Abstract
TAFRO syndrome is a rare clinicopathologic variant of idiopathic multicentric Castleman disease characterized by Thrombocytopenia, Ascites (anasarca), myeloFibrosis, Renal dysfunction, and Organomegaly. Here, we report a case of TAFRO syndrome in an HIV-negative young Caucasian male who presented with fever, normocytic anemia, thrombocytopenia, and acute renal insufficiency. The serum interleukin-6 (IL-6) level was elevated. Chest and abdominal CT revealed bilateral pleural effusion, ascites, splenomegaly, and multiple mildly enlarged lymph nodes. An excisional biopsy of inguinal lymph node showed a few atrophic follicles and expansion of interfollicular areas by marked vascular proliferation and polytypic plasmacytosis. HHV-8 was negative. Subsequent bone marrow biopsy was normocellular with moderately increased megakaryocytes and occasional megakaryocytic emperipolesis. His signs and symptoms improved after treatment with methylprednisolone and tocilizumab (anti-IL-6 receptor antibody). Our study confirms the distinctive nature of this syndrome, which should allow for better recognition and appropriate therapy.
1. Introduction
Multicentric Castleman's disease (MCD) is a systemic inflammatory disorder caused by excessive proinflammatory cytokines, especially interleukin-6 (IL-6). Most cases of MCD are linked to infection by HHV-8/KSHV, and the virus was identified as the cause of hypercytokinemia in MCD patients. In the past few years, a group of HHV-8 and HIV negative MCD cases with similar clinicopathologic features, namely idiopathic MCD (iMCD), were reported [1]. In 2010, Takai et al. described three cases of iMCD with the following constellation of symptoms: thrombocytopenia, anasarca, fever, reticulin fibrosis, and organomegaly [2]. The term “TAFRO syndrome” was proposed at the Fukushima and Nagoya meetings to delineate this disorder [3]. Interestingly, the lymph nodes from TAFRO syndrome patients appeared to show features of iMCD. To date, >30 cases have been reported mostly in Japan and two cases in Europe [4,5], one in South America [6], and two in North America [7,8]. Here we present a new case of TAFRO syndrome in the United States and review recent data on this newly recognized systemic disease.
2. Case presentation
The patient was a 32-year-old Caucasian male who presented with fever, fatigue, abdominal distension, anorexia, and shortness of breath. Complete blood count (CBC) showed mild leukocytosis, mild normocytic and normochromic anemia, and severe thrombocytopenia. Acute kidney injury was demonstrated by increased creatinine and blood urea nitrogen (BUN), elevated anion gap and hyperkalemia. The workup for multiple autoantibodies and viruses were all negative. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were both increased. IL-6 level in serum was increased to 43.6 pg/mL (0–15.5 pg/mL). Serum protein electrophoresis showed no evidence of monoclonal immunoglobulinemia. Chest and abdominal CT showed splenomegaly, ascites, as well as multiple prominent retroperitoneal, iliac chain, and inguinal lymph nodes. The largest one measured up to 8 mm. Given the thrombocytopenia and acute renal insufficiency, the possibility of thrombotic thrombocytopenic purpura (TTP) was clinically suspected. The patient symptoms temporarily improved after treatment with high dose steroid and plasmapheresis.
The patient returned shortly after discharge due to shortness of breath and fatigue. Repeated CT showed progressive ascites and bilateral pleural effusion. An inguinal lymph node biopsy showed small atrophic follicles with regressed germinal center surrounded by concentric rings of mantle zone. Occasional vessels penetrating through the germinal centers were noted (Fig. 1A-C). Interfollicular areas were markedly expanded by polytypic plasma cells and vascular proliferation with plump endothelial cells (Fig. 1D and E). IgG4 positive plasma cells were not increased. HHV-8 and Epstein–Barr virus-encoded small RNAs (EBER) were both negative (Fig. 2). The histopathologic features were reminiscent of multicentric Castleman disease.
Fig. 1.
Biopsy of an inguinal lymph node shows regressed germinal center with surrounded mantle zone with onion skinning change and penetrating blood vessel (A–C). Abundant histiocytes and plasma cells (D) and prominent vascular proliferation with plump endothelial cells are present in the expanded interfollicular zone (E). Bone marrow biopsy shows moderate hyperplasia of megakaryocytes (F) with occasional emperipolesis (F, inserts). A, ×40; B, ×100; C and E, ×200; D and F, ×400.
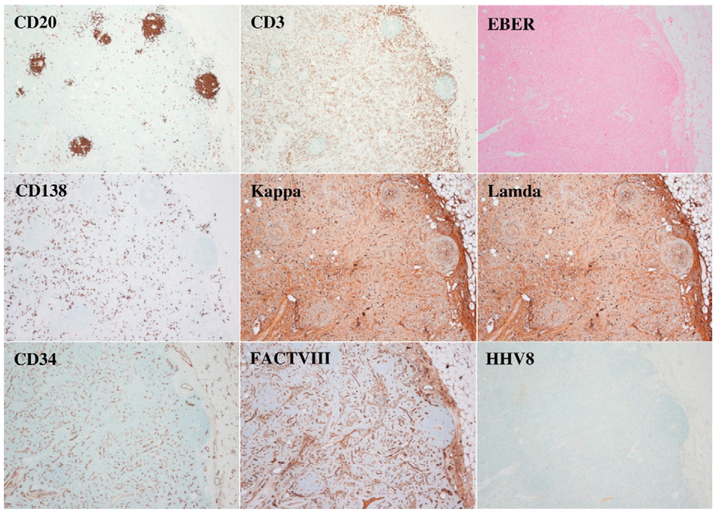
Fig. 2.
Immunohistochemistry staining, ×40. CD20 shows B-cells in small follicles and CD3 highlights the unremarkable paracortical T-cells. CD138, Kappa, and Lambda show polyclonal plasmacytosis. CD34 and Factor VIII-related antigen highlight the massive proliferation of small vessels. HHV-8 and EBER are negative.
PCR studies showed polyclonal T-cell receptor beta and gamma gene rearrangements. B-cell receptor gene rearrangement studies showed clonal gene rearrangement in the IgH FRIII region (one positive peak arising in a polyclonal background). The rest of the frameworks showed a polyclonal pattern. The significance of the PCR result is uncertain, and there was no evidence of monoclonality by immunohistochemistry. Also, the patient did not have other evidence of lymphoma.
A subsequent bone marrow biopsy was performed and showed normocellular marrow with moderately increased megakaryocytes. Occasional megakaryocytic emperipolesis was noted (Fig. 1F). Reticulin stain did not reveal myeloid fibrosis. Given the history of pleural effusion, ascites, anemia, thrombocytopenia, mild lymphadenopathy, splenomegaly, and renal dysfunction, the overall findings were compatible with TAFRO syndrome.
The patient was then initiated on high-dose methylprednisolone and tocilizumab. After four doses of tocilizumab, leukocytosis, anemia, and thrombocytopenia were all resolved. Electrolytes and kidney function returned to normal. Repeated CT showed both pleural effusion and ascites were improved. He was then treated with maintenance steroid and tocilizumab every 14 days. Ten months later, the patient presented to the emergency department due to worsening joint pain and body ache. Imaging studies revealed mild ascites and splenomegaly with no lymphadenopathy. BUN and creatinine were normal. Serum IL-6 level ranged between 26 and 56 pg/mL. ESR was also elevated. The patient was referred to an outside health care center for a second opinion, and additional laboratory tests showed IgG4 subclass and soluble IL-2 receptor (sIL-2R) were within the normal range. Since rituximab has shown some effect in treating HHV8-negative, HIV-negative Castleman disease [9], rituximab was added weekly for four weeks. Cyclosporine A was also attempted for a short period of time and subsequently was discontinued due to severe skin rash reaction. In the meantime, the patient continued to complain extensive bone pain with symptoms of bowel obstruction, narcotics abuse, and drug-seeking behavior were suspected. Currently, the patient is treated by Rituxan, etoposide, and tocilizumab based on a recent study [10]. His most recently laboratory tests showed mild anemia, elevated sIL-2R to 1199 U/mL (reference range: 223–710 U/mL), and normal IL-6 and vascular endothelial growth factor (VEGF) levels. Image studies did not show significant findings.
3. Discussion
TAFRO is a recently described syndrome representing a rare form of iMCD. To better facilitate the diagnosis of TAFRO syndrome, modified criteria were proposed in the second Japanese TAFRO meeting in October 2015 [11]. The diagnosis of TAFRO syndrome required fulfillment of three major criteria and two of four minor criteria (see Table 1). The major criteria were defined as thrombocytopenia (≤100 × 103/μL), anasarca (pleural effusion, ascites or general edema), and systemic inflammation (temperature above 37.5 °C and/or serum CRP ≥ 2 mg/dL). The four minor criteria included (1) histopathologic features of Castleman disease in lymph node; (2) myelofibrosis and/or hyperplasia of megakaryocytes in bone marrow; (3) mild organomegaly (hepatomegaly, splenomegaly, and lymphadenopathy, usually <1.5 cm); (4) progressive renal insufficiency. The current case report meets all the major and minor diagnostic criteria for TAFRO syndrome. Interestingly, although not listed as a recognized histological feature, megakaryocytic emperipolesis was reported recently [12] and also present in this case. The significance of this finding is unclear.
Table 1.
Proposed diagnostic criteria for TAFRO syndrome [11].
| Major criteria (all required) | Minor criteria (at least two of four required) |
|---|---|
| 1. Anasarca, including pleural effusion, ascites, and general edema | 1. Lymph node biopsy shows Castleman's disease-like histopathologic features |
| 2. Thrombocytopenia; defined as a pretreatment platelet count ≤100,000/μL | 2. Bone marrow shows reticulin myelofibrosis and/or increased number of megakaryocytes |
| 3. Systemic inflammation: fever of unknown etiology above 37.5 °C and/or serum CRP ≥ 2 mg/dL | 3. Mild organomegaly, including hepatomegaly, splenomegaly, and lymphadenopathy |
| 4. Progressive renal insufficiency |
A scoring system also was established to predict disease severity based on the evaluation and grading of four major categories: anasarca, thrombocytopenia, fever and renal insufficiency [11]. The treatment strategy [11] proposed by the 2015 Japanese meeting lists high-dose glucocorticoid or methylprednisolone as a first line approach and cyclosporine A as a second line. Tocilizumab or rituximab (anti-CD20 antibody) is recommended in patients with renal insufficiency in whom cyclosporine A is contraindicated. Plasma exchange, cyclophosphamide, and chemotherapy, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) have also been used in the selected patients with success. Elevated levels of IL-6 are considered a key element leading to the clinical findings. Notably, initial treatment with plasmapheresis relieved symptoms in our patient for a short period. It is thought that plasmapheresis helps to lower the concentration of the proinflammatory cytokines in serum including IL-6. The fact that sIL-2R was elevated in our patient when IL-6 and VEGF levels returned to normal suggests that T cell activation also plays a role in Castleman disease and also explains why cyclosporin A works in a subset of cases.
One of the differential diagnosis of Castleman disease is POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy and skin changes), which is also related to changes in the levels of cytokines, including IL-1, IL-6, TNF-α and VEGF. Although our patient fulfilled two minor diagnostic criteria for POEMS syndrome: organomegaly and extravascular volume overload, lack of two major mandatory criteria, including polyneuropathy and monoclonal plasma cell proliferative disorder, makes the diagnosis of POEMS unlikely.
It is still controversial whether TAFRO syndrome should be an independent entity or classified as a subvariant of iMCD. In the study conducted by Liu AY et al., a subset of iMCD patients (19%) had features of TAFRO [13]. The 2-year survival rate of these patients appeared to be lower than the group without symptoms characteristic of TAFRO (85% vs. 92%, p = 0.16) [13]. Increased mortality was also seen in patients with TAFRO features compared with non-TAFRO iMCD patients within the first six months after diagnosis [13].
4. Conclusion
Despite the growing knowledge of TAFRO syndrome, the pathogenesis and optimal clinical management still need to be elucidated. To better understand this disease, a multicenter on-line patient registry has been initiated in Japan recently [11]. Since the clinicopathologic characteristics have been elucidated in recent literature, we expect more TAFRO cases to be reported in the future. This will advance the understanding of the pathogenesis and management of this unique syndrome.
Abbreviation:
- iMCD
idiopathic multicentric Castleman's disease
- IL-6
interleukin-6
- TAFRO
Thrombocytopenia, Ascites (anasarca), myeloFibrosis, Renal dysfunction, and Organomegaly
Footnotes
Conflict of interest
The authors have no conflict of interest to report.
References
- [1].Fajgenbaum DC, van Rhee F, Nabel CS, HHV-8-negative, idiopathic multicentric Castleman disease: novel insights into biology, pathogenesis, and therapy, Blood 123 (19) (2014) 2924–2933. [DOI] [PubMed] [Google Scholar]
- [2].Takai K, et al. , Thrombocytopenia with mild bone marrow fibrosis accompanied by fever, pleual effusion, ascites and hepatosplenomegaly, Jpn.J. Clin. Hematol 51 (5) (2010) 320–325. [PubMed] [Google Scholar]
- [3].Kawabata H, et al. , Castleman-Kojima disease (TAFRO syndrome): a novel systemic inflammatory disease characterized by a constellation of symptoms, namely, thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly: a status report and summary of Fukushima (6 June, 2012) and Nagoya meetings (22 September, 2012), J. Clin. Exp. Hematop 53 (1) (2013) 57–61. [DOI] [PubMed] [Google Scholar]
- [4].Tedesco S, et al. , Successful treatment of a Caucasian case of multifocal Castleman's disease with TAFRO syndrome with a pathophysiology targeted therapy - a case report, Exp. Hematol. Oncol 4 (1) (2015) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lynn Antoun Abdo, Clement Philippe Morin, Rocco Paolo Collarino, Jean Paul Cabane, Marc Albert Gatfosse, First European case of TAFRO syndrome associated with Sjogren disease, Am.J. Intern. Med 2 (6) (2014) 102–105. [Google Scholar]
- [6].Contardo Damian, Finocchietto Paola, Uehara Tatiana, Papini Claudia, Deligiannis Natalia, Darderes Enrique, Castroagudin Augusto, Cabral Cecilia, Horacio di Fonzo, TAFRO syndrome in a patient of South-Amerian Descent, Eur. J. Case Rep. Intern. Med 2 (2015). [Google Scholar]
- [7].Hawkins Jennifer M., Pillai Vinodh, TAFRO syndrome or Castleman-Kojima syndrome: a variant of multicentric Castleman disease, Blood 126 (18) (2015) 2163. [DOI] [PubMed] [Google Scholar]
- [8].Jain P, et al. , Durable remission with rituximab in a patient with an unusual variant of Castleman's disease with myelofibrosis-TAFRO syndrome, Am. J. Hematol 90(11) (2015) 1091–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ide M, et al. , Long-term remission in HIV-negative patients with multicentric Castleman's disease using rituximab, Eur. J. Haematol 76 (2) (2006) 119–123. [DOI] [PubMed] [Google Scholar]
- [10].Simons Malorie, Apor Emmanuel, Butera James N., Treaba Diana O., TAFRO syndrome associated with EBV and successful triple therapy treatment: case report and review of the literature, Case Rep. Hematol 2016 (2016) (Article ID 4703608). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Masaki Y, et al. , Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version, Int. J. Hematol. 103 (6) (2016) 686–692. [DOI] [PubMed] [Google Scholar]
- [12].Wang HW, Pittaluga S, Jaffe ES, Multicentric Castleman disease: where are we now? Semin. Diagn. Pathol 33 (5) (2016) 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu AY, et al. , Idiopathic multicentric Castleman's disease: a systematic literature review, Lancet Haematol. 3 (4) (2016) e163–e175. [DOI] [PubMed] [Google Scholar]