Abstract
The aim of the present study was to screen differentially expressed miRNAs in vulvar squamous cell carcinoma (VSCC), observe the role of microRNA-4712-5p in VSCC and investigate its targets and regulatory mechanism. Differentially expressed miRNAs in human VSCC tissues were screened. microRNA-4712-5p was selected and its expression level was verified in clinical tissue samples and the VSCC cell line A431 by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis. The overexpression vector of microRNA-4712-5p was prepared and transfected into A431 cells; subsequently, cell invasion and metastasis were examined by Cell Counting Kit-8 and Transwell migration assays. Furthermore, the target gene of miRNA-4712-5p was predicted by bioinformatics and verified by The Dual-Luciferase® Reporter (DLR™) Assay System. The expression of phosphatase and tensin homologue (PTEN) and its downstream proteins, such as protein kinase B (PKB; AKT), glycogen synthase kinase (GSK)3β and cyclin D1, were detected by western blot assays. The expression level of microRNA-4712-5p in VSCC tissues and the A431 cell line was found to be significantly increased, promoting proliferation and invasion of VSCC. The DLR™ assay indicated that PTEN was a target of miR-4712-5p. RT-qPCR revealed that PTEN expression was markedly lower in VSCC tissues compared with that in adjacent tissues. After A431 cells were transfected with the miRNA-4712-5p overexpression vector, phospho-AKT (p-AKT) and cyclin D1 expression were notably increased, but miRNA-4712-5p-targeted PTEN and phospho-GSK3β (p-GSK3β) protein markedly decreased. Therefore, microRNA-4712-5p can reduce the expression of PTEN, further affecting its downstream p-AKT, p-GSK3β and cyclin D1 signaling pathways, promoting the proliferation and invasion of VSCC.
Keywords: vulvar squamous cell carcinoma, microRNA-4712-5p, proliferation, phosphatase and tensin homologue
Introduction
Vulvar cancer is a malignant tumor that poses a threat to women's health (1). The most common subtype is vulvar squamous cell carcinoma (VSCC), accounting for 80–90% of all vulvar malignant tumors. VSCC is very common among women aged >60 years (2). In recent years, its incidence has increased. The cause for vulvar cancer development has not been fully elucidated. Human papillomavirus has been reported to be associated with a subset of vulvar cancers (3). It has been demonstrated that surgically treated vulvar intraepithelial neoplasia (VIN) has a high rate of recurrence (3.8%) and untreated VIN in women aged >30 years has an appreciable invasive potential (4). Non-neoplastic lesions in the vulvar epithelium include squamous epithelial hyperplasia and lichen sclerosus (5). At present, epidemiological studies have reported that the malignant rate is 1–5% (6). It is not clear in molecular biology whether there is a malignant tendency in vulvar intraepithelial tumor-like lesions and vulvar intraepithelial non-tumor-like lesions. Further investigating the mechanism of tumorigenesis and progression of VSCC may uncover novel targets and help design novel therapeutic methods for VSCC treatment.
An increasing number of studies have proven that microRNAs play important roles in carcinogenesis (7–9). MicroRNAs are regulatory RNAs, 18–23 nucleotides in size (10). Although they have a low molecular weight, they play key roles at the transcriptional level of human cells, regulating several important biological functions, such as tumor cell proliferation, invasion and migration (11). A number of studies have demonstrated that microRNAs are abnormally expressed in different tumors, and they act as tumor suppressors or tumor-promoting factors (12,13). Yang and Guo reported that miR-3147 can participate in the epithelial-to-mesenchymal transition of VSCC cells by inhibiting Smad4 (14). Mir-30c and let-7a were found to be reciprocally correlated with the expression of HMGA2 (15), which promotes diverse tumorigenic processes in VSCC (16). The upregulation of miR-590-5p was found to promote cellular malignant behavior in VSCC via the target gene TGFβRII (17). However, to the best of our knowledge, the role of miR-4712-5p in VSCC has not been reported to date.
The aim of the present study was to determine whether miR-4712-5p is dysregulated in VSCC, and whether it plays a role in the occurrence and development of VSCC, as well as to investigate the underlying mechanism.
Materials and methods
Tissue collection
Three pairs of freshly frozen VSCC samples and adjacent non-cancerous tissues were used for microarray assay (Table I). A total of 30 freshly frozen VSCC samples and adjacent non-cancerous tissues were examined to confirm miRNA expression and validate the targeting. All the tissues were obtained from the Department of Gynecology at the First Affiliated Hospital of China Medical University between January 2011 and January 2018. All the lesions were staged using the new vulvar cancer classification system (18). The frozen specimens were examined pathologically. Clinical records were retrospectively reviewed. Patients who had not undergone chemotherapy or radiation treatment prior to surgery were enrolled. The protocol of the present study was approved by the Ethics Committee of the First Affiliated Hospital of China Medical University and informed consent was also obtained from the patients.
Table I.
Characteristics of the patients in the microarray study.
| Sample name | Age (years) | FIGO stage | Tumor differentiation | Lymph node metastasis |
|---|---|---|---|---|
| A exp | 48 | IIIA | Moderate | Yes |
| a ctrl | 48 | – | – | – |
| B exp | 85 | IB | High | No |
| b ctrl | 85 | – | – | – |
| F exp | 64 | IA | High | No |
| f ctrl | 64 | – | – | – |
FIGO, International Federation of Gynecology and Obstetrics.
Cell culture
Human VSCC A431 cells and the human normal vaginal epithelial cell line HNVEC/HL-016 (VECs) were purchased from Shanghai Cell Bank. Cells were cultured with DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum in a 5% CO2 incubator at 37°C and saturated humidity.
Vector construction and cell transfection
The vector with miR-4712-5p overexpression and corresponding negative control (NC) were synthesized at GenePharma. The protocol of cell transfection was as follows: A431 cells were seeded at a density of 1×105 cells/well in 6-well culture plates. As the cells were in a good condition and the confluence was >70%, transfection was performed. After removal of the culture medium, the cells were washed with PBS, followed by addition of 1 ml medium to each well. siRNA and Lipofectamine 2000 (siRNA final concentration, 50 nM; Lipofectamine 2000, 1:50) was dissolved with Opti-MEM. The diluted siRNA and Lipofectamine 2000 were mixed and allowed to form a complex for 20 min. The complex was added to the cell culture plate (200 µl per well) and incubated in a 5% CO2 incubator at 37°C overnight. The medium was replaced every 4–6 h. After 48 h, the transfection efficiency was determined by polymerase chain reaction (PCR) analysis.
miR-4712-5p mimics and inhibitor sequences were cloned into the green fluorescent lentivirus (GeneChem), which was designed upon request. A431 cells were transfected with lentivirus and screened with puromycin to increase transfection efficiency. The transfection efficiency was examined using PCR.
Reverse transcription-quantitative (RT-q)PCR analysis
VSCC tissues and A431 cells were treated with TRIzol to extract RNA using the SYBR Prime Script miRNA RT-PCR Kit (TAKARA) reaction system as follows: SYBR Premix Ex Taq II (2X), 10 µl; PCR forward primer (10 µM), 0.8 µl; Uni-miR qPCR primer (10 µM), 0.8 µl; ROX Reference Dye II (50X), 0.4 µl; cDNA template, 2 µl; ddH2O, 6 µl. In the fluorescence qPCR device, the reaction conditions were as follows: Pre-denaturation at 95°C for 1 min, denaturation at 95°C for 15 sec, annealing at 60°C for 40 sec, and extension at 72°C for 15 sec for a total of 40 cycles. The melting curve was analyzed. The expression of miRNA-124-3p was calculated by the 2−ΔΔCq method (19), and U6 snRNA was used as a relative quantitative internal reference. We defined its negative value as relative low expression of miR-4712-5p and its positive value as relative high expression of miR-4712-5p (VSCC compared with adjacent normal tissues). The primers used for qPCR are listed in Table II.
Table II.
Gene primers used for reverse transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer (5′→3′) |
|---|---|
| miR-4712-5p | Forward: AAATGACGAGTTACTGGATGACATA |
| Reverse: ATCCAGTAACTCGTCATTTTATGTC | |
| RNU48 | Forward: AGTGATGATGACCCCAGGTAA |
| Reverse: CTCTTGAGTGTGTCGCTGATG | |
| PTEN | Forward: GGACGAACTGGTGTAATGATATG |
| Reverse: TCTACTGTTTTTGTGAAGTACAGC | |
| GAPDH | Forward: GCATGATGCCGGCAGCTTT |
| Reverse: CAGCAACTGAATGAGGCCA |
Transwell migration assay
An artificial basement membrane was formed by Matrigel. The cells were collected, washed once with PBS, and diluted to 1×105/ml with complete medium. A total of 0.5 ml of the cell mixture was added to the upper chamber, and 0.75 ml of DMEM supplemented with 10% fetal bovine serum was added to the lower chamber. After incubation at 37°C for 48 h, all samples were stained with hematoxylin and eosin. At an original magnification of ×100 under a light microscope, three fields were randomly selected. The number of cells which traversed the artificial basement membrane was counted, and the mean value was calculated.
Cell Counting Kit-8 (CCK-8) assay
A431 cells in the logarithmic growth phase were collected, and prepared into a single-cell suspension with cell culture medium. Cells were seeded in 96-well culture plates (100 µl/well) under sterile conditions (3×l03−5×l03/well). After 24 h of incubation, the medium was replaced. After treatment with siRNA and blank control, the cells were further cultured for 48 h. CCK-8 solution (10 µl) was added to each well and further incubated for 2–4 h. Absorbance values were measured at 450 nm with a microplate reader (MR-96A, Mindray).
Animal tumor transplantation experiment
A total of 16 nude mice, aged 4 weeks, were purchased from China Medical University. All animals were maintained in individual cages under controlled ambient temperature (24±2°C) and 40–70% humidity, with a 12-h light/dark cycle. Standard pelleted chow and drinking water were available ad libitum. The mice were allowed to acclimatize for at least 1 week before the experiments. The health and behavior of mice were observed daily by animal husbandry staff at our facility. To reduce animal suffering and distress, animal tools and nesting material were provided to the mice. A431 and A431/miR-4712-5p cells (0.1 ml; 5×106/ml) were subcutaneously injected into the right axilla (8 mice per group). The mice were examined every 3 days to observe and record the growth of the tumor. After tumor development, the weight of the mice was recorded and the length and width of the tumor were measured to calculate the tumor volume. Two weeks later, all animals were sacrificed by cervical dislocation, and the tumors were resected and weighed. The experiment was approved by the Department of Laboratory Animal of China Medical University and the Animal Experimental Protection Agency.
Dual luciferase reporter gene analysis
The PTEN 3′-untranslated region (UTR) sequence was cloned into the pMIR-REPORTTM vector (Ambion). 293T cells were grown in 24-well plates until the density reached 50% and co-transfected with 200 ng wild-type or mutant firefly luciferase reporter plasmid and 10 ng pMIR-REPORTTM control plasmid, 100 nM miR-4712-5p or NC miRNA. After 48 h of culture, the cells were harvested and examined with the luciferase activity system (Promega Corporation).
Immunohistochemistry
The tumors from patients and nude mice were sectioned for pathological examination. Antigen was retrieved with citric acid at 120°C and 100 kPa. The sections were washed with PBS, incubated with H2O2 for 30 min, blocked with 5% bovine serum albumin for 30 min, then 10% goat serum for 30 min. Primary anti-PTEN antibody (rabbit monoclonal, 1:800 dilution, ab32199, Abcam) was added and incubated at 4°C overnight. After washing with PBS, secondary antibody was added and incubated at 37°C for 40 min, followed again by washing with PBS. Horseradish peroxidase-labeled streptavidin/avidin was added and incubated at 37°C for 40 min. After washing with PBS, the sections were developed with 3,3′-diaminobenzidine.
Western blot assay
Tissues of patients and cells collected after transfection were lysed in RIPA lysate containing protease inhibitor. Following centrifugation at 12,000 × g at 4°C for 20 min, the supernatant was collected. The protein concentration was determined using the bicinchoninic acid assay. The proteins were separated by 10% SDS-PAGE and transferred onto a polyvinylidene fluoride membrane. The membrane was blocked with skimmed milk, and incubated with antibodies against p-AKT (1:200 ab38449, Abcam), cyclin D1 (1:200 ab134175, Abcam), PTEN (1:200 ab32199, Abcam), CDK4 (1:200 ab199728, Abcam), p-GSK3β (1:200 ab68476, Abcam) and CDK6 (1:200 ab131439, Abcam) at 4°C overnight. The membrane was washed with TBST, incubated with secondary antibody at room temperature for 2 h, visualized with an ECL kit (32106; Invitrogen; Thermo Fisher Scientific, Inc.), and photographed with a gel imaging system. The results were analyzed by absorbance using ImageJ software (National Institutes of Health).
Statistical analysis
All results were analyzed using SPSS 19.0 (SPSS, Inc.). Measurement data are expressed as the mean ± standard error of the mean. The difference between groups was compared using t-test. The difference among multiple groups was compared using one-way analysis of variance) with Tukey's multiple comparison post hoc test. A value of P<0.05 was considered to indicate statistically significant differences.
Results
MicroRNA expression profile of VSCC
We determined the miRNA expression profile of VSCC. We selected the miRNAs which were overexpressed or underexpressed by >2-fold in bioinformatics analysis and identified 90 upregulated and 67 downregulated miRNAs in the cancer samples (Fig. 1).
Figure 1.
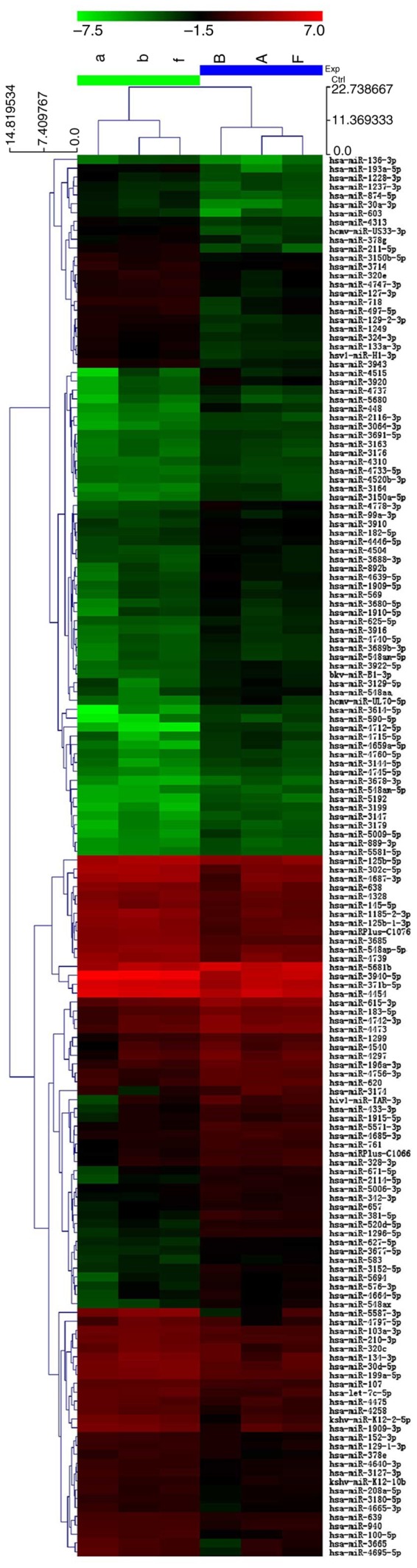
miRNA expression profile in VSCC. The miRNAs that were overexpressed or underexpressed by >2-fold for the bioinformatics analysis and 90 upregulated and 67 downregulated miRNAs were identified in the cancer samples. VSCC, vulvar squamous cell carcinoma.
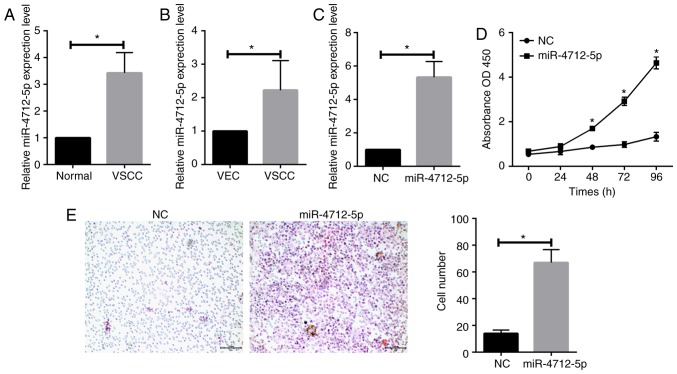
MicroRNA-4712-5p is upregulated in VSCC tissues and cells
RT-qPCR was conducted to determine the expression levels of miR-4712-5p in 56 pairs of VSCC and adjacent normal tissues. The results revealed that the expression level of miR-4712-5p was significantly upregulated in VSCC tissues (P<0.05; Fig. 2A). RT-qPCR was further used to detect the expression levels of miR-4712-5p in VSCC cells and VECs. The results demonstrated that the expression of miR-4712-5p in VSCC cells was significantly higher compared with that in VECs (P<0.05; Fig. 2B).
Figure 2.
MicroRNA-4712-5p is upregulated in VSCC tissues and cells and promotes the proliferation and invasion of VSCC. RT-qPCR was conducted to determine the expression levels of miR-4712-5p; CCK-8 assay was used to detect the effect of miR-4712-5p on the proliferation of VSCC cells; Transwell assay demonstrated the invasiveness of cell lines transfected with miR-4712-5p mimics. (A) Expression levels of miR-4712-5p in 56 pairs of VSCC tissue and adjacent normal tissue; (B) expression levels of miR-4712-5p in VSCC cells and VECs; (C) transfection efficiency of A431 cells transfected with miR-4712-5p mimics; (D) effect of miR-4712-5p on the proliferation of VSCC cells; (E) invasiveness of cell lines transfected with miR-4712-5p mimics (magnification ×200, scale bar=100 µm); *P<0.05. VSCC, vulvar squamous cell carcinoma; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; VECs, vaginal epithelial cells; NC, negative control.
MicroRNA-4712-5p promotes the proliferation and invasion of VSCC
To further study the role of miR-4712-5p in VSCC, A431 cells were separately transfected with miR-4712-5p mimics and NC, and the transfection efficacy was verified by RT-qPCR (Fig. 2C). The CCK-8 assay was used to detect the effect of miR-4712-5p on the proliferation of VSCC cells. On the fifth day, the proliferation rate of cells transfected with miR-4712-5p was significantly increased compared with day 3 of culture (P<0.05; Fig. 2D). The Transwell assay demonstrated that the invasiveness of cells transfected with miR-4712-5p mimics was significantly higher compared with that in the negative control group (P<0.05; Fig. 2E). These results suggested that miR-4712-5p can affect cell proliferation and invasion.
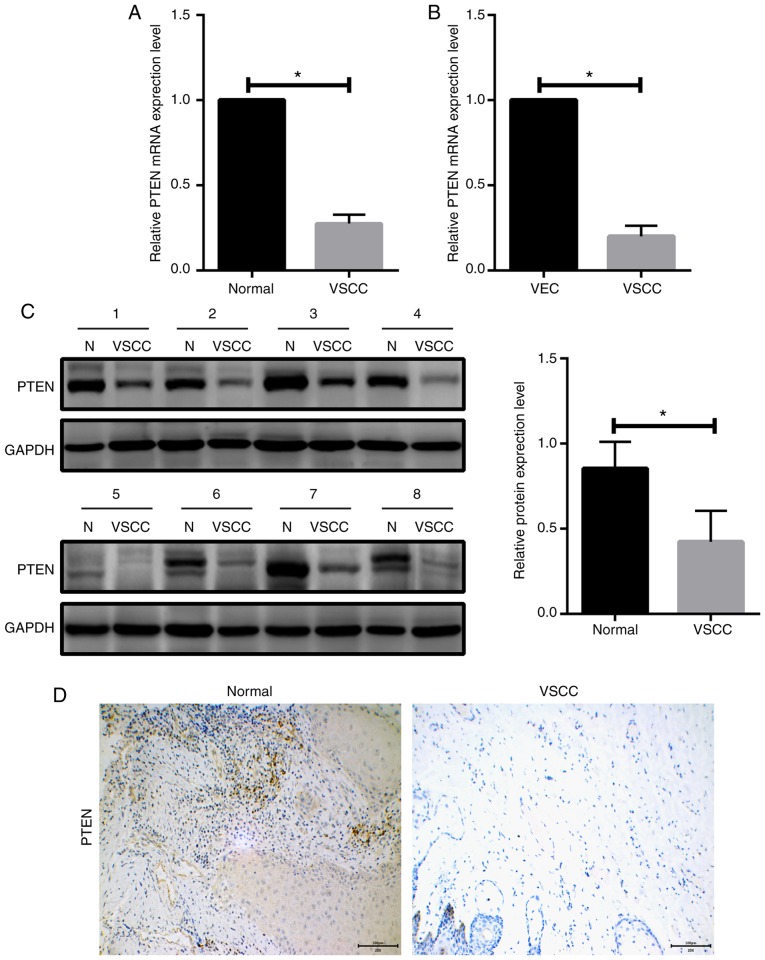
PTEN is downregulated in VSCC tissues and cells
To examine the association between miR-4712-5p and PTEN, we first analyzed the mRNA expression levels of PTEN in 56 paired human VSCC samples and adjacent normal tissues by RT-qPCR. The results demonstrated that the mRNA expression level of PTEN in VSCC tissues was significantly downregulated compared with that in normal tissues (P<0.05; Fig. 3A). PTEN mRNA expression levels in VSCC cells and VECs were determined by RT-qPCR. The results demonstrated that the expression of PTEN in VSCC cells was significantly lower compared with that in VECs (P<0.05; Fig. 3B). We next examined PTEN expression in VSCC tissues and adjacent normal tissues in 8 clinicopathological samples by western blot assay. The results revealed that the expression level of PTEN in VSCC tissues was significantly lower compared with that in adjacent normal tissues (P<0.05; Fig. 3C). Immunohistochemical examination yielded the same results (Fig. 3D).
Figure 3.
PTEN is downregulated in VSCC tissues and cells. RT-qPCR was conducted to determine the expression levels of miR-4712-5p. PTEN expression was detected by western blot assay and immunohistochemical analysis. (A) mRNA expression levels of PTEN in 56 paired human VSCC samples and adjacent normal tissues; (B) PTEN mRNA expression levels in VSCC cells and VECs; (C) PTEN expression in VSCC tissues and adjacent normal tissues of 8 clinicopathological tissue samples via western blot assay; (D) PTEN expression in VSCC tissues and adjacent normal tissues via immunohistochemistry (magnification ×200, scale bar=100 µm); *P<0.05. PTEN, phosphatase and tensin homologue; VSCC, vulvar squamous cell carcinoma; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; VECs, vaginal epithelial cells; N, normal.
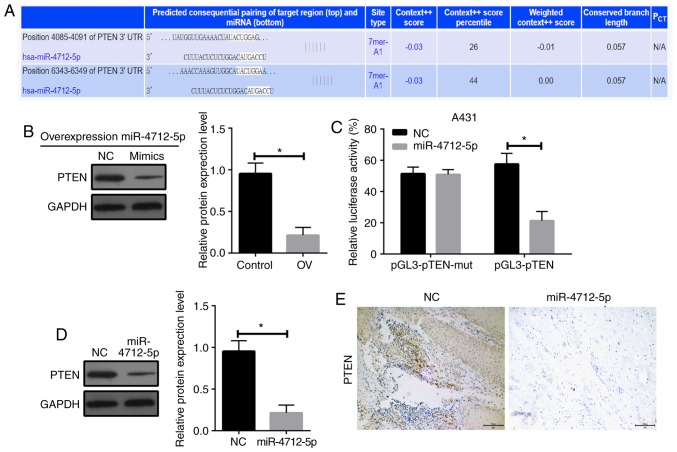
PTEN is a direct target of microRNA-4712-5p
To investigate the potential mechanism of miR-4712-5p affecting the proliferation of VSCC cells, we used the public databases microRNA (http://www.microrna.org/microrna/getdownloads.do), miRDB (http://mirdb.org) and TargetScan (http://www.targetscan.org/vert_71/) for bioinformatics analysis (Fig. 4A). The results revealed that PTEN has a conserved sequence that may bind to miR-4712-5p. It was hypothesized that miR-4712-5p promotes tumor growth by downregulating the expression of PTEN. The expression of PTEN protein following miR-4712-5p expression alterations was determined by western blot assay. The results demonstrated that the overexpression of miR-4712-5p significantly downregulated the expression of PTEN (P<0.05; Fig. 4B). The luciferase reporter gene assay revealed that the luciferase activity of A431 cells co-transfected with miR-4712-5p mimics and pGL3-PTEN vector was significantly lower compared with that of cells transfected with NC (P<0.05). The luciferase activity of the same cells co-transfected with miR-4712-5p mimics and pGL3-PTEN-mut vector did not differ significantly from that of cells transfected with NC (P>0.05; Fig. 4C). These results indicate that the PTEN gene may be one of the direct targets of miR-4712-5p.
Figure 4.
PTEN is a direct target of microRNA-4712-5p. The expression of PTEN protein was detected by western blot assay and immunohistochemical analysis. The luciferase reporter gene assay was used to detect the luciferase activity of co-transfected A431 cells of miR-4712-5p mimics and pGL3-PTEN vector. (A) Bioinformatics analysis; (B) expression of PTEN protein after miR-4712-5p expression was altered; (C) luciferase activity of co-transfected A431 cells of miR-4712-5p mimics and pGL3-PTEN vector; (D) expression level of PTEN protein in A431 cells transfected with miR-4712-5p via western blot assay; (E) expression level of PTEN protein in A431 cells transfected with miR-4712-5p via immunohistochemistry (magnification ×200, scale bar=100 µm); *P<0.05. PTEN, phosphatase and tensin homologue; NC, negative control; OV, overexpression vector.
The expression of PTEN in the A431 cells transfected with miR-4712-5p was compared with the blank control group. The A431 cells transfected with miR-4712-5p exhibited significantly decreased expression of PTEN (P<0.05) (Fig. 4D), as shown by western blot assay. This result suggests that there is a significant correlation between the high expression of miR-4712-5p and the low expression of the PTEN protein. The results of the immunohistochemical examination further proved these findings (Fig. 4E). These results indicate that PTEN may be a target of miRNA-4712-5p.
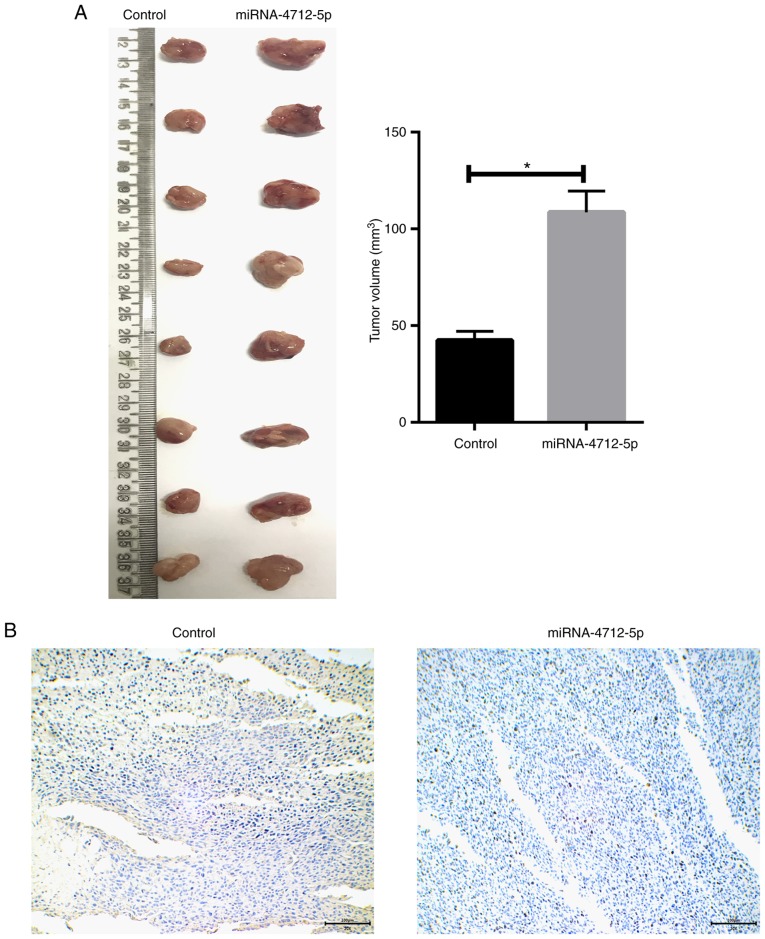
MicroRNA-4712-5p promotes tumor growth in mouse xenograft models
The role of miRNA-4712-5p expression in a nude mouse model was further investigated. The results demonstrated that the tumor volume of the transfected miRNA-4712-5p cells was significantly larger compared with that of the blank control group (P<0.05) (Fig. 5A). We prepared and observed the tissue sections of xenograft tumors. The immunohistochemical results demonstrated that transfection of miRNA-4712-5p significantly downregulated the expression of PTEN in xenograft tumors (Fig. 5B).
Figure 5.
MicroRNA-4712-5p promotes tumor growth of A431 cells in nude mice. (A) Tumor volume; (B) immunohistochemical results of tissue section preparation of transplanted tumors (magnification ×200, scale bar=100 µm); *P<0.05.
MicroRNA-4712-5p regulates cyclin D1 expression via PTEN
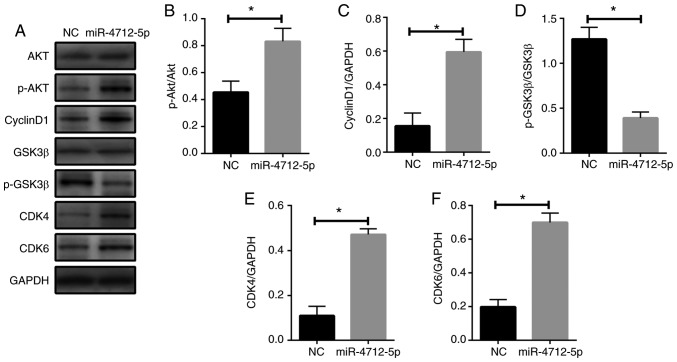
To further investigate the mechanism of miR-4712-5p affecting VSCC cell proliferation in vitro and in vivo by targeting PTEN, the expression of p-AKT, cyclin D1, p-GSK3β, CDK4 and CDK6 in VSCC cells was detected by western blot assay. The results demonstrated that the protein expression of p-AKT, cyclin D1, CDK4 and CDK6 in miRNA-4712-5p cells was significantly increased, and the expression of the p-GSK3β protein was significantly decreased (P<0.05) (Fig. 6A-F). These results indicate that miRNA-4712-5p affects the proliferation of VSCC cells and this effect may be mediated by the PTEN/AKT/p-GSK3β/cyclin D1 signaling pathway.
Figure 6.
MicroRNA-4712-5p regulates cyclin D1 via PTEN. The expression levels of (A and B) p-AKT, (A and C) cyclin D1, (A and D) p-GSK3β, (A and E) CDK4 and (A and F) CDK6 in VSCC cells were detected by western blot assay. *P<0.05. PTEN, phosphatase and tensin homologue; AKT, protein kinase B; GSK, glycogen synthase kinase; CDK, cyclin-dependent kinase; VSCC, vulvar squamous cell carcinoma; NC, negative control.
Discussion
Vulvar cancer belongs to the group of gynecological malignant tumors (18). Vulvar intraepithelial neoplasia is a type of atypical hyperplasia of the vulva (19). Studies on VSCC are relatively rare, even more so in China; however, its incidence has been increasing in recent years, and it is becoming a major gynecological concern.
miRNAs are small non-coding RNAs that regulate post-transcriptional gene expression by interfering with the translation of one or more target mRNAs (20). Despite their low molecular weight, they play a key role in regulating a number of important human biological functions at the transcriptional level, such as tumor cell proliferation, invasion, and metastasis (21–23). Deregulation of miRNAs is a major factor in almost all types of cancer (24). miRNAs are also important regulators of hematopoietic function, through controlling the gene expression of several transcription factors necessary for the formation, differentiation and apoptosis of hematopoietic stem cells, so specific miRNAs may represent a potential therapeutic target for acute lymphoblastic leukemia (25). In the present study, qPCR was used to detect differences in RNA levels between VSCC and adjacent normal tissues. The expression of miR-4712-5p was found to be obviously higher in cancer tissues compared with that in adjacent normal tissues. Histological differences suggest that the presence of relevant microRNAs may exert some effect on the biological properties of the tumor. To the best of our knowledge, the mechanism of action of miR-4712-5p in VSCC has not yet been reported. Therefore, based on the histological differences, we propose that miR-4712-5p may act as a carcinogenic factor and promote VSCC growth and invasion.
PTEN is a potent tumor suppressor and loss of its function is often observed in hereditary and sporadic cancers (26). PTEN has phosphatase-dependent and phosphatase-independent activities in cells and controls a variety of biological processes, including maintenance of genomic stability, cell survival, migration, proliferation and metabolism (27–31). Even a subtle reduction in PTEN levels and activity may increase cancer susceptibility and contribute to tumor progression (32). Therefore, PTEN has become a hotspot of cancer research. Liu et al have demonstrated that miR-19b plays a carcinogenic role in the progression of ovarian cancer and promotes metastasis and invasion of ovarian cancer cells by inhibiting the PTEN/AKT signaling pathway (33). Chen et al found that the expression of miR-1297 is low in human cervical cancer tissues, and the overexpression of miR-1297 may activate PTEN, increasing proliferation and decreasing apoptosis of HeLa cells (34). Cyclin D1 is an important member of the cyclin family (35). In normal proliferating cells, the level of cyclin D1 is extremely low, whereas its overexpression leads to uncontrolled cell proliferation, and eventually tumor formation; thus, it is often defined as an oncoprotein (36). When cyclin D1 is overexpressed, it often combines with CDK4 and CDK6 to form a complex under the stimulation of some external factors, resulting in cell cycle abnormalities and tumorigenesis (37). Cyclin D1 protein is highly expressed in several malignant tumors and participates in their occurrence and development (38,39). Worsley et al considered that the positive expression rate of cyclin D1 in ovarian borderline tumors and well-differentiated malignant tumors was >70%, and the positive expression in poorly differentiated tumors was ~15%, while negative expression was found in normal ovarian and benign tumor tissues (40). Cyclin D1 expression abnormalities are considered an early event in ovarian cancer and are associated with tumor proliferative activity. These findings indicate that, when cyclin D1 is overexpressed, it may enhance the proliferative ability of the tumor.
The present study demonstrated that miR-4712-5p targeted the PTEN protein, and A431 cells transfected with miR-4712-5p exhibited markedly reduced expression and inhibition of PTEN, with increased proliferative and invasive abilities of the tumor. The luciferase activity assay was used to confirm the direct correlation between miR-4712-5p and PTEN. As described above, the 3′-UTR of miR-4712-5p directly binds to the PTEN protein, suggesting that miR-4712-5p acts as a carcinogen in VSCC through the PTEN signaling pathway axis. The PTEN/AKT protein signaling pathway has been shown to affect the proliferative capacity of cancer cells through periodic pathways in other cancers. Our experiments also confirmed that miR-4712-5p targeted the PTEN protein, thereby affecting the proliferation of VSCC cells, and this action was dependent on the PTEN/Akt/p-GSK3β/cyclin D1 signaling pathway. Following transfection with miR-4712-5p, the expression of PTEN and p-GSK3β was markedly downregulated, while that of p-Akt and cyclin D1 was upregulated. These results validate our previous hypothesis that miR-4712-5p targets PTEN and promotes the proliferation of VSCC cells through a periodic pathway. The present study may help achieve a better understanding of the molecular mechanisms underlying the development and progression of VSCC and identify a new therapeutic target for vulvar cancer.
In conclusion, microRNA-4712-5p reduces the expression of PTEN, thereby affecting the downstream p-AKT, p-GSK3β and cyclin D1 signaling pathways and promoting the proliferation and invasion of VSCC.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science Foundation of China (30973190) and Liaoning science and technology program (2014021057).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
SY and XW conceived and designed the study and drafted the manuscript. SY, YZ, YL, LW, CL, ZF, HS and WZ performed the experiments and interpreted the results. SY, CL, ZF, HS and WZ analyzed the data. XW contributed to acquisition of funding support. All authors have read and approved the final version of this manuscript.
Ethics approval and consent to participate
The protocol of the present study was approved by the Ethics Committee of the First Affiliated Hospital of China Medical University and informed consent was obtained from the patients.
Patent consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Suh DH, Kim M, Kim HJ, Lee KH, Kim JW. Major clinical research advances in gynecologic cancer in 2015. J Gynecol Oncol. 2016;27:e53. doi: 10.1016/j.ygyno.2016.04.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clancy AA, Spaans JN, Weberpals JI. The forgotten woman's cancer: Vulvar squamous cell carcinoma (VSCC) and a targeted approach to therapy. Ann Oncol. 2016;27:1696–1705. doi: 10.1093/annonc/mdw242. [DOI] [PubMed] [Google Scholar]
- 3.Yap ML, Allo G, Cuartero J, Pintilie M, Kamel-Reid S, Murphy J, Mackay H, Clarke B, Fyles A, Milosevic M. Prognostic significance of human papilloma virus and p16 expression in patients with vulvar squamous cell carcinoma who received radiotherapy. Clin Oncol (R Coll Radiol) 2018;30:254–261. doi: 10.1016/j.clon.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: Aspects of the natural history and outcome in 405 women. Obstet Gynecol. 2005;106:1319–1326. doi: 10.1097/01.AOG.0000187301.76283.7f. [DOI] [PubMed] [Google Scholar]
- 5.Hantschmann P, Sterzer S, Jeschke U, Friese K. P53 expression in vulvar carcinoma, vulvar intraepithelial neoplasia, squamous cell hyperplasia and lichen sclerosus. Anticancer Res. 2005;25:1739–1745. [PubMed] [Google Scholar]
- 6.Jancárková N, Freitag P, Zivný J. Vulvar carcinoma-retrospective study of 47 cases (epidemiology, etiology and long-term results. Ceska Gynekol. 2002;67:78–82. (In Czech) [PubMed] [Google Scholar]
- 7.Zhao L, Zhang Y, Zhang Y. Long noncoding RNA CASC2 regulates hepatocellular carcinoma cell oncogenesis through miR-362-5p/Nf-κB axis. J Cell Physiol. 2018;233:6661–6670. doi: 10.1002/jcp.26446. [DOI] [PubMed] [Google Scholar]
- 8.Huang GH, Du L, Li N, Zhang Y, Xiang Y, Tang JH, Xia S, Zhang EE, Lv SQ. Methylation-mediated miR-155-FAM133A axis contributes to the attenuated invasion and migration of IDH mutant gliomas. Cancer Lett. 2018;432:93–102. doi: 10.1016/j.canlet.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Luo Y, Cao X, Chen J, Gu J, Zhao J, Sun J. MicroRNA-224 suppresses osteoblast differentiation by inhibiting SMAD4. J Cell Physiol. 2018;233:6929–6937. doi: 10.1002/jcp.26596. [DOI] [PubMed] [Google Scholar]
- 10.Lekka E, Hall J. Non-coding RNAs in disease. FEBS Lett. 2018;592:2884–2900. doi: 10.1002/1873-3468.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao L, Zhang CY, Guo L, Li X, Han NN, Zhou Q, Liu ZL. MicroRNA-497 accelerates apoptosis while inhibiting proliferation, migration, and invasion through negative regulation of the MAPK/ERK signaling pathway via RAF-1. J Cell Physiol. 2018;233:6578–6588. doi: 10.1002/jcp.26272. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H, Zhao H, Zhang Y, Zhou Y. MicroRNA199b promotes cell proliferation and invasion in Wilms' tumour by directly targeting runt-related transcription factor 3. Mol Med Rep. 2018;18:1812–1819. doi: 10.3892/mmr.2018.9096. [DOI] [PubMed] [Google Scholar]
- 13.Liang Y, Zhang P, Li S, Li H, Song S, Lu B. MicroRNA-873 acts as a tumor suppressor in esophageal cancer by inhibiting differentiated embryonic chondrocyte expressed gene 2. Biomed Pharmacother. 2018;105:582–589. doi: 10.1016/j.biopha.2018.05.152. [DOI] [PubMed] [Google Scholar]
- 14.Yang XH, Guo F. miR3147 serves as an oncomiR in vulvar squamous cell cancer via Smad4 suppression. Mol Med Rep. 2018;17:6397–6404. doi: 10.3892/mmr.2018.8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agostini A, Brunetti M, Davidson B, Trope CG, Heim S, Panagopoulos I, Micci F. Expressions of miR-30c and let-7a are inversely correlated with HMGA2 expression in squamous cell carcinoma of the vulva. Oncotarget. 2016;7:85058–85062. doi: 10.18632/oncotarget.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hetland TE, Holth A, Kærn J, Flørenes VA, Tropé CG, Davidson B. HMGA2 protein expression in ovarian serous carcinoma effusions, primary tumors, and solid metastases. Virchows Arch. 2012;460:505–513. doi: 10.1007/s00428-012-1228-9. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Wu X. miRNA expression profile of vulvar squamous cell carcinoma and identification of the oncogenic role of miR-590-5p. Oncol Rep. 2016;35:398–408. doi: 10.3892/or.2015.4344. [DOI] [PubMed] [Google Scholar]
- 18.Sznurkowski JJ. Vulvar cancer: Initial management and systematic review of literature on currently applied treatment approaches. Eur J Cancer Care (Engl) 2016;25:638–646. doi: 10.1111/ecc.12455. [DOI] [PubMed] [Google Scholar]
- 19.Preti M, Scurry J, Marchitelli CE, Micheletti L. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2014;28:1051–1062. doi: 10.1016/j.bpobgyn.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Paul P, Chakraborty A, Sarkar D, Langthasa M, Rahman M, Bari M, Singha RS, Malakar AK, Chakraborty S. Interplay between miRNAs and human diseases. J Cell Physiol. 2018;233:2007–2018. doi: 10.1002/jcp.25854. [DOI] [PubMed] [Google Scholar]
- 21.Zhang LL, Zhang LF, Guo XH, Zhang DZ, Yang F, Fan YY. Downregulation of miR-335-5p by long noncoding RNA ZEB1-AS1 in gastric cancer promotes tumor proliferation and invasion. DNA Cell Biol. 2018;37:46–52. doi: 10.1089/dna.2017.3926. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Zhou L, Shi M, Kuang Y, Fang L. Downregulation of miRNA-15a and miRNA-16 promote tumor proliferation in multiple myeloma by increasing CABIN1 expression. Oncol Lett. 2018;15:1287–1296. doi: 10.3892/ol.2017.7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu L, Wu H, Wan X, Liu L, He Y, Zhu L, Liu S, Yao H, Zhu Z. MicroRNA-585 suppresses tumor proliferation and migration in gastric cancer by directly targeting MAPK1. Biochem Biophys Res Commun. 2018;499:52–58. doi: 10.1016/j.bbrc.2018.03.116. [DOI] [PubMed] [Google Scholar]
- 24.Rupaimoole R, Calin GA, Lopez-Berestein G, Sood AK. miRNA deregulation in cancer cells and the tumor microenvironment. Cancer Discov. 2016;6:235–246. doi: 10.1158/2159-8290.CD-15-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ultimo S, Martelli AM, Zauli G, Vitale M, Calin GA, Neri LM. Roles and clinical implications of microRNAs in acute lymphoblastic leukemia. J Cell Physiol. 2018;233:5642–5654. doi: 10.1002/jcp.26290. [DOI] [PubMed] [Google Scholar]
- 26.Pulido R. PTEN inhibition in human disease therapy. Molecules. 2018;23:E285. doi: 10.3390/molecules23020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukherjee A, Karmakar P. Attenuation of PTEN perturbs genomic stability via activation of Akt and down-regulation of Rad51 in human embryonic kidney cells. Mol Carcinog. 2013;52:611–618. doi: 10.1002/mc.21903. [DOI] [PubMed] [Google Scholar]
- 28.Cheng D, Zhang L, Yang G, Zhao L, Peng F, Tian Y, Xiao X, Chung RT, Gong G. Hepatitis C virus NS5A drives a PTEN-PI3K/Akt feedback loop to support cell survival. Liver Int. 2015;35:1682–1691. doi: 10.1111/liv.12733. [DOI] [PubMed] [Google Scholar]
- 29.Tian K, Di R, Wang L. MicroRNA-23a enhances migration and invasion through PTEN in osteosarcoma. Cancer Gene Ther. 2015;22:351–359. doi: 10.1038/cgt.2015.27. [DOI] [PubMed] [Google Scholar]
- 30.Howitt J, Low LH, Putz U, Doan A, Lackovic J, Goh CP, Gunnersen J, Silke J, Tan SS. Ndfip1 represses cell proliferation by controlling Pten localization and signaling specificity. J Mol Cell Biol. 2015;7:119–131. doi: 10.1093/jmcb/mjv020. [DOI] [PubMed] [Google Scholar]
- 31.Worby CA, Dixon JE. PTEN. Annu Rev Biochem. 2014;83:641–669. doi: 10.1146/annurev-biochem-082411-113907. [DOI] [PubMed] [Google Scholar]
- 32.Bera A, Ghosh-Choudhury N, Dey N, Das F, Kasinath BS, Abboud HE, Choudhury GG. NFκB-mediated cyclin D1 expression by microRNA-21 influences renal cancer cell proliferation. Cell Signal. 2013;25:2575–2586. doi: 10.1016/j.cellsig.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu DT, Yao HR, Li YY, Song YY, Su MY. MicroRNA-19b promotes the migration and invasion of ovarian cancer cells by inhibiting the PTEN/AKT signaling pathway. Oncol Lett. 2018;16:559–565. doi: 10.3892/ol.2018.8695. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Chen Z, Zhang M, Qiao Y, Yang J, Yin Q. MicroRNA-1297 contributes to the progression of human cervical carcinoma through PTEN. Artif Cells Nanomed Biotechnol. 2018;46(Suppl 2):S1120–S1126. doi: 10.1080/21691401.2018.1479711. [DOI] [PubMed] [Google Scholar]
- 35.John RR, Malathi N, Ravindran C, Anandan S. Mini review: Multifaceted role played by cyclin D1 in tumor behavior. Indian J Dent Res. 2017;28:187–192. doi: 10.4103/ijdr.IJDR_697_16. [DOI] [PubMed] [Google Scholar]
- 36.Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med (Berl) 2016;94:1313–1326. doi: 10.1007/s00109-016-1475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozar K, Sicinski P. Cell cycle progression without cyclin D-CDK4 and cyclin D-CDK6 complexes. Cell Cycle. 2005;4:388–391. doi: 10.4161/cc.4.3.1551. [DOI] [PubMed] [Google Scholar]
- 38.Hong Y, Huang X, An L, Ye H, Ma K, Zhang F, Xu Q. Overexpression of COPS3 promotes clear cell renal cell carcinoma progression via regulation of Phospho-AKT(Thr308), Cyclin D1 and Caspase-3. Exp Cell Res. 2018;365:163–170. doi: 10.1016/j.yexcr.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 39.Sun W, Guo F, Liu M. Upregulated WDR5 promotes gastric cancer formation by induced cyclin D1 expression. J Cell Biochem. 2018;119:3304–3316. doi: 10.1002/jcb.26491. [DOI] [PubMed] [Google Scholar]
- 40.Worsley SD, Ponder BA, Davies BR. Overexpression of cyclin D1 in epithelial ovarian cancers. Gynecol Oncol. 1997;64:189–195. doi: 10.1006/gyno.1996.4569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.