Abstract
Case summary
A 5-year-old neutered male domestic shorthair cat presented with an 18-month history of facial tics, and progressive general ataxia, weakness, lethargy and anorexia of 2 weeks’ duration. MRI of the brain showed a well-defined heterogeneous hyperintense mass on T1-weighted and T2-weighted images, with central hypointensity in the rostral commissure and septum pellucidum, and perilesional hyperintensity in fluid-attenuated inversion recovery, suggestive of perilesional oedema. Gross examination in a transverse section of the brain at the level of the septum pellucidum revealed a 0.2 cm brown soft mass. Histopathological examination identified a biphasic neoplastic proliferation of mesenchymal and neuroepithelial cell populations. Fusiform cells were predominately distributed in bundles showing a high degree of anisocytosis and marked immune-positive reaction to vimentin immunochemistry, confirming a sarcomatous origin. Additionally, high numbers of astrocytic cells were identified by an intense immunopositive reaction to glial fibrillary acidic protein and negative reaction to oligodendrocyte transcription factor 2 immunochemistry. Vascular invasion of the neoplasia into the wall of a medium branch of the rostral cerebral artery was present (secondary Scherer structures). Based on these characteristics, the tumour was defined as a gliosarcoma. Gliosarcoma is a recognised astrocytoma grade IV anaplastic glial cell tumour with sarcomatous differentiation.
Relevance and novel information
To our knowledge, this is the first report describing a cerebral gliosarcoma in a cat including clinical, MRI, macroscopic and histopathological features and immunolabelling characteristics.
Keywords: Brain, neoplasia, glioma, histopathology, immunohistochemistry, Scherer structures, MRI
Introduction
The reported incidence of feline intracranial neoplasia is 3.5 in 100,000 cats, or as high as 2.2%.1 Glial cell tumours are reported infrequently, with an incidence of 7.5% of all intracranial tumours, being the fourth most common central nervous system (CNS) neoplasia in cats.2 Astrocytomas are considered the most frequent type of feline glial cell tumour.3
Gliosarcoma is classified by the World Health Organization as a subtype of glioblastoma grade IV astrocytoma.4 Gliosarcoma is an infrequently reported primary CNS neoplasm in people and has only been described in one dog in the veterinary literature.5 It has a bimorphic population characterised by diffuse anaplastic astrocytic neoplastic cells with sarcomatous differentiation.
This case report describes a cerebral gliosarcoma in a cat presenting with MRI, macroscopic and histopathological features, and immunolabelling characteristics.
Case description
A 5-year-old neutered male domestic shorthair cat presented with an 18-month history of facial twitches, generalised ataxia and abnormal behaviour characterised by reduced interaction with the family members. The cat deteriorated acutely 2 weeks prior to presentation, showing progressive generalised weakness, which was worse in the pelvic limbs, lethargy and weight loss. Water intake was restricted 5 days prior to presentation, as the cat would only drink from the tap but was unable to reach it.
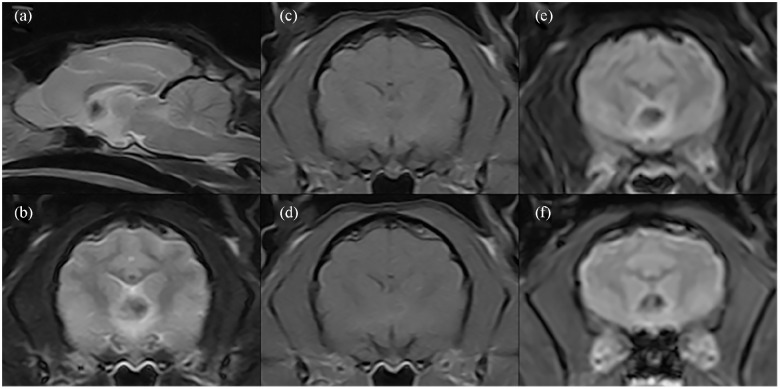
Clinical examination revealed 5% dehydration, mild obtundation, generalised weakness, cervical ventroflexion, intermittent head tremors and ambulatory tetraparesis with reduced postural reactions in all limbs, indicative primarily of forebrain dysfunction. A metabolic disorder was considered. A complete blood count and serum biochemistry showed mild erythrocytosis (50%; reference interval [RI] 24–45%), mild neutrophilic leukocytosis (13.1 K/μl; RI 2.5–12.5 K/μl), uraemia (114.9 mg/dl; RI 18–60 mg/dl), raised alkaline phosphatase (205 U/l; RI 0–80 U/l) and severe hypernatraemia (>189 mmol/l; RI 150–160 mmol/l). A feline leukaemia virus and feline immunodeficiency virus SNAP test was negative. Abdominal ultrasound and urinalysis were within normal limits and urine culture was negative. Intravenous fluid therapy was used to correct the dehydration. Empirical therapy with clindamycin (12.5 mg/kg IV q12h) was added to treat a potential viral infection (toxoplasmosis) alongside a gastric protector used preventively (omeprazole 1 mg/kg IV q24h). The following day, the cat’s mental and hydration status had normalised and the cervical ventroflexion resolved, yet the marked hindlimb ataxia, ambulatory paraparesis and head tremor persisted. A repeat blood analysis was within normal limits. The cat was anaesthetised and underwent MRI of the brain using a 0.25 T unit (Imagovet). MRI showed a fairly well-defined 0.5 cm heterogeneous hyperintense mass on T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI) in the rostral commissure and septum pellucidum with central hypointensity in T1WI, T2WI, fluid-attenuated inversion recovery and signal void in T2* gradient echo images. Marginal ring enhancement surrounding the mass lesion was observed following intravenous gadolinium (0.01 mmol/kg) administration. The imaging features were suggestive of neoplasia or a congenital vascular malformation (Figure 1).
Figure 1.
MRI of the brain. Mid-line well-defined heterogeneous mass at the level of the rostral commissure and septum pellucidum. The mass is hypointense in (a,b) T2-weighted imaging and (c) T1-weighted imaging (T1WI), with (d) marginal contrast enhancement after gadolinium administration. The mass showed perilesional hyperintensity in (e) fluid-attenuated inversion recovery and (f) marginal T1WI ring signal void in T2* gradient echo images
Methylprednisolone (2 mg/kg IV followed by PO treatment q24h) was added as palliative treatment for perilesional oedema resulting in partial improvement characterised by increased appetite and a brighter mental status. A couple of weeks later, further deterioration occurred, characterised by obtundation, generalised weakness and inappetence. Considering the poor prognosis, the owner requested euthanasia. Permission for macroscopic post-mortem examination and histological examination of the brain was given.
Gross findings of the brain showed a 0.2 cm diameter brownish mass with a soft-to-gelatinous consistency located at the level of the septum pellucidum. No macroscopic abnormalities were found on examination of the thoracic and abdominal cavities. Histological study of the brain confirmed the mass as a bimorphic neoplastic cellular proliferation located in the rostral cerebral hemispheres at the level of the septum pellucidum (Figure 2). Areas of haemorrhage were not identified, contrary to the possible haemorrhage suggested by findings on the MRI gradient echo sequence.
Figure 2.
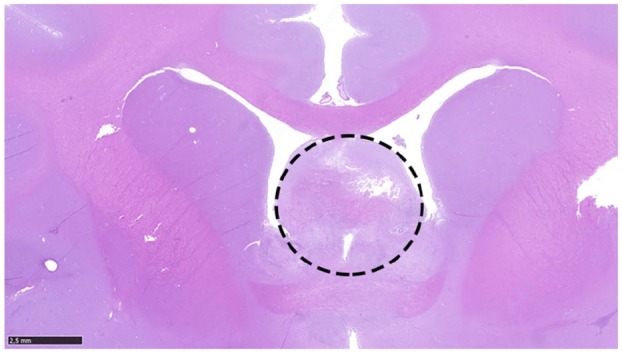
Neoplastic cellular proliferation, indicated by the dashed circle, affecting the rostral cerebral hemispheres at the level of the septum pellucidum. Haematoxylin and eosin
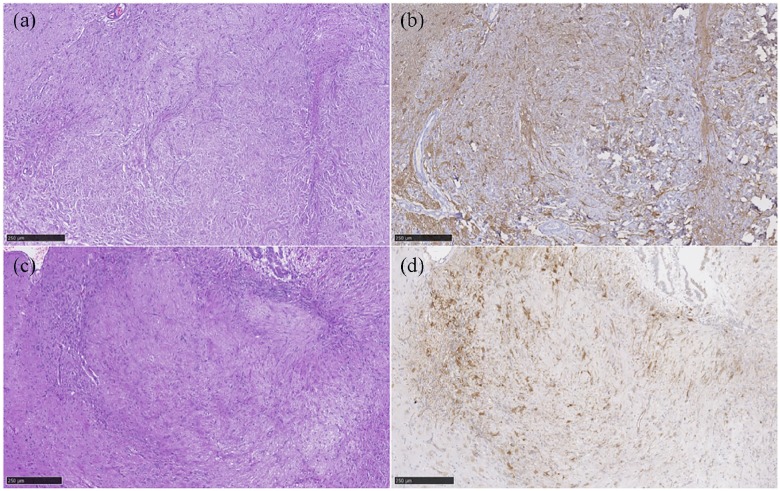
The bimorphic neoplastic population consisted of two cell types. One population was characterised by fusiform cells predominately distributed in bundles showing a high degree of anisocytosis and anisokaryosis with a low mitotic index. The identification of mitotic figures was facilitated by Ki-67 immunohistochemistry (IHC). This population showed a high immunopositive reaction to vimentin IHC and a weak reaction to smooth muscle actin (SMA) IHC alongside collagen fibres stained with Masson’s trichrome stain. These histopathological features are specific for a mesenchymal sarcomatous cell origin (Figure 3).
Figure 3.
Gliosarcoma. Mixed neoplastic population of two different and intermingled cell types. (a) A distinct neoplastic cell population characterised by astrocytic-like cells (haematoxylin and eosin). (b) Astrocytic cells reacting positively to glial fibrillary acidic protein immunohistochemistry (IHC). (c) The second population was characterised by fusiform cells distributed in bundles (haematoxylin and eosin). (d) Mesenchymal origin is defined by positive immune reaction to vimentin IHC
The second neoplastic population was defined as astrocytic in origin as it involved high numbers of astrocytic-like cells. This glial cell type was characterised by large round-to-polygonal cells with eosinophilic cytoplasm showing a moderate degree of anisocytosis and anisokaryosis with a moderate mitotic index. They showed a high immunopositive reaction to glial fibrillary acidic protein (GFAP) IHC not only in the periphery, but also widely inside the neoplastic population (see Figure 3). Oligodendrocyte transcription factor 2 was negative for the neoplastic population. Gemistocytes were commonly seen mixed within the astrocytic component.
Neoplastic proliferation was further observed in continuity from the mass invading the adventitia and middle tunica of a medium branch of the cerebral rostral artery. Immunolabelling characteristics showed a positive reaction to vimentin (Figure 4), and was negative to GFAP and SMA IHC.
Figure 4.
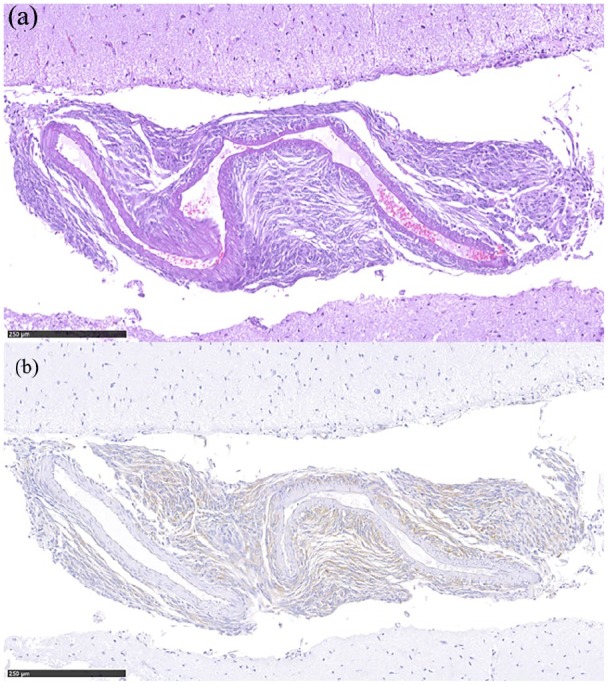
(a) Perivascular neoplastic infiltration of a medium-sized branch of the rostral cerebral artery (haematoxylin and eosin). (b) Sarcomatous origin of the neoplastic population is defined by the immune-positive reaction to vimentin immunohistochemistry
Based on these characteristic histopathological features, the neoplasm was characterised as cerebral gliosarcoma with perivascular involvement.
Discussion
Astrocytomas are reported infrequently in the feline literature.2,6–12 Four different subtypes – pilocytic, protoplasmic, gemistocytic and fibrillary astrocytomas – are described in feline intracranial astrocytoma.12 This case report identified gliosarcoma as an additional, previously unreported subtype of intracranial astrocytoma in a feline patient.
Gliosarcoma is a rare primary neoplasm of the CNS, classified as a subtype of glioblastoma defined as a grade IV astrocytoma.4 This tumour type is extremely rare, reported once in dogs and unreported in cats.5 In people, gliosarcoma constitutes 1–8% of all glioblastomas and <0.5% of all intracranial tumours. Primary gliosarcoma (ie, intracranial) is the most common presentation; however, it has been sporadically reported as a secondary neoplasia occurring after radiation of a previous glioblastoma.13
The pathogenesis of gliosarcoma is much debated. Originally, it was believed that the sarcomatous component was the result of a neoplastic transformation of the endothelial cells in the vessels traversing the glial tumour. However, this theory was discarded as no endothelial markers could be found.14,15 Other groups have suggested the possibility of a malignant transformation of intratumoural proliferating vessels or of glial cells surrounding a pre-existing sarcoma or simply an unfortunate coexistence given the originally low number of cases with this rare entity.16,17 The most recent theory describes a common monoclonal cell origin of glial and sarcomatous cells supported by the finding of identical genetic alterations in both tumour elements as two distinct malignant cell populations (glial and sarcomatous).13,18–20
Gliosarcoma not only shares genetic similarities with glioblastoma, but also clinical, therapeutic and prognostic features, making them almost indistinguishable from each other. Imaging features reveal variable appearances, including a homogeneous, well-circumscribed, strongly enhancing mass often abutting the dura, simulating the appearance of meningiomas, as well as a heterogeneous, ill-defined mass with either patchy or peripheral ring enhancement, as seen in this feline patient.21 Considering the different imaging descriptions, MRI cannot be used to reliably differentiate gliosarcoma from other glial tumours or non-neoplastic CNS pathology, and histopathological examination is essential for its definitive diagnosis.
Vascular malformation such as a hamartoma was also considered in this cat given the long clinical history and the signal void on gradient echo sequences. Diffusion-weighted imaging was not performed and the histopathological examination failed to confirm the presence of haemorrhage or hemosiderin within the neoplasm. The signal void seen may have been an acquisition artefact in this low-field MRI study. Additional acquisition planes and, ideally, a high-field MRI study would have been helpful for further identification but were not performed.
In people, the biological behaviour of gliosarcoma is slightly different to glioblastoma, which may be seen as a different clinicopathological entity. Gliosarcomas have a predisposition for supratentorial cerebral lobes (especially temporal lobe) and a tendency for extraneural metastasis.21 Metastatic disease secondary to a gliosarcoma is reported in 11% of the cases via haematogenous spread to bone, lymphatic system, lungs, kidney, liver and, less frequently, spinal cord, and thus extended imaging is commonly recommended.22 While we cannot completely exclude metastatic disease in our cat, given that the MRI and histopathological examination was limited to the brain, clinical examination and investigations did not suggest the involvement of other organs.
Histopathological features of gliosarcomas involve a mixture of two morphologically distinct neoplastic components, a glial and a sarcomatous component.23 The glial element is more frequently astrocytic in nature, although neoplastic oligodendrocytes have also been described.12,24 The second component is sarcomatous and must resemble a spindle cell sarcoma, yet it can also be a fibrosarcoma, an angiosarcoma, a soft tissue sarcoma or a rhabdomyosarcoma.25 Both components were present in this cat’s diagnosis as the mass was a gliosarcoma with a subjectively stronger sarcomatous component. Cartilaginous, bony or mesenchymal components can also be seen in a gliosarcoma, but these were not present in this case.
IHC identified these two distinct neoplastic patterns in the cat. The sarcomatous component was naturally arranged in bundles and immunostained for vimentin, which is specific for mesenchymal tumours. The second neoplastic component consisted of astrocytic-like cells, which immunostained for GFAP, the principal marker for astrocytes. Another significant characteristic of glial cell neoplasia present in this cat was the presence of perivascular infiltration of sarcomatous cell component in continuity with the neoplastic mass, which is a histopathological feature included in the structures of Scherer. The Scherer structures originally reflect the highly malignant behaviour of glial cell tumours denoted by its upgraded migratory and invasive capability. Scherer described four distinct microanatomical features of malignant cell gliomas – perivascular involvement, perineuronal and perivascular satellitosis, and subpial space spread of the neoplastic cells – all of which were seen in our cat.26 This perivascular pattern could indicate a possible vascular origin of this tumour.
The survival time associated with gliosarcoma is unknown in animals and poor in people. The mean reported survival time of 11 patients treated surgically with adjunctive radiotherapy or chemotherapy was 6 months.13 However, if imaging and intraoperative features showed a well-demarcated, enhancing, intraoperatively firm mass, a longer survival of 8 months was achieved.13 Similarly, histopathological findings characterised by a more than 50% sarcomatous component showed a better survival in patients.13,27 Surprisingly, longer survival times have been described in two people with intraventricular gliosarcoma after surgical excision combined with radiotherapy and chemotherapy.28 The cat in this report had a long clinical history prior to diagnosis. The histopathological examination showed a subjective predominance of a fibrous/bundle pattern compatible with a larger proportion of sarcomatous population over the glial neoplastic population. As in people, this feature may have been associated with a longer survival time in this cat. Moreover, the tumour location (septum pellucidum) may have allowed better accommodation by adjacent cerebral tissue.
Conclusions
Gliosarcoma is a previously unreported subtype of astrocytoma in cats and should be considered in the differential diagnosis of intracranial tumours. IHC is required to confirm the mixed glial and sarcomatous neoplastic populations. Although the longer-term prognosis is poor, the clinical course may be long and >1 year. Further studies are essential to gain a better understanding of this rare tumour.
Acknowledgments
We gratefully acknowledge Ester Blasco and Tamara Rivero for their technical assistance.
Footnotes
Accepted: 9 September 2019
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work involved the use of non-experimental animals only (owned or unowned), and followed established internationally recognized high standards (‘best practice’) of individual veterinary clinical patient care. Ethical approval from a committee was not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work for the procedure(s) undertaken. No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
References
- 1. Vandevelde M. Brain tumours in domestic animals: an overview. Proceedings: Brain tumors in man and animals; 1984 MONTH DAY–DAY; 1984 Sept 5-6; Research Triangle Park, NC, USA. National Institute of Environmental Sciences, 1984. [Google Scholar]
- 2. Troxel MT, Vite CH, Van Winkle TJ, et al. Feline intracranial neoplasia: retrospective review of 160 cases (1985– 2001). J Vet Intern Med 2003; 17: 850–859. [DOI] [PubMed] [Google Scholar]
- 3. Rissi DR, Miller AD. Feline glioma: a retrospective study and review of the literature. J Feline Med Surg 2017; 19: 1307–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016; 131: 803–820. [DOI] [PubMed] [Google Scholar]
- 5. Fadda A, Vajtai I, Lang J, et al. Cerebral high-grade oligodendroglioma with sarcomatous transdifferentiation (“oligosarcoma”) in a Boxer dog. J Vet Intern Med 2014; 28: 1881–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Komarnisky MD. Astrocytoma in a cat. Can Vet J 1985; 26: 237–240. [PMC free article] [PubMed] [Google Scholar]
- 7. Sarfaty D, Carrillo JM, Patnaik AK. Cerebral astrocytoma in four cats: clinical and pathologic findings. J Am Vet Med Assoc 1987; 191: 976–978. [PubMed] [Google Scholar]
- 8. Sato T, Nakamura A, Shibuya H, et al. Cerebral high-grade astrocytoma (glioblastoma) in a cat. J Vet Med A Physiol Pathol Clin Med 2003; 50: 269–271. [DOI] [PubMed] [Google Scholar]
- 9. Duniho S, Schulman FY, Morrison A, et al. A subependymal giant cell astrocytoma in a cat. Vet Pathol 2000; 37: 275–278. [DOI] [PubMed] [Google Scholar]
- 10. Sant’Ana FJ, Serakides R, Graca DL. Pilocytic astrocytoma in a cat. Vet Pathol 2002; 39: 759–761. [DOI] [PubMed] [Google Scholar]
- 11. Tomek A, Cizinauskas S, Doherr M, et al. Intracranial neoplasia in 61 cats: localisation, tumour types and seizure patterns. J Feline Med Surg 2002; 8: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Demierre S, Bley T, Botteron C, et al. Intracranial astrocytomas in eight cats: clinical and pathological findings [article in German]. Schweiz Arch Tierheilkd 2002; 144: 66–73. [DOI] [PubMed] [Google Scholar]
- 13. Singh G, Das KK, Sharma P, et al. Cerebral gliosarcoma: analysis of 16 patients and review of literature. Asian J Neurosurg 2015; 10: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feigen IH, Gross SW. Sarcoma arising in glioblastoma of the brain. Am J Pathol 1955; 31: 633–653. [PMC free article] [PubMed] [Google Scholar]
- 15. Haddad SF, Moore SA, Schelper RL, et al. Smooth muscle can comprise the sarcomatous component of gliosarcomas. J Neuropathol Exp Neurol 1992; 51: 493–498. [DOI] [PubMed] [Google Scholar]
- 16. Lalitha VS, Rubinstein LJ. Reactive glioma in intracranial sarcoma: a form of mixed sarcoma and glioma (‘sarcoglioma’): report of eight cases. Cancer 1979; 43: 246–257. [DOI] [PubMed] [Google Scholar]
- 17. Schittenhelm J, Erdmann T, Maennlin S, et al. Gliosarcoma with chondroid and osseous differentiation. Neuropathology 2007; 27: 90–94. [DOI] [PubMed] [Google Scholar]
- 18. Romero-Rojas AE, Diaz-Perez JA, Ariza-Serrano LM, et al. Primary gliosarcoma of the brain: radiologic and histopathologic features. Neuroradiol J 2013; 26: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Biernat W, Aguzzi A, Sure U, et al. Identical mutations of the p53 tumor suppressor gene in the gliomatous and the sarcomatous components of gliosarcomas suggest a common origin from glial cells. J Neuropathol Exp Neurol 1995; 54: 651–656. [DOI] [PubMed] [Google Scholar]
- 20. Reis RM, Konu-Lebleblicioglu D, Lopes JM, et al. Genetic profile of gliosarcomas. Am J Pathol 2000; 156: 425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huo Z, Yang D, Shen J, et al. Primary gliosarcoma with long-survival: report of two cases and review of literature. Int J Clin Exp Pathol 2014; 7: 6323–6332. [PMC free article] [PubMed] [Google Scholar]
- 22. Demirci S, Akalin T, Islekel S, et al. Multiple spinal metastases of cranial gliosarcoma: a case report and review of the literature. J Neurooncol 2008; 88: 199–204. [DOI] [PubMed] [Google Scholar]
- 23. Meis JM, Martz Kl, Nelson JS. Mixed glioblastoma multiforme and sarcoma. A clinicopathologic study of 26 radiation therapy oncology group cases. Cancer 1991; 67: 2342–2349. [DOI] [PubMed] [Google Scholar]
- 24. Rodriguez FJ, Scheithauer BW, Jenkins R, et al. Gliosarcoma arising in oligodendroglial tumors (“oligosarcoma”): a clinicopathologic study. Am J Surg Pathol 2007; 31: 351–362. [DOI] [PubMed] [Google Scholar]
- 25. Shintaku M, Miyaji K, Adachi Y. Gliosarcoma with angiosarcomatous features: a case report. Brain Tumor Pathol 1998; 15: 101–105. [DOI] [PubMed] [Google Scholar]
- 26. Scherer HJ. The forms of growth in gliomas and their practical significance. Brain 1940; 63: 1–35. [Google Scholar]
- 27. Salvati M, Caroli E, Raco A, et al. Gliosarcomas: analysis of 11 cases do two types exist? J Neurooncol 2005; 74: 59–63. [DOI] [PubMed] [Google Scholar]
- 28. Huo Z, Yang D, Shen J, et al. Primary gliosarcoma with long-survival: report of two cases and review of literature. Int J Clin Exp Pathol 2014; 7: 6323–6332. [PMC free article] [PubMed] [Google Scholar]