Abstract
The oily wastewater generated in the industrial field is adversely affecting the environment, while the current methods for oil–water separation are complex and costly. Therefore, it is significant to use low cost and environmentally friendly materials to prepare a smart responsive superhydrophobic coating for the effective separation of oil–water mixtures. In this paper, a fluorine-free copolymer with pH responsiveness was fabricated by a solution impregnation method, and it was compounded by silica nanoparticles/polydimethylsiloxane to prepare a superhydrophobic coating on the paper and cotton fabric. The prepared superhydrophobic coating remained in the superhydrophobic state after the alkali treatment, while it would be converted into the hydrophilic state after the acid treatment. Therefore, the pH-responsive superhydrophobic coating will be applied in controlled selective oil–water separation.
1. Introduction
A lot of oily wastewater is produced in the fields of printing, textile, food processing, leather, and petrochemical industries. The separation of oil–water mixtures has become an urgent problem to be solved in industrial production, so it is very important to design materials for effective separation of oil–water mixtures.1−4 The current methods for oil–water separation are complex, time-consuming, costly, and prone to secondary pollution. In addition, there is no switchable wettability in these materials, which limits their practical and commercial applications to some extent. Therefore, it is significant to design smart materials with switchable surface wettability.5−8
Smart response materials refer to the materials whose surface energy and surface microstructure will be changed under the stimulation of pH, temperature, light, electric potential, and so on, resulting in transition in surface wettability.9−18 These materials can be used in the separation of oil–water mixtures via single-phase selective absorption or filtration. Therefore, the development of smart response materials has become a hotspot of present research. Liu et al. prepared a ZnO nanorod/epoxy composite membrane which achieved reversible transition of wettability by the UV-induced photocatalytic effect.19 Geissler et al. developed superhydrophobic paper with temperature response characteristics using cellulose stearoyl ester solutions and nanoparticles.20 Zheng et al. fabricated a nanostructured polyaniline that can induce oil–water separation by electrical selective penetration.21 However, most of these smart response surfaces require high cost and complicated processes, which limit their applications. The pH responsiveness is more favored because of easy operation, fast response, and there is no need for additional equipment. There are protonatable groups including carboxyl groups, pyridines, and amines in usual pH-responsive materials. Xu et al. fabricated a superamphiphobic coating using coordination chemistry, showing a special capability to control oil–water separation and remove water from bulk oil selectively.22 Wang et al. successfully prepared pH-responsive fabrics by in situ-growing Ag nanocrystals on the fabric and modifying them with a mixture of methyl-terminated thiol and carboxyl-terminated thiol.23 However, most of these coatings are prepared using precious materials such as Ag or fluorine-containing substances which are considered to be harmful to the environment. Therefore, it is an inevitable trend to use environmental friendly materials with low cost to prepare smart responsive superhydrophobic coatings.
In our work, a random copolymer is synthesized by solution-processable free radical polymerization using inexpensive fluorine-free monomers. Silica nanoparticles/polydimethylsiloxanes (SiO2 NPs/PDMS) are compounded to build a high-roughness surface because of the strong interaction between SiO2 NPs/PDMS and the covalent linkage of the triblock copolymer. The paper and cotton fabric are then selected as the substrate for the superhydrophobic coating because of their low cost, environmental friendliness, and permeability, and they are coated with the prepared pH responsive copolymer to obtain a smart response superhydrophobic capability. The test of surface wettability proves that the coating is superhydrophobic in both neutral and alkali environments. In an acidic environment, the coating will change from a superhydrophobic state to a hydrophilic state, which can be used for efficient selective oil–water separation to meet the demand in different environments. The superhydrophobic coating prepared by the method in this paper is considered to have a good application prospect.
2. Results and Discussion
2.1. Surface Morphology Analysis
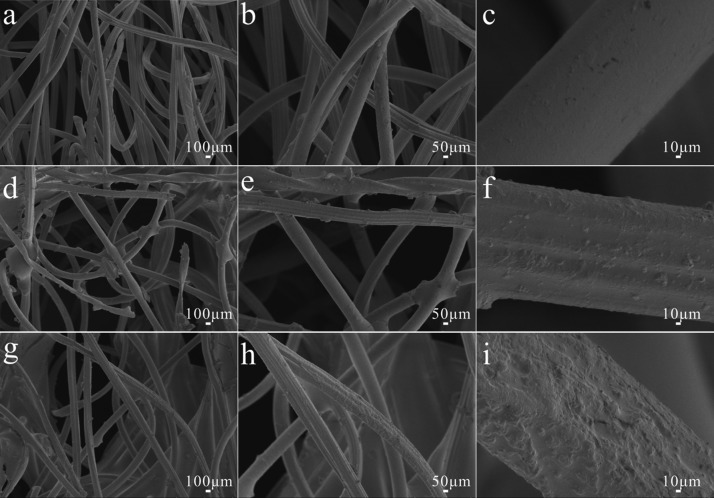
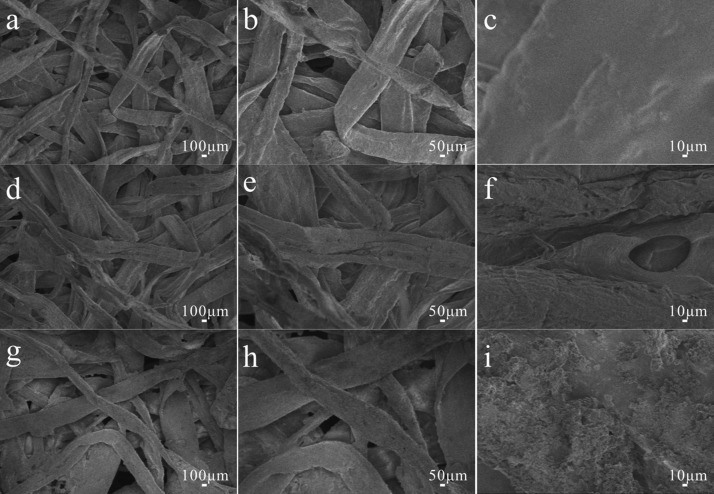
In order to study the effect of the prepared copolymer and SiO2 NPs on the surface morphology of the samples, the surface morphologies of the original samples, the samples coated with the copolymer, and the samples coated with copolymer and SiO2 NPs/PDMS are observed and analyzed by scanning electron microscope (SEM) images. For the original samples (Figures 1a–c and 2a–c), it can be intuitively seen that the native striations of the fiber structure are obvious and the surface is flat without an uneven structure. For the samples coated with a pH-responsive copolymer (Figures 1d–f and 2d–f), it can be seen that the surface of the fiber is covered with a thin coating of copolymer with certain roughness, which is conducive to enhancing the hydrophobic properties of the substrate.24−26 For the samples modified by the copolymer compounded by the SiO2 NPs/PDMS (Figures 1g–i and 2g–i), it can be clearly observed that SiO2 NPs and pH-responsive copolymer coating are deposited on the surface, showing a state of unevenness. Compared with the above two kinds of samples, the surface roughness is greatly promoted after adding SiO2 nanoparticles into the copolymer.
Figure 1.
Different magnifications of SEM images of (a–c) original cotton fabric, (d–f) the copolymer-coated cotton fabric, and (g–i) copolymer–SiO2 NPs/PDMS-coated cotton fabric.
Figure 2.
Different magnifications of SEM images of (a–c) original paper, (d–f) the copolymer-coated paper, and (g–i) copolymer–SiO2 NPs/PDMS-coated paper.
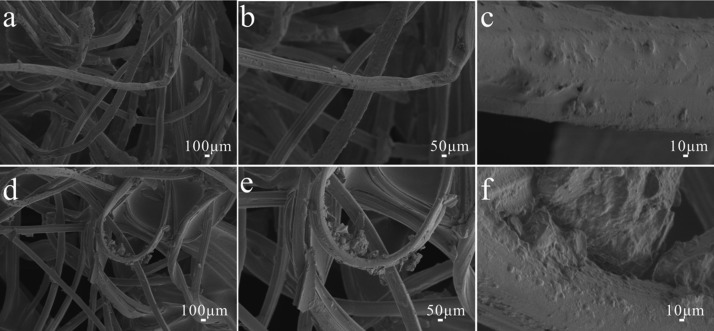
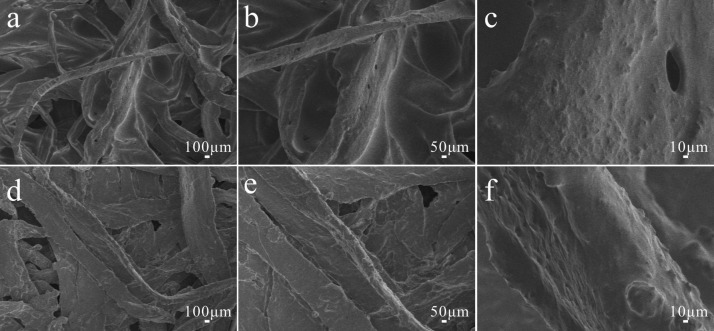
In addition, the effects of acid and alkali on the surface morphology of fibers are also studied, as shown in Figures 3 and 4. It can be seen from the figures that there is no significant change in the surface structure of modified paper and cotton fabric treated by the acid and alkali. The original rough structure is still maintained. It turns out that different pH solutions have no obvious effect on the surface of modified paper and cotton fabric.
Figure 3.
Different magnifications of SEM images of modified cotton fabric treated by (a–c) acid and (d–f) alkali.
Figure 4.
Different magnifications of SEM images of modified paper treated by (a–c) acid and (d–f) alkali.
2.2. Structure Characterization
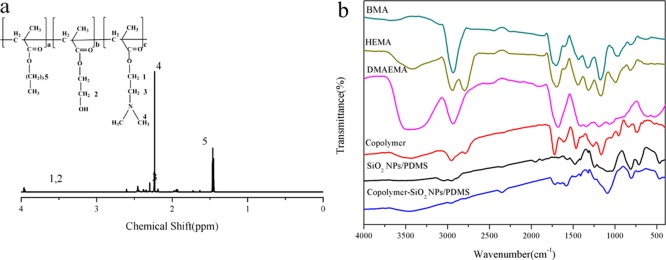
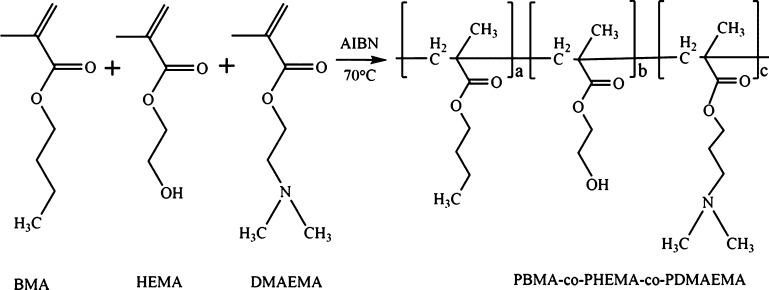
The molecular structure of pH-responsive copolymer was characterized by 1H nuclear magnetic resonance (1H NMR) (Figure 5a). The −CH2 peak of poly(butyl methacrylate) (PBMA) was observed at 1.4 ppm. The −N–(CH3)2 and −N–CH2 peaks of poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) were located at 2.23 and 2.3 ppm. The groups appeared at 3.97 ppm were contributed to −CH2OH and −CH2 linked to an ester group of PDMAEMA. The Fourier transform infrared spectroscopy (FT-IR) studies (Figure 5b) were carried out on the copolymer monomers, prepared copolymer, SiO2 NPs/PDMS, and copolymer compounded by SiO2 NPs/PDMS to identify the chemical structure of the synthesized products. The band around 3427 cm–1 was contributed to the stretching vibration of −OH in poly(2-hydroxyethyl methacrylate) (PHEMA). The absorption at 2954 cm–1 was ascribed to the C–H stretching of PBMA. The peaks at 1720 and 1166 cm–1 corresponded to the C=O and C–N stretching peaks in PDMAEMA segments, respectively. All the characteristic absorption peaks could be observed in the spectra of the corresponding monomers with the same wave numbers.27,28 For SiO2/PDMS, C–H bond was observed at around 2958 cm–1. The characteristic peak assigned to Si–CH3 of PDMS was located near 800 cm–1. The Si–O–Si stretching vibration appeared at 1087 cm–1. All of the above characteristic absorption peaks were found in the spectrum of pH-responsive copolymer compounded by SiO2 NPs/PDMS. The 1H NMR and FT-IR results showed that the copolymer was synthesized successfully and SiO2 NPs was introduced into the pH-responsive copolymer.29
Figure 5.
(a) 1H NMR spectrum of the pH-responsive copolymer. (b) FT-IR spectra of BMA, HEMA, DMAEMA, copolymer, SiO2 NPs/PDMS, and the copolymer compounded by SiO2 NPs/PDMS.
2.3. XPS Analysis
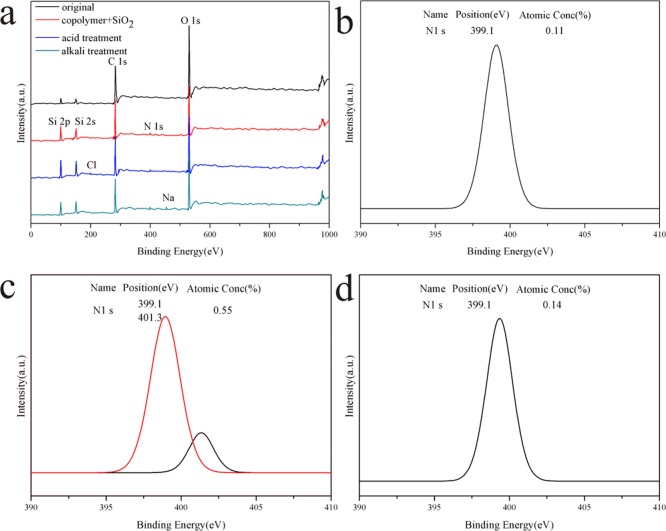
X-ray photoelectron spectroscopy (XPS) shown in Figure 6 was analyzed to verify the successful loading of the copolymer on the paper and the mechanism of pH responsiveness. For original paper, Figure 6a showed that only C and O elements emerged at 283 and 530 eV, respectively. Compared with the original paper, the N 1s peak at 399.1 eV appeared on the surface of copolymer–SiO2 NPs/PDMS-coated paper, which was ascribed to the tertiary amine of PDMAEMA. After immersing in a pH 1 solution, a distinctive peak was attributed to Cl 2p. The N 1s peaks at 399.1 and 401.3 eV (Figure 6c) corresponded to the tertiary amine and the quaternary amine, indicating that the PDMAEMA chain was protonated. When treated by using alkali, the Na peak appeared. The N 1s peak was only observed at 399.1 eV (Figure 6d), which was due to the deprotonation of PDMAEMA.30−32 In terms of atomic content, the N atom content of the modified paper without the acid/alkali treatment was 0.11%. After the acid treatment, the N atom content on the surface of the paper increased to 0.55%. This was caused by the quaternary amines produced by the protonation of PDMAEMA. After the alkali treatment, the content of the N atom on the surface of the paper did not substantially change, corresponding to the tertiary amine in the PDMAEMA segment. This result further demonstrated that the wettability of the paper could be altered by the protonation and deprotonation processes of the PDMAEMA segment in different pH solutions.
Figure 6.
(a) XPS spectra. N 1s core level of (b) copolymer–SiO2 NPs/PDMS-coated paper, (c) modified paper treated by using acid, and (d) modified paper treated by using alkali, respectively.
There are ionizable tertiary amine groups in PDMAEMA, which can be protonated and deprotonated, leading to changes in the wettability of pH-responsive coatings as shown in Figure 7. When in an acidic environment, the tertiary amine groups in PDMAEMA will be protonated to form positively charged ammonium groups. Molecular hydrogen bonds between the groups bind to surrounding water molecules, turning the coating into a hydrophilic state. When in a neutral or alkaline condition, tertiary amine groups will be deprotonated and Cl– in the quaternary ammonium will be removed, which will keep the coating in a superhydrophobic state.
Figure 7.
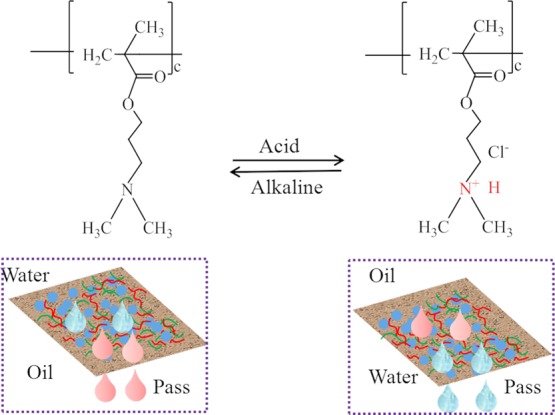
Mechanism of pH-responsive coating. At low pH values, the coating transitions to a superhydrophilic state. At high pH, the coating remains in a superhydrophobic state.
2.4. TG Analysis
It could be seen from Figure 8 that the original paper lost approximately 90% weight within the temperature range. The weight loss of the copolymer-coated paper and copolymer–SiO2 NPs/PDMS-coated paper was about 80 and 76%, respectively, indicating that the paper was modified successfully. The amount of the copolymer and SiO2 NPs/PDMS was about 10 and 4 wt %, respectively. This phenomenon demonstrated that the copolymer interacted with the micro–nanostructure to create a superhydrophobic surface.31,33,34
Figure 8.
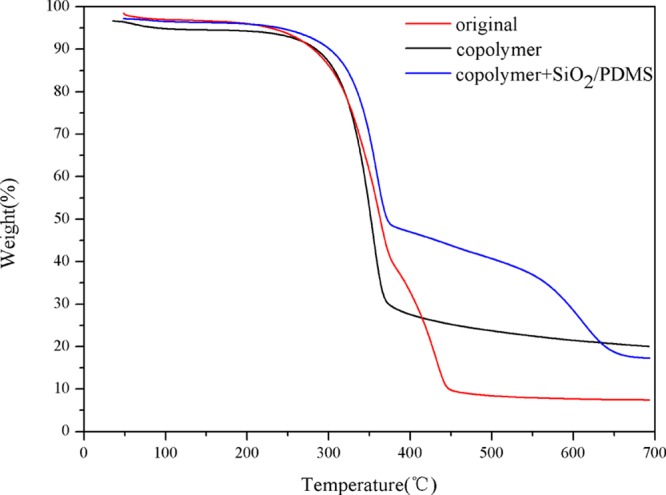
TGA curves of original paper, copolymer-coated paper, and copolymer–SiO2 NPs/PDMS-coated paper.
2.5. Surface Wettability and pH-Responsiveness
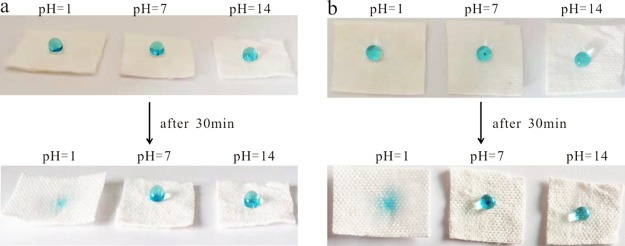
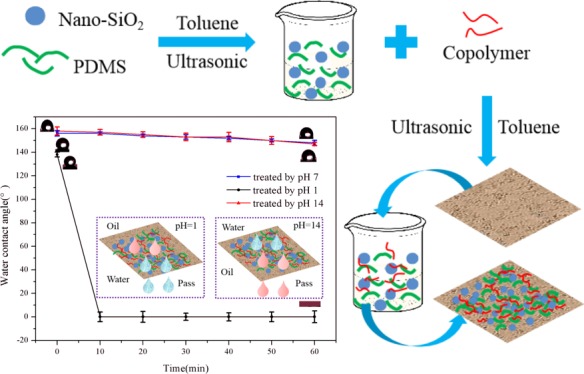
The water contact angle (WCA) of the coating was measured by selecting several points of the same sample randomly at ambient temperature. To observe the superhydrophobic stability and pH response of the coating, methylene blue-dyed deionized water was randomly dropped on the surface of the modified paper, acid-treated paper, and alkali-treated paper. As shown in Figure 9, the paper treated differently was initially in a superhydrophobic state. The surface of the acid treated paper was gradually infiltrated by the droplets, indicating that the paper was converted to a hydrophilic state. However, for the modified paper and alkali-treated paper, the shape of droplets remained unchanged after 30 min.
Figure 9.
Photographs of the droplets from the (a) front view and (b) top view on the surface of modified paper, acid-treated paper, and alkali-treated paper.
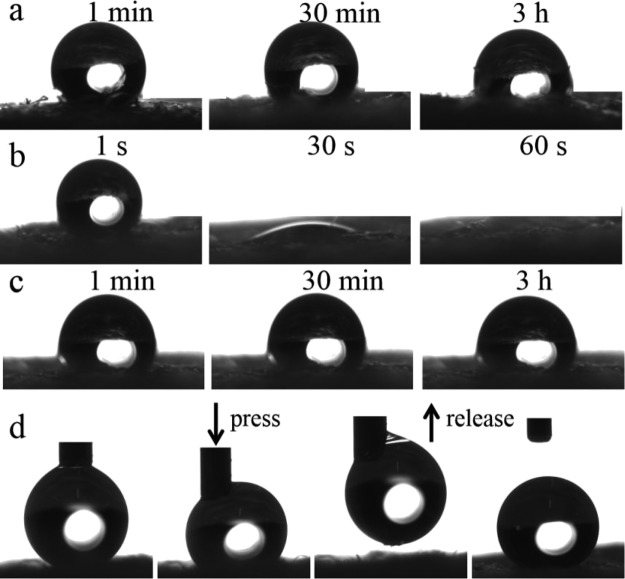
As shown in Figure 10, the WCA of the modified paper and alkali-treated paper reached above 150° and remained substantially constant. The WCA of the acid-treated paper was initially larger than 150°, but it began to decrease until it become 0° after 30 min. The modified paper was affected by amine protonation and deprotonation, resulting in the change of surface wettability. In addition, a graph describing the change of WCA with time was also drawn in Figure 11. The coated paper still remained superhydrophobic after 3 h. The result further illustrated the stability and pH responsiveness of the prepared superhydrophobic coating.35−37
Figure 10.
Time-dependence of the contact angle of (a) modified paper, (b) acid-treated paper, (c) alkali-treated paper, and (d) photographs of the water droplets on the modified paper.
Figure 11.
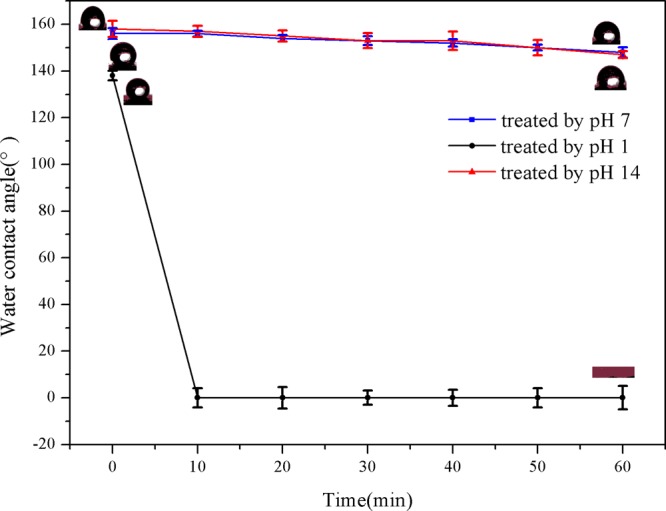
Evolution of WCA of different paper with time.
2.6. Mechanical Durability Test
The mechanical durability was characterized by the abrasion test. The superhydrophobic paper was placed face-down to the sandpaper with a weight of 200 g and pulled 20 cm back and forth along the horizontal line as a cycle, which was shown in Figure 12a. The paper was dragged at different cycles to determine the change in the contact angle. Figure 12b demonstrated that the WCA of the superhydrophobic surface changed with the increase of the pulling cycles. It could be seen that the contact angle ranged from 165° to 151° and it still was more than 150° after 30 cycles. The result indicated that there was a strong bonding force between the superhydrophobic coating and the substrate.
Figure 12.
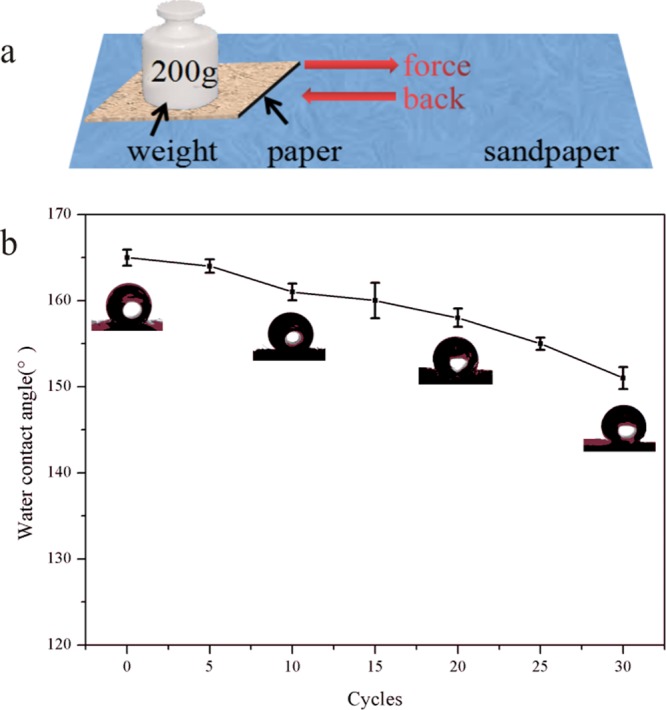
(a) Schematic of the abrasion test for the as-prepared paper. (b) Plot of the WCA after abrasion cycles.
2.7. Oil–Water Separation
The oil–water separation experiment was carried out on a device as shown in Figure 13 to verify the function of superhydrophobic paper. The copolymer–SiO2 NPs/PDMS-coated paper was fixed in the middle of two glass containers. A mixture of methylene blue-dyed water and oil red O-dyed oil (hexane, 1,2-dichloroethane, and toluene) was poured into the upper half of the glassware. Taking 1,2-dichloroethane as an example, because of the hydrophobic and lipophilic nature of the modified paper, the bottom heavy oil 1,2-dichloroethane rapidly penetrated the paper into the lower half of the glassware while the water remained on the responsive paper.38
Figure 13.

Oil–water separation process of copolymer–SiO2 NPs/PDMS-coated paper (taking 1,2-dichloroethane as an example).
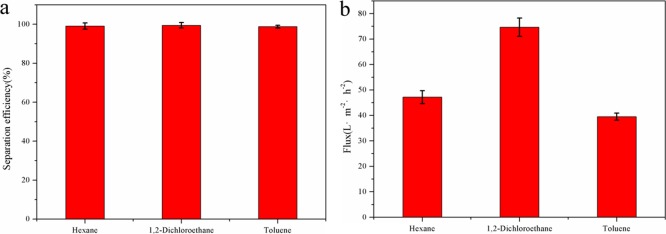
It can be concluded that the separation efficiency of the superhydrophobic paper can reach more than 98.5% for various oil–water mixtures, indicating that the superhydrophobic paper possesses excellent separation performance for oil–water mixtures (Figure 14a). Separation flux is also a key parameter to characterize the separation performance of superhydrophobic paper. The separation fluxes of as-prepared paper for various mixtures are between 39 and 74 L·m–2·h–2 through calculation (Figure 14b). The recycle performance of superhydrophobic paper was tested. As shown in Figure 15, after 30 separation cycles, the separation efficiency of superhydrophobic paper can still reach more than 98%.39−42
Figure 14.
(a) Separation efficiency and (b) separation flux of superhydrophobic paper for various oil–water mixtures.
Figure 15.
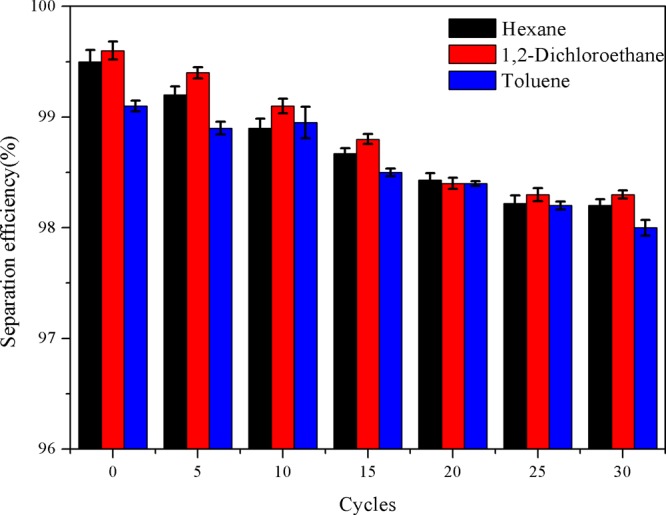
Separation efficiency of superhydrophobic paper varies with the number of cycles.
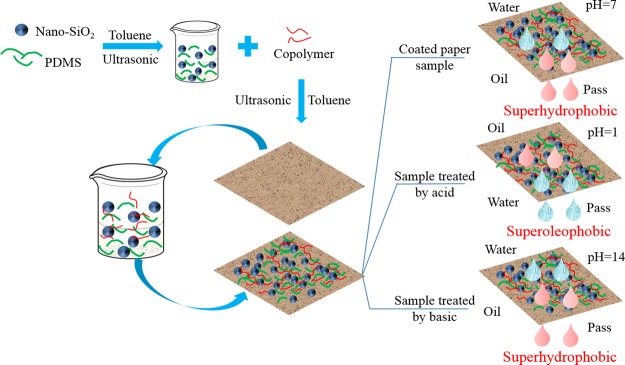
In order to further investigate the effect of pH on the performance of oil–water separation, the test of oil–water separation was conducted on acid-/alkali-treated paper. For the acid-treated paper, a mixture of methylene blue-dyed water and oil red O dyed hexane was poured into the upper of the glass container. Because the modified paper was converted to the superoleophobicity state under the acidic condition, water permeated through the paper into the lower glass vessel, while hexane was collected in the upper vessel (Figure 16a). A mixture of 1,2-dichloroethane (oil red O dyeing) and water (methylene blue dyeing) was used to verify the oil–water separation ability of the alkali-treated paper (Figure 16b). It could be found that the wettability of the paper treated by using alkali was completely in contrast to that treated by using acid. When the coated paper was treated by using alkali, 1,2-dichloroethane passed through the paper driven by gravity while water was retained. Therefore, the selective oil–water separation was achieved by altering the wettability of the pH-responsive coating.
Figure 16.

Oil–water separation process of (a) acid-treated paper and (b) alkali-treated paper.
3. Conclusions
In this paper, a pH responsive random copolymer is prepared by a simple solution impregnation method, and it is compounded by SiO2 NPs/PDMS to prepare a fluorine-free superhydrophobic coating. The results of characterizations such as SEM and FT-IR show that the prepared copolymer compounded by SiO2 NPs/PDMS is successfully coated on the substrate. The tests of WCA and oil–water separation show that the prepared coating successfully obtains pH-responsive superhydrophobic capability. The WCA of pH-responsive coating reaches more than 150° in both neutral and alkali environments. After the acidic treatment, the coating transitions to a superhydrophilic state because of the protonation of the responsive monomer PDMAEMA. The pH-responsive superhydrophobic coating prepared in this paper can be applied well to both paper and cotton fabric. Therefore, it has a good application prospect in effective oil–water separation in industries.
4. Experimental Section
4.1. Materials
Butyl methacrylate (BMA), 2-hydroxyethyl methacrylate (HEMA), and 2-(dimethylamino)ethyl methacrylate (DMAEMA) were all purchased from Ivkeyan Chemical Reagent Co., Ltd., Shanghai, China. PDMS was purchased from Sigma-Aldrich. All the above regents were used as received. 2,2-Azobisisobutyronitrile (AIBN) obtained from Damao Chemical Reagent Factory, Tianjin, China was recrystallized from ethanol for further use. The paper and cotton fabric used for modification were purchased from a local store.
4.2. Synthesis of pH-Responsive Copolymer
As shown in Scheme 1, the copolymer was prepared by polymerization under anhydrous and oxygen-free conditions. BMA (5.68 g, 0.04 mol), HEMA (0.52 g, 0.004 mol), and DMAEMA (6.28 g, 0.04 mol) were dissolved in anhydrous toluene (30 g) and then AIBN (0.1 g) was added as the initiator. The mixture was placed in a three-necked round-bottom flask. Nitrogen gas was introduced into the flask for 40 min to get rid of the oxygen to facilitate the polymerization. The sealed reaction flask was placed in a 70 °C constant temperature oil bath with stirring for 6 h. Then, the obtained solution was cooled to room temperature and purified by rotary evaporation to remove the solvent. The resultant copolymer was dissolved in acetone and precipitated in hexane several times and dried under vacuum at 50 °C.
Scheme 1. Synthesis Route of Poly(butyl methacrylate-co-hydroxyethyl methacrylate-co-2-dimethylaminoethyl methacrylate) (PBMA-co-PHEMA-co-PDMAEMA).
4.3. Preparation of pH-Responsive Coating Solution
Silica nanoparticles (SiO2 NPs) were first dissolved in 20 g of anhydrous toluene with subsequent addition of PDMS and the mixture was ultrasonicated for 30 min. Then, the prepared pH-responsive copolymer was added and the solution was dispersed under ultrasonic conditions for 30 min to form a uniform and stable solution.
4.4. Fabrication of pH-Responsive Paper
The preparation of the pH-responsive paper is illustrated in Scheme 2. The paper and cotton fabric to be used were cut into a size of 3 cm × 3 cm and washed with acetone, absolute ethanol, and distilled water, respectively, to remove impurities. The clean paper and cotton fabric were separately immersed in the prepared copolymer solution and the pH-responsive solution containing SiO2 NPs/PDMS for 30 min. After the completion of soaking, the paper and cotton fabric were dried in an oven at 100 °C for 1 h to complete the cross-linking reaction.
Scheme 2. Preparation Procedure of pH-Responsive Coating and Its Application in Oil–Water Separation after the Acid/Alkali Treatment.
4.5. Selective Oil–Water Separation Experiments
Different oil–water mixtures were prepared to test the selective oil–water separation function of the pH-responsive coating. The separation performances of the superhydrophobic paper were characterized by the separation efficiency, separation flux, and cycle usage. The separation efficiency is calculated by formula 1 as follows
| 1 |
where V1 is the volume of oil collected in the oil–water separation experiment and V0 is the volume of oil before the separation experiment. Separation flux can be obtained from formula 2
| 2 |
where V is the volume of oil passing the superhydrophobic paper in time t and S is the effective contact area between the oil–water mixture and the superhydrophobic paper.
4.6. Characterization
The surface morphologies of all the coated paper and cotton fabric were examined by a SEM (SU-8010, Hitachi, Japan). FT-IR was recorded using an infrared spectrometer (8400S, Shimadzu, Japan) at room temperature. The structure of the copolymer dissolved in CDCl3 was analyzed by 1H NMR (AVANCE III HD 600 MHz, Bruker, Germany) and all chemical shifts were recorded in ppm. XPS (Axis Ultpa, Kratos analytical, UK) with an Al Kα X-ray source at 10 kV and 10 mA was conducted to determine the surface element of samples. Thermogravimetric analysis (TGA, Q600SDT, TA Instruments, USA) was performed on the original sample, copolymer-coated sample, and copolymer–SiO2 NPs/PDMS-coated sample under nitrogen gas with a heating rate of 10 °C/min and scanned temperature ranging from ambient temperature to 700 °C. The surface wettability and pH responsiveness of the coating were characterized by WCA measured on goniometer (SDC-200, Sindin, China). The bonding force between the coating and substrate was tested by measuring the change of the contact angle after the abrasion test on the surface of superhydrophobic paper.
Acknowledgments
We acknowledge the support by NSF of the Science and Technology Department of Shaanxi Province under grant no. 2019JM-122, NSF of the Science and Technology Department of Shaanxi Province under grant no. 2018JQ5100, NSF of the Key Laboratory of Shaanxi Provincial Department of Education under grant no. 15JS075, Doctoral Research Initiation Fund of Xi’an University of Technology under grant no. 108-451119007, and Shaanxi Collaborative Innovation Center of Green Intelligent Printing and Packaging.
The authors declare no competing financial interest.
References
- Zhang L.; Zhang Z.; Wang P. Smart surfaces with switchable superoleophilicity and superoleophobicity in aqueous media: Toward controllable oil/water separation. NPG Asia Mater. 2012, 4, e8 10.1038/am.2012.14. [DOI] [Google Scholar]
- Zhou X.; Kong J.; Sun J.; Li H.; He C. Stable Superhydrophobic Porous Coatings from Hybrid ABC Triblock Copolymers and Their Anti-Corrosive Performance. ACS Appl. Mater. Interfaces 2017, 9, 30056–30063. 10.1021/acsami.7b08482. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Fan X.; He C. Hybrid Starlike Block Copolymer POSS-(PDMAEMA-b-PNIPAm)8:Thermal Gelation and Its Blends with Poly(vinyl alcohol). Macromolecules 2016, 49, 4236–4244. 10.1021/acs.macromol.6b00534. [DOI] [Google Scholar]
- Cheng Z.; Lai H.; Du M.; Zhu S.; Zhang N.; Sun K. Super-hydrophobic surface with switchable adhesion responsive to both temperature and pH. Soft Matter 2012, 8, 9635–9641. 10.1039/c2sm26399h. [DOI] [Google Scholar]
- Cheng Z.; Lai H.; Du Y.; Fu K.; Hou R.; Li C.; Zhang N.; Sun K. pH-Induced Reversible Wetting Transition between the Underwater Superoleophilicity and Superoleophobicity. ACS Appl. Mater. Interfaces 2014, 6, 636–641. 10.1021/am4047393. [DOI] [PubMed] [Google Scholar]
- Cao Y.; Liu N.; Fu C.; Li K.; Tao L.; Feng L.; Wei Y. Thermo and pH Dual-Responsive Materials for Controllable Oil/Water Separation. ACS Appl. Mater. Interfaces 2014, 6, 2026–2030. 10.1021/am405089m. [DOI] [PubMed] [Google Scholar]
- Du C.; Wang J.; Chen Z.; Chen D. Durable superhydrophobic and superoleophilic filter paper for oil–water separation prepared by a colloidal deposition method. Appl. Surf. Sci. 2014, 313, 304–310. 10.1016/j.apsusc.2014.05.207. [DOI] [Google Scholar]
- Cheng Q.-Y.; Liu M.-C.; Li Y.-D.; Zhu J.; Du A.-K.; Zeng J.-B. Biobased super-hydrophobic coating on cotton fabric fabricated by spraycoating for efficient oil/water separation. Polym. Test. 2018, 66, 41–47. 10.1016/j.polymertesting.2018.01.005. [DOI] [Google Scholar]
- Xu Q. F.; Liu Y.; Lin F.-J.; Mondal B.; Lyons A. M. Superhydrophobic TiO2-polymer nanocomposite surface with UV-induced reversible wettability and self-cleaning properties. ACS Appl. Mater. Interfaces 2013, 5, 8915–8924. 10.1021/am401668y. [DOI] [PubMed] [Google Scholar]
- Yin Y.; Guo N.; Wang C.; Rao Q. Alterable Superhydrophobic–Superhydrophilic Wettability of Fabric Substrates Decorated with Ion–TiO2 Coating via Ultraviolet Radiation. Ind. Eng. Chem. Res. 2014, 53, 14322–14328. 10.1021/ie502338y. [DOI] [Google Scholar]
- Tian D.; Zhang X.; Tian Y.; Wu Y.; Wang X.; Zhai J.; Jiang L. Photo-induced water-oil separation based on switchable superhydrophobicity-superhydrophilicity and underwater superoleophobicity of the aligned ZnO nanorod array-coated mesh films. J. Mater. Chem. 2012, 22, 19652–19657. 10.1039/c2jm34056a. [DOI] [Google Scholar]
- Xue B.; Gao L.; Hou Y.; Liu Z.; Jiang L. Temperature Controlled Water/Oil Wettability of a Surface Fabricated by a Block Copolymer: Application as a Dual Water/Oil On-Off Switch. Adv. Mater. 2013, 25, 273–277. 10.1002/adma.201202799. [DOI] [PubMed] [Google Scholar]
- Xiong D.; Cui G.; Wang J.; Wang H.; Li Z.; Yao K.; Zhang S. Reversible Hydrophobic-Hydrophilic Transition of Ionic Liquids Driven by Carbon Dioxide. Angew. Chem. 2015, 127, 7373–7377. 10.1002/ange.201500695. [DOI] [PubMed] [Google Scholar]
- Pernites R. B.; Ponnapati R. R.; Advincula R. C. Superhydrophobic–Superoleophilic Polythiophene Films with Tunable Wetting and Electrochromism. Adv. Mater. 2011, 23, 3207–3213. 10.1002/adma.201100469. [DOI] [PubMed] [Google Scholar]
- Ju G.; Cheng M.; Shi F. A pH-responsive smart surface for the continuous separation of oil/water/oil ternary mixtures. NPG Asia Mater. 2014, 6, e111 10.1038/am.2014.44. [DOI] [Google Scholar]
- Liu C.-T.; Liu Y.-L. pH-Induced Switches of the Oil- And Water-Selectivity of Crosslinked Polymeric Membranes for Gravity-Driven Oil-Water Separation. J. Mater. Chem. A 2016, 4, 13543–13548. 10.1039/c6ta05968f. [DOI] [Google Scholar]
- Cheng Z.; Du M.; Fu K.; Zhang N.; Sun K. pH-Controllable Water Permeation through a Nanostructured Copper Mesh Film. ACS Appl. Mater. Interfaces 2012, 4, 5826–5832. 10.1021/am3014746. [DOI] [PubMed] [Google Scholar]
- Wang B.; Guo Z. pH-responsive bidirectional oil–water separation material. Chem. Commun. 2013, 49, 9416–9418. 10.1039/c3cc45566a. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Lin Z.; Lin W.; Moon K. S.; Wong C. P. Reversible Superhydrophobic–Superhydrophilic Transition of ZnO Nanorod/Epoxy Composite Films. ACS Appl. Mater. Interfaces 2012, 4, 3959–3964. 10.1021/am300778d. [DOI] [PubMed] [Google Scholar]
- Geissler A.; Loyal F.; Biesalski M.; Zhang K. Thermo-responsive superhydrophobic paper using nanostructured cellulose stearoyl ester. Cellulose 2014, 21, 357–366. 10.1007/s10570-013-0160-8. [DOI] [Google Scholar]
- Zheng X.; Guo Z.; Tian D.; Zhang X.; Jiang L. Electric Field Induced Switchable Wettability to Water on the Polyaniline Membrane and Oil/Water Separation. Adv. Mater. Interfaces 2016, 3, 1600461. 10.1002/admi.201600461. [DOI] [Google Scholar]
- Xu Z.; Zhao Y.; Wang H.; Wang X.; Lin T. A Superamphiphobic Coating with an Ammonia-Triggered Transition to Superhydrophilic and Superoleophobic for Oil-Water Separation. Angew. Chem. 2015, 127, 4610–4613. 10.1002/ange.201411283. [DOI] [PubMed] [Google Scholar]
- Wang B.; Guo Z.; Liu W. pH-responsive smart fabrics with controllable wettability in different surroundings. RSC Adv. 2014, 4, 14684–14690. 10.1039/c3ra48002j. [DOI] [Google Scholar]
- Vasiljević J.; Tomšič B.; Jerman I.; Orel B.; Jakša G.; Simončič B. Novel multifunctional water- and oil-repellent, antibacterial, and flame-retardant cellulose fibres created by the sol–gel process. Cellulose 2014, 21, 2611–2623. 10.1007/s10570-014-0293-4. [DOI] [Google Scholar]
- Piltan S.; Seyfi J.; Hejazi I.; Davachi S. M.; Khonakdar H. A. Superhydrophobic filter paper via an improved phase separation process for oil/water separation: study on surface morphology, composition and wettability. Cellulose 2016, 23, 3913–3924. 10.1007/s10570-016-1059-y. [DOI] [Google Scholar]
- Li H.; Yang J.; Li P.; Lan T.; Peng L. A facile method for preparation superhydrophobic paper with enhanced physical strength and moisture-proofing property. Carbohydr. Polym. 2017, 160, 9–17. 10.1016/j.carbpol.2016.12.018. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Jin B.; Wang B.; Fu Y.; Zhan X.; Chen F. Fabrication of a Highly Stable Superhydrophobic Surface with Dual-Scale Structure and Its Antifrosting Properties. Ind. Eng. Chem. Res. 2017, 56, 2754–2763. 10.1021/acs.iecr.6b04650. [DOI] [Google Scholar]
- Fu Y.; Jiang J.; Zhang Q.; Zhan X.; Chen F. Robust Liquid-repellent Coatings Based on Polymer Nanoparticles with Excellent Self-cleaning and Antibacterial Performances. J. Mater. Chem. A 2017, 5, 275–284. 10.1039/c6ta06481g. [DOI] [Google Scholar]
- Zhou X.; He C. Tailoring the surface chemistry and morphology of glass fiber membranes for robust oil/water separation using poly(dimethylsiloxanes) as hydrophobic molecular binders. J. Mater. Chem. A 2018, 6, 607–615. 10.1039/c7ta09411f. [DOI] [Google Scholar]
- Chen K.; Gu K.; Qiang S.; Wang C. Environmental stimuli-responsive self-repairing waterbased superhydrophobic coatings. RSC Adv. 2017, 7, 543–550. 10.1039/c6ra25135h. [DOI] [Google Scholar]
- Dang Z.; Liu L.; Li Y.; Xiang Y.; Guo G. In Situ and Ex Situ pH-Responsive Coatings with Switchable Wettability for Controllable Oil/Water Separation. ACS Appl. Mater. Interfaces 2016, 8, 31281–31288. 10.1021/acsami.6b09381. [DOI] [PubMed] [Google Scholar]
- Lee C. H.; Kang S. K.; Lim J. A.; Lim H. S.; Cho J. H. Electrospun smart fabrics that display pH-responsive tunable wettability. Soft Matter 2012, 8, 10238–10240. 10.1039/c2sm26625c. [DOI] [Google Scholar]
- Kwon G.; Kota A. K.; Li Y.; Sohani A.; Mabry J. M.; Tuteja A. On-Demand Separation of Oil-Water Mixtures. Adv. Mater. 2012, 24, 3666–3671. 10.1002/adma.201201364. [DOI] [PubMed] [Google Scholar]
- Xue C.-H.; Zhang L.; Wei P.; Jia S.-T. Fabrication of superhydrophobic cotton textiles with flame retardancy. Cellulose 2016, 23, 1471–1480. 10.1007/s10570-016-0885-2. [DOI] [Google Scholar]
- Xu Z.; Zhao Y.; Wang H.; Zhou H.; Qin C.; Wang X.; Lin T. Fluorine-Free Superhydrophobic Coatings with pH-induced Wettability Transition for Controllable Oil–Water Separation. ACS Appl. Mater. Interfaces 2016, 8, 5661–5667. 10.1021/acsami.5b11720. [DOI] [PubMed] [Google Scholar]
- Wang N.; Xiong D.; Pan S.; Deng Y.; Shi Y.; Wang K. Superhydrophobic paper with superior stability against deformations and humidity. Appl. Surf. Sci. 2016, 389, 354–360. 10.1016/j.apsusc.2016.07.110. [DOI] [Google Scholar]
- Xu Z.; Zhao Y.; Dai L.; Lin T. Multi-Responsive Janus Liquid Marbles: The Effect of Temperature and Acidic/Basic Vapors. Part. Part. Syst. Charact. 2014, 31, 839–842. 10.1002/ppsc.201400009. [DOI] [Google Scholar]
- Fu Y.; Jin B.; Zhang Q.; Zhan X.; Chen F. pH-Induced Switchable Superwettability of Efficient Antibacterial Fabrics for Durable Selective Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 30161–30170. 10.1021/acsami.7b09159. [DOI] [PubMed] [Google Scholar]
- Long Y.; Shen Y.; Tian H.; Yang Y.; Feng H.; Li J. Superwettable Coprinus comatus coated membranes used toward the controllable separation of emulsified oil/water mixtures. J. Membr. Sci. 2018, 565, 85–94. 10.1016/j.memsci.2018.08.013. [DOI] [Google Scholar]
- Wang X.; Li M.; Shen Y.; Yang Y.; Feng H.; Li J. Facile preparation of loess-coated membranes for multifunctional surfactant-stabilized oil-in-water emulsion separation. Green Chem. 2019, 21, 3190–3199. 10.1039/c9gc00747d. [DOI] [Google Scholar]
- Hu J.; Li X.; Dong J. Development of Highly Efficient Oil–Water Separation Carbon Nanotube Membranes with Stimuli-Switchable Fluxes. ACS Omega 2018, 3, 6635–6641. 10.1021/acsomega.8b00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M.; Huang Y.; Xu A.; Zhang T.; Zhan C.; Hong L. On-Demand Oil–Water Separation by Environmentally Responsive Cotton Fabrics. ACS Omega 2019, 4, 12333–12341. 10.1021/acsomega.9b01235. [DOI] [PMC free article] [PubMed] [Google Scholar]