herning M, Skriver EB, Lassen NA. Blood flow and vascular reactivity in collaterally perfused brain tissue. Evidence of an ischemic penumbra in patients with acute stroke. Stroke. 1983;14:332–341. [DOI] [PubMed] [Google Scholar]
- 9.Petersen NH, Ortega-Gutierrez S, Reccius A, Masurkar A, Huang A, Marshall RS. Dynamic cerebral autoregulation is transiently impaired for one week after large-vessel acute ischemic stroke. Cerebrovasc. Dis. 2015;39:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treurniet KM, Berkhemer OA, Immink RV, Lingsma HF, Ward-van der Stam VMC, Hollmann MW, et al. A decrease in blood pressure is associated with unfavorable outcome in patients undergoing thrombectomy under general anesthesia. J Neurointerv Surg. 2018; 10:107–111. [DOI] [PubMed] [Google Scholar]
- 11.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 12.Sulter G, Steen C, Jacques De Keyser. Use of the Barthel Index and Modified Rankin Scale in Acute Stroke Trials. Stroke. 1999;30:1538–1541. [DOI] [PubMed] [Google Scholar]
- 13.Campbell BCV, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke. 2011;42:3435–3440. [DOI] [PubMed] [Google Scholar]
- 14.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, Kummer von R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis MJ, Menon BK, Baghirzada LB, Campos-Herrera CR, Goyal M, Hill MD, et al. Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology. 2012;116:396–405. [DOI] [PubMed] [Google Scholar]
- 16.Löwhagen Hendén P, Rentzos A, Karlsson J-E, Rosengren L, Sundeman H, Reinsfelt B, et al. Hypotension During Endovascular Treatment of Ischemic Stroke Is a Risk Factor for Poor Neurological Outcome. Stroke. 2015;46:2678–2680. [DOI] [PubMed] [Google Scholar]
- 17.Jagani M, Brinjikji W, Rabinstein AA, Pasternak JJ, Kallmes DF. Hemodynamics during anesthesia for intra-arterial therapy of acute ischemic stroke. J Neurointerv Surg. 2016;8:883–888. [DOI] [PubMed] [Google Scholar]
- 18.Whalin MK, Lopian S, Wyatt K, Sun C-HJ, Nogueira RG, Glenn BA, et al. Dexmedetomidine: a safe alternative to general anesthesia for endovascular stroke treatment. J Neurointerv Surg. 2014;6:270–275. [DOI] [PubMed] [Google Scholar]
- 19.Kummer von R, Dzialowski I. Imaging of cerebral ischemic edema and neuronal death. Neuroradiology. 2017;59:545–553. [DOI] [PubMed] [Google Scholar]
- 20.Whalin MK, Halenda KM, Haussen DC, Rebello LC, Frankel MR, Gershon RY, et al. Even Small Decreases in Blood Pressure during Conscious Sedation Affect Clinical Outcome after Stroke Thrombectomy: An Analysis of Hemodynamic Thresholds. American Journal of Neuroradiology. 2017;38:294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talke PO, Sharma D, Heyer EJ, Bergese SD, Blackham KA, Stevens RD. Society for Neuroscience in Anesthesiology and Critical Care Expert consensus statement: anesthetic management of endovascular treatment for acute ischemic stroke*: endorsed by the Society of NeuroInterventional Surgery and the Neurocritical Care Society. J Neurosurg Anesthesiol. 2014;26:95–108. [DOI] [PubMed] [Google Scholar]
- 22.Mulder MJHL, Ergezen S, Lingsma HF, Berkhemer OA, Fransen PSS, Beumer D, et al. Baseline Blood Pressure Effect on the Benefit and Safety of Intra-Arterial Treatment in MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands). Stroke. 2017; 2017;48:1869–1876. [DOI] [PubMed] [Google Scholar]
- 23.Goyal N, Tsivgoulis G, Iftikhar S, Khorchid Y, Fawad Ishfaq M, Doss VT, et al. Admission systolic blood pressure and outcomes in large vessel occlusion strokes treated with endovascular treatment. J Neurointerv Surg. 2017;9:451–454. [DOI] [PubMed] [Google Scholar]
- 24.Rocha M, Jovin TG. Fast Versus Slow Progressors of Infarct Growth in Large Vessel Occlusion Stroke: Clinical and Research Implications. Stroke. 2017;48:2621–2627. [DOI] [PubMed] [Google Scholar]
- 25.Lima FO, Furie KL, Silva GS, Lev MH, Camargo ECS, Singhal AB, et al. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke. 2010;41:2316–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liebeskind DS, Starkman S, Jo KD, Ohanian AG, Sayre JW, Yun S, et al. Blood pressure in acute stroke is inversely related to the extent of collaterals. Stroke. 2008; 39:538–538. [Google Scholar]
- 27.Rasmussen M, Espelund US, Juul N, Yoo AJ, Sorensen LH, Sorensen KE, et al. The influence of blood pressure management on neurological outcome in endovascular therapy for acute ischaemic stroke. British Journal of Anaesthesia. 2018;120:1287–1294. [DOI] [PubMed] [Google Scholar]
- 28.Simonsen CZ, Sørensen LH, Juul N, Johnsen SP, Yoo AJ, Andersen G, et al. Anesthetic strategy during endovascular therapy: General anesthesia or conscious sedation? (GOLIATH - General or Local Anesthesia in Intra Arterial Therapy) A single-center randomized trial. Int J Stroke. 2016;11:1045–1052. [DOI] [PubMed] [Google Scholar]
- 29.Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CBLM, Dippel DW, et al. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA. 2016;316:1279–1288. [DOI] [PubMed] [Google Scholar]
- 30.Goyal M, Jadhav AP, Bonafe A, Diener H, Mendes Pereira V, Levy E, et al. Analysis of Workflow and Time to Treatment and the Effects on Outcome in Endovascular Treatment of Acute Ischemic Stroke: Results from the SWIFT PRIME Randomized Controlled Trial. Radiology. 2016;279:888–897. [DOI] [PubMed] [Google Scholar]
- 31.Rinaldo L, Brinjikji W, McCutcheon BA, Bydon M, Cloft H, Kallmes DF, et al. Hospital transfer associated with increased mortality after endovascular revascularization for acute ischemic stroke. J Neurointerv Surg. 2016; 9:1166–1172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.