Abstract
The synthesized Fe3O4@l-arginine showed strong catalytic performance in the one-pot synthesis of spiropyranopyrazoles via the reactions of hydrazines, β-keto esters, isatins, and malononitrile or ethyl cyanoacetate under solvent-free conditions. The biologically active heterocyclic compounds including spiropyranopyrazole derivatives were efficiently synthesized in short reaction times and excellent yields in the presence of Fe3O4/l-arginine at room temperature. The highlighted features of the Fe3O4@l-arginine nanocomposite are highly stable, easy to separate, low loading, cost-effective with easy preparation and reusability of the catalyst. The heterogeneous nanocomposite was fully characterized by SEM, EDX, FT-IR, XRD and TEM analysis.
Keywords: Multi-component reactions, Fe3O4@l-arginine, Nanocomposite, Spiropyranopyrazoles, Amino acid, Catalyst
Introduction
One-pot multi-component reactions (MCRs), is an interesting synthetic strategy for the synthesis of small-molecule libraries with various degrees of structural variety because various organic moieties are joined in one step for carbon–carbon and carbon-heteroatom bond formation [1, 2]. They offer considerable advantages over ordinary linear step synthesis by decreasing time, saving money, energy, and crude materials. Therefore, they result in both economical and environmental benefits. At the same time, variety can be gained from building up libraries by differing each component [3, 4]. In recent years, there has been massive development in three- and four-component reactions, and great efforts continue to be made to expand new MCRs [5]. Spirocyclic compounds are considered as significant building canton for the easy availability of a diversity of cyclic products by a sequential reaction due to their steric strain attendant with the quaternary carbon [6]. Expansion of new procedures for producing spirocyclic compounds is an interesting and challenging function in organic synthesis [7]. One of the significant multi-component reactions is the synthesis of spiropyranopyrazole derivatives which exhibit high medicinal attributes and biological activities. Spiropyranopyrazoles area class of nitrogen heterocyclic compounds with considerable and well- offered biological activities that consist of antimicrobial [8], anti-inflammatory [9], anticancer [10] and molluscicidal activities [11]. Spiropyranopyrazoles are important heterocyclic compounds due to their variety and pharmaceutical biological activities [12]. Concerning the arithmetic of the significance of oxindole parts in organic compounds, as well as the intrinsic complexity of isatins as heterocyclic substrates, it is not amazing that many diverse and elegant MCRs have been introduced for the synthesis of various heterocyclic and spiroheterocyclic products by using isatins as a core component [13]. Therefore, different synthetic approaches for the synthesis of spirooxindole-fused heterocycles have been reported and reviewed [14]. Previous studies have described procedures to synthesize of pyrano[2,3-c]pyrazoles using several catalysts such as cerium ammonium nitrate [15], l-proline [16], piperidine [17] and cobalt NPs [18].
Nevertheless, there are only two reported methods in the literature for the synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives via four-component reaction of hydrazines, ethyl acetoacetate, isatins, and malononitrile or ethyl cyanoacetate which have been done in the presence of piperidine [19], and Et3N [20].
Magnetic organic–inorganic nanocomposites have recently been the subject of intense research as magnetic catalysts in both industrial and academic settings. These magnetic nanoparticle catalysts can be used for investigating the reusing and seclusion problems that occur in several homogenous and heterogeneous catalytic reactions. Supported magnetic metal nanoparticles as new class of nanocatalysts have received much attention in diverse fields. The main feature of these particles is their high surface area that leads to their higher catalytic activity in comparison with traditional heterogeneous acid catalysts [21–27].
One of the outstanding procedures for preventing particle aggregation is coating nanoparticles with various targeting factors, taking into account their biocompatibility. Among the chemicals that can be used for achieving this target, amino acids are appropriate because of their crucial role in the body [28]. Amino acids react with the nanoparticles’ surface via the carboxyl groups and side chains [29]. Amino-functionalized materials demonstrate excellent ability to remove an extensive range of heavy metal ions from aqueous solutions because of the potent affinity between the nitrogen atom and metal cations [30]. Among different nanocomposite, Fe3O4/amino acid has received great attention in different fields because of their unique attributes and potential functions [31]. Some crucial characteristics of these catalysts include high catalytic activity, facile separation through an external magnet with no need for filtration, eco-friendliness, and non-toxicity. Recently, functionalized magnetic nanoparticles have been utilized as a useful catalytic system in numerous chemical processes such as synthesis of α-amino nitriles [32], bis(indolyl)methane derivatives [33], indazolo[2,1-b]phthalazine-triones and pyrazolo[1,2-b]phthalazine-diones [34], 3,4-dihydropyrimidin-2(1H)-ones [35], 1,8-dioxo-octa hydro xanthene derivatives [36], 2-amino-4H-chromen-4-yl phosphonates [37], 1,4-dihydropyridines [38] and pyrrole synthesis [39].
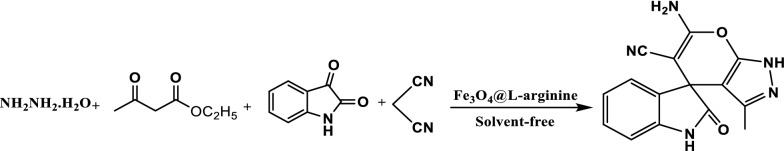
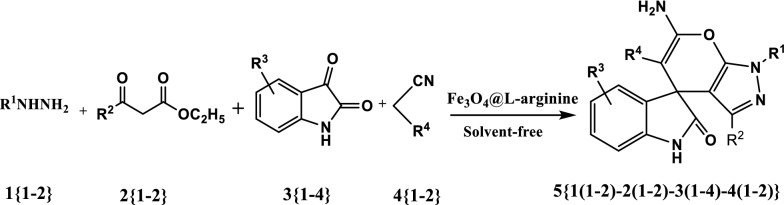
In continuation of our study in the synthesis of heterocyclic compounds using heterogeneous nanostructures [40–44], herein we describe a highly efficient and straightforward method for the synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazoles] via multi-component reaction of hydrazines, β-keto esters, isatin and malononitrile or ethyl cyanoacetate using Fe3O4@l-arginine as a green, economic, available and environmentally benign nanocatalyst under solvent-free conditions (Fig. 1).
Fig. 1.
Synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazoles] using Fe3O4@l-arginine nanocomposite under solvent-free conditions
Results
Catalyst characterization
In the preliminary experiments Fe3O4@l-arginine nanoparticles were prepared and characterized by SEM, EDX, FT-IR and XRD spectroscopy tenchniques.
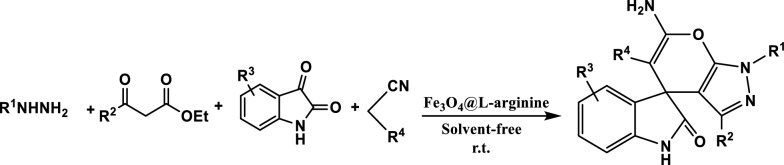
The earned lattice parameter of the nanoparticle Fe3O4@l-arginine using XRD technique coincided to the standard parameters of magnetite. The pattern of the Fe3O4@l-arginine nanocomposite is depicted in Fig. 2. It could be seen that the strong diffraction peaks at 2θ of 30.1°, 35.4°, 43.2°, 53.7°, 56.9° and 62.9° belong to the peaks of (220), (311), (400), (422), (511) and (440) of the Fe3O4, which is similar to the bare Fe3O4 nanoparticles [40, 45].
Fig. 2.
The X-ray diffraction pattern of the Fe3O4@l-arginine nanocomposite
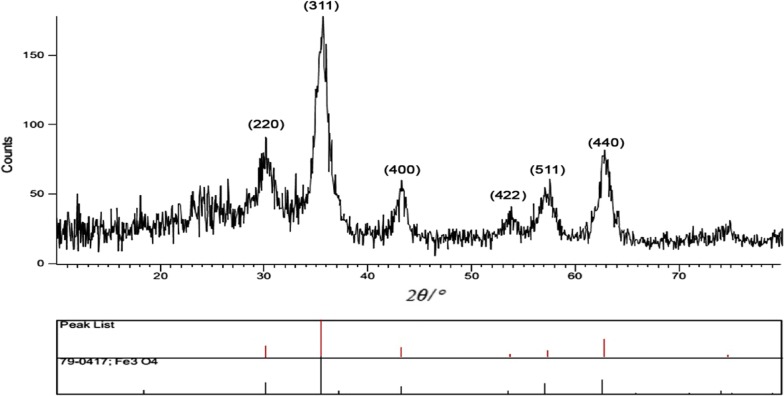
The chemical purity of the sample, as well as their stoichiometry, was tested by EDX study. Figure 3 shows that the elemental compositions of Fe3O4@l-arginine are Fe, O, C, H, and N.
Fig. 3.
EDX spectrum of the Fe3O4@l-arginine nanocomposite
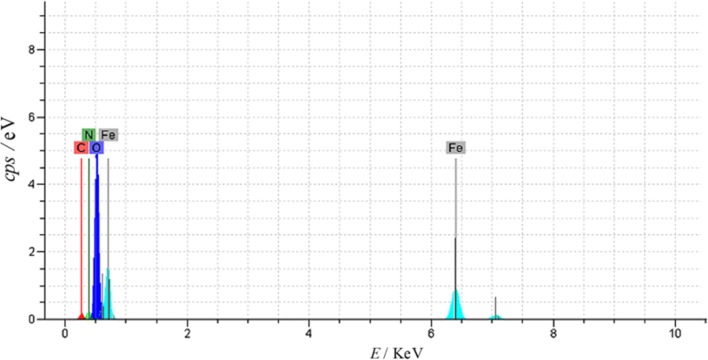
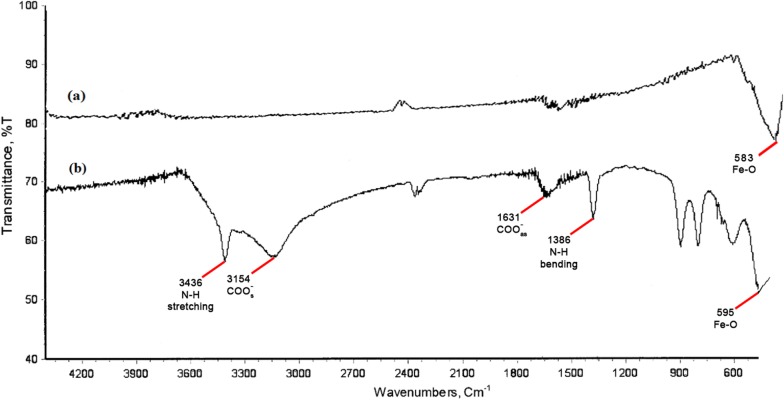
The FT-IR spectra of the bare Fe3O4 and Fe3O4@l-arginine nanocomposite are presented in Fig. 4. Bare magnetite nanoparticles are easily distinguished by strong absorption peaks at 583 cm−1 because of the stretching vibration of the Fe–O band (Fig. 4a). Figure 4b shows the FT-IR spectrum of Fe3O4@l-arginine nanocomposite. The existence of Fe3O4 NPs is determined by the strong adsorption band at 595 cm−1 related to the Fe–O vibrations. In the case of Fe3O4@l-arginine, the additional adsorption peaks at 1386, 1631 and 3154, 3436 cm−1 are due to bending vibration of N–H, asymmetric and symmetric stretching vibrations of COO−, and stretching vibrations of N–H, respectively, which indicate the presence of bonded arginine on the surface of magnetite nanoparticles. Furthermore, the connections and interactions between COO− groups and metal atoms are completely according to pervious literature [45–48].
Fig. 4.
The comparative FT-IR spectra of the Fe3O4 (a) and Fe3O4@l-arginine (b)
In order to investigate the morphology and particle size of nanoparticles, SEM image of the mesoporous is illustrated in Fig. 5. The SEM image of the magnetite nanoparticles modified with arginine indicate spherical shape with an average diameter about 10–15 nm.
Fig. 5.

SEM image of the Fe3O4 @l-arginine nanocomposite
The morphology and particle size of Fe3O4@l-arginine were investigated using transmission electron microscopy (TEM) (Fig. 6). The TEM image of this nanocomposite shows that the average particle size of Fe3O4@l-arginine is around 10–20 nm which was confirmed by the SEM image.
Fig. 6.

TEM image of Fe3O4@l-arginine
Discussions
Initially, to obtain the best reaction conditions, we selected reaction of hydrazine, ethyl acetoacetate, isatin and malononitrile as model reaction. Different catalysts, solvents and temperatures were examined in the four-component preparation of spiro[indoline-3,4′- pyrano[2,3-c]pyrazole] (Fig. 7).
Fig. 7.
The model reaction for the synthesis of spiro[indoline-3,4′- pyrano[2,3-c]pyrazole]
Firstly, the model study was performed in various solvents including EtOH, DMF, H2O, CH3CN and PhCH3 under reflux conditions and also under solvent-free conditions using Fe3O4@l-arginine nanocomposite. The summarized results of Table 1 show that the best results were obtained under solvent-free conditions. To further improve the yield and decrease the reaction time, we used the different reaction temperatures under solvent-free conditions. Further increase in temperature from room temperature to 80 °C in the model study did not have any remarkable influence on the reaction time and production yield (Table 1).
Table 1.
The effect of solvents on the model reaction in the presence of Fe3O4@l-arginine
| Entry | Solvent | Time (min) | Yield (%)a |
|---|---|---|---|
| 1 | EtOH (reflux) | 70 | 60 |
| 2 | DMF (reflux) | 120 | 50 |
| 3 | H2O (reflux) | 130 | 35 |
| 4 | CH3CN (reflux) | 120 | 45 |
| 5 | Toluene (reflux) | 240 | 25 |
| 6 | Solvent-free (r.t.) | 60 | 96 |
| 7 | Solvent-free (40 °C) | 60 | 95 |
| 8 | Solvent-free (80 °C) | 60 | 96 |
Reaction conditions: hydrazine monohydrate, isatin, ethyl acetoacetate, malononitrile (molar ratio: 1:1: 1.2:1) using (0.01 g) of Fe3O4@l-arginine
aIsolated yields
Afterward, the model was performed using several catalysts including ZnO, CuI, MgO, Na2CO3, Et3N, piperidine, Fe3O4, CaO, SiO2, and Fe3O4@l-arginine under solvent-free conditions. As can be seen from Table 2, no product was afforded in the absence of a catalyst (Table 2, entry 1). Also, it is noticed that Fe3O4@l-arginine has a significant effect in the yield of the corresponding product and reaction time (Table 2, entry 11). Next, various amounts of the Fe3O4@l-arginine were used in the model reaction. As shown in Table 2, the best experimental operation conditions included 8 mol % of the Fe3O4@l-arginine. With increasing the amount of nanocatalyst, no considerable change was observed in the product yield and reaction time. In comparison, a decrease in the catalyst amount cause to decrease the product yield. Hence, 8 mol % Fe3O4@l-arginine was selected as the optimum amount in the model reaction (Table 2).
Table 2.
The model study catalyzed in the presence of various catalysts
| Entry | Catalyst | Catalyst loading (mol %) | Time (min) | Yield(%)a |
|---|---|---|---|---|
| 1 | None | – | 120 | 0 |
| 2 | ZnO | 15 | 120 | 35 |
| 3 | CuI | 15 | 120 | 42 |
| 4 | MgO | 15 | 100 | 65 |
| 5 | Na2CO3 | 15 | 80 | 72 |
| 6 | Et3N | 15 | 60 | 70 |
| 7 | Piperidine | 15 | 80 | 55 |
| 8 | Fe3O4 | 15 | 120 | 40 |
| 9 | CaO | 15 | 80 | 65 |
| 10 | SiO2 | 15 | 120 | 35 |
| 11 | Fe3O4@l-arginine | 15 | 60 | 96 |
| 12 | Fe3O4@l-arginine | 10 | 60 | 96 |
| 13 | Fe3O4@l-arginine | 8 | 60 | 96 |
| 14 | Fe3O4@l-arginine | 5 | 90 | 70 |
Reaction conditions: hydrazine monohydrate (1 mmol), isatin (1 mmol), ethyl acetoacetate (1 mmol) and malononitrile (1 mmol) under solvent-free at room temperature
aIsolated yields
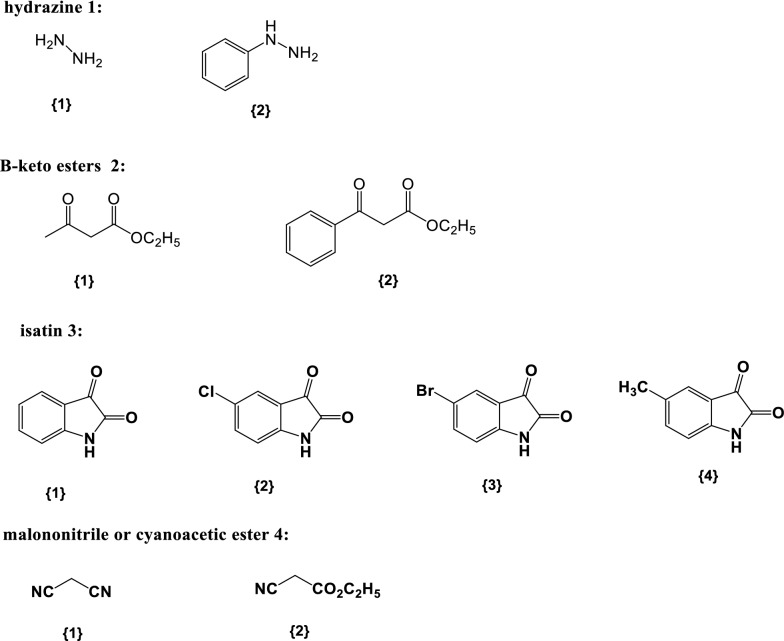
The optimized reaction conditions were tested for library constructions with two hydrazines 1{1–2}, β-keto esters 2{1–2}, four isatins 3{1–4}, and two acetonitrile derivatives 4{1–2} (Figs. 8 and 9).
Fig. 8.
Synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives
Fig. 9.
Diversity of the reagents
The corresponding spiro-[indoline-3,4′-pyrano [2,3-c]pyrazole] derivatives 5 were obtained in good yields at room temperature under solvent-free conditions (Table 3). The protocol was effective with isatins containing either electron-withdrawing (halides) or electron-donating (alkyl) groups.
Table 3.
Synthesis of spiro[indoline-3,4′- pyrano[2,3-c]pyrazole] derivatives using Fe3O4@l-arginine under solvent-free conditions
| Entry | Product | Yield (%)a | M.p. °C | Lit. M.p. °C |
|---|---|---|---|---|
| 1 | 5{1,1,1,1} | 96 | 286–288 | 285–286 [18] |
| 2 | 5{1,1,2,1} | 91 | 297–298 | 297–298 [18] |
| 3 | 5{1,1,3,1} | 95 | 283–285 | 282–283 [18] |
| 4 | 5{2,1,1,1} | 90 | 225–227 | 227–229 [18] |
| 5 | 5{1,2,3,1} | 91 | 257–259 | 256–257 [18] |
| 6 | 5{1,2,1,1} | 90 | 282–284 | 280–281 [18] |
| 7 | 5{1,2,1,2} | 87 | 240–242 | 242–243 [18] |
| 8 | 5{1,2,3,2} | 89 | 256–258 | 257–259 [18] |
| 9 | 5{1,2,2,2} | 85 | 266–268 | 265–267 [18] |
| 10 | 5{1,1,4,1} | 94 | 278–280 | 279–281 [18] |
| 11 | 5{1,2,4,1} | 86 | 246–248 | 247–249 [18] |
| 12 | 5{1,2,4,2} | 87 | 262–264 | 260–263 [18] |
| 13 | 5{2,1,4,1} | 89 | 220–222 | 222–224 [18] |
| 14 | 5{2,1,3,2} | 92 | 212–214 | –b |
| 15 | 5{2,1,2,2} | 90 | 279–281 | –b |
| 16 | 5{2,1,4,2} | 97 | 198–200 | –b |
aIsolated yield
bNew Products
The model study was run several times using recycled magnetic nanocomposite to consider the recoverability level and lifetime of the Fe3O4@l-arginine nanocomposite. The results showed that the recovered magnetic nanocomposite can be utilized for five successive runs with a negligible decrease in its activity (Table 4).
Table 4.
The catalyst reusability for the synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]
| Cycle | First | Second | Third | Fourth | Fifth |
|---|---|---|---|---|---|
| Yield (%)a | 96 | 94 | 93 | 89 | 88 |
aYields refer to the isolated pure product
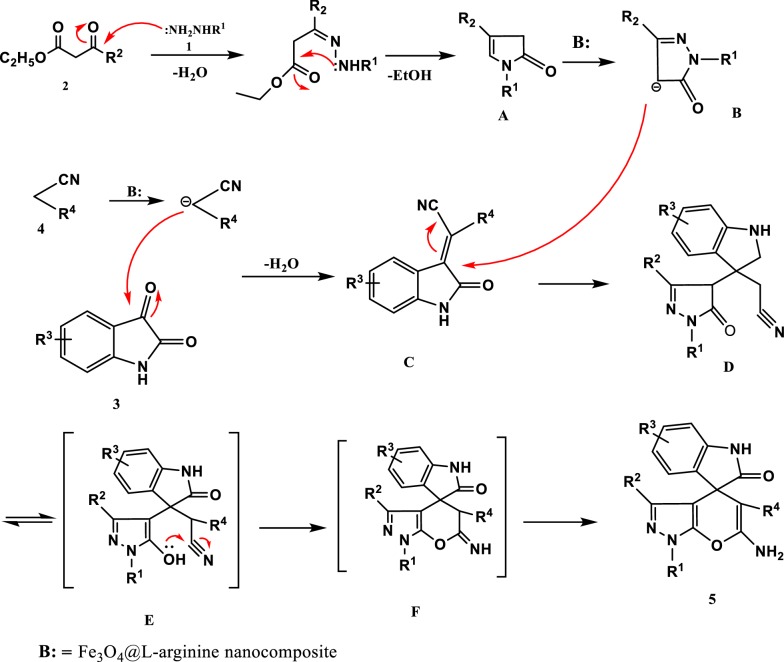
A possible mechanism for the synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazoles] using Fe3O4@l-arginine MNPs is presented in Fig. 10. This mechanism is based on the results of our experiment and some literature [19]. According to this mechanism, a condensation of hydrazine 1 with β-keto esters 2 is offered to give the intermediate A. Next, A Knoevenagel condensation of isatin 3 with malononitrile or ethyl cyanoacetate 4 is presented to provide the intermediate C. Next, the Michael addition of the intermediate A to C catalyzed by Fe3O4@l-arginine which provided the intermediate D. Then, the intermediate F was prepared via the intramolecular cyclization of intermediate D. Eventually, the intermediate F is tautomerized to product 5 (Fig. 10).
Fig. 10.
The proposed mechanism for the synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazoles] catalyzed by Fe3O4@l-arginine nanocomposite
Conclusions
In conclusion, we have demonstrated that Fe3O4@l-arginine nanocomposite is an effective catalyst for the MCRs of hydrazine, β-keto esters, isatins, and malononitrile or ethyl cyanoacetate under solvent-free conditions at room temperature. The heterocyclic compounds including spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives were obtained in high yields. The catalyst can be recovered and reused at least up to five runs for the synthesis of corresponding product. The one-pot nature and the use of heterogeneous solid Brønsted basic catalyst as an eco-friendly structure make it an interesting alternative to multi-step approaches.
Experimental section
Chemicals and apparatus
Chemicals were purchased from the Sigma-Aldrich and Merck in high purity. All of the materials were of commercial reagent grade and were used without further purification. The synthesis and characterization of the studied compounds were previously reported [49]. Melting points of products were determined by Electro thermal 9200. 1H NMR and 13C NMR spectra were obtained on Bruker 400 MHz spectrometer with DMSO-d6 as solvent using TMS as an internal standard. FT-IR spectrum was recorded on Magna-IR, spectrometer 550. The elemental analyses (C, H, N) were obtained from a Carlo ERBA Model EA 1108 analyzer. Powder X-ray diffraction (XRD) was carried out on a Philips diffractometer of X’pert Company with mono chromatized Cu Kα radiation (λ = 1.5406 Å). Microscopic morphology of products was visualized by SEM (LEO 1455VP). The compositional analysis was done by energy dispersive analysis of X-ray (EDX, Kevex, Delta Class I). Transmission electron microscopy (TEM) was performed with a Jeol JEM-2100UHR, operated at 200 kV.
Preparation of Fe3O4@l-arginine nanocomposite
Fe3O4@l-arginine was prepared according to previous report in the literature with some modifications [50]. In a typical experiment, FeCl3·6H2O (13 g, 0.048 mol), FeCl2·4H2O (4.8 g, 0.024 mol) and arginine (16.7 g, 0.096 mol) were dissolved in 100 mL deionized water. Then, the solution pH was adjusted to 11 with NaOH solution (2 M) to form a black suspension. Next, the reaction mixture was reflux under Ar atmosphere for 12 h. Finally, the prepared nanocomposite was separated from the reaction media by an external magnet and washed several times with deionized water and dried in an oven overnight to yield Fe3O4@l-arginine (Fig. 11).
Fig. 11.
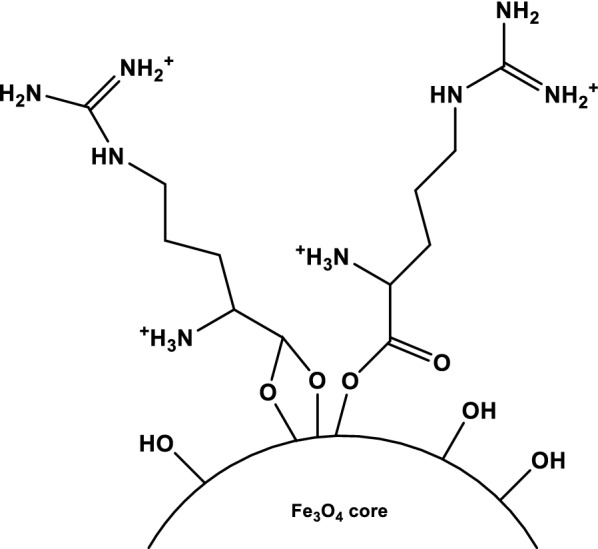
Possible structure for l-arginine coating on Fe3O4 nanoparticles, the inset is showing the molecular structure of Fe3O4
General procedure for the synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]
Fe3O4@l-arginine (8 mol %) was added to a solution of hydrazine (1 mmol), β-keto esters (1 mmol), isatin derivatives (1 mmol), and malononitrile/ethyl cyanoacetate (0.06 g, 1 mmol). The reaction mixture was stirred under solvent-free conditions at room temperature for the appropriate times. After completion of the reaction [as determined by thin layer chromatography (TLC)], the reaction mixture was dissolved in dichloromethane and the catalyst was separated magnetically. The solvent was evaporated and the residue was recrystallized from ethanol to afford the pure product.
All of the products were characterized and identified with m.p., 1H NMR, 13C NMR and FT-IR spectroscopy techniques. Spectral data of the new products are given below.
Spectral data of new compounds
Ethyl-6′-amino-5-bromo-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (5{2,1,3,2})
White solid, m.p. 212–214 °C. IR (KBr) (νmax/cm−1): 3396 (NH2), 3143 (NH), 1714 (CO); 1H NMR (400 MHz, DMSO-d6) δ: 0.92 (t, 3H, J = 7.6, CH3CH2OCO), 1.23 (s, 3H, CH3), 3.70 (q, 2H, J = 7.8, CH3CH2OCO), 6.93 (d, 1H, J = 7.6, ArH(isatin)), 7.34 (d, 1H, J = 7.4, ArH (isatin)), 7.51–7.58 (m, 3H, ArH(PhNHNH2)), 7.61 (s, 2H, NH2), 7.74 (s, 1H, ArH(isatin)), 7.78 (d, 2H, J = 7.8, ArH (PhNHNH2)), 10.90 (s, 1H, NH); 13C NMR (100 MHz, DMSO-d6) δ: 11.2, 14.8, 26.8, 57.2, 91.2, 113.1, 119.9, 124.5, 126.6, 129.1, 129.7, 131.2, 131.9, 135.2, 139.6, 148.7, 152.1, 161.8, 162.9, 171.2, 177.5; Anal. Calcd for C23H19BrN4O4 (Mr = 495.33) (%): C 55.77, H 3.87, N 11.31. Found (%): C 55.87, H 3.79, N 11.36.
Ethyl-6′-amino-5-chloro-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (5{2,1,2,2})
White solid, m.p. 279–281 °C. IR (KBr) (νmax/cm−1): 3377 (NH2), 3185(NH), 1712 (CO); 1H NMR (400 MHz, DMSO-d6) δ: 1.09 (t, 3H, J = 7.8, CH3CH2OCO), 1.24 (s, 3H, CH3), 3.72 (q, 2H, J = 7.6, CH3CH2OCO), 6.82 (d, 1H, J = 7.4, ArH(isatin)), 7.21 (d, J = 7.6, ArH(isatin)), 7.48–7.53 (m, 3H, J = 7.7 ArH(PhNHNH2)), 7.68 (s, 2H, NH2), 7.72 (s, 1H, ArH(isatin)), 7.81 (d, 2H, J = 7.8 ArH (PhNHNH2)), 10.93 (s, 1H, NH); 13C NMR (100 MHz, DMSO-d6) δ: 10.8, 15.1, 23.9, 55.2, 93.2, 113.1, 120.1, 123.5, 125.1, 128.7, 129.9, 132.1, 133.1, 135.1, 140.2, 147.1, 151.4, 162.4, 162.9, 174.1, 178.1; Anal. Calcd for C23H19ClN4O4 (Mr = 450.88) (%): C 61.27, H 4.25, N 12.43. Found (%): C 61.20, H 4.33, N 12.39.
Ethyl-6′-amino-3′,5-dimethyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (5{2,1,4,2})
White solid, m.p 198–200 °C. IR (KBr) (νmax/cm−1): 3370 (NH2), 3181 (NH), 1709 (CO); 1H NMR (400 MHz, DMSO-d6) δ: 1.13 (t, 3H, J = 7.6, CH3CH2OCO), 1.22 (s, 3H, CH3), 2.25 (s, 3H, CH3), 3.78 (q, 2H, J = 7.7, CH3CH2OCO), 7.08 (d, 1H, 2H, J = 7.8, ArH (isatin)), 7.16 (d, 1H, 2H, J = 7.6, ArH (isatin)), 7.34–7.41 (m, 3H, m, p, ArH (PhNHNH2)), 7.45 (s, 1H, ArH (isatin)), 7.66 (s, 2H, NH2), 7.68 (d, 2H, 2H, J = 7.6, ArH (PhNHNH2)), 10.78 (s, 1H, NH); 13C NMR (100 MHz, DMSO-d6) δ: 11.6, 14.8, 21.4, 25.7, 56.1, 92.7, 115.4, 117.9, 121.2, 123.4, 125.8, 126.9, 128.6, 129.1, 129.9, 137.1, 147.1, 148.3, 159.4, 162.6, 173.2, 176.8; Anal. Calcd for C24H22N4O4 (Mr = 430.46) (%): C 66.97, H 5.15, N 13.02. Found (%): C 66.91, H 5.19, N 12.97.
Acknowledgements
The authors would like to thank Islamic Azad University, Qom Branch, Qom, I. R. Iran.
Abbreviations
- MCR
multi-component reactions
- EtOH
ethanol
- SEM
scanning electron microscope
- TEM
transmission electron microscopy
- FT-IR
Fourier transform infrared spectroscopy
- XRD
powder X-ray diffraction
- EDX
energy dispersive analysis of X-ray
- NMR
Nuclear Magnetic Resonance
- TLC
thin layer chromatography
Authors’ contributions
MAG, BME and MHAB designed and performed the research. BME and MHAB did the sample collection. MAG, BME and MHAB analyzed the data and interpreted the results. MAG and BME wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was financially supported by the Islamic Azad University, Qom Branch, Iran, based on Grant Number [2014-13929].
Availability of data and materials
The datasets generated and analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu J, Bienayme H. Multicomponent reactions. Weinheim: Wiley; 2005. [Google Scholar]
- 2.Zhang Mo FuQY, Ge Gao, He HY, Zhang Y, Wu YS, Zhang ZH. Catalyst-free, visible-light promoted one-pot synthesis of spirooxindole-pyran derivatives in aqueous ethyl lactate. ACS Sustain Chem Eng. 2017;5:6175–6182. doi: 10.1021/acssuschemeng.7b01102. [DOI] [Google Scholar]
- 3.Domling A, Ugi I. Multicomponent reactions with isocyanides. Angew Chem Int Ed. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 4.Alam MM, Mubarak AT, Assiri MA, Ahmed SM, Fouda AM. A facile and efficient synthesis of 1,8-dioxodecahydroacridines derivatives catalyzed by cobalt-alanine metal complex under aqueous ethanol media. Bioorg Med Chem. 2019;13:40–50. doi: 10.1186/s13065-019-0545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomha SM, Edrees MM, Faty RAM, Muhammad ZA, Mabkhot YN. Microwave-assisted one pot three-component synthesis of some novel pyrazole scaffolds as potent anticancer agents. Chem Cent J. 2017;11:37–48. doi: 10.1186/s13065-017-0266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rousseau G, Robert F, Schenk K, Landais Y. Rearrangement of spirocyclic oxindoles with lithium amide bases. Org Lett. 2008;10:4441–4444. doi: 10.1021/ol8016885. [DOI] [PubMed] [Google Scholar]
- 7.Huang J, Frontier A. Development of a nazarov cyclization/wagner-meerwein rearrangement sequence for the stereoselective synthesis of spirocycles. J Am Ceram Soc. 2007;129:8060–8061. doi: 10.1021/ja0716148. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Rahman AH, Keshk E, Hanna MA, El-Bady SM. Synthesis and evaluation of some new spiro indoline-based heterocycles as potentially active antimicrobial agents. Med Chem. 2004;12:2483–2488. doi: 10.1016/j.bmc.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 9.Zaki MEA, Soliman HA, Hiekal OA, Rashad AE. Pyrazolopyranopyrimidines as a class of anti-inflammatory agents. Naturforsch C Bio Sci. 2006;61c:1–5. doi: 10.1515/znc-2006-1-201. [DOI] [PubMed] [Google Scholar]
- 10.Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Natl Acad Sci USA. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdelrazek FM, Metz P, Kataeva O, Jaeger A, El-Mahrouky SF. Synthesis and molluscicidal activity of new chromene and pyrano[2,3-c]pyrazole derivatives. Arch Pharm. 2007;340:543–548. doi: 10.1002/ardp.200700157. [DOI] [PubMed] [Google Scholar]
- 12.Wu H, Zhang LL, Tian ZQ, Huang YD, Wang YM. Highly efficient enantioselective construction of bispirooxindolescontaining three stereocenters through an organocatalytic cascademichael-cyclization reaction. Chem Eur J. 2013;19:1747–1753. doi: 10.1002/chem.201203221. [DOI] [PubMed] [Google Scholar]
- 13.Isambert N, Lavilla R. Heterocycles as key substrates in multicomponent reactions: the fast lane towards molecular complexity. Chem Eur J. 2008;14:8444–8454. doi: 10.1002/chem.200800473. [DOI] [PubMed] [Google Scholar]
- 14.Borad MA, Bhoi MN, Prajapati NP, Patel HD. Review of synthesis of multi spiro heterocyclic compounds from isatin. Synth Commun. 2014;44:1043–1057. doi: 10.1080/00397911.2013.858361. [DOI] [Google Scholar]
- 15.Dandia A, Saini D, Bhaskaran S, Saini DK. Ultrasound promoted green synthesis of spiro [pyrano [2, 3-c] pyrazoles] as antioxidant agents. Med Chem Res. 2014;23:725–734. doi: 10.1007/s00044-013-0671-8. [DOI] [Google Scholar]
- 16.Mecadon H, Rohman MR, Kharbangar I, Laloo BM, Kharkongor L, Rajbangshi IM, Myrboh B. l-Proline as an efficicent catalyst for the multi-component synthesis of 6-amino-4-alkyl/aryl-3-methyl-2,4-dihydropyrano [2, 3-c] pyrazole-5-carbonitriles in water. Tetrahedron Lett. 2011;52:3228–3231. doi: 10.1016/j.tetlet.2011.04.048. [DOI] [Google Scholar]
- 17.Liu X, Xu X, Wang X, Yang W, Qian Q, Zhang M. Facile and convenient way to functionalized trifluoromethylated spirocyclic [indole-3,4-pyrano[2,3-c]pyrazole] derivatives. Tetrahedron Lett. 2013;54:4451–4455. doi: 10.1016/j.tetlet.2013.06.038. [DOI] [Google Scholar]
- 18.Sabaghi Mianai R, Ghasemzadeh MA, Zand Monfared MR. Green fabrication of cobalt NPs using aqueous extract of antioxidant rich zingiber and their catalytic Applications for the synthesis of pyrano[2,3-c]pyrazoles. Comb Chem High Throughput Screen. 2019;22:18–26. doi: 10.2174/1386207322666190307160354. [DOI] [PubMed] [Google Scholar]
- 19.Zou Y, Hu Y, Liu H, Shi D. Rapid and efficient ultrasound-assisted method for the combinatorial synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives. ACS Comb Sci. 2011;14:38–43. doi: 10.1021/co200128k. [DOI] [PubMed] [Google Scholar]
- 20.Litvinov YM, Shestopalov AA, Rodinovskaya LA, Shestopalov AM. New convenient four-component synthesis of 6-amino-2,4-dihydropyrano[2,3-c] pyrazol-5-carbonitriles and one-pot synthesis of 6′-aminospiro[(3H)-indol-3, 4′pyrano[2,3-c]pyrazol]-(1H)-2-on-5′-carbonitriles. J Comb Chem. 2009;11:914–919. doi: 10.1021/cc900076j. [DOI] [PubMed] [Google Scholar]
- 21.Naeimi H, Babaei Z. MnO2 nanoparticles as efficient oxidant for ultrasound-assisted synthesis of 2-substituted benzimidazoles under mild conditions. Polycycl Aromat Compd. 2016;36:490–505. doi: 10.1080/10406638.2015.1014970. [DOI] [Google Scholar]
- 22.Mo Z, Liu YH, Shang ZR, Hu HC, Zhang ZH. Supported molybdenum on graphene oxide/Fe3O4: an efficient, magnetically separable catalyst for one-pot construction of spiro-oxindole dihydropyridines in deep eutectic solvent under microwave irradiation. Catal Commun. 2017;88:39–44. doi: 10.1016/j.catcom.2016.09.028. [DOI] [Google Scholar]
- 23.Chen MN, Mo LP, Cui ZS, Zhang ZH. Magnetic nanocatalysts: synthesis and application in multicomponent reactions. Curr Opin Green Sustain Chem. 2019;15:27–37. doi: 10.1016/j.cogsc.2018.08.009. [DOI] [Google Scholar]
- 24.Mo Z, Liu P, Liu YH, Shang ZR, Hu HC, Zhang ZH. Magnetically separable graphene oxide anchored sulfonic acid: a novel, highly efficient and recyclable catalyst for one-pot synthesis of 3,6-di(pyridin-3-yl)-1H-pyrazolo[3,4-b]pyridine-5-carbonitriles in deep eutectic solvent under microwave irradiation. RSC Adv. 2016;6:106160–106170. doi: 10.1039/C6RA19579B. [DOI] [Google Scholar]
- 25.Zhao XN, Hu HC, Zhang FJ, Zhang ZH. Magnetic CoFe2O4 nanoparticle immobilized N-propyl diethylenetriamine sulfamic acid as an efficient and recyclable catalyst for the synthesis of amides via the Ritter reaction. Appl Catal A Gen. 2014;482:258–265. doi: 10.1016/j.apcata.2014.06.006. [DOI] [Google Scholar]
- 26.Zhang HY, Hao XP, Mo LP, Liu Sh, Zhang WB, Zhang ZH. A magnetic metal–organic framework as a highly active heterogeneous catalyst for one-pot synthesis of 2-substituted alkyl and aryl (indolyl) kojic acid derivatives. New J Chem. 2017;41:7108–7115. doi: 10.1039/C7NJ01592E. [DOI] [Google Scholar]
- 27.Lu AH, Salabas EL, Schuth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed. 2007;46:1222–1244. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- 28.Culita DC, Marinescu G, Patron L, Carp O, Cizmas CB, Diamandescu L. Preparation and characterization of a silica-based magnetic nanocomposite and its application as a recoverable catalyst for the one-pot multicomponent synthesis of quinazolinone derivatives. Mater Chem Phys. 2008;15:381–385. doi: 10.1016/j.matchemphys.2008.04.033. [DOI] [Google Scholar]
- 29.Maleki A, Rabbani M, Shahrokh S. Preparation and characterization of a silica-based magnetic nanocomposite and its application as a recoverable catalyst for the one-pot multicomponent synthesis of quinazolinone derivatives. Appl Organometal Chem. 2015;29:809–814. doi: 10.1002/aoc.3373. [DOI] [Google Scholar]
- 30.Ramadevi P, Singh R, Jana SS, Devkar R, Chakraborty D. Mixed ligand ruthenium arene complexes containing N-ferrocenyl amino acids: biomolecular interactions and cytotoxicity against MCF7 cell line. J Organomet Chem. 2017;833:80–87. doi: 10.1016/j.jorganchem.2017.01.020. [DOI] [Google Scholar]
- 31.Ghorbani-Choghamarani A, Azadi G. Fe3O4@SiO2@l-arginine@Pd(0): a new magnetically retrievable heterogeneous nanocatalyst with high efficiency for C–C bond formation. Appl Organometal Chem. 2016;30:247–252. doi: 10.1002/aoc.3424. [DOI] [Google Scholar]
- 32.Kassaee MZ, Masrouri H, Movahedi F. Sulfamic acid-functionalized magnetic Fe3O4 nanoparticles as an efficient and reusable catalyst for one-pot synthesis of α-amino nitriles in water. Appl Catal A Gen. 2011;395:28–33. doi: 10.1016/j.apcata.2011.01.018. [DOI] [Google Scholar]
- 33.Ghafuri H, Ghorbani B, Esmaili Zand HR. Synthesis of Bis(indolyl)methane derivatives catalyzed by recyclable nano Fe3O4@ZrO2/SO42. Lett Org Chem. 2018;15:295–301. doi: 10.2174/1570178614666170707151649. [DOI] [Google Scholar]
- 34.Kiasat AR, Davarpanah J. Fe3O4@silica sulfuric acid nanoparticles: an efficient reusable nanomagnetic catalyst as potent solid acid for one-pot solvent-free synthesis of indazolo[2,1-b]phthalazine-triones and pyrazolo[1,2-b]phthalazine-diones. J Mol Catal A Chem. 2013;373:46–54. doi: 10.1016/j.molcata.2013.03.003. [DOI] [Google Scholar]
- 35.Zamani F, Izadi E. Synthesis and characterization of sulfonated-phenylacetic acid coated Fe3O4 nanoparticles as a novel acid magnetic catalyst for Biginelli reaction. Catal Commun. 2013;42:104–108. doi: 10.1016/j.catcom.2013.08.006. [DOI] [Google Scholar]
- 36.Naeimi H, Nazifi ZS. A highly efficient nano-Fe3O4 encapsulated-silica particles bearing sulfonic acid groups as a solid acid catalyst for synthesis of 1,8-dioxo-octahydroxanthene derivatives. J Nanopart Res. 2013;15:2026–2037. doi: 10.1007/s11051-013-2026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohammadi R, Kassaee MZ. Sulfochitosan encapsulated nano-Fe3O4 as an efficient and reusable magnetic catalyst for green synthesis of 2-amino-4H-chromen-4-yl phosphonates. J Mol Catal A Chem. 2013;380:152–158. doi: 10.1016/j.molcata.2013.09.027. [DOI] [Google Scholar]
- 38.Gawande MB, Bonifácio VDB, Varma RS, Nogueira ID, Bundaleski N, Amjad C, Ghumman A, Teodoro MND, Branco PS. Magnetically recyclable magnetite-ceria (Nanocat-Fe-Ce) nanocatalyst- applications in multicomponent reactions under benign conditions. Green Chem. 2013;15:1226–1231. doi: 10.1039/c3gc40375k. [DOI] [Google Scholar]
- 39.Li B, Zhang M, Hu H, Du X, Zhang Z. Nano-CoFe2O4 supported molybdenum as an efficient and magnetically recoverable catalyst for a one-pot, four-component synthesis of functionalized pyrroles. New J Chem. 2014;38:2435–2442. doi: 10.1039/c3nj01368e. [DOI] [Google Scholar]
- 40.Ghasemzadeh MA, Safaei-Ghomi J, Molaei H. Fe3O4 nanoparticles: as an efficient, green and magnetically reusable catalyst for the one-pot synthesis of 1, 8-dioxo-decahydroacridine derivatives under solvent-free conditions. C R Chimie. 2012;15:969–974. doi: 10.1016/j.crci.2012.08.010. [DOI] [Google Scholar]
- 41.Ghasemzadeh MA, Abdollahi-Basir MH, Mirhosseini-Eshkevari B. Multi-component Synthesis of Spiro [diindeno[1,2-b:2′,1′-e]pyridine-11,3′-indoline]-triones using Zinc Terephthalate Metal-Organic Frameworks. Green Chem Lett Rev. 2018;11:47–53. doi: 10.1080/17518253.2018.1434565. [DOI] [Google Scholar]
- 42.Ghasemzadeh MA, Mirhosseini-Eshkevari B, Abdollahi-Basir MH. MIL-53(Fe) Metal-Organic Frameworks (MOFs) as an Efficient and Reusable Catalyst for the One-Pot Four-Component Synthesis of Pyrano[2,3-c]-pyrazoles. Appl Organomet Chem. 2018;33:e4679. doi: 10.1002/aoc.4679. [DOI] [Google Scholar]
- 43.Ghasemzadeh MA, Mirhosseini-Eshkevari B, Abdollahi-Basr MH. Rapid and efficient one-pot synthesis of 3,4-dihydroquinoxalin-2-amine derivatives catalyzed by Co3O4@SiO2 core-shell nanoparticles under ultrasound irradiation. Comb Chem High Throughput Screen. 2016;19:592–601. doi: 10.2174/1386207319666160524141831. [DOI] [PubMed] [Google Scholar]
- 44.Safaei-Ghomi J, Ghasemzadeh MA. Silver iodide nanoparticle as an efficient and reusable catalyst for the one-pot synthesis of benzofurans under aqueous conditions. J Chem Sci. 2013;125:1003–1008. doi: 10.1007/s12039-013-0451-5. [DOI] [Google Scholar]
- 45.Azizi K, Karimi M, Shaterianb HR, Heydari A. Ultrasound irradiation for the green synthesis of chromenes using l-arginine-functionalized magnetic nanoparticles as a recyclable organocatalyst. RSC Adv. 2014;4:42220–42225. doi: 10.1039/C4RA06198E. [DOI] [Google Scholar]
- 46.Zhang L, He R, Gu HC. Oleic acid coating on the monodisperse magnetite nanoparticles. Appl Surf Sci. 2006;253:2611–2617. doi: 10.1016/j.apsusc.2006.05.023. [DOI] [Google Scholar]
- 47.Eftekhari A, Foroughifar N, Hekmati M, Mahdi Khobi. Fe3O4@l-arginine magnetic nanoparticles: a novel and magnetically retrievable catalyst for the synthesis of 10 3-diaryl-2 N-azaphenalene. J Chin Chem Soc. 2019;66:761–768. doi: 10.1002/jccs.201800274. [DOI] [Google Scholar]
- 48.Ghasemzadeh MA, Abdollahi-Basir MH, Elyasi Z. Fe3O4@l-arginine as a reusable catalyst for the synthesis of polysubstituted 2-pyrrolidinones. Curr Organocatalysis. 2019;6:61–68. doi: 10.2174/2213337206666181211125226. [DOI] [Google Scholar]
- 49.Mamaghani M, Hossein Nia R. A review on the recent multicomponent synthesis of pyranopyrazoles. Polycycl Aromat Comp. 2019 doi: 10.1080/10406638.2019.1584576. [DOI] [Google Scholar]
- 50.Zamani F, Hosseini SM. Palladium nanoparticles supported on Fe3O4/amino acid nanocomposite: highly active magnetic catalyst for solvent-free aerobic oxidation of alcohols. Catal Commun. 2014;43:164–168. doi: 10.1016/j.catcom.2013.09.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study available from the corresponding author on reasonable request.