Abstract
Background
Dental implants offer one way to replace missing teeth. Patients who have undergone radiotherapy and those who have also undergone surgery for cancer in the head and neck region may particularly benefit from reconstruction with implants. Hyperbaric oxygen therapy (HBO) has been advocated to improve the success of implant treatment in patients who have undergone radiotherapy but this remains a controversial issue.
Objectives
To compare the success, morbidity, patient satisfaction and cost effectiveness of dental implant treatment carried out with and without HBO in irradiated patients.
Search methods
The following electronic databases were searched: Cochrane Oral Health's Trials Register (to 17 June 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 5), MEDLINE via OVID (1946 to 17 June 2013) and EMBASE via OVID (1980 to 17 June 2013). No restrictions were placed on the language or date of publication when searching the electronic databases. We checked the bibliographies of relevant clinical trials and review articles for studies outside the searched journals. We wrote to authors of the identified randomised controlled trials (RCTs) and to more than 55 oral implant manufacturers; we used personal contacts and we made a request on an internet discussion group in an attempt to identify unpublished or ongoing RCTs.
Selection criteria
Randomised controlled trials (RCTs) of HBO therapy for irradiated patients requiring dental implants.
Data collection and analysis
Screening of eligible studies, assessment of the methodological quality of the trials and data extraction were conducted in duplicate and independently by two review authors. Results were analysed using random‐effects models to determine mean differences for continuous outcomes and risk ratios for dichotomous outcomes, with 95% confidence intervals.
Main results
Only one RCT, providing very low‐quality evidence, was identified and included. Thirteen patients received HBO therapy while another 13 did not. Two to six implants were placed in people with fully edentulous mandibles to be rehabilitated with bar‐retained overdentures. One year after implant loading, four patients had died from each group. One patient, treated with HBO, developed an osteoradionecrosis and lost all implants so the prosthesis could not be provided. Five patients in the HBO group had at least one implant failure versus two in the control group. There were no statistically significant differences for prosthesis and implant failures, postoperative complications and patient satisfaction between the two groups.
Authors' conclusions
Despite the limited amount of clinical research available, it appears that HBO therapy in irradiated patients requiring dental implants may not offer any appreciable clinical benefits. There is a definite need for more RCTs to ascertain the effectiveness of HBO in irradiated patients requiring dental implants. These trials ought to be of a high quality and reported as recommended by the CONSORT statement (www.consort‐statement.org/). Each clinical centre may have limited numbers of patients and it is likely that trials will need to be multicentred.
Plain language summary
Interventions for replacing missing teeth: hyperbaric oxygen therapy for irradiated patients who require dental implants
Review question
This review, carried out by authors of Cochrane Oral Health, was produced to compare the success of dental implant treatment carried out with and without hyperbaric oxygen therapy (HBO) in patients who had previously had radiation treatment.
Background
Missing teeth can affect eating and speaking as well as appearance. Dental implants offer one way to replace missing teeth. Patients who have undergone radiotherapy and those who have also undergone surgery for cancer in the head and neck region may particularly benefit from reconstruction with implants. Dental implants into the bone of the jaw offer support for replacement teeth, and sometimes for replacements for parts of the mouth (prosthetics) that have been removed following surgery for cancer or as a result of damage to the bone (osteonecrosis) caused by radiation treatment.
Hyperbaric oxygen therapy (which requires patients to breath pure oxygen under pressure in a specially designed chamber on several occasions) has been advocated to improve the success of implant treatment. It has been suggested that HBO therapy will improve the healing of the bone and tissues around dental implants in patients who have undergone radiotherapy, but this remains a controversial issue.
Study characteristics
The evidence on which this review is based was up‐to‐date as of 1 July 2013. One small study carried out at a head and neck cancer clinic based at a university in the Netherlands was found. The study included 26 adults who had been treated for head and neck cancer either with radiotherapy or a combination of radiotherapy and surgery. All participants were missing all their teeth in the lower jaw and were experienced problems retaining a denture. The participants were split into two groups, 13 of them were treated with HBO and the other 13 were not.
Key results
Only one small trial that was at high risk of bias compared treatment with HBO with treatment without HBO. The results failed to determine a benefit for HBO therapy in preventing failure of dental implants or other serious complications such as the death of bone in the jaw caused by radiotherapy treatment. More reliable studies are needed to provide the final answer to this question.
Quality of the evidence
The quality of evidence was very low as it was based on one small trial at high risk of bias.
Summary of findings
Summary of findings for the main comparison. Hyperbaric oxygen (HBO) versus no HBO.
| Hyperbaric oxygen (HBO) versus no HBO | ||||||
|
Patient or population: people requiring dental implants Settings: specialist centre Intervention: HBO Comparison: no HBO | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Without HBO | HBO | |||||
| Prosthetic failure | Low risk population | RR 3.00 (0.13, 67.06) | 24 (1) | ⊕⊝⊝⊝2 very low quality | ||
| 10 per 10001 | 30 per 1000 (1 to 670) | |||||
| High risk population | ||||||
| 100 per 1000 | 300 per 1000 (13 to 1000) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
1The risk in the control arm was 0% so low risk of 1% and high risk of 10% assumed. 2 Downgraded due to one small study at high risk of bias.
CI = confidence interval; RR = risk ratio.
GRADE Working Group grades of evidence. High quality (⊕⊕⊕⊕): further research is very unlikely to change our confidence in the estimate of effect. Moderate quality (⊕⊕⊕⊝): further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality (⊕⊕⊝⊝): further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality (⊕⊝⊝⊝): we are very uncertain about the estimate.
Background
Missing teeth and supporting oral tissues have traditionally been replaced with dentures or bridges, permitting restoration of masticatory function, speech and aesthetics. Since the 1970s dental implant supported prostheses have offered an alternative. Dental implants are surgically inserted into the jaw bone and are retained due to the intimacy of bone growth onto their surface, termed osseointegration (Brånemark 1977). Osseointegrated dental implants have undoubtedly been one of the most significant scientific breakthroughs in dentistry over the past 40 years.
Description of the condition
Teeth are lost due to dental diseases or trauma, or may be congenitally absent. In addition, there are some patients who have lost extensive oral and facial tissues following surgery for cancer. Reconstruction of these tissues can be difficult but implant therapy offers an improvement to previous treatment modalities (Franzen 1995). Some cancer patients have undergone radiotherapy as an adjunct to surgery whereas others have only had radiotherapy treatment. Complications of radiotherapy treatment include oral mucosal damage (mucositis), dry mouth (xerostomia) as a result of salivary gland damage, and damage to bone (osteoradionecrosis). Osteoradionecrosis is the most serious complication as it is difficult to treat and may require partial jaw resection. It commonly affects the mandible although it may also affect other bones (sternum, skull, pelvis). Any surgical treatment involving the jaws that follows radiotherapy may show compromised healing or even lead to osteonecrosis, hence dental implant treatment for such patients has been considered as a relative contraindication. Conversely, implant therapy is of significant benefit to this group of patients who can have trouble wearing dentures due to a dry mouth and since ulcerations of the oral mucosa below the dentures are common and impair their eating capacity and may lead to serious infections or osteonecrosis.
Description of the intervention
Hyperbaric oxygen (HBO) therapy gained strong support, for positive effects on compromised tissue following irradiation, after its introduction in the 1970s (Marx 1984). HBO therapy consists of exposing a patient in a special chamber to intermittent, short term 100% oxygen inhalation at a pressure greater than one atmosphere. A typical protocol developed for osteoradionecrosis is the Marx‐University of Miami protocol (Marx 1984), which requires a patient to receive 20 HBO treatments of 100% oxygen at 2.4 atmospheres for 90 minutes before surgery, followed by a further 10 HBO treatments of 100% oxygen at 2.4 atmospheres for 90 minutes after surgery.
How the intervention might work
It has been proposed that HBO therapy may improve osseointegration of implants (Granström 1992).
Why it is important to do this review
The question as to whether HBO therapy affects implant success in irradiated patients is important because HBO therapy is not without risk of adverse effects. HBO therapy is time consuming, expensive, and requires significant patient compliance. Side effects include the most common temporary trauma to the ears and sinuses or barotraumas due to pressure changes. Mild ear pain is normal and is not of concern. Patients may also experience blurry vision or fatigue while in the pressurized chamber. Complications and serious side effects are uncommon. Occasionally patients may experience oxygen toxicity while in the chamber, which can cause seizures and serious respiratory illness. Particular attention should be given to avoiding flammable items and accidental sparks since a 100% saturated oxygen environment is highly flammable. Despite several authors supporting HBO therapy (Larsen 1997; Granström 1999; Feldmeier 2002; Granström 2005), it remains a controversial issue and some clinicians consider HBO ineffective (Keller 1997). Those who advocate HBO therapy have based their conclusions on clinical experience, retrospective case control studies (Granström 1999) and experimental animal studies (Larsen 1997). On the other hand, oral implant rehabilitation of irradiated patients has been shown to be successful without adjunctive hyperbaric oxygen (Franzen 1995).
The aim of this review was to compare dental implant treatment carried out with and without HBO therapy in irradiated patients.
Objectives
To compare the success, morbidity, patient satisfaction and cost effectiveness of dental implant treatment carried out with and without HBO in irradiated patients.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Patients who have had radiotherapy and who have missing teeth that require replacement with osseointegrated dental implants.
Types of interventions
Hyperbaric oxygen (HBO) therapy compared with no HBO therapy.
Types of outcome measures
Outcome measures of interest were the following.
Prosthesis failure if secondary to implant failure.
Implant failure: mobility, and removal of stable implants dictated by progressive marginal bone loss.
Radiographic marginal bone level changes on intraoral radiographs taken with a parallel technique.
Preimplantation complications: all complications that occurred after initiation of HBO therapy but prior to implant placement (eustachian tube dysfunction, tympanic membrane rupture, ear or sinus or tooth pain, pneumothorax, etc.).
Postimplantation complications: all complications that occurred after implant placement (mucosa ulceration, osteoradionecrosis, etc.).
Patient satisfaction.
Cost effectiveness.
Search methods for identification of studies
For the identification of studies included or considered for this review, we developed detailed search strategies for each database searched. These were based on the search strategy developed for MEDLINE (OVID) but revised appropriately for each database. The search strategy used a combination of controlled vocabulary and free text terms and was linked with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials (RCTs) in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) (Higgins 2011). Details of the MEDLINE search are provided in Appendix 1. The search of EMBASE was linked to the Cochrane Oral Health Group filter for identifying RCTs.
Electronic searches
We searched the following electronic databases.
Cochrane Oral Health's Trials Register (to 17 June 2013) (Appendix 2)
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 5) (Appendix 3)
MEDLINE via OVID (1946 to 17 June 2013) (Appendix 1)
EMBASE via OVID (1980 to 17 June 2013) (Appendix 4).
There were no restrictions on language in the searches of the electronic databases.
Searching other resources
Unpublished studies
We wrote to all the authors of the identified RCTs, we checked the bibliographies of all identified RCTs and relevant review articles, and we used personal contacts in an attempt to identify unpublished or ongoing RCTs. In the first version of this review we also wrote to more than 55 oral implant manufacturers and we requested information on trials through an Internet discussion group (implantology@yahoogroups.com), however we discontinued this due to poor yield.
Handsearching
Only handsearching done as part of the Cochrane Worldwide Handsearching Programme and uploaded to CENTRAL was included (see the Cochrane Masterlist for details of journal issues searched to date).
Data collection and analysis
Selection of studies
The titles and abstracts (when available) of all reports identified through the electronic searches were scanned independently by two review authors. For studies appearing to meet the inclusion criteria, or for which there were insufficient data in the title and abstract to make a clear decision, the full report was obtained. The full reports obtained from all the electronic and other methods of searching were assessed independently by two review authors to establish whether the studies did meet the inclusion criteria or not. Disagreements were resolved by discussion. Where resolution was not possible, a third review author was to be consulted. All studies meeting the inclusion criteria then underwent validity assessment and data extraction. Studies rejected at this or subsequent stages were recorded in the 'Characteristics of excluded studies' table, and the reasons for exclusion were recorded.
Data extraction and management
Data were independently extracted by two review authors using specially designed data extraction forms. The data extraction forms were piloted and modified as required before use. Any disagreement was discussed and a third review author consulted where necessary. All authors were contacted for clarification or missing information. Data were excluded until further clarification became available if agreement could not be reached.
For each trial the following data were recorded.
Year of publication and country of origin.
Details of the participants including demographic characteristics and criteria for inclusion.
Details of the type of intervention.
Details of the outcomes reported, including method of assessment and time intervals.
Assessment of risk of bias in included studies
This was conducted using the recommended approach for assessing risk of bias in studies included in Cochrane reviews (Higgins 2011). It is a two‐part tool addressing the six specific domains (namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and 'other bias'). Each domain includes one specific entry in a 'Risk of bias' table. Within each entry, the first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry, either 'low risk', 'high risk' or, where there is insufficient information on which to base a judgement, 'unclear risk'.
The risk of bias assessment of the included trials was completed independently and in duplicate by two review authors as part of the data extraction process. On occasions when the review authors were also authors of trial reports that needed to be assessed, the reports were independently evaluated only by review authors who had not been involved in the trials.
Summarising risk of bias for a study
After taking into account the additional information provided by the authors of the trials, studies were grouped into the following categories. We assumed that the risk of bias was the same for all outcomes and each study was assessed as follows.
| Risk of bias | Interpretation | Within a study | Across studies |
| Low risk of bias | Plausible bias unlikely to alter the results seriously | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
Measures of treatment effect
For dichotomous outcomes, the estimates of effect of an intervention were expressed as risk ratios together with 95% confidence intervals. For continuous outcomes, mean differences and standard deviations were used to summarise the data for each group.
Unit of analysis issues
The statistical unit was the patient and not the implant.
Dealing with missing data
Trial authors were contacted to retrieve missing data where necessary. If agreement could not be reached, data were excluded until further clarification was available. Methods for estimating missing standard deviations in section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions would have been used if required (Higgins 2011). An intention‐to‐treat (ITT) analysis was undertaken where data were available and appropriate.
Assessment of heterogeneity
The significance of any discrepancies in the estimates of the treatment effects from the different trials was to be assessed by means of Cochran's test for heterogeneity and the I2 statistic. Chi2 assesses the percentage total variation across studies that is due to heterogeneity rather than chance. Heterogeneity would have been considered to be significant if the P value was less than 0.1. The I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance, was used to quantify heterogeneity with an I2 value over 50% indicating moderate to high heterogeneity.
Assessment of reporting biases
If there had been a sufficient number of trials (more than 10) in any meta‐analysis we would have assessed publication bias according to the recommendations on testing for funnel plot asymmetry (Egger 1997) as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If asymmetry had been identified we would have examined possible causes.
Data synthesis
A meta‐analysis was to be performed only if there were studies with similar comparisons that reported the same outcome measures. Risk ratios were combined for dichotomous data, using random‐effects models, provided there were more than three studies in the meta‐analysis. Numbers needed to treat for an additional harm (NNTH) were to be calculated for participants affected by implant failures. The recommendations of the Cochrane Handbook for Systematic Reviews of Interventions were followed for studies with zero‐cell counts (Higgins 2011). The fixed value of 0.5 was added to all cells with zero‐cell counts and risk ratios calculated with the Review Manager (RevMan) software (RevMan 2013). If there were no events in both arms, no calculations were undertaken because in this situation the study does not provide any indication of the direction or magnitude of the relative treatment effect.
Subgroup analysis and investigation of heterogeneity
Clinical heterogeneity was to be assessed by examining the types of participants and interventions for all outcomes in each study.
The following subgroup analyses were planned, however there were insufficient studies to undertake them. (1) Whether the implants were placed in mandibles or maxillae. (2) Whether the implants were placed in augmented bone or not. (3) Whether radiotherapy was hyperfractionated (total dose over more than 12 fractions) or not. (4) Whether the cumulative dose was > 60 grays or less. (5) Whether one year or more passed after the radiotherapy end and implant placement or not. (6) Whether the pressure (dose) of oxygen received was to 2.5 atmospheres or less.
Sensitivity analysis
We planned to undertake sensitivity analyses to examine the effect of the study quality assessment on the overall estimates of effect. In addition, the effect of including unpublished literature on the review's findings was to be examined, but there were insufficient trials to undertake this.
Results
Description of studies
Results of the search
The search for this review was part of a wider search for all eligible trials for the series of Cochrane Reviews on dental implants. This search is conducted every six months and has so far included about 8600 records.
Included studies
One potentially eligible trial was identified and included (Schoen 2007).
Characteristics of the trial setting and investigators
The included study was conducted at the Head and Neck Oncology Group of the Groningen University Medical Center, the Netherlands, and included only adults.
Characteristics of interventions
Both groups received antibiotic prophylaxis with broad‐spectrum antibiotics (cephradine 1 g, three times daily during two weeks) starting one day before implant surgery. The test group received 20 hyperbaric oxygen (HBO) treatments of 100% oxygen at 2.5 atmospheres for 80 minutes (four periods of 20 minutes) before implant surgery, and 10 HBO identical treatments after implant surgery. In all patients Brånemark implants were placed in the interforaminal region of the mandible according to a one‐stage procedure. After six months, bar‐retained overdentures were delivered.
Characteristics of outcome measures
The following data were presented: prosthesis failures as implant failures, radiographic bone level changes (but not used since extrapolated from oblique lateral radiographs), preimplantation complications, postimplantation complications, patient satisfaction. We used the 'overall denture satisfaction', however one patient from the HBO group who lost all the implants during the healing period and could not receive an implant‐supported overdenture was excluded from the trial. This was not correct since the treatment was clearly a failure, but its potentially negative consequences were not accounted for. Cost effectiveness data were not presented but it was possible to extrapolate this.
Main inclusion criteria
Patients with completely edentulous mandibles treated for a first malignancy in the head and neck region (squamous cell carcinoma of the tongue, floor of the mouth, mandibular gingiva, buccal mucosa or oropharynx) with either radiotherapy or a combination of surgery and radiotherapy, and having problems in retaining of their lower dentures.
Main exclusion criteria
None were described.
Comparability of control and treatment groups at entry
The groups appeared to be comparable at entry.
The agreed quality of the included trial after having incorporated the information provided by the author is summarised in Figure 1. The trial was rated as at high risk of bias.
1.
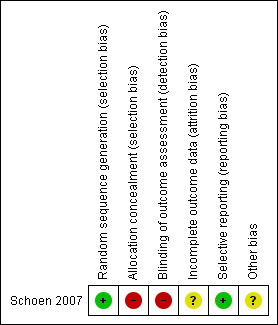
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Sample size
No sample size calculation was performed.
Excluded studies
No excluded studies were identified.
Risk of bias in included studies
Allocation
After considering the reply from the author, the method of allocation concealment was considered inadequate and this domain was assessed as high risk of bias.
Blinding
After considering the reply from the author, the outcome assessors were not blinded so this domain was assessed as high risk of bias.
Incomplete outcome data
After considering the reply from the author, the reasons for drop‐outs were clear, however one patient who lost all implants was excluded from the analysis so this domain was assessed as at unclear risk of bias.
Selective reporting
All outcomes had been adequately reported so this domain was assessed as low risk of bias.
Other potential sources of bias
There were no apparent other sources of bias so this domain was assessed as unclear
Overall risk of bias
The risk of bias is summarised in Figure 1. This study has been assessed as at high risk of bias.
Effects of interventions
See: Table 1
One study (Schoen 2007) included 13 patients treated with hyperbaric oxygen (HBO) therapy and 13 patients who did not receive HBO therapy. Two patients, one from each group, died during the healing period of the implants. One year after placement of the overdentures three additional patients in each group were no longer alive. Eight implants failed in five patients subjected to HBO therapy versus three implants in two patients in the control group. Two postoperative complications (one osteoradionecrosis and some minor soft tissue complications) developed in two patients subjected to HBO therapy. The patient affected by osteoradionecrosis lost all implants, the implant‐supported prosthesis could not be fabricated and patient satisfaction was not evaluated. One year after delivery of the prosthesis there were no statistically significant differences for any of the outcome measures (Analysis 1.1; Analysis 1.2).
1.1. Analysis.
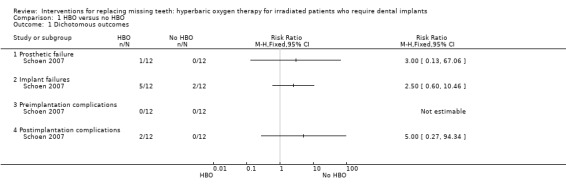
Comparison 1 HBO versus no HBO, Outcome 1 Dichotomous outcomes.
1.2. Analysis.
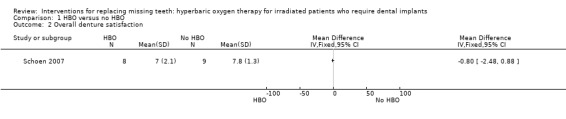
Comparison 1 HBO versus no HBO, Outcome 2 Overall denture satisfaction.
Discussion
The question of whether or not hyperbaric oxygen (HBO) therapy is effective for implant success in irradiated patients is important. HBO therapy requires significant patient compliance and involves substantial financial expense in terms of cost per patient treatment and the equipment. It is not without risk of adverse effects.
Readers should be aware that the 'evidence' on this matter remains highly controversial. For instance, another Cochrane systematic review suggested a limited advantage of HBO therapy in reducing the chances of osteoradionecrosis in irradiated tooth sockets following dental extractions (Bennett 2012).
Summary of main results
Only one trial at high risk of bias compared HBO with no HBO and the results failed to determine a benefit for HBO therapy in preventing failures of dental implants or other serious complications such as osteoradionecrosis of the mandible (Schoen 2007) (Table 1).
Despite the increased number of implant losses, irradiation therapy cannot be considered an absolute contraindication to dental implants in the mandible. In fact, only three out of the 26 patients included in the trial (Schoen 2007) did not benefit from their implant‐supported overdentures. Two patients died during the implant healing period, and all implants failed in another patient because of osteoradionecrosis so that the overdenture could not be fabricated. From the trial it clearly emerges that implant‐retained mandibular overdentures improve life quality in terms of oral function and denture satisfaction in patients treated for head and neck cancer (Schoen 2007).
Overall completeness and applicability of evidence
It is disappointing that there is only one trial at high risk of bias looking at the potential benefits of hyperbaric oxygen for people with dental implants.
Quality of the evidence
The body of evidence is very low as it is based on one small study at high risk of bias.
Agreements and disagreements with other studies or reviews
There are many scientific papers written about this subject, including a number of review articles (Esposito 1998; Granström 1998), but only one randomised controlled clinical trial (Schoen 2007) including a limited number of participants. It is interesting that since this review was last published in 2007 there is still controversy over the benefit of using hyperbaric oxygen in the treatment of patients with implants. Some authors still strongly recommend its use (Anderson 2013).
Authors' conclusions
Implications for practice.
Despite the limited amount of research available, it appears that hyperbaric oxygen (HBO) therapy in irradiated patients requiring dental implants may not offer any appreciable clinical benefits.
Implications for research.
There is a definite need for more randomised controlled trials (RCTs) to ascertain the effectiveness of HBO therapy in irradiated patients requiring dental implants. These trials ought to be of a high quality and reported as recommended by the CONSORT statement (www.consort‐statement.org/). Each clinical centre may have limited numbers of patients and it is likely that trials will need to be multicentred.
What's new
| Date | Event | Description |
|---|---|---|
| 10 October 2019 | Review declared as stable | This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 3, 2002
| Date | Event | Description |
|---|---|---|
| 25 September 2013 | New search has been performed | Search updated to June 2013. |
| 25 September 2013 | New citation required but conclusions have not changed | Background and methods sections updated. Summary of findings table included. No new included or excluded studies. Conclusions not changed. |
| 12 June 2008 | Amended | Converted to new review format. |
| 1 November 2007 | New citation required and conclusions have changed | Substantive amendment. One randomised controlled trial (RCT) has been identified and included. Conclusions changed. |
Notes
This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future.
Acknowledgements
We wish to thank Anne Littlewood (Cochrane Oral Health) for her assistance with literature searching; Luisa Fernandez Mauleffinch and Phil Riley (Cochrane Oral Health) for their help with the preparation of this review; Pieter Schoen for providing us with information on his trial. We wish to thank Paul Coulthard, Asbjørn Jokstad, Maria Gabriella Grusovin and Satya Patel for the contribution they gave for earlier versions of this review. We would also like to thank the following referees who have reviewed various versions of this review: Gösta Granström, Steve Thomas, Pieter Schoen and Sylvia Bickley.
Appendices
Appendix 1. MEDLINE (OVID) search strategy
exp Dental Implants/
exp Dental Implantation/ or dental implantation
exp Dental Prosthesis, Implant‐Supported/
((osseointegrated adj implant$) and (dental or oral))
dental implant$
(implant$ adj5 dent$)
(((overdenture$ or crown$ or bridge$ or prosthesis or restoration$) adj5 (Dental or oral)) and implant$)
"implant supported dental prosthesis"
("blade implant$" and (dental or oral))
((endosseous adj5 implant$) and (dental or oral))
((dental or oral) adj5 implant$)
OR/1‐11
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011] (Higgins 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 2. The Cochrane Oral Health Group's Trials Register search strategy
Updated searches were undertaken using the Cochrane Register of Studies and the search strategy below from January 2013:
#1 ("dental implant*" or "oral implant*" or "implant support*" or "endosseous implant*" or "blade implant*") AND (INREGISTER) #2 ((implant* and (oral or dental))) AND (INREGISTER) #3 ("subperiosteal implant*") AND (INREGISTER) #4 ((implant* AND overdenture*)) AND (INREGISTER) #5 (((overdenture* OR crown* OR bridge* OR prosthesis OR prostheses OR restoration*) AND ("dental implant*" OR "Oral implant" OR (zygoma* AND implant*)))) AND (INREGISTER) #6 (#1 or #2 or #3 or #4 or #5) AND (INREGISTER)
Previous searches of the Register were undertaken using the Procite software and the search strategy below: (dental‐implants OR "dental implant*" OR "oral implant*" OR dental‐implantation OR dental‐prosthesis‐implant‐supported OR "implant supported" OR "implant supported prosthesis" OR dental‐implantation‐endosseous‐endodontic OR "endosseous implant*" OR blade‐implantation OR "blade implant*" OR (implant* AND (oral OR dental)) or dental‐implantation‐subperiosteal OR "subperiosteal implant" OR (implant* AND overdenture*) OR ((overdenture* OR crown* OR bridge* OR prosthesis OR prostheses OR restoration*) AND ("dental implant*" OR "Oral implant" OR (zygoma* AND implant*))))
Appendix 3. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 DENTAL IMPLANTS explode all trees (MeSH) #2 DENTAL IMPLANTATION explode all trees (MeSH) #3 DENTAL PROSTHESIS IMPLANT‐SUPPORTED single term (MeSH) #4 ((osseointegrat* near implant*) and (dental* or oral*)) #5 (dental next implant*) #6 (implant* near dent*) #7 dental‐implant* #8 ((overdenture* near dental*) and implant*) #9 ((overdenture* near oral*) and implant*) #10 ((crown* near dental*) and implant*) #11 ((crown* near oral*) and implant*) #12 ((bridge* near dental*) and implant*) #13 ((bridge* near oral*) and implant*) #14 ((prosthesis near dental*) and implant*) #15 ((prosthesis near oral*) and implant*) #16 ((prostheses near dental*) and implant*) #17 ((prostheses near oral*) and implant*) #18 ((restoration* near dental*) and implant*) #19 ((restoration* near oral*) and implant*) #20 (implant next supported next dental next prosthesis) #21 (blade next implant*) #22 ((endosseous near implant*) and dental) #23 ((endosseous near implant*) and oral*) #24 ((dental* near implant*) or (oral* near implant*)) #25 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24)
Appendix 4. EMBASE (OVID) search strategy
1. tooth implantation/ 2. ((implant‐supported or implant$) adj support$).mp. 3. ((osseointegrated adj implant$) and (dental or oral)).mp. 4. ((dental implant$ or dental‐implant or implant$) adj (dent$ or oral or tooth)).mp. 5. (((overdenture$ or crown$ or bridge$ or prosthesis or prostheses or restoration$) adj5 (dental or oral)) and implant$).mp. 6. "implant supported dental prosthesis".mp. 7. ("blade implant$" and (dental or oral or tooth or teeth)).mp. 8. ((endosseous adj5 implant$) and (dental or oral or tooth or teeth)).mp. 9. ((dental or oral or tooth or teeth) and implant$).mp. 10. or/1‐9
The above subject search was linked to the Cochrane Oral Health Group filter for identifying RCTs in EMBASE via OVID:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 16. 14 NOT 15
Data and analyses
Comparison 1. HBO versus no HBO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dichotomous outcomes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Prosthetic failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Implant failures | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Preimplantation complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Postimplantation complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Overall denture satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Schoen 2007.
| Methods | 1‐year follow‐up, randomised, parallel group study. Outcome assessor was
not blinded. 8 patients (4 from each group) died at 1 year. 2 patients
actually died during the healing period of the implants (1 from each group).
They were considered as withdrawals. Recruited between 1990 and 2000. |
|
| Participants | Patients with completely edentulous mandibles, treated for a first malignancy in the head and neck region (squamous cell carcinoma of the tongue, floor of the mouth, mandibular gingiva, buccal mucosa or oropharynx) with either radiotherapy or a combination of surgery and radiotherapy, having problems in retaining of their lower dentures. Exclusion criteria were not specified. Adults treated at the Head and Neck Oncology Group of the Groningen University Medical Center, the Netherlands. 26 enrolled (13 in each group) and results given for 24. | |
| Interventions | The control group received antibiotic prophylaxis (cephradine 1 g, 3 times daily during 2 weeks) starting 1 day before implant surgery, while the test group received 20 HBO treatments of 100% oxygen at 2.5 atmospheres for 80 minutes (4 periods of 20 minutes), before implant surgery, and 10 HBO identical treatments after implant surgery in addition to the antimicrobial prophylaxis as applied to the control group. Brånemark implants were placed in the interforaminal region of the mandible according to a 1‐stage procedure. After 6 months bar‐retained overdentures were delivered. | |
| Outcomes | Prosthesis and implant failures, per implant marginal bone level changes on oblique lateral radiographs, postimplantation complications, plaque index, calculus, bleeding index, gingiva index, probing pocket depths, width of the attached gingiva, Periotest, functional assessment and quality of life, denture satisfaction, subjective chewing ability. Outcomes were assessed preoperatively when feasible, and 6 weeks and 1 year after placement of the prostheses. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer program was used for randomization of the patients". The author replied: "The randomization was performed by using a computer program with regard to age, gender, site and stage of the primary tumour, reconstructive procedure and total dose of irradiation. The computer program takes care of a balanced allocation between groups". |
| Allocation concealment (selection bias) | High risk | Not stated. The author replied: "We didn't use a blinded or double‐blinded design, so the surgeon was aware of the group allocation before placement of the implants". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessor was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | From the 26 patients included for our study, 8 patients passed away during
the study and in one patient no prosthesis could be made because of loss of
all implants related to development of osteoradionecrosis. So, the 1 year
results for overall denture satisfaction were based on 17 patients, 8 in the
HBO group and 9 in the non‐HBO group. The patient who did not receive a
prosthesis was excluded (Comment: this should have been counted as a
failure). 26 recruited, data presented on 24. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Unclear. |
HBO = hyperbaric oxygen.
Differences between protocol and review
None.
Contributions of authors
Conceiving, designing and co‐ordinating the review (Marco Esposito (ME)). Developing search strategy and undertaking searches (Anne Littlewood, ME). Screening search results and retrieved papers against inclusion criteria (ME). Appraising risk of bias (ME, Helen Worthington (HW)). Extracting data from papers (ME, HW). Writing to authors for additional information (ME). Data management for the review and entering data into RevMan (HW, ME). Analysis and interpretation of data (ME, HW). Writing the review (ME, HW).
Sources of support
Internal sources
Division of Dentistry, The University of Manchester, UK.
-
Manchester Academic Health Sciences Centre (MAHSC), UK.
Cochrane Oral Health is supported by MAHSC and the NIHR Manchester Biomedical Research Centre
External sources
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; and the Swiss Society for Endodontology, Switzerland.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed are those of the authors and not necessarily those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Declarations of interest
Marco Esposito: no interests to declare. Helen Worthington: no interests to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Schoen 2007 {published data only}
- Schoen PJ, Raghoebar GM, Bouma J, Reintsema H, Vissink A, Sterk W, et al. Rehabilitation of oral function in head and neck cancer patients after radiotherapy with implant‐retained dentures: effects of hyperbaric oxygen therapy. Oral Oncology 2007;43(4):379‐88. [DOI] [PubMed] [Google Scholar]
Additional references
Anderson 2013
- Anderson L, Meraw S, Al‐Hezaimi K, Wang HL. The influence of radiation therapy on dental implantology. Implant Dentistry 2013;22:31‐8. [DOI] [PubMed] [Google Scholar]
Bennett 2012
- Bennett MH, Feldmeier J, Hampson N, Smee R, Milross C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD005005.pub3] [DOI] [PubMed] [Google Scholar]
Brånemark 1977
- Brånemark PI, Hansson BO, Adell R, Breine U, Lindstrom J, Hallen O, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10‐year period. Scandinavian Journal of Plastic and Reconstructive Surgery. Supplementum 1977;16:1‐132. [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esposito 1998
- Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (II). Etiopathogenesis. European Journal of Oral Sciences 1998;106(3):721‐64. [DOI] [PubMed] [Google Scholar]
Feldmeier 2002
- Feldmeier JJ, Hampson NB. A systematic review of the literature reporting the application of hyperbaric oxygen prevention and treatment of delayed radiation injuries: an evidence based approach. Undersea and Hyperbaric Medicine 2002;29(1):4‐30. [PubMed] [Google Scholar]
Franzen 1995
- Franzen L, Rosenquist JB, Rosenquist KI, Gustafsson I. Oral implant rehabilitation of patients with oral malignancies treated with radiotherapy and surgery without adjunctive hyperbaric oxygen. International Journal of Oral and Maxillofacial Implants 1995;10(2):183‐7. [PubMed] [Google Scholar]
Granström 1992
- Granström G, Jacobsson M, Tjellstrom A. Titanium implants in irradiated tissue: benefits from hyperbaric oxygen. International Journal of Oral and Maxillofacial Implants 1992;7(1):15‐25. [PubMed] [Google Scholar]
Granström 1998
- Granström G. Hyperbaric oxygen therapy as a stimulator of osseointegration. Advances in Otorhinolaryngology 1998;54:33‐49. [DOI] [PubMed] [Google Scholar]
Granström 1999
- Granström G, Tjellström A, Brånemark PI. Osseointegrated implants in irradiated bone: a case‐controlled study using adjunctive hyperbaric oxygen therapy. Journal of Oral and Maxillofacial Surgery 1999;57(5):493‐9. [DOI] [PubMed] [Google Scholar]
Granström 2005
- Granström G. Osseointegration in irradiated cancer patients: an analysis with respect to implant failures. Journal of Oral and Maxillofacial Surgery 2005;63(5):579‐85. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Keller 1997
- Keller EE. Placement of dental implants in the irradiated mandible: a protocol without adjunctive hyperbaric oxygen. Journal of Oral and Maxillofacial Surgery 1997;55(9):972‐80. [DOI] [PubMed] [Google Scholar]
Larsen 1997
- Larsen PE. Placement of dental implants in the irradiated mandible: a protocol involving adjunctive hyperbaric oxygen. Journal of Oral and Maxillofacial Surgery 1997;55(9):967‐71. [DOI] [PubMed] [Google Scholar]
Marx 1984
- Marx RE. Osteoradionecrosis of the jaws: a review and update. Hyperbaric Oxygen Review 1984;5:78‐126. [Google Scholar]
RevMan 2013 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2013.
References to other published versions of this review
Coulthard 2002
- Coulthard P, Esposito M, Worthington HV, Jokstad A. Interventions for replacing missing teeth: hyperbaric oxygen therapy for irradiated patients who require dental implants. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003603] [DOI] [PubMed] [Google Scholar]
Coulthard 2003
- Coulthard P, Esposito M, Worthington HV, Jokstad A. Therapeutic use of hyperbaric oxygen for irradiated dental implant patients: a systematic review. Journal of Dental Education 2003;67(1):64‐8. [PubMed] [Google Scholar]
Esposito 2008
- Esposito M, Grusovin MG, Patel S, Worthington HV, Coulthard P. Interventions for replacing missing teeth: hyperbaric oxygen therapy for irradiated patients who require dental implants. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD003603.pub2] [DOI] [PubMed] [Google Scholar]


