Abstract
Here we conducted a retrospective study to examine the risk of cardiovascular events (CVEs) relative to that of end-stage renal disease (ESRD) in patients with primary membranous nephropathy, in a discovery cohort of 404 patients. The cumulative incidence of CVEs was estimated in the setting of the competing risk of ESRD with risk factors for CVEs assessed by multivariable survival analysis. The observed cumulative incidences of CVEs were 4.4%, 5.4%, 8.2%, and 8.8% at 1, 2, 3, and 5 years respectively in the primary membranous nephropathy cohort. In the first 2 years after diagnosis, the risk for CVEs was similar to that of ESRD in the entire cohort, but exceeded it among patients with preserved renal function. Accounting for traditional risk factors and renal function, the severity of nephrosis at the time of the event (hazard ratio 2.1, 95% confidence interval 1.1 to 4.3) was a significant independent risk factor of CVEs. The incidence and risk factors of CVEs were affirmed in an external validation cohort of 557 patients with primary membranous nephropathy. Thus early in the course of disease, patients with primary membranous nephropathy have an increased risk of CVEs commensurate to, or exceeding that of ESRD. Hence, reduction of CVEs should be considered as a therapeutic outcome measure and focus of intervention in primary membranous nephropathy.
Keywords: cardiovascular disease, glomerulonephritis, membranous nephropathy
Primary membranous nephropathy (MN) is one of the most common causes of nephrotic syndrome in adults.1 The goals of therapy of MN have primarily focused on the prevention of ESRD, an event that generally occurs after several years,2 whereas other complications of the nephrotic syndrome may occur early in the course of disease. A well-recognized early complication of MN are venous thromboembolic events,3–7,8 with hypoalbuminemia being the most important independent risk factor.9 While the increased risk of venous thromboembolic events is well characterized, the risk of arterial thromboembolic events, primarily consisting of CVEs (acute myocardial ischemic events and infarction, ischemic cerebrovascular events, and peripheral artery occlusive disease), has only been described to a limited extent by Mahmoodi et al.10 Small cohort studies11–13 have reported on the risk of CVEs in primary MN, but the data relative to their incidence, timing, and risk factors are scant. We hypothesized that primary MN is associated with a high risk of CVEs, and that there may be a temporal pattern favoring events early in the course of disease given the thrombophilic state associated with MN. We further hypothesized that, early in the course of disease, the risk of major CVEs is commensurate to or exceeds that of ESRD and represents an important cause of morbidity. If confirmed, the prevention of early CVEs should be considered as part of the early goals of therapy of the nephrotic syndrome of primary MN.
In this study, we present data on the cumulative incidence rate of CVEs in a large inception cohort of primary MN from the Glomerular Disease Collaborative Network (GDCN cohort) of the University of North Carolina at Chapel Hill.9 We performed a detailed analysis of risk factors of CVEs with a focus on the severity of nephrotic syndrome. We validated our findings by comparing them to those derived from an external independent cohort of patients with primary MN from the Toronto Glomerulonephritis Registry (TGNR cohort) of the University of Toronto.14
RESULTS
Incidence of cardiovascular events in primary MN
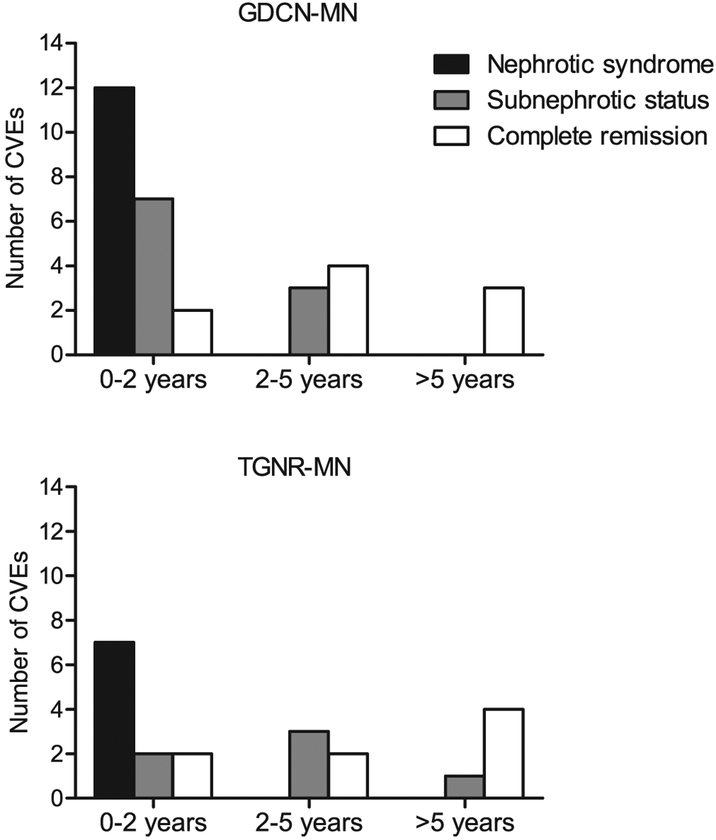
A total of 404 patients with primary MN identified from the GDCN registry constituted the study cohort to evaluate the risk of CVE. Table 1 shows the detailed baseline characteristics of the study and validation cohorts. Patients in the GDCN study cohort (60% men, mean age 55 years) presented with a mean proteinuria of 8.7 ± 6.2 g/d and a mean serum albumin of 2.5 ± 0.8 g/dl. Eighty-eight percent presented with nephrotic syndrome (defined as proteinuria >3.5 g/d and serum albumin <3.2 g/dl), and 62% had an estimated glomerular filtration rate (eGFR) greater than 60 ml/min per 1.73 m2. During the median follow-up of 24.3 months (interquartile range 9.9–52.7 months), 31 patients had a CVE, 58 progressed to ESRD, and 6 died of causes other than CVEs. Of the 31 CVEs, 22 were acute coronary syndrome, 8 were acute ischemic cerebrovascular events, and 1 was an acute peripheral arterial thromboembolic event. The distribution of CVEs according to times after biopsy and the status of nephrotic syndrome at the time of event is shown in Figure 1. The majority of total CVEs (21 of 31 events) occurred within 2 years after diagnosis, with 57% (12 of 21 events) of them occurring while the patient had severe proteinuria (mean 7.6 ± 4.1 g/d) hypoalbuminemia (mean 2.5 ± 0.7 g/dl). In contrast, about one-third of total CVEs (10 of 31 events) occurred beyond 2 years after diagnosis. These late CVEs occurred in 7 patients in complete remission and in 3 patients with subnephrotic proteinuria at the time of the event (Figure 1). The proteinuria (2.2 ± 1.5 g/d, P < 0.001) and hypoalbuminemia (3.6 ± 0.4 g/dl, P < 0.001) at the time of the event in patients with late CVEs were significantly less severe compared to those of patients who experienced CVEs early within 2 years of kidney biopsy (7.6 ± 4.1 g/d and 2.5 ± 0.7 g/dl, respectively) (Table 2).
Table 1 |.
Baseline characteristics of the GDCN study cohort and TGNR validation cohort
| GDCN (n = 404) | TGNR (n = 557) | |
|---|---|---|
| Age (years)a | 51.4 ± 15.5 | 46.7 ± 16.7 |
| Male gender (%) | 60 | 65 |
| Race,a white (%) | 71 | 73 |
| Black | 20 | 7 |
| Asian/Others | 9 | 20 |
| Diabetes (%)a | 9 | 3 |
| Smoking | ||
| Ever (%) | 33 | 31 |
| Current (%) | 21 | NA |
| Previous history of CVE (%) | 12 | 13 |
| Serum creatinine (mg/dl)a | 1.5 ± 1.3 | 1.1 ± 0.7 |
| eGFR (ml/min/1.73 m2)a | 68.9 ± 33.5 | 78.9 ± 36.7 |
| eGFR >60 (%) | 62 | 72 |
| eGFR 45–60 (%) | 16 | 15 |
| eGFR <45 (%)a | 22 | 13 |
| Proteinuria (g/d)a | 8.7 ± 6.2 | 7.7 ± 6.6 |
| Serum albumin (g/dl) | 2.5 ± 0.8 | 2.5 ± 0.7 |
| Serum total cholesterol (mg/dl)b | 337.9 ± 107.7 | 326.9 ± 100 |
| < 200 (%) | 7 | 9 |
| 200–300 (%) | 33 | 34 |
| ≥300 (%) | 60 | 57 |
| Nephrotic syndrome (%)a | 88 | 74 |
| Medication usec | ||
| Aspirin (%) | 21 | 17 |
| Corticosteroids (%)a | 76 | 50 |
| CNI (%) | 7 | 10 |
| Cyclophosphamide (%) | 22 | 15 |
| Statin (%) | 40 | 22 |
| Follow-up duration (months)a | 24.3 (9.9–52.7) | 52 (21.3–96.2) |
Data presented as mean ± SD or median (interquartile range) for continuous variables and as percentages for categorical variables.
CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate by modified MDRD equation; GDCN, Glomerular Disease Collaborative Network; MN, membranous nephropathy; TGNR, Toronto Glomerulonephritis Registry.
Significant difference with P value <0.05 by t-test for continuous variables and χ2 test for categorical variables.
Number of patients with valid laboratory values was limited: GDCN, 270 of 404 (67%); TGNR, 341 of 557(61%).
Number of patients with valid information was limited in TGNR cohort: aspirin and statin use, 352 of 557 (63%).
Figure 1 |. Number of cardiovascular events observed according to the duration of follow-up and the status of nephrotic syndrome in primary MN cohorts.
CVE, cardiovascular event; GDCN, Glomerular Disease Collaborative Network; MN, membranous nephropathy; TGNR, Toronto Glomerulonephritis Registry.
Table 2 |.
Comparison of the characteristics at the time of CVE between early and late CVEs in GDCN and TGNR cohorts
| Characteristics at the time of CVEs | GDCN (N = 31) | ||
|---|---|---|---|
| Early CVE (N = 21) | Late CVE (N = 10) | P value | |
| Age (years) | 64 ± 11 | 62 ± 11 | 0.71 |
| eGFR (ml/min/1.73 m2) | 47 ± 24 | 57 ± 27 | 0.40 |
| Proteinuria (g/d) | 7.6 ± 4.1 | 2.2 ± 1.5 | <0.001 |
| Serum albumin (g/dl) | 2.5 ± 0.7 | 3.6 ± 0.4 | <0.001 |
| Characteristics at the time of CVEs | TGNR (N = 21) | ||
| Early CVE (N = 11) | Late CVE (N = 10) | P value | |
| Age (years) | 62 ± 11 | 59 ± 9 | 0.61 |
| eGFR (ml/min/1.73 m2) | 50 ± 27 | 51 ± 19 | 0.81 |
| Proteinuria (g/d) | 7.5 ± 2.8 | 2.9 ± 1.8 | <0.001 |
| Serum albumin (g/dl) | 2.7 ± 0.9 | 3.5 ± 0.6 | 0.02 |
Early CVE defined as occurrence ≤2 years from time of biopsy and late CVE defined as an occurrence >2 years from time of biopsy.
CVE, cardiovascular event; eGFR, estimated glomerular filtration rate; GDCN, Glomerular Disease Collaborative Network; MN, membranous nephropathy; PY, person-years; TGNR, Toronto Glomerulonephritis Registry.
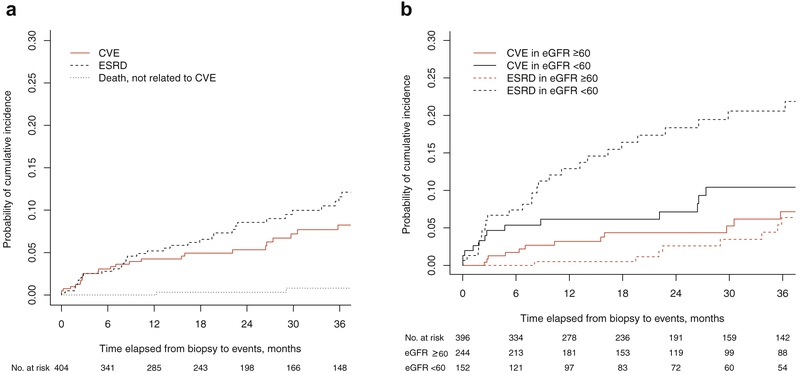
The cumulative incidence rates of newly diagnosed CVEs in competing risk analysis were 4.4%, 5.4%, and 8.2%, at 1, 2, and 3 years after biopsy, respectively. At the same time points, the cumulative incidence rates of ESRD were 5.6%, 8.9%, and 11.9%, respectively (Figure 2a). Among patients with baseline eGFR ≥ 60 ml/min per 1.73 m2, the estimated incidence of CVEs exceeded that of ESRD during the first 3 years after diagnosis (cumulative incidence rates of 2.6%,3.7%, and 6.4% for CVEs vs. 0.5%, 2.5%, and 6.1% for ESRD at 1, 2, and 3 years, respectively) (Figure 2b). In contrast, among patients with eGFR less than 60 ml/min per 1.73 m2 at baseline, the cumulative incidence of ESRD exceeded that of CVEs beyond the first 6 months after kidney biopsy (cumulative incidence of 7.1%, 8.0%, and 11.0% for CVEs and 11.9%, 16.9%, and 18.9% for ESRD at 1, 2, and 3 years, respectively) (Figure 2B). Nevertheless, even in this subgroup of patients, a trend of relatively high incidence of CVEs early in the course of disease was observed.
Figure 2 |. Estimates of cumulative incidence functions.
(a) Cumulative incidences of CVEs, ESRD, and death unrelated to CVE in the GDCN cohort. The cumulative incidence rate of CVE was calculated by accounting for the influence of competing risks of ESRD and death unrelated to CVE. (b) The incidence of CVEs is commensurate to or exceeds that of ESRD in the early period of follow-up for each category of baseline eGFR. Cumulative incidence probabilities of CVEs and ESRD were calculated by competing risk analysis. In patients with eGFR ≥60 ml/min per 1.73 m2 at baseline (red lines), the incidence rate of CVEs (red solid line) is higher than that of ESRD (red dotted line) throughout the follow-up period. For patients with eGFR <60 ml/min per 1.73 m2 at baseline, the cumulative incidences of CVEs (black solid line) and ESRD (black dotted line) are similar during the first 6 months after diagnosis. Later in the course of disease, the cumulative incidence of ESRD exceeds that of CVEs among patients with baseline eGFR <60 ml/min per 1.73 m2. CVE, cardiovascular event; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; GDCN, Glomerular Disease Collaborative Network.
Risk factors for CVEs in primary MN
The risk factors for CVEs were evaluated based on the GDCN cohort (Table 3). By univariable analysis, older age (hazard ratio [HR] 1.8 per decade increase, 95% confidence interval [CI] 1.3–2.5, P < 0.001), history of previous CVE (HR 6.0, 95% CI 2.9–12.5, P < 0.001), and diabetes mellitus (HR 4.4, 95% CI 1.9–10.4, P < 0.001) were significantly associated with CVEs. Neither current smoking (HR 1.1, 95% CI 0.4–2.5, P = 0.868) nor a history of smoking (ever smoker) (HR 1.5, 95% CI 0.6–3.8, P = 0.439) was statistically significantly associated with an increased occurrence of CVEs. In addition to the previously established CVE risk factors, severe renal dysfunction at baseline (by 3 categories of eGFR <45, 45–60, and >60 ml/min per 1.73 m2) was also associated with increased likelihood of CVEs (HR 1.7 per increase of the category, 95% CI 1.1–2.5, P = 0.011). During the period of observation, changes in renal function and severity of nephrotic syndrome influenced the incidence of CVEs as observed in Figure 1. Therefore, in addition to the baseline characteristics, we considered these 2 time-varying characteristics as important covariates of CVE-risk estimation models; we assigned varying categories during follow-up for the severity of nephrotic status (nephrotic syndrome vs. subnephrotic or complete remission) and for renal dysfunction (as described above). Time-dependent status of nephrotic syndrome (HR 2.2, 95% CI 1.1–4.3, P = 0.027) and severe renal dysfunction (eGFR <45 ml/min per 1.73 m2) (HR 1.6, 95% CI 1.1–2.3, P = 0.023) were significantly associated with the increased hazards of CVEs in each univariable model (Table 3). In addition to smoking (not a significant but a recognized risk factor for CVEs and thrombosis), all the characteristics identified below the cutoff of variable selection criteria in univariable models were considered potential explanatory variables in the final multivariable model. Four independent risk factors were selected as statistically significant after adjusting for covariates incorporated in the final model: the time-dependent severity of nephrotic syndrome increased the potential of CVEs 2.1-fold (95% CI 1.1–4.3, P= 0.032); 3 traditional risk factors, older age (HR 1.6), diabetes (HR 3.0), and history of previous CVE (HR 2.7), also remained independent predictors of CVEs (Table 3).
Table 3 |.
Risk factors for cardiovascular events in the GDCN cohort
| Hazard ratio | 95% CI | P value | |
|---|---|---|---|
| Univariable model | |||
| Baseline variables | |||
| Age at biopsy (per decade increase from <30 years old) | 1.8 | 1.3–2.5 | <0.001 |
| Sex, male | 1.0 | 0.5–2.1 | 0.973 |
| Diabetes | 4.4 | 1.9–10.4 | <0.001 |
| Previous history of CVE | 6.0 | 2.9–12.5 | <0.001 |
| Smoking | |||
| Ever (vs. never) | 1.5 | 0.6–3.8 | 0.439 |
| Current (vs. ever or no smoking) | 1.1 | 0.4–2.5 | 0.868 |
| Lower eGFR category (eGFR <45 vs. 45–60 vs. >60 ml/min/1.73 m2) | 1.7 | 1.1 −2.5 | 0.011 |
| Nephrotic syndrome (vs. subnephrotic) | 2.4 | 0.6–10.2 | 0.232 |
| Medication use | |||
| Aspirin | 1.8 | 0.8–4.0 | 0.112 |
| Corticosteroid | 0.6 | 0.3–1.2 | 0.157 |
| CNI | 0.3 | 0.03–1.5 | 0.143 |
| Cyclophosphamide | 0.5 | 0.2–1.1 | 0.101 |
| Statin | 0.5 | 0.3–1.1 | 0.096 |
| Severity of hypercholesterolemia (≥300 vs. 200–300 vs. <200 mg/dl) | 1.5 | 0.7–3.2 | 0.311 |
| Time-varying variablesa | |||
| Lower eGFR category | 2.2 | 1.1 −4.3 | 0.027 |
| Severity of nephrotic syndrome (nephrotic vs. subnephrotic vs. normal) | 1.6 | 1.1 −2.3 | 0.023 |
| Multivariable modelb | |||
| Age at biopsy (decades) | 1.6 | 1.2–2.2 | 0.003 |
| Diabetes | 3.0 | 1.2–7.7 | 0.025 |
| Previous history of CVE | 2.7 | 1.2–6.3 | 0.02 |
| Time-varying severity of nephrotic syndrome | 2.1 | 1.1 −4.3 | 0.032 |
CI, confidence interval; CNI, calcineurin inhibitor; CVE, cardiovascular event; eGFR, estimated glomerular filtration rate calculated by MDRD equation; MN, membranous nephropathy.
Time-varying values of eGFR category and severity of nephrotic syndrome over the follow-up times were taken into account as explanatory variables.
Current smoking, statin use, and baseline and time-varying lower eGFR categories were removed from the final model by backward elimination.
Validation of the risk for CVEs in the TGNR cohort of patients with primary MN
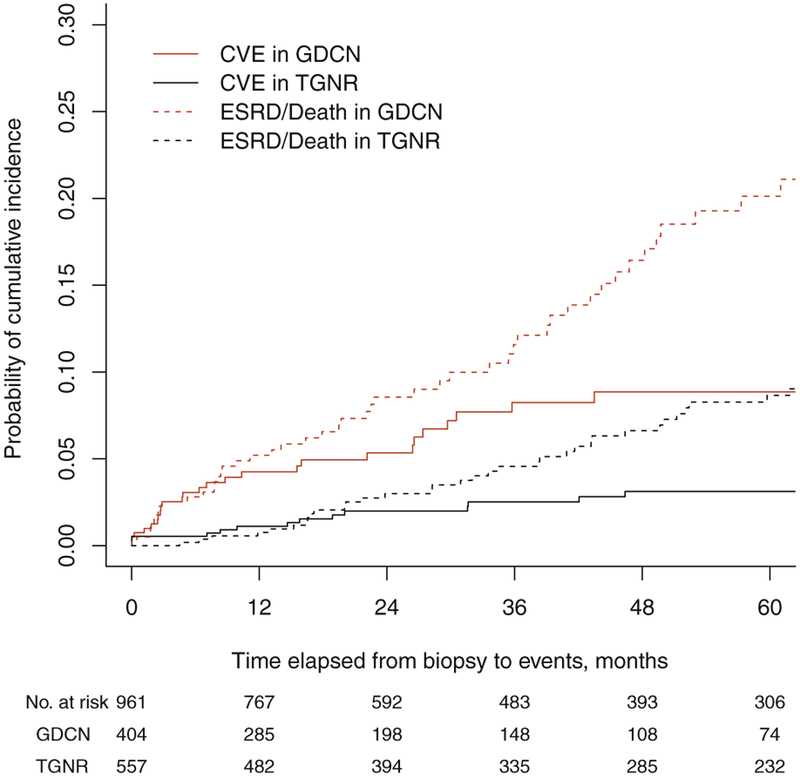
To validate the findings of the increased risk and the pattern of CVEs associated with disease activity in patients with primary MN, we examined the cumulative incidence of CVEs and assessed risk factors in an independent cohort of 557 patients with primary MN recruited from the TGNR (Table 1).15 Compared to the GDCN cohort, patients in the TGNR cohort were younger (mean age of 47 years vs. 51 years in GDCN, P < 0.05), more frequently of Pacific Asian descent, and presented with better-preserved renal function and less severe disease: 72% of the TGNR patients presented with an eGFR less than 60 ml/min per 1.73 m2 (vs. 62% in GDCN, P < 0.05), and 74% had nephrotic syndrome (vs. 88% in GDCN, P < 0.05). During the median follow-up of 52 months, 21 CVEs were observed, while 57 patients progressed to ESRD. The cumulative incidence of CVE and ESRD were 1.1% and 0.8% at 1 year; 2.0% and 3.0% at 2 year; 2.5% and 4.6% at 3 years; and 3.1% and 6.6% at 5 years, respectively (Figure 3). Similar to the findings in the GDCN cohort, patients in the TGNR cohort who suffered a CVE within the first 2 years after diagnosis had significantly more severe proteinuria (7.5 ± 2.8 g/d vs. 2.9 ± 1.8 g/d, P < 0.001) and hypoalbuminemia (2.7 ± 0.9 g/d vs. 3.5 ± 0.6 g/dl, P = 0.02) at the time of CVE compared to patients with later events (Table 2). In the univariable risk prediction models, older age (HR 1.6), diabetes (HR 4.6), prior cardiovascular disease (HR 11.3), and severe renal dysfunction (HR 1.7) were also significantly associated with CVEs in the TGNR cohort just as they were in the GDCN cohort (Table 4). In the multivariable survival analysis model, time-dependent severity of nephrotic status (HR 2.3, 95% CI 1.0–5.2, P = 0.04), older age (HR 1.4, 95% CI 1.1–2.0, P = 0.024), and history of previous CVE (HR 4.3, 95% CI 1.5–7.3, P < 0.001) were independently associated with CVEs, similar to the findings in the GDCN model (Table 4). These risk factors remained independently associated with CVEs in a multivariate model of the combined GDCN + TGNR cohort (Table 4).
Figure 3 |. Comparison of outcomes between GDCN and TGNR cohorts.
CVE, cardiovascular event; ESRD, end-stage renal disease; GDCN, Glomerular Disease Collaborative Network; TGNR, Toronto Glomerulonephritis Registry.
Table 4 |.
Risk factors for CVEs in TGNR cohort and GDCN + TGNR combined cohort
| Hazard ratio | 95% CI | P value | |
|---|---|---|---|
| Univariable model with TGNR cohorta | |||
| Baseline variables | |||
| Age decade (per decade increase from <30 years old) | 1.6 | 1.2–2.2 | 0.003 |
| Sex, male | 3.2 | 0.8–10.9 | 0.084 |
| Diabetes | 4.6 | 1.1 −18.4 | 0.004 |
| Previous history of CVE | 11.3 | 4.3–21.5 | <0.001 |
| Ever smoker | 1.1 | 0.5–2.8 | 0.948 |
| Lower eGFR category (eGFR <45 vs. 45–60 vs. >60 ml/min/1.73 m2) | 1.7 | 1.0–2.9 | 0.042 |
| Nephrotic syndrome (vs. subnephrotic) | 3.1 | 0.6–15.2 | 0.114 |
| Time-varying variables | |||
| Lower eGFR category | 2.2 | 1.1 −4.3 | 0.027 |
| Severity of nephrotic syndrome (nephrotic vs. subnephrotic vs. normal) | 1.6 | 1.1 −2.3 | 0.023 |
| Multivariable model with TGNR cohorta | |||
| Age decades at biopsy | 1.5 | 1.1 −2.0 | 0.022 |
| Previous history of CVE | 11.5 | 3.4–26.3 | <0.001 |
| Time-varying severity of nephrotic syndrome | 2.3 | 1.0–5.2 | 0.045 |
| Multivariable model with combined cohort (GDCN+TGNR)b | |||
| Age decades at biopsy | 1.6 | 1.3–1.9 | <0.001 |
| Previous history of CVE | 4.5 | 2.2–9.0 | <0.001 |
| Time-varying severity of nephrotic syndrome | 2.4 | 1.4–4.0 | 0.002 |
| TGNR cohort (vs. GDCN cohort) | 0.5 | 0.3–0.9 | 0.014 |
Sex, diabetes, lower eGFR category at baseline, and time-varying lower eGFR were eliminated in the final model. Aspirin use and statin use were not tested due to missing information in 33% of cohort.
Diabetes, nephrotic status at baseline, baseline and time-varying lower eGFR categories, and interaction term between cohort identifier and time-varying severity of nephrotic syndrome were eliminated in the final model.
DISCUSSION
Patients with primary MN have an established increased risk of venous thromboembolic events, greater than that attributable to the nephrotic syndrome alone.8 In this study, we present new evidence that primary MN is also associated with increased risk of arterial thromboembolic events as measured by CVEs. Early in the course of disease, the risk of CVEs is commensurate to or exceeds the risk of ESRD.
Previous reports on arterial thromboembolic events are relatively scarce and limited in scope.11–13 Prior retrospective cohort studies of patients with nephrotic syndrome of various causes reported an increased risk for coronary artery disease15 and an incidence rate of CVEs similar to the one we observed in the GDCN cohort.10
The risk of CVEs in MN is related to the severity of nephrotic syndrome as measured by proteinuria, hypoalbuminemia, or both, as it has previously been reported for venous thromboembolic events.8–10 Indeed the majority of CVEs occurred within the first 2 years after diagnosis of MN in the setting of severe nephrotic syndrome. However, unlike with venous thromboembolic events, where the events occurred almost exclusively during periods of severe hypoalbuminemia,9 our study reveals that some CVEs occur late in the course of disease, in the setting of subnephrotic or even complete remission of proteinuria. Interestingly, our data reveal 2 patterns of CVEs: “early” events associated with severe proteinuria, hypoalbuminemia, or both; and “late” events (beyond 2 years after diagnosis) not associated with nephrosis (Figure 1 and Table 2). This dichotomous pattern suggests that the early and late events may result from different pathophysiologic mechanisms. It is conceivable that the early events may be more directly related to the thrombophilic state associated with the nephrotic syndrome, whereas the late events may be primarily attributable to more “classic” pathogenic mechanisms of atherosclerosis (in addition to thrombus formation). While it is plausible that the latter mechanism is accelerated by the profound hyperlipidemia of the nephrotic syndrome,16 we cannot ascertain this possibility from our data. However, our results must be interpreted cautiously. The relatively small number of patients with full nephrotic syndrome beyond the 2-year time point (n = 92 in the GDCN cohort) has likely limited our ability to detect late CVEs associated with it.
The strong association with severe proteinuria and hypoalbuminemia supports a predominantly “thrombotic” cause of CVEs in the early phase of primary MN (Table 2). Indeed, the severity of nephrotic status remained an independent predictor of CVEs, after adjusting for other risk factors. The mechanism accounting for the increased risk of thrombosis in MN is currently unknown. In support of the “atherosclerotic” mechanism of CVEs is the occurrence of late events in the setting of mild or no proteinuria and near-normal serum albumin levels (Table 2). The fact that the “classic” risk factors for cardiovascular disease—age, diabetes, and prior history of CVE—are retained as independent risk factors of CVEs in the MN population also brings support to the “atherosclerotic” pathway of CVEs in this disease. We did not identify hyperlipidemia as a risk factor of CVEs in the GDCN cohort. This result can be explained by the fact that 96% of patients with MN and full nephrotic syndrome also had severe hypercholesterolemia (>300 mg/dl) (compared to only 4% of patients with subnephrotic proteinuria) (Supplementary Table S1 online). This extremely high prevalence and the close correlation between these variables make it difficult to tease out an association between severe hypercholesterolemia and CVEs independently from nephrotic syndrome. GFR was identified as a risk factor of CVEs by univariable analysis but was not retained as an independent risk factor in the multivariable model. This may be a reflection of the relatively small number of CVEs in our study cohorts, and does not exclude the possibility that GFR is indeed an independent risk factor of CVEs as reported by Mahmoodi et al. in patients with nephrotic syndrome of various causes.10
Surprisingly, neither current smoking nor a history of smoking was statistically significantly associated with increased risk of CVEs in our cohort with primary MN. This may be explained by the relatively limited categorical analysis (current, ever, never smoker) rather than a quantitative analysis of the intensity and duration of smoking exposure (e.g., pack-year of smoking). Likewise, we found no statistically significant association between CVEs and the use of various medications such as corticosteroids, calcineurin inhibitors, cyclophosphamide, or statins. The unexpected direction of hazard of CVEs associated with aspirin use is likely attributable to the fact that more than 30% of aspirin-treated patients had a prior CVE, suggesting that aspirin was used for secondary rather than primary prophylaxis. Although exposure to corticosteroids could be associated with increased cardiovascular disease in the general population,17 this was not detected in our cohort with primary MN. Instead, corticosteroid use appears to be associated with a reduced risk of CVEs, although it did not reach statistical significance. Likewise, the use of other immunosuppressants (in combination with corticosteroids) or statins appears to be associated with a decreased risk of CVEs. These associations did not reach statistical significance, possibly due to the relatively small number of patients treated with these medications.
The high incidence of CVEs relative to that of ESRD early in the course of MN has significant implications for the treatment of patients and may prove important to the design of clinical trials. Our findings suggest that particular attention to the prevention of CVEs should be paid early in the course of MN, especially during periods of severe nephrosis. In addition to treatment with lipid-lowering agents,18–20 perhaps measures to reduce arterial thrombotic events (e.g., treatment with aspirin) should be considered.
A strength of our study is the validation of results derived from the GDCN cohort with those of an independent large cohort of patients with primary MN from the TGNR. Multivariable analysis of the risk factors from the TGNR cohort confirmed that severity of nephrotic status, older age, and history of previous CVE are independently associated with CVEs, with similar HRs as derived from the GDCN cohort (Table 4). In the time-to-event analysis, the early occurrence of CVEs was not as pronounced in the TGNR cohort as was observed in the GDCN cohort. This may be, in part, related to baseline differences between the 2 cohorts in race and the proportion of patients with severe nephrotic syndrome. In addition, the median follow-up was significantly longer in the TGNR than the GDCN cohort (52 months [interquartile range 21.3–96.2] vs. 24.3 [interquartile range 9.9–52.7], P < 0.001). This longer follow-up favors the capture of late events, as well as events associated with relapses of MN. Despite the difference in the timing of CVEs, the strong association of early events with severe proteinuria and hypoalbuminemia compared to late events was almost identical in the TGNR and GDCN cohorts (Table 2). Additionally, these findings remained consistent in the combined cohort analysis (Table 4).
Our study builds on and complements previous studies of CVEs in nephrotic syndrome. Our finding of a dichotomous “early-versus-late” pattern of CVEs may explain the inconsistent results reported by previous cohort studies on patients with nephrotic syndrome of various causes. Mahmoodi et al. reported that arterial thromboembolic events occurred most frequently in the first 6 to 12 months after diagnosis,10 whereas the mean time to CVEs was 5.6 years in the study by Ordonez et al.15 Likewise our finding of classic risk factors (age, diabetes, and history of previous events) as independently associated with CVEs is similar to the findings of Mahmoodi et al.10 However, that study did not find an association between hypoalbuminemia or proteinuria and CVEs, which contrasts with our findings. This difference may be attributable to the cohort size, and the heterogeneity of causes of nephrotic syndrome in their study.
Our study has limitations stemming from its retrospective nature. Both the GDCN and the TGNR registry were prospectively collected over more than 30 years during which the standard of care and the completeness of data collection have evolved. This has resulted in relatively limited data on use of certain medications (e.g., aspirin and lipid-lowering agents), which may affect the results of our analysis. We may not have been fully able to detect an effect of immunosuppressive, antithrombotic, or lipid-lowering therapy on the risk of CVEs. Similarly, we were not able to evaluate the effect of renin–angiotensin blockers on the risk of CVEs. We have analyzed the risk factors of CVEs based on a composite definition that includes cardiac, cerebrovascular, and peripheral arterial events because of the relatively small number of events. A separate analysis of each type of event would have required much larger study cohorts. Finally, our combined analyses of the 2 cohorts may have been influenced by unmeasured differences between the 2 populations.
In addition to describing the incidence, timing, and risk factors of CVEs in patients with MN, our findings have important implications for current practice and clinical trial design. The primary concerns of practice and clinical trial in MN have largely focused on the reduction of proteinuria as a surrogate end point for ESRD,21–24 while few studies were adequately powered and of long enough duration to report directly on the impact of treatment on renal survival.25–27 Our study detected that the incidence of CVEs is at least as high as that of ESRD in the first 2 years after diagnosis, and indeed exceeds that of ESRD among patients with preserved eGFR at baseline. Considering the risks, morbidity, long-term sequelae, and impact on quality of life of cardiac and cerebrovascular events, our findings suggest that the prevention of CVEs should be considered an important early (<2 years) therapeutic target of practice and a part of composite “hard” outcome measures in the design of clinical trials in MN.
MATERIALS AND METHODS
Description of study cohorts, explanatory variables, and outcomes
We identified 483 adult patients (>18 years of age) who were diagnosed with MN by native kidney biopsy from 1980 to 2011 and enrolled in the GDCN registry. Briefly, the GDCN is a longitudinal registry of patients with glomerular disease who are followed at the University of North Carolina and by nephrologists in private practice in the Southeastern United States.11 A total of 79 patients were excluded: 24 patients with disease deemed secondary to cancer or viral hepatitis; 29 patients with insufficient follow-up information; and 26 patients receiving therapeutic anticoagulation for venous thromboembolic events, as such treatment would impact the risk of cardiovascular events. Likewise, no patient included in this cohort received prophylactic anticoagulation with warfarin. A total of 404 patients with primary MN were retained for the analysis. All patients provided informed consent for review and analysis of their medical records. The Institutional Review Board of the University of North Carolina at Chapel Hill approved this study.
The baseline date of the analysis was the date of biopsy. Clinical information at baseline was collected: demographics (age, sex, and race), past medical history of CVEs, diabetes, renal function, and nephrotic status (defined by serum albumin and degree of proteinuria). Medication use including aspirin, statins, corticosteroids, or immunosuppressants was defined by whether patients had any exposure to these medications before the CVE or the end of follow-up. The outcomes of interest were ESRD, death unrelated to CVE, and CVE. CVE was defined as any first event, including acute coronary syndrome (acute myocardial infarction and unstable angina requiring any procedural coronary intervention including bypass surgery), thrombotic ischemic stroke, and acute thrombotic event from peripheral arterial occlusive disease. We also collected information on changes of chronic kidney disease stage (eGFR >60, 45–60, <45 ml/min per 1.73 m2) and severity of nephrotic status (nephrotic syndrome, subnephrotic state, and complete remission) at each clinic visit during the observation period to reflect the disease characteristics at the time of events. Nephrotic syndrome was defined as proteinuria >3.5 g/d and hypoalbuminemia (<3.2 g/dl). Patients with hypoalbuminemia <2.5 g/dl were also included in this category even if the proteinuria was between 3 and 3.5 g/d. Subnephrotic state was defined as proteinuria >0.3 g/d and <3.5 g/d. Complete remission was defined as proteinuria <0.3 g/d.
Validation study in TGNR cohort with primary MN
To confirm our findings from the GDCN cohort, we conducted a validation study in the TGNR cohort that comprised 557 patients with primary MN. The cohort was previously described in detail.10 Baseline characteristics and time-varying variables (levels of lower eGFR and severity of nephrotic syndrome) were reported using the same methods as for the GDCN cohort. We first constructed a separate survival analysis model in the TGNR cohort alone to identify risk factors of CVEs. We then performed an analysis of the combined GDCN + TGNR cohorts with all significant risk factors found in each separate cohort analysis, along with an interaction term between cohort identifier and covariate of interest.
Statistical analysis
Summary statistics for continuous variables are reported as either mean ± SD or median with interquartile ranges for skewed data. Categorical variables are reported as percentages. In order to estimate the absolute incidence of CVEs in primary MN before the progression to ESRD, we took into consideration the incidence of ESRD or death unrelated to CVE as competing risks. We chose this methodology because patients with ESRD are no longer at risk of CVEs purely related to primary MN and because patients who died without CVEs would overestimate the incidence of CVEs from censoring.28 The cumulative incidence function was calculated for the absolute incidence of CVEs considering ESRD and non-CVE death as competing risks for CVE occurrence in the setting of primary MN.29
To assess risk factors for CVEs, we conducted time-to-first-event analyses using Cox proportional hazards regression models. Baseline variables at the time of biopsy were incorporated into the model, including sex, race, diabetes, previous history of CVE, and presence of full nephrotic syndrome, each represented as a binary variable. Age categorized by decades was compared to all individuals less than 30 years of age. The severity of nephrotic syndrome (nephrotic syndrome, subnephrotic state, and complete remission) and the level of renal dysfunction (eGFR <45, 45–60, >60 ml/min per 1.73 m2) were treated as ordinal categorical variables. When screening explanatory variables in univariable analyses, the level of significance with P < 0.1 was used for the cutoff value for variable selection criteria for inclusion in multivariable models. After incorporating all significant covariates from univariable models, the final explanatory variables in multivariable analysis were selected by backward elimination using an α-level of 0.05. The linearity assumption for ordinal measures was tested by visual examination of residuals. Since the severity of nephrotic syndrome and renal function change with the clinical course of MN, we incorporated these as time-varying variables to capture disease status at the time the outcome occurred. The proportionality assumption of time-independent covariates in the final model was evaluated by observing the Schoenfeld residuals of independent variables and with statistical testing as well. Statistical significance was considered at a 2-tailed value of P < 0.05. SAS version 9.3.1 (SAS Institute, Cary, NC) and R version 2.15.2 (www.r-project.org) were used for statistical analysis and plotting.
Supplementary Material
Table S1. Correlation between hypercholesterolemia and status of nephrotic syndrome at baseline in each study cohort.
ACKNOWLEDGMENTS
We are indebted to the contribution of the nephrologists of the Greater Toronto Area and of the Glomerular Disease Collaborative Network. This work would not be possible without the contribution of registrars N. Ryan, P. Lam, and P. Ling. TL was supported by the UNC Kidney Center. VKD was supported by the NephCure Foundation, Nephrotic Syndrome Study Network Career Development Award (U-54-DK-083912). Investigator-initiated support from Amgen Canada contributed to support of the TGNR. HNR’s work is supported by the Gabor Zellerman Chair in Nephrology Research.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.kidney-international.org.
REFERENCES
- 1.Hull RP, Goldsmith DJ. Nephrotic syndrome in adults. BMJ. 2008;336: 1185–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cattran DC, Pei Y, Greenwood C. Predicting progression in membranous glomerulonephritis. Nephrol Dial Transplant. 1992;7(suppl 1):48–52. [PubMed] [Google Scholar]
- 3.Kerlin BA, Blatt NB, Fuh B, et al. Epidemiology and risk factors for thromboembolic complications of childhood nephrotic syndrome: a Midwest Pediatric Nephrology Consortium (MWPNC) study. J Pediatr. 2009;155:105–110.e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagoner RD, Stanson AW, Holley KE, et al. Renal vein thrombosis in idiopathic membranous glomerulopathy and nephrotic syndrome: incidence and significance. Kidney Int. 1983;23:368–374. [DOI] [PubMed] [Google Scholar]
- 5.Glassock RJ. Prophylactic anticoagulation in nephrotic syndrome: a clinical conundrum. J Am Soc Nephrol. 2007;18:2221–2225. [DOI] [PubMed] [Google Scholar]
- 6.Crew RJ, Radhakrishnan J, Appel G. Complications of the nephrotic syndrome and their treatment. Clin Nephrol. 2004;62:245–259. [DOI] [PubMed] [Google Scholar]
- 7.Radhakrishnan J. Venous thromboembolism and membranous nephropathy: so what’s new? Clin J Am Soc Nephrol. 2012;7:3–4. [DOI] [PubMed] [Google Scholar]
- 8.Barbour SJ, Greenwald A, Djurdjev O, et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int. 2012;81:190–195. [DOI] [PubMed] [Google Scholar]
- 9.Lionaki S, Derebail VK, Hogan SL, et al. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol. 2012;7: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahmoodi BK, ten Kate MK, Waanders F, et al. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: results from a large retrospective cohort study. Circulation. 2008;117:224–230. [DOI] [PubMed] [Google Scholar]
- 11.Parag KB, Somers SR, Seedat YK, et al. Arterial thrombosis in nephrotic syndrome. Am J Kidney Dis. 1990;15:176–177. [DOI] [PubMed] [Google Scholar]
- 12.Fuh JL, Teng MM, Yang WC, et al. Cerebral infarction in young men with nephrotic syndrome. Stroke. 1992;23:295–297. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki Y, Raita Y, Uehara G, et al. Carotid thromboembolism associated with nephrotic syndrome treated with dabigatran. Case Rep Nephrol Urol. 2014;4:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troyanov S, Wall CA, Miller JA, et al. Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int. 2004;66:1199–1205. [DOI] [PubMed] [Google Scholar]
- 15.Ordonez JD, Hiatt RA, Killebrew EJ, et al. The increased risk of coronary heart disease associated with nephrotic syndrome. Kidney Int. 1993;44:638–642. [DOI] [PubMed] [Google Scholar]
- 16.Radhakrishnan J, Appel AS, Valeri A, et al. The nephrotic syndrome, lipids, and risk factors for cardiovascular disease. Am J Kidney Dis. 1993;22:135–142. [DOI] [PubMed] [Google Scholar]
- 17.Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med. 2004;141:764–770. [DOI] [PubMed] [Google Scholar]
- 18.Nickolas TL, Radhakrishnan J, Appel GB. Hyperlipidemia and thrombotic complications in patients with membranous nephropathy. Semin Nephrol. 2003;23:406–411. [DOI] [PubMed] [Google Scholar]
- 19.Kong X, Yuan H, Fan J, et al. Lipid-lowering agents for nephrotic syndrome. Cochrane Database Syst Rev. 2013;12:CD005425. [DOI] [PubMed] [Google Scholar]
- 20.Thomas ME, Harris KP, Ramaswamy C, et al. Simvastatin therapy for hypercholesterolemic patients with nephrotic syndrome or significant proteinuria. Kidney Int. 1993;44:1124–1129. [DOI] [PubMed] [Google Scholar]
- 21.Hogan SL, Muller KE, Jennette JC, et al. A review of therapeutic studies of idiopathic membranous glomerulopathy. Am J Kidney Dis. 1995;25: 862–875. [DOI] [PubMed] [Google Scholar]
- 22.Ponticelli C, Passerini P, Salvadori M, et al. A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis. 2006;47:233–240. [DOI] [PubMed] [Google Scholar]
- 23.Ponticelli C, Altieri P, Scolari F, et al. A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol. 1998;9:444–450. [DOI] [PubMed] [Google Scholar]
- 24.Praga M, Barrio V, Juarez GF, et al. Tacrolimus monotherapy in membranous nephropathy: a randomized controlled trial. Kidney Int. 2007;71:924–930. [DOI] [PubMed] [Google Scholar]
- 25.Jha V, Ganguli A, Saha TK, et al. A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol. 2007;18:1899–1904. [DOI] [PubMed] [Google Scholar]
- 26.Ponticelli C, Zucchelli P, Passerini P, et al. A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int. 1995;48:1600–1604. [DOI] [PubMed] [Google Scholar]
- 27.Cattran DC, Greenwood C, Ritchie S, et al. A controlled trial of cyclosporine in patients with progressive membranous nephropathy. Canadian Glomerulonephritis Study Group. Kidney Int. 1995;47: 1130–1135. [DOI] [PubMed] [Google Scholar]
- 28.Noordzij M, Leffondre K, van Stralen KJ, et al. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28:2670–2677. [DOI] [PubMed] [Google Scholar]
- 29.Gaynor JJ, Feuer EJ, Tan CC, et al. On the use of cause-specific failure and conditional failure probabilities—examples from clinical oncology data. J Am Stat Assoc. 1993;88:400–409. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Correlation between hypercholesterolemia and status of nephrotic syndrome at baseline in each study cohort.