Abstract
Circadian clocks, biological timekeepers that are present in almost every cell of our body, are complex systems whose disruption is connected to various diseases. Controlling cellular clock function with high temporal resolution in an inducible manner would yield an innovative approach for the circadian rhythm regulation. In the present study, we present structure-guided incorporation of photoremovable protecting groups into a circadian clock modifier, longdaysin, which inhibits casein kinase I (CKI). Using photodeprotection by UV or visible light (400 nm) as the external stimulus, we have achieved quantitative and light-inducible control over the CKI activity accompanied by an accurate regulation of circadian period in cultured human cells and mouse tissues, as well as in living zebrafish. This research paves the way for the application of photodosing in achieving precise temporal control over the biological timing and opens the door for chronophotopharmacology to deeper understand the circadian clock system.
Introduction
Circadian clocks are self-sustaining, feedback loop-based biochemical oscillators that regulate rhythmic aspects of behavior and physiology.1 Through these oscillators, biological processes are synchronized with the daily environmental changes caused by the rotation of the Earth around the Sun. Keeping all of the cellular circadian clocks perfectly synchronized within one organism is crucial for its normal and healthy functioning. It has been shown that disruption of circadian clock function promotes a wide variety of illnesses, such as Alzheimer’s, cardiovascular, gastrointestinal, psychological, and other diseases.2,3 As suggested previously,3 there are three major strategies in treating circadian rhythm disorders: optimizing the circadian lifestyle (“training the clock”), optimizing timing of therapies (“clocking the drugs”), and targeting specific circadian clock components (“drugging the clock”).
The crucial role of the circadian clocks in health and disease led to the emergence of strategies to control their function with small molecules.4 Chemical screening, based on cell-based circadian assays with luminescent readout, has been extensively used in discovery of small molecule modifiers of the circadian clock.5−10 Alongside chemical screening, synthetic approaches have emerged as methods for development and optimization of small molecules that are used as a powerful tool for better understanding of clock regulation.11−16 Despite breakthroughs in developing such clock modifiers, achieving exact, externally regulated time-control poses a general challenge for both in vitro and in vivo systems. Enabling fine temporal control by means of clock modifiers over the circadian rhythm will enhance their utility in the investigation of the underlying clock regulation mechanisms as well as their therapeutic application.
The application of photoremovable protecting groups (PPG; also known as photocages or photocleavable groups) is an attractive approach to achieve precise regulation of bioactivity that is employed in photopharmacology.17,18 This emerging field of chemical biology relies on the use of light as an ideal external stimulus that offers high spatiotemporal resolution and bioorthogonality without causing any contamination in comparison to the other stimuli, such as pH and redox changes, metal addition, etc.19 Furthermore, having control over properties of light allows precise control over release or activation of a drug,20,21 which is an ideal solution for obtaining a fine modulation over circadian time of biological clocks and has been employed in other oscillating systems.22
The intrinsic period of the circadian rhythm is modulated by posttranslational modifications.23 The enzyme casein kinase I (CKI) is a clock regulatory kinase, known to play a crucial role in determining the speed of the circadian rhythm. CKI phosphorylates the period (PER) protein and promotes its degradation through a proteasomal pathway.24,25 Genetic mutations of CKI-dependent phosphorylation site of PER2 and CKIδ, an isoform of CKI, lead to the “familial advanced sleep phase” caused by shortening of the circadian period.26,27 The key role of CKI in establishing the period length has also been demonstrated pharmacologically, where CKI inhibitors, such as longdaysin,5 drastically lengthen the period.6,7,28−30
Here, we present quantitative and inducible control of the cellular circadian time by a photopharmacological approach using photocaged longdaysin, a purine-based inhibitor of CKIα and CKIδ that shows a strong period lengthening effect. We have designed photocleavable derivatives DK325 and DK359 for a light-dependent control of CKI activity, which enabled the regulation of the circadian period in human U2OS cells, mouse tissue explants, and zebrafish, by choosing the wavelength and duration of light irradiation.
Results
Design of the Caged Longdaysin
Our photopharmacological approach utilizes photoremovable protecting groups (PPGs), which upon incorporation into the structure of bioactive compounds are able to either fully deactivate or significantly suppress their potency and enable the release of the active molecule by light illumination (Figure 1A).21,31,32 In order to reversibly suppress the activity of longdaysin by rationally designed incorporation of a PPG, it was necessary to recognize the most important binding interaction of longdaysin with CKI. Since a cocrystal structure of CKI–longdaysin complex was not reported, we performed molecular docking simulations. The study revealed crucial interactions of longdaysin with the hinge region of CKIα and CKIδ, forming two hydrogen bonds (Figure 1B). This interaction with the hinge region has also been observed in ADP binding (e.g., PDB entry 5X17),33 indicating a competitive inhibition mechanism of longdaysin. Based on this observation, we targeted the secondary amine at the C6 carbon of the purine scaffold, which formed a hydrogen bond with Leu93 backbone (Figure 1B) for incorporation of a PPG to efficiently disrupt the interaction between longdaysin and CKIα/δ. Thus, two molecules (DK325 and DK359) were designed by incorporating 2-nitrobenzyl and NVOC (6-nitroveratryloxycarbonyl) PPGs, respectively, at this position (Figure 2A).
Figure 1.
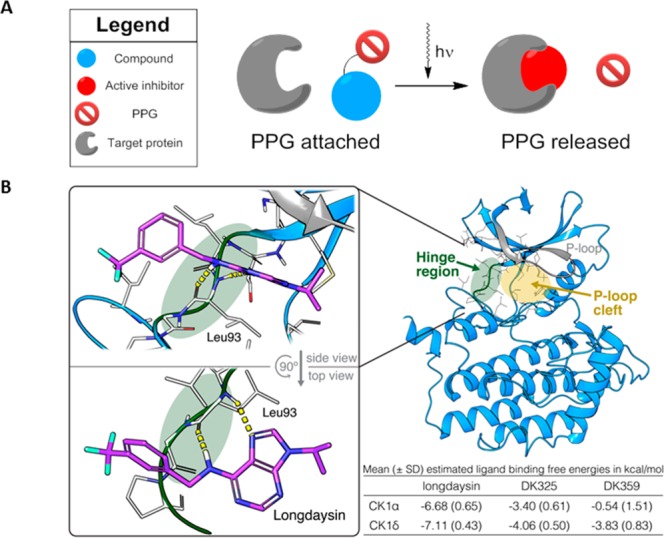
General scheme of the photocleavable approach and putative binding mode of CK1α-longdaysin complex. (A) Schematic representation of the photocleavage approach, where light is used to remove the photoremovable protecting group (PPG) for the release of an active compound. (B) Ligand-binding site of CKIα is characterized by a hinge region (green) and an adjacent cavity formed by the P-loop (yellow area; gray represents P-loop). Docking simulations indicated interaction of the purine scaffold of longdaysin with the hinge region (two hydrogen bonds with Leu93 backbone, indicated by yellow line) (glide XP docking score: −7.39 kcal/mol). The table provides mean estimated ligand binding free energies from molecular docking simulations of longdaysin, DK325, and DK359 with CKIα and CKIδ. SD = standard deviation.
Figure 2.
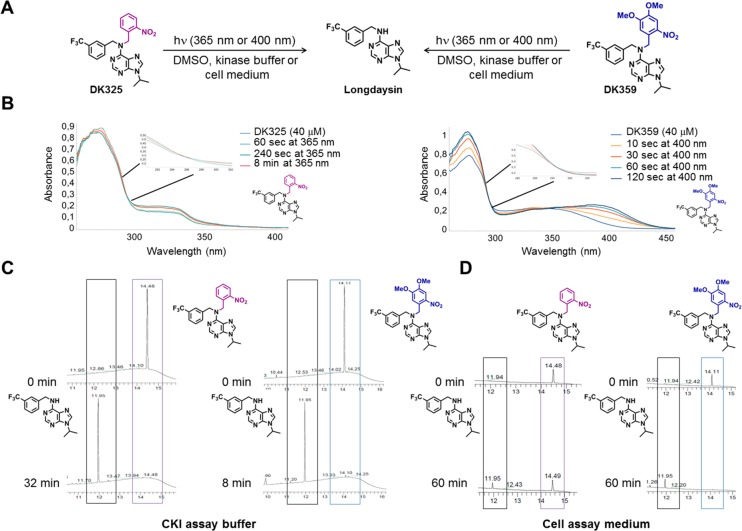
Photodeprotection studies of DK325 and DK359. (A) Photodeprotection of DK325 and DK359 using UV (λ = 365 nm) and violet light (λ = 400 nm). (B) UV–vis spectroscopy analysis of photodeprotection of DK325 and DK359 (40 μM in DMSO, 30 °C) showing clear isosbestic points upon irradiation of DK325 with UV light and DK359 with 400 nm light. (C) UPLC traces for monitoring the deprotection of DK325 (left) and DK359 (right) (40 μM in CKI assay buffer) with UV light. Retention time (min) is shown on the x-axis. Shown are the peaks of longdaysin (11.95 min, black box), DK325 (14.49 min, purple box), and DK359 (14.11 min, blue box). (D) UPLC traces for monitoring the deprotection of DK325 (left) and DK359 (right) (40 μM in cellular assay medium) with 400 nm light. Retention time (min) is shown on the x-axis. Shown are the peaks of longdaysin (11.95 min, black box), DK325 (14.49 min, purple box), and DK359 (14.11 min, blue box).
To predict the differences in potential CKI interactions among longdaysin, DK325, and DK359, molecular docking simulations were performed (Figures 1B and S1). In terms of protein–ligand interaction energies, longdaysin docking conformations ranked significantly lower than DK325 and DK359 in CKIα and CKIδ (Figures 1B and S1). Moreover, while longdaysin conformations were predominantly found at the hinge region forming multiple hydrogen bonds with the Leu93 backbone, DK325 and DK359 were placed more diffusely in the adjacent cavity formed by the P-loop in CKIα and CKIδ. No hinge region interactions were observed for DK325 and DK359 in CKIα. The scarcity of prominent interactions and steric fit of DK325 and DK359 with the proteins and the less favorable protein–ligand interaction energies (indicated by higher docking scores) suggested severely reduced binding of DK325 and DK359 with CKI.
With the molecular-docking-inspired design of the PPG–longdaysin in hand, we used our previously published strategy34 to develop an efficient two-step synthetic route to produce differently protected longdaysin analogues (Supporting Information). For DK325, we employed 2-nitrobenzyl group (Figure 2A), which belongs to a class of widely applied photocleavable groups introduced by Barltrop et al. in 1966.35 Generally, this class of PPGs can be removed under irradiation with UV light. For DK359, the two methoxy groups in the 6-nitroveratryloxycarbonyl (NVOC) derivative were introduced to induce a bathochromic shift of the absorption spectrum that improves the photochemical properties, such as the wavelength required for photocleavage, as well as photodeprotection efficiency.31,36,37 These two PPGs are structurally small and do not significantly interfere with the water solubility provided by the purine core (50–60 μM).
Photochemical Properties of the Caged Longdaysin
Photochemical properties of the protected longdaysin derivatives were assessed by means of UV–vis absorption spectroscopy (Figure 2B) and ultraperformance liquid chromatography mass spectrometry (UPLC–MS; Figures 2C,D). The photocleavage process in DMSO and kinase assay buffer was monitored by UV–vis spectrometry, which showed clear isosbestic points for both DK325 and DK359, indicating single product formation (Figures 2B and S2). UV light was used for photocleavage of DK325, while 400 nm light was applied for DK359 due to an extended absorption in visible region as the result of additional methoxy groups (Figure S3A). Furthermore, the photocleavage in kinase assay buffer and cellular assay medium was examined to make an unambiguous comparison between photocleavage and biological activity. The deprotection rate of DK359 was faster than that of DK325 in kinase assay buffer under the same 365 nm light irradiation conditions, i.e., lamp distance and photon flux (Figures 2C and S3). Using chemical actinometry, the quantum yields (Φ365 nm) of the photocleavable reaction were determined to be 0.22 for both compounds (Figure S4). Despite the same quantum yield, faster deprotection rate of DK359 can be explained by much higher extinction coefficient at 365 nm (Figure S5). The photodeprotection was also performed in the cellular assay medium that contains luciferin, a compound that absorbs light significantly at λ = 365 nm (Figures 2D, S3A, and S6). Since luciferin largely reduced photodeprotection efficiency of UV light, we applied 400 nm light, which surprisingly cleaved both compounds despite very low extinction coefficients of DK325 at this wavelength (Figure S3A). Whereas longdaysin release from DK325 was slower (Figure 2D, left panel; Figure S3B right panel), DK359 showed nearly full deprotection after 30–60 min of irradiation (Figure 2D, right panel; Figure S3C right panel).
The Photodosing of CKI Inhibition
Furthermore, we attempted the light-dependent control of CKI activity in vitro. The photocleavage of DK325 and DK359 was induced by UV and violet light irradiation during the assay with different irradiation times (Figure 3A) in order to analyze the correlation between light dosimetry and CKIα inhibition level. The starting concentration of DK325 and DK359 was 40 μM, which was 7 times higher than the IC50 value of longdaysin (5.6 μM).5 DK325 and DK359 did not show kinase inhibition under the dark conditions (Figure 3B,C, 0 min irradiation). This result validates our rational molecular design that aimed at preventing the interactions with CKI by the incorporation of PPGs on the secondary amine (Figure 1B). Upon increase of irradiation time (UV light in Figure 3B; 400 nm light in Figure 3C), the activity of CKIα was reduced in a dose-dependent manner, reaching the maximum level of inhibition after approximately 30 min for DK325 and 10 min for DK359. As a control, irradiation with UV or 400 nm light during the 60 min period in the absence of compounds did not affect the kinase activity (Figure 3D). These results are in perfect correlation with the UPLC analysis, which showed more efficient uncaging of DK359 than DK325 under the same conditions—light and distance (Figures 2C and S3). The minimal activity of CKIα reached is around 40%. DK359 also repressed the activity of CKIδ, and the minimal activity reached was 29% upon irradiation with 400 nm (Figure S7). This indicates a gradual release of longdaysin over the irradiation time allowing CKIα and CKIδ to consume ATP before the whole amount of DK325 or DK359 was photodeprotected. These results demonstrate tuning of CKIα and CKIδ activity by light.
Figure 3.
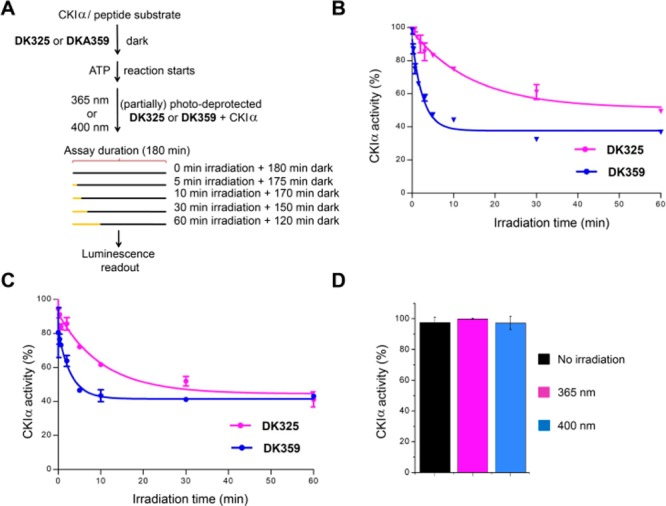
Inhibition of CK1α in a light-dependent manner. (A) Compounds DK325 and DK359 (40 μM final concentration) were applied to CKIα-reaction mixture. The release of longdaysin was controlled by different irradiation duration (0–60 min) after the reaction was initiated by the addition of ATP and peptide substrate. (B and C) In situ irradiation results. Degree of CK1α inhibition was plotted against irradiation time of UV light, λ = 365 nm (B) and with visible light, λ = 400 nm (C). ATP consumption in DMSO control samples, containing the enzyme and peptide substrate without inhibitor, was set at 100% enzyme activity. (D) Effects of 1 h light irradiation on CKIα activity. Showing a nonirradiated (black), UV-light-irradiated (red; λ = 365 nm) and visible light-irradiated (blue, λ = 400 nm) samples. Results are mean ± SD (n = 2) (B–D).
Inducible Period Control of the Circadian Clock by Photodosing in Human U2OS Cells
Next, we tried to control cellular circadian rhythms by targeting CKI proteins inside the cells (cytosol and nucleus) with photodosing. The experiments were designed to release longdaysin in highly controlled manner by tuning wavelength as well as the irradiation duration of light applied to the cells. This allowed us to analyze whether cellular time changes are dependent on properties of the applied light. The experiments were conducted using the bioluminescent circadian assay in human U2OS cells with a Bmal1-dLuc reporter that consists of Bmal1 gene promoter followed by coding sequence of destabilized luciferase.28 We treated the cells with various concentrations of DK325 and DK359 (six points of the 3-fold dilution series), as well as different irradiation durations (0–30 min), and then measured luminescence rhythms. Period parameter represents time required for the circadian clock to run one cycle and was determined from luminescence rhythms by curve fitting. Longdaysin lengthened the period as reported previously,5 and the effect was independent of UV light irradiation (Figure 4A, purple lines). In agreement with the kinase assay, both DK325 and DK359 exhibited almost no effect on circadian period in the dark, indicating that the potent period effect of longdaysin was successfully suppressed by incorporation of 2-nitrobenzyl and NVOC groups also in cell culture conditions. DK325-treated cells exhibited 3–4 h period lengthening by 30 min UV light irradiation, while DK359-treated cells showed this period lengthening in less than 10 min irradiation (Figure 4A). Moreover, the longest irradiation (30 min) in case of DK359 was able to slow the cellular clock period by 10 h.
Figure 4.
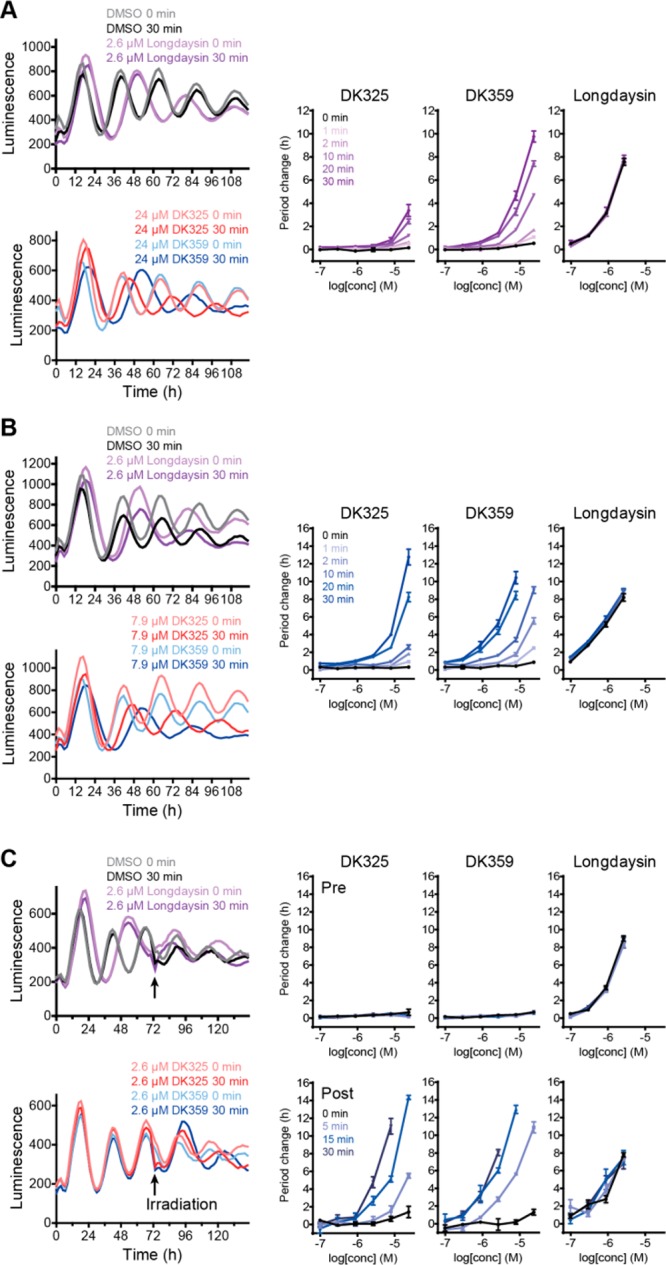
Irradiation-dependent effects of DK325 and DK359 on circadian rhythms in human U2OS cells. (A) Effect of UV light. Bmal1-dLuc reporter cells were treated with various concentrations of compound (six points of 3-fold dilution series in DMSO) and irradiated with 365 nm light for 0–30 min. Luminescence rhythms were then monitored (the left panel, mean of n = 4). Rhythms of DMSO and longdaysin controls are also shown. Period changes compared to a DMSO control are plotted in the right panels (n = 4); p values are summarized in Table S1. (B, C) Effect of visible light (λ = 400 nm). Bmal1-dLuc reporter cells were treated with compounds and irradiated with 400 nm light for 0–30 min at the beginning (B) or in the middle (C, indicated by arrows) of luminescence monitoring. In (C), period changes pre- and postirradiation are plotted in the top right and bottom right panels, respectively.
Using visible light instead of UV light has multiple advantages in photopharmacology, including deeper tissue penetration and lower cytotoxicity.38 For both compounds, a shorter irradiation time was required for period lengthening by 400 nm light compared with UV light, and the potency was enhanced (Figure 4B). This effect is presumably due to a high concentration of luciferin (0.2 mM) in the cell culture medium. Luciferin has a significant absorption at 365 nm and thus can interfere upon photodeprotection with UV light, while its absorption at 400 nm is negligible and enables better efficiency of visible light (Figures S3A and S6). Also, to confirm that the photodeprotection side products have no effect on the circadian period modulation, we designed photocaged acetate with 2-nitrobenzyl (DK491) and NVOC (DK492) groups (Figure S8A). Photodeprotection of these compounds releases acetate and the same side products as DK325 and DK359. Cells treated with DK491 and DK492 did not show period change upon irradiation with both wavelengths (Figures S8B and S8C), confirming that effect from DK325 and DK359 originates only from the release of longdaysin. With these irradiation experiments, we showed that it is possible to adjust cellular circadian period with high temporal precision using light as a privileged external stimulus.
We further tried to control pre-existing rhythms 3 days after the addition of the compounds (Figure 4C) in order to examine the cellular stability of photocaged molecules and confirm light-initiated modulation of circadian period. The cells were treated with compounds, and then luminescence rhythms were measured without light irradiation. Before irradiation, DK325 and DK359 showed almost no effect on the period (“pre”, Figure 4C, right top panels). On the third day, the cells were exposed to visible light (λ = 400 nm) for 0–30 min, and the luminescence rhythms were monitored for three more days (“post”, Figure 4C, right bottom panels). Interestingly, the period lengthening effects were much stronger than those observed with irradiation from the beginning (Figure 4B), possibly due to increased cellular concentration of the compounds during three-day incubation. These results confirm high stability of DK325 and DK359 in the cellular medium with cells present and light-induced uncaging during the assay.
Ex Vivo Manipulation of the Circadian Period by Light in Mouse Tissues
Following experiments in cells, we tested the principle of light-dependent period control at the tissue level using spleen explant of Per2::Luc knock-in reporter mice (Figure 5). The mice express PER2-luciferase fusion protein under control of the endogenous Per2 promoter.39 According to rhythmic activation of the Per2 promoter, the tissue explants show circadian changes of luminescence intensity (Figure 5A, gray line). The explants were treated with compounds and irradiated with 400 nm light, and then luminescence rhythms were measured. Consistent with the cellular assay results, DK325 and DK359 showed period lengthening in a concentration- and irradiation-duration-dependent manner with a stronger effect of DK359 in comparison to DK325, while 400 nm light showed no influence on the effect of longdaysin (Figure 5A–D). In addition to the peripheral clock in spleen, we investigated the effect of DK359 on the central clock in the hypothalamic suprachiasmatic nucleus (SCN) that controls behavioral rhythms. The compound showed no effect in the dark and induced period lengthening upon 400 nm light irradiation (Figure 5E,F). Together, DK359 enabled light-dependent quantitative and inducible control of the circadian period at both cellular and tissue levels.
Figure 5.
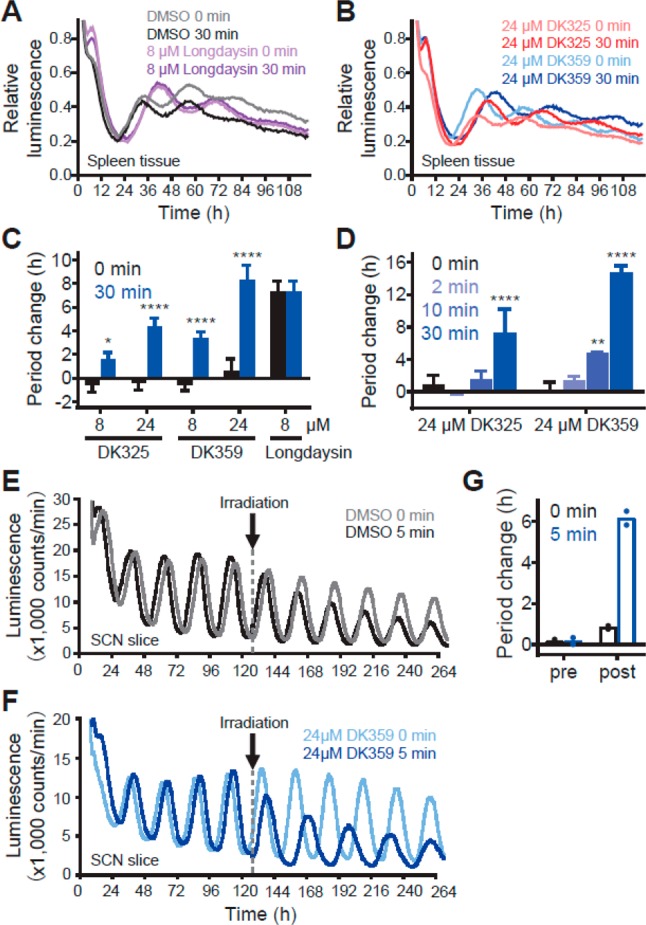
Irradiation-dependent effects of DK325 and DK359 on circadian rhythms in mouse tissue explants. Spleen tissue (A–D) and the SCN (E–G) of the Per2::Luc knock-in reporter mice were treated with various concentrations of compound and irradiated with λ = 400 nm light for 0–30 min. Luminescence rhythms were then monitored and shown in (A) and (B) (mean of n = 3–4) and in (E) and (F) (representative result). Period changes compared to a DMSO control are plotted in (C) for concentration-dependent period lengthening, in (D) for irradiation-duration-dependent period lengthening (n = 2–4), and in (G) for the SCN (n = 2). ****p < 0.0001, **p < 0.01, *p < 0.05 against the dark control.
In Vivo Manipulation of the Circadian Period by Light
In addition to mouse tissue explant, we tested living zebrafish larva containing the Per3:Luc reporter for circadian activity monitoring. Previous results showed that the larva circadian rhythms exhibit a sensitivity to longdaysin for period lengthening similar to human cell lines.5 The larvae were exposed to four cycles of light/dark cycles and transferred to constant darkness for circadian monitoring. The DK359 treatment were performed 6 h following the start of the circadian time (CT6) and immediately followed by 400 nm light treatment (0–10 min). In the absence of DK359 (DMSO control), a short 400 nm light treatment at CT6 did not affect the phase and period of the luminescence rhythms despite every cell being light sensitive in this organism (Figure 6A). In the presence of DK359, the period was substantially lengthened in an irradiation-duration-dependent manner (Figure 6B). The shorter irradiation time needed for zebrafish in vivo in comparison to cell and tissue culture experiments can be explained by lack of vitamins, FBS, and other light-absorbing components in solution for zebrafish maintenance.
Figure 6.
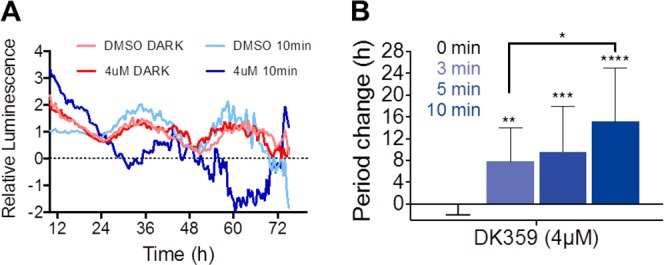
Irradiation-dependent effects of DK359 on circadian rhythms in zebrafish larva. (A) Per3::Luc zebrafish larvae were treated with compound DK359 (4 μM) or DMSO at CT6 and irradiated with λ = 400 nm light for 0 and 10 min. Luminescence rhythms were then monitored for 3 d (mean of n = 3). Data are baseline subtracted for detrending. (B) Period changes compared to a DMSO control for 0, 3, 5, and 10 min light exposure are plotted (n = 13–15). ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05 against the dark control.
Discussion
Kinases play an important role in a wide variety of diseases,40−42 thus being one of the most interesting pharmaceutical targets.43 In the circadian clock function, kinases are also essential regulators in every cell throughout the body.23 To enable inducible regulation of clock function via kinase activity control, the effect of longdaysin, a circadian modulator inhibiting casein kinase I (CKIα and CKIδ), was silenced with PPGs. This has enabled the reactivation of silenced longdaysin by light irradiation with high temporal control, allowing for conditional modulation of the circadian rhythm with light, from the enzymatic level to a living organism.
On the basis of the rational molecular design, facilitated by molecular docking, photocleavable groups (2-nitrobenzyl and NVOC) were incorporated at the 6-NH position of longdaysin, which is crucial for the interaction with the hinge region of CKIα and CKIδ. As a result, CKI inhibitory activity of protected longdaysin was entirely suppressed. This approach features several advantages. First, a straightforward two-step synthesis of the modulators was established that emphasizes the generality of this approach, which may potentially allow for incorporation of red-shifted PPGs31,44−46 that would facilitate low energy and deeper tissue penetrating light. Furthermore, the water solubility of longdaysin was not compromised by introducing small and rather polar PPGs. Finally, photochemical properties showed quantitative photodeprotection of both modulators. DK359 exhibited much faster deprotection under the same irradiation conditions that makes it more suitable for a quick period adjustment. The circadian period was successfully modified by using both UV and visible light (400 nm) by exploring variable concentration and photodose. A fine and conditional tuning of the period length in cellulo, ex vivo, and in zebrafish was achieved within (sub)minute range of light irradiation, which presents an ideal system for further mechanistic and in vivo studies.
So far, the common mechanism of the circadian clock in each cell of the body prevented the use of clock modifiers in precise spatiotemporal control. As a result of the suppressed activity of protected longdaysin toward CKI and period lengthening, we believe that our approach will be applicable also to control tissue-specific clocks with spatial resolution limited only by the ability of light delivery. Therefore, future research should focus on formulation and development of the photocaged derivatives that enable spatially controlled activation. Together with our work on temporally controlled regulation, this will enable us to study the circadian organization in mammals, to identify the relationship between clock disruption and disease development, as well as to study the potential use of this chronophotopharmacology approach in chronotherapy in the future.
Acknowledgments
We gratefully acknowledge generous support from The Netherlands Organization for Scientific Research (NWO-CW, Top grant to B.L.F. and VIDI Grant No. 723.014.001 to W.S.), the Royal Netherlands Academy of Arts and Sciences Science (KNAW), the Ministry of Education, Culture and Science (Gravitation program 024.001.035), the European Research Council (Advanced Investigator Grant No. 227897 to B.L.F), PRESTO Grant JPMJPR14LA from JST (T.H), Grant-in-Aid for Young Scientists (A) 15H05590, Scientific Research (B) 18H02402, and Challenging Research (Exploratory) 18K19171 from JSPS (T.H), Senri Life Science Foundation (T.H), and Mochida Memorial Foundation for Medical and Pharmaceutical Research (T.H). D.K. acknowledges the receipt of a fellowship from the Dositeja Fund for Young Talents for international studies. We thank ing. P. van der Meulen for help with the NMR studies, ing. T. Tiemersma-Wegman for ESI-MS analyses (both from Stratingh Institute for Chemistry, University of Groningen), Dr. Kaori Goto for cellular circadian assays, and Dr. Joseph S. Takahashi for Per2::Luc knock-in mice.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b05445.
Experimental procedures, assays, photochemistry, and characterization data for all new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Takahashi J. S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18 (3), 164. 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J.; Lazar M. A. Circadian time signatures of fitness and disease. Science 2016, 354 (6315), 994. 10.1126/science.aah4965. [DOI] [PubMed] [Google Scholar]
- Sulli G.; Manoogian E. N. C.; Taub P. R.; Panda S. Training the Circadian Clock, Clocking the Drugs, and Drugging the Clock to Prevent, Manage, and Treat Chronic Diseases. Trends Pharmacol. Sci. 2018, 39 (9), 812. 10.1016/j.tips.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.; Yoo S.-H.; Takahashi J. S. Development and Therapeutic Potential of Small-Molecule Modulators of Circadian Systems. Annu. Rev. Pharmacol. Toxicol. 2018, 58 (1), 231. 10.1146/annurev-pharmtox-010617-052645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T.; Lee J. W.; Lewis W. G.; Zhang E. E.; Breton G.; Liu X.; Garcia M.; Peters E. C.; Etchegaray J.-P.; Traver D.; Schultz P. G.; Kay S. A. High-Throughput Chemical Screen Identifies a Novel Potent Modulator of Cellular Circadian Rhythms and Reveals CKIα as a Clock Regulatory Kinase. PLoS Biol. 2010, 8 (12), e1000559. 10.1371/journal.pbio.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. W.; Hirota T.; Peters E. C.; Garcia M.; Gonzalez R.; Cho C. Y.; Wu X.; Schultz P. G.; Kay S. A. A small molecule modulates circadian rhythms through phosphorylation of the period protein. Angew. Chem., Int. Ed. 2011, 50 (45), 10608. 10.1002/anie.201103915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.; Yoo S.-H.; Park Y.-S.; Kim K.-H.; Wei S.; Buhr E.; Ye Z.-Y.; Pan H.-L.; Takahashi J. S. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (1), 101. 10.1073/pnas.1118034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T.; Lee J. W.; St. John P. C.; Sawa M.; Iwaisako K.; Noguchi T.; Pongsawakul P. Y.; Sonntag T.; Welsh D. K.; Brenner D. A.; Doyle F. J.; Schultz P. G.; Kay S. A. Identification of Small Molecule Activators of Cryptochrome. Science 2012, 337 (6098), 1094. 10.1126/science.1223710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T.; Kay S. A. Identification of small-molecule modulators of the circadian clock. Methods Enzymol. 2015, 551, 267. 10.1016/bs.mie.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Oshima T.; Niwa Y.; Kuwata K.; Srivastava A.; Hyoda T.; Tsuchiya Y.; Kumagai M.; Tsuyuguchi M.; Tamaru T.; Sugiyama A.; Ono N.; Zolboot N.; Aikawa Y.; Oishi S.; Nonami A.; Arai F.; Hagihara S.; Yamaguchi J.; Tama F.; Kunisaki Y.; Yagita K.; Ikeda M.; Kinoshita T.; Kay S. A.; Itami K.; Hirota T. Cell-based screen identifies a new potent and highly selective CK2 inhibitor for modulation of circadian rhythms and cancer cell growth. Sci. Adv. 2019, 5 (1), eaau9060. 10.1126/sciadv.aau9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura L.; Swanson T.; Adamowicz W.; Adams J.; Cianfrogna J.; Fisher K.; Holland J.; Kleiman R.; Nelson F.; Reynolds L.; St. Germain K.; Schaeffer E.; Tate B.; Sprouse J. An inhibitor of casein kinase I epsilon induces phase delays in circadian rhythms under free-running and entrained conditions. J. Pharmacol. Exp. Ther. 2007, 322 (2), 730. 10.1124/jpet.107.122846. [DOI] [PubMed] [Google Scholar]
- Walton K. M.; Fisher K.; Rubitski D.; Marconi M.; Meng Q.-J.; Sládek M.; Adams J.; Bass M.; Chandrasekaran R.; Butler T.; Griffor M.; Rajamohan F.; Serpa M.; Chen Y.; Claffey M.; Hastings M.; Loudon A.; Maywood E.; Ohren J.; Doran A.; Wager T. T. Selective inhibition of casein kinase 1 epsilon minimally alters circadian clock period. J. Pharmacol. Exp. Ther. 2009, 330 (2), 430. 10.1124/jpet.109.151415. [DOI] [PubMed] [Google Scholar]
- Solt L. A.; Wang Y.; Banerjee S.; Hughes T.; Kojetin D. J.; Lundasen T.; Shin Y.; Liu J.; Cameron M. D.; Noel R.; Yoo S.-H.; Takahashi J. S.; Butler A. A.; Kamenecka T. M.; Burris T. P. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 2012, 485 (7396), 62. 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima T.; Yamanaka I.; Kumar A.; Yamaguchi J.; Nishiwaki-Ohkawa T.; Muto K.; Kawamura R.; Hirota T.; Yagita K.; Irle S.; Kay S. A.; Yoshimura T.; Itami K. C-H activation generates period-shortening molecules that target cryptochrome in the mammalian circadian clock. Angew. Chem., Int. Ed. 2015, 54 (24), 7193. 10.1002/anie.201502942. [DOI] [PubMed] [Google Scholar]
- Lee J. W.; Hirota T.; Kumar A.; Kim N.-J.; Irle S.; Kay S. A. Development of Small-Molecule Cryptochrome Stabilizer Derivatives as Modulators of the Circadian Clock. ChemMedChem 2015, 10 (9), 1489. 10.1002/cmdc.201500260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries P. S.; Bersot R.; Kincaid J.; Mabery E.; McCluskie K.; Park T.; Renner T.; Riegler E.; Steinfeld T.; Turtle E. D.; Wei Z.-L.; Willis E. Carbazole-containing amides and ureas: Discovery of cryptochrome modulators as antihyperglycemic agents. Bioorg. Med. Chem. Lett. 2018, 28 (3), 293. 10.1016/j.bmcl.2017.12.051. [DOI] [PubMed] [Google Scholar]
- Velema W. A.; Szymanski W.; Feringa B. L. Photopharmacology: Beyond Proof of Principle. J. Am. Chem. Soc. 2014, 136 (6), 2178. 10.1021/ja413063e. [DOI] [PubMed] [Google Scholar]
- Broichhagen J.; Frank J. A.; Trauner D. A Roadmap to Success in Photopharmacology. Acc. Chem. Res. 2015, 48 (7), 1947. 10.1021/acs.accounts.5b00129. [DOI] [PubMed] [Google Scholar]
- Gorostiza P.; Isacoff E. Y. Optical switches for remote and noninvasive control of cell signaling. Science 2008, 322 (5900), 395. 10.1126/science.1166022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Correia A.; Weyel X. M. M.; Heckel A. Four Levels of Wavelength-Selective Uncaging for Oligonucleotides. Org. Lett. 2013, 15 (21), 5500. 10.1021/ol402657j. [DOI] [PubMed] [Google Scholar]
- Velema W. A.; Van Der Berg J. P.; Szymanski W.; Driessen A. J. M.; Feringa B. L. Orthogonal Control of Antibacterial Activity with Light. ACS Chem. Biol. 2014, 9 (9), 1969. 10.1021/cb500313f. [DOI] [PubMed] [Google Scholar]
- Glock P.; Broichhagen J.; Kretschmer S.; Blumhardt P.; Mücksch J.; Trauner D.; Schwille P. Optical Control of a Biological Reaction–Diffusion System. Angew. Chem., Int. Ed. 2018, 57, 2362. 10.1002/anie.201712002. [DOI] [PubMed] [Google Scholar]
- Partch C. L.; Green C. B.; Takahashi J. S. Takahashi, Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24 (2), 90. 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide E. J.; Woolf M. F.; Kang H.; Woolf P.; Hurst W.; Camacho F.; Vielhaber E. L.; Giovanni A.; Virshup D. M. Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation. Mol. Cell. Biol. 2005, 25 (7), 2795. 10.1128/MCB.25.7.2795-2807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchegaray J.-P.; Machida K. K.; Noton E.; Constance C. M.; Dallmann R.; Di Napoli M. N.; DeBruyne J. P.; Lambert C. M.; Yu E. A.; Reppert S. M.; Weaver D. R. Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol. Cell. Biol. 2009, 29 (14), 3853. 10.1128/MCB.00338-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh K. L.; Jones C. R.; He Y.; Eide E. J.; Hinz W. A.; Virshup D. M.; Ptacek L. J.; Fu Z. H. An hPer2 Phosphorylation Site Mutation in Familial Advanced Sleep Phase Syndrome. Science 2001, 291 (5506), 1040. 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Padiath Q. S.; Shapiro R. E.; Jones C. R.; Wu S. C.; Saigoh N.; Saigoh K.; Ptáček L. J.; Fu Y.-H. Functional consequences of a CKIδ mutation causing familial advanced sleep phase syndrome. Nature 2005, 434 (7033), 640. 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- Hirota T.; Lewis W. G.; Liu A. C.; Lee J. W.; Schultz P. G.; Kay S. A. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3beta. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (52), 20746. 10.1073/pnas.0811410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isojima Y.; Nakajima M.; Ukai H.; Fujishima H.; Yamada R. G.; Masumoto K.; Kiuchi R.; Ishida M.; Ukai-Tadenuma M.; Minami Y.; Kito R.; Nakao K.; Kishimoto W.; Yoo S.-H.; Shimomura K.; Takao T.; Takano A.; Kojima T.; Nagai K.; Sakaki Y.; Takahashi J. S.; Ueda H. R. CKIepsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (37), 15744. 10.1073/pnas.0908733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q.-J.; Maywood E. S.; Bechtold D. A.; Lu W.-Q.; Li J.; Gibbs J. E.; Dupré S. M.; Chesham J. E.; Rajamohan F.; Knafels J.; Sneed B.; Zawadzke L. E.; Ohren J. F.; Walton K. M.; Wager T. T.; Hastings M. H.; Loudon A. S. I. Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (34), 15240. 10.1073/pnas.1005101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klán P.; Šolomek T.; Bochet C. G.; Blanc A.; Givens R.; Rubina M.; Popik V.; Kostikov A.; Wirz J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113 (1), 119. 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. J.; Velema W. A.; Lerch M. M.; Szymanski W.; Feringa B. L. Wavelength-selective cleavage of photoprotecting groups: strategies and applications in dynamic systems. Chem. Soc. Rev. 2015, 44 (11), 3358. 10.1039/C5CS00118H. [DOI] [PubMed] [Google Scholar]
- Shinohara Y.; Koyama Y. M.; Ukai-Tadenuma M.; Hirokawa T.; Kikuchi M.; Yamada R. G.; Ukai H.; Fujishima H.; Umehara T.; Tainaka K.; Ueda H. R. Temperature-Sensitive Substrate and Product Binding Underlie Temperature-Compensated Phosphorylation in the Clock. Mol. Cell 2017, 67 (5), 783. 10.1016/j.molcel.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Kolarski D.; Szymanski W.; Feringa B. L. Two-step, one-pot synthesis of visible-light-responsive 6-azopurines. Org. Lett. 2017, 19 (19), 5090. 10.1021/acs.orglett.7b02361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barltrop J. A.; Plant P. J.; Schofield P. Photosensitive protective groups. Chem. Commun. 1966, 822–823. 10.1039/c19660000822. [DOI] [Google Scholar]
- Bochet C. G. Photolabile protecting groups and linkers. J. Chem. Soc. Perkin Trans. 1 2002, 125. 10.1039/B009522M. [DOI] [Google Scholar]
- Patchornik A.; Amit B.; Woodward R. B. Photosensitive protecting groups. J. Am. Chem. Soc. 1970, 92 (21), 6333. 10.1021/ja00724a041. [DOI] [Google Scholar]
- Sandell J. L.; Zhu T. C. A review of in-vivo optical properties of human tissues and its impact on PDT. J. Biophotonics 2011, 4 (11–12), 773. 10.1002/jbio.201100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.-H.; Yamazaki S.; Lowrey P. L.; Shimomura K.; Ko C. H.; Buhr E. D.; Siepka S. M.; Hong H.-K.; Oh W. J.; Yoo O. J.; Menaker M.; Takahashi J. S. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U. S. A. 2004, 101 (15), 5339. 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A. Protein Kinase Inhibitors as a Therapeutic Modality. Acc. Chem. Res. 2003, 36 (6), 462. 10.1021/ar0201207. [DOI] [PubMed] [Google Scholar]
- Müller S.; Chaikuad A.; Gray N. S.; Knapp S. The ins and outs of selective kinase inhibitor development. Nat. Chem. Biol. 2015, 11, 818. 10.1038/nchembio.1938. [DOI] [PubMed] [Google Scholar]
- Byrne D. P.; Foulkes D. M.; Eyers P. A. Pseudokinases: update on their functions and evaluation as new drug targets. Future Med. Chem. 2017, 9 (2), 245. 10.4155/fmc-2016-0207. [DOI] [PubMed] [Google Scholar]
- Ferguson F. M.; Gray N. S. Kinase inhibitors: the road ahead. Nat. Rev. Drug Discovery 2018, 17 (5), 353. 10.1038/nrd.2018.21. [DOI] [PubMed] [Google Scholar]
- Sitkowska K.; Feringa B. L.; Szymański W. Green-Light-Sensitive BODIPY Photoprotecting Groups for Amines. J. Org. Chem. 2018, 83 (4), 1819. 10.1021/acs.joc.7b02729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami P. P.; Syed A.; Beck C. L.; Albright T. R.; Mahoney K. M.; Unash R.; Smith E. A.; Winter A. H. BODIPY-derived photoremovable protecting groups unmasked with green light. J. Am. Chem. Soc. 2015, 137 (11), 3783. 10.1021/jacs.5b01297. [DOI] [PubMed] [Google Scholar]
- Peterson J. A.; Wijesooriya C.; Gehrmann E. J.; Mahoney K. M.; Goswami P. P.; Albright T. R.; Syed A.; Dutton A. S.; Smith E. A.; Winter A. H. Family of BODIPY Photocages Cleaved by Single Photons of Visible/Near-Infrared Light. J. Am. Chem. Soc. 2018, 140 (23), 7343. 10.1021/jacs.8b04040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.