Abstract
The advent of monoclonal antibody therapies has revolutionised inflammatory bowel disease (IBD) treatment and delivered great benefits to patients. The optimal use of this class of drugs requires careful management and a clear understanding of their properties. In this review article, we consider how to maximise the benefit of our most novel biological agents, vedolizumab and ustekinumab. For each agent, we consider practical aspects including dose flexibility, evidence for use in combination with a conventional immunomodulator and the potential role of therapeutic drug monitoring. We also address positioning of the various mechanisms and agents in treatment algorithms as well as important aspects of managing patients receiving monoclonal antibodies, such as disease reassessment. Finally, we look ahead to the future of monoclonal antibodies, where not only have biosimilars increased the number of agents available but there are also a range of novel mechanisms currently in late phase clinical trials.
Keywords: iInflammatory bowel disease, ulcerative colitis, Crohn’s disease, vedolizumab, ustekinumab
Introduction
There is no doubt that the advent of biological therapy with monoclonal antibodies has provided significant benefit for patients with ulcerative colitis (UC) and Crohn’s disease (CD). Their use has resulted in improvement in clinical symptoms, endoscopic evidence of inflammation and quality of life. Moreover, the magnitude of the benefit they can deliver has increased with our collective understanding of how best to use them. For example, it was quickly realised that to derive their maximum benefit these agents should be used in a continuous manner with ‘maintenance’ dosing, rather than an as part of an ‘on-demand’ episodic regimen, as was originally practised. Evidence demonstrating their diminishing benefit when used later in the disease course has also driven earlier introduction than was previously the case. More recently, technology has evolved that allows the measurement of serum drug concentrations. The combination of evidence demonstrating an exposure–response relationship along with the ability to carry out therapeutic drug monitoring (TDM) has therefore opened up entirely new avenues for treatment optimisation.
In recent years, the range of agents and mechanisms of action has expanded rapidly (figure 1). In addition to the long-standing anti-tumour necrosis factor (TNF) agents, infliximab and adalimumab, a third agent, golimumab was added to the class, receiving National Institute for Health and Care Excellence (NICE) approval for the treatment of UC in 2015. At the same time, NICE approved the selective leucocyte adhesion molecule inhibitor, vedolizumab (VDZ), for use in both UC and CD. Most recently in 2017, ustekinumab (UST), a monoclonal antibody targeting the p40 subunit of interleukin-12 and interleukin-23, received NICE approval for use in CD. Although this increase in the range of available options has delivered clear improvement in outcomes for many patients, it has also greatly added to the complexity of decision-making and range of knowledge now required of inflammatory bowel disease (IBD) clinicians. There now exist multiple uncertainties regarding the optimal use and positioning of these newer drugs, including but not limited to: should they be used as first-line biological agents in preference to anti-TNF?; what role, if any, does TDM have in their use?; is dose escalation effective (figure 2)?; and should they be used in combination with an immunomodulator (figure 3)? In this review we aim to address such issues and consider commonly arising practical aspects of using the more novel biological agents, VDZ and UST.
Figure 1.
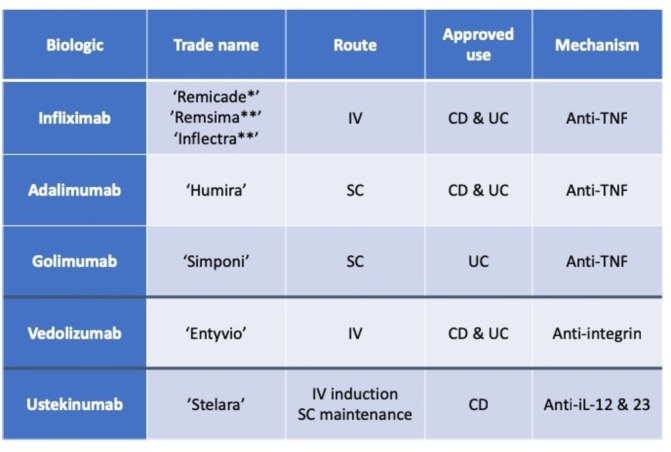
Summary of approved monoclonal antibodies for the treatment of IBD (*originator infliximab, **biosimilar infliximab). CD, Crohn’s disease; IBD, inflammatory bowel disease; IL, interleukin; IV, intravenous; SC, subcutaneous; TNF, tumour necrosis factor; UC, ulcerative colitis.
Figure 2.

Monoclonal antibody dosing regimen and dose flexibility. CD, Crohn’s disease IV, intravenous; N/A, not applicable; SC, subcutaneous.
Figure 3.

Degree of available evidence for monoclonal antibody exposure–response relationships, evidence of benefit of use in combination with an immunosuppressant and for dose escalation.
Vedolizumab
VDZ binds to alpha-4 beta-7 integrin molecules expressed on gut-specific lymphocytes, preventing their migration into the gastrointestinal parenchyma and the resultant intestinal inflammation. This gut-selective mechanism of action is key to differentiating it from the other biologics which all have a systemic immunosuppressive effect.
Effectiveness
The US VICTORY consortium (Vedolizumab health outcomes in Inflammatory Bowel Diseases) has published the largest real-world observational effectiveness cohort to date. Among 212 patients with CD, the reported rate of clinical remission at 12 months was 35%. In addition, this remission rate should be understood in the context of this cohort’s high (90%) prior anti-TNF inhibitor exposure rate.1 In their UC cohort (n=321), the authors reported corticosteroid-free clinical remission rates of 37% (with 73% having prior exposure to anti-TNF therapy).2 The relatively high rates of prior anti-TNF use, compared with the rates in the phase III GEMINI trials of VDZ (48% in GEMINI I and 62% in GEMINI II),3 4 are a marker of the complexity of patients seen in current clinical practice and may explain the marginally lower clinical response rates in observational studies compared with randomised controlled trials (RCTs). High prior anti-TNF exposure has also been described in numerous other ‘real-world’ cohorts.5
Dose flexibility
VDZ is given as a 300 mg fixed dose intravenous infusion at weeks 0, 2, 6 and then every 8 weeks thereafter. Patients with CD may benefit from an extra dose at week 10 if they have not yet had an adequate response at week 6. This is supported by analyses of the week 52 clinical remission rates from GEMINI II and the week 10 clinical remission rates from GEMINI III.4 6 It is important to have processes in place to ensure that the potential for this dose is considered in sufficient time for it to be delivered. Practically, this is likely to involve review of clinical response, ideally with objective biomarkers, shortly after the week 6 infusion. In cases of equivocal response, we would recommend administering the additional week 10 dose.
There is also scope to escalate maintenance therapy, to every 4 or 6 weeks, in those who experience secondary loss of response to VDZ. A recent meta-analysis and systematic review reported on the effectiveness of dose intensification (interval shortening to 4 or 6 weeks) to recapture response to VDZ. The data, pooled from four studies, were jointly reported for patients with UC and CD and showed that 56 of 111 (50%) secondary non-responders recaptured response with dose intensification.7 A recent cohort study also showed that dose intensification resulted in significant falls in clinical (Simple Clinical Colitis Activity Index (SCCAI)) and biochemical (C reactive protein (CRP)) measures of disease activity.8
Combination or monotherapy?
The rates of immunogenicity among patients treated with VDZ are remarkably low (<5%) and most antibodies appear to be transient.9 The rate of antidrug antibody development in GEMINI I was 3.7%, but only 1% were persistent. For GEMINI II the rate was 4.1%, with persistent antibodies in 0.4%, and in GEMINI III the rate was 1% and persistent antibodies were found in no patients. A caveat to these low rates is that these tests were performed using a drug-sensitive assay, making it more difficult to detect antibodies in the presence of drug. However, the low rates of immunogenicity have been corroborated in subsequent studies, even when testing samples with drug-tolerant assays.10 However, another study using a drug-tolerant assay reported the finding that antibodies to VDZ occur during the induction phase and disappear over time. Testing at weeks 2, 6 and 14 identified antibodies in 7 of 41 patients (17%, 3 of whom still responded and 4 who did not) but testing during maintenance identified antibodies in just 2 of 60 patients (3%).11
At odds with experience gained from the use of anti-TNF agents, the use of concomitant immunomodulation did not affect VDZ clearance or concentration in a study analysing population pharmacokinetics and pharmacodynamics.12 This reinforces the notion that the use of concomitant immunomodulators solely to prevent immunogenicity is not necessary with VDZ. However, if concomitant immunomodulation is to be withdrawn when switching to VDZ, we recommend this is delayed until after the induction period. This is in view of its somewhat slower onset of action when compared with other biological agents. This aspect of VDZ use can pose a clinical problem, particularly among patients who are steroid refractory or intolerant. The calcineurin inhibitors, tacrolimus or ciclosporin, have recently been demonstrated to be a safe and effective when used as a ‘bridge’ to VDZ effect in a small cohort of steroid refractory patients.13
Therapeutic drug monitoring
Post hoc analysis of the GEMINI studies demonstrated an exposure–response relationship for VDZ in both UC and CD. Quartile analyses of drug levels showed that those in the highest quartiles had significantly higher rates of clinical response and remission during the induction and maintenance periods when compared with those in the lowest quartiles.3 4 6 Studies have suggested that week 6 trough levels below 19 μg/mL predicted the need for subsequent dose escalation and that levels of 18 μg/mL and above correlated with higher rates of mucosal healing at 1 year.14 Although these thresholds offer some guidance, they require further validation and there is currently insufficient evidence to recommend a target therapeutic window. Until further data are available, VDZ dosing should be empirically adjusted based on objective measures of disease activity.
Ustekinumab
UST, a monoclonal antibody to the p40 subunit of interleukin 12 and interleukin 23, is the most recently NICE-approved monoclonal antibody for the treatment of CD. Approval was granted in July 2017 based on proven efficacy, both in anti-TNF-naive and anti-TNF-exposed patients, generated in the UNITI trial programme.15
Effectiveness
Probably the best characterised real-world CD cohort comes from Canada, which includes patients who started treatment as long ago as 201116. Among a large cohort (n=167) of anti-TNF-refractory patients, clinical response rates of 39% and 60% were observed at 3 and 6 months, respectively. The correlating clinical remission rates were 15% and 25%. These figures may be of use in counselling patients regarding likelihood of benefit and certainly offer reason for a degree of optimism, even in the face of anti-TNF non-response.
Dose flexibility
The dosing regimen for UST requires an assessment of response to induction therapy in order to guide maintenance dosing.17 Although, in a positive sense, this encourages ‘good’ practice, which should involve review of treatment effect, it poses certain practical challenges that deserve discussion. The induction doses are fixed: an intravenous administration equating to approximately 6 mg/kg at week 0 followed by a subcutaneous 90 mg dose at week 8. Thereafter, 90 mg subcutaneous maintenance dosing is given 8 or 12 weekly depending on response. It is recommended that patients are reviewed in the week/fortnight running up to week 16; for those achieving an adequate response, 12-weekly dosing should be commenced (next administration will therefore be at week 20), while those only achieving a partial response should be given 8-weekly dosing (next administration at week 16) (figure 4). In patients with no response whatsoever (or deterioration), treatment withdrawal could be considered. However, as options in this scenario are often limited and late response is a recognised phenomenon,18 continuing 8-weekly dosing for another dosing cycle beyond week 16 may also be appropriate. Judging the adequacy of response may be aided by the use of paired Harvey-Bradhaw Index (HBI) scores and objective markers of inflammation (CRP and faecal calprotectin (FC)) at baseline and pre-week 16.
Figure 4.
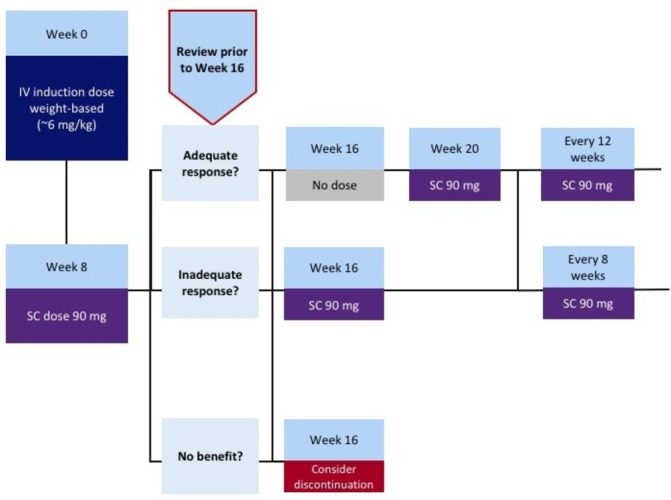
Recommended algorithm for managing the transition from ustekinumab induction treatment to maintenance dosing. IV, intravenous; SC, subcutaneous.
If there is uncertainty regarding dosing frequency, there is good rationale to err on the side of 8-weekly dosing as this appears likely to result in higher rates of endoscopic response and healing than 12-weekly dosing.19 20 At any point onward, the dosing interval can be altered, either from 12 to 8 weekly for loss of response (with both post hoc RCT18 20 and observational21 evidence to support this) or from 8 to 12 weekly in sustained remission (although the effect of this has not been well characterised). Escalation to 4-weekly dosing is also described as part of observational studies21 and is currently being investigated in a phase IV RCT.
Combination or monotherapy?
A comprehensive pharmacokinetic and pharmacodynamic post hoc analysis of the UNITI trials has been carried out, which offers valuable practical insights into several aspects of the best use of UST.20 First, even using a drug-tolerant assay (which is, therefore, able to detect antidrug antibodies in the presence of drug), UST appears to result in very low rates of immunogenicity; just 2.3% among 1366 patients over a year of treatment. Second, perhaps due to the observed low rate of immunogenicity and again in contrast to anti-TNF agents, the use of concomitant immunomodulation did not significantly impact UST drug levels.20 On this basis, where immunomodulators have failed and are being used only to reduce immunogenicity, it may be more appropriate to discontinue their use and give UST as monotherapy.
Therapeutic drug monitoring
The clearly observed exposure–response relationship for UST means that TDM is almost certainly going to become a useful clinical tool in the future, and therapeutic thresholds have already been postulated.20 However, there currently exists no widely available, validated ELISA kit to measure UST concentrations and therefore, dose adjustments are currently carried out on an empiric basis only.
Reassessment of patients commencing monoclonal antibodies
Although the reassessment of patients receiving monoclonal antibody therapy need not necessarily be particularly different to that of patients commencing other treatments, it does warrant particular consideration for several reasons. First, by nature of their positioning in most treatment algorithms (including those recommended by NICE), they are generally administered to our most refractory and/or severely affected group of patients. There is, therefore, an entirely appropriate desire to ensure that an ‘adequate’ treatment response has been achieved, and if not, that an alternative strategy can be instituted. This leads onto the second reason: their high cost which also supports the discontinuation of an ineffective treatment in the face of sustained non-response.
In an attempt to define, for the first time, universally applicable criteria that could be used to judge the adequacy of treatment response, the IOIBD (International Organisation for the study of IBD) ran the STRIDE (Selecting Therapeutic Targets in Inflammatory Bowel Disease) initiative. These targets are by no means limited to biological therapies, and the timelines and goals they set out are pragmatic (figure 5).22
Figure 5.
Treatment targets proposed by STRIDE (Selecting Therapeutic Targets in Inflammatory Bowel Disease)22 for use in a treat-to-target strategy.
From a practical point of view, when carrying out serial endoscopies to assess response to treatment the use of a validated index will help to standardise reporting between endoscopists.23 24 For UC, the endoscopic component of the Mayo score or the ulcerative colitis endoscopic index of severity (UCEIS) are easy to use. For CD, the simple endoscopic score for CD (SES-CD) is probably the easiest index to use although, at a minimum, the presence/absence of ulceration should be reported.22 While the STRIDE recommendations are useful and appropriate, particularly in their recognition of the need to demonstrate improvement in symptoms and objective markers of inflammation, repeated endoscopic assessment may not be always be feasible and/or acceptable to patients. The use of faecal calprotectin as a surrogate of mucosal inflammation has the benefit of being cheap and widely available with increasingly acceptable turnaround times.
Positioning of monoclonal antibodies
The choice of mechanism and agent for first-line biological treatment is an ongoing ‘hot-topic’ for debate. Although some such trials are currently underway, there exists no prospective, head-to-head RCT data to demonstrate the benefit of one approach over another. Even if it existed, this type of data would not necessarily be informative in terms of predicting the response of individual patients to each agent. Retrospective approaches to comparing treatment outcomes, such as network meta-analyses25 26 and the use of propensity score matching in real-world cohorts,27 28 also have considerable limitations. Until robust biochemical or pharmacogenetic markers to predict treatment response to individual agents become available, our practice is to discuss each patient commencing biological therapy in a multidisciplinary setting. The appropriate choice of biological drugs is often a matter of nuance that incorporates multiple factors including disease-specific factors, such as the predominance of extraintestinal manifestations or perianal disease (where anti-TNF may be preferred), as well as medical comorbidities such as predisposition to or history of malignancy or infection (where VDZ may be preferred). Whether biological treatment is being administered as monotherapy or in combination with an immunomodulator will also influence decision-making. In addition, other factors such as patient preference and route of administration should be considered.
Generally speaking, most patients commencing their first monoclonal antibody for the treatment of active CD are commenced on anti-TNF agents. This class has been shown to be highly effective in day-to-day clinical practice, has robust data of benefit for extraintestinal manifestations and perianal disease, can be optimised using TDM and in the case of biosimilars offers significant cost saving. However, as experience grows with using UST or VDZ first line, and TDM becomes available, this paradigm may change. In some cases, second-line treatment will be clearly guided by first-line experience; patients who respond well to one anti-TNF but have a pharmacokinetic loss of response will generally do well on a different anti-TNF agent.29 However, where first-line therapy with an anti-TNF has failed despite adequate levels, a switch out of class is generally recommended.30
For UC, positioning of first-line treatment is less clear, with VDZ demonstrating good tolerance, robust mucosal healing data,31 longevity of effect32 and evidence of limited immunogenicity.9 It should be appreciated that this is a rapidly moving field and that the next wave of biologics and small molecules (eg, tofacitinib and ozanimod; figure 6) will add further complexity.
Figure 6.

Recently licensed and late-stage pipeline drugs for the treatment of inflammatory bowel disease. CD, Crohn’s disease; UC, ulcerative colitis. *In Japan only; **not licensed in Europe. (Modified from P Hindryckx et al.; The Expanding Therapeutic Armamentarium for Inflammatory Bowel Disease: How to Choose the Right Drug[s] for Our Patients?, Journal of Crohn’s and Colitis, Volume 12, Issue 1, 5 January 2018, Pages 105–119).
Future of monoclonal antibodies
The rapid expansion of available monoclonal antibodies is set to continue with a range of novel agents already in the later stages of clinical trials (figure 6). One such drug, etrolizumab, inhibits lymphocyte trafficking by blocking β7 resulting in disruption of both α4β7 and αeβ7 with their target molecules. This approach is mechanistically similar to that of VDZ and it remains to be seen whether the relatively subtle differences between the molecules will alter efficacy. Following this will be a wave of agents that target the p19 subunit of interleukin 23. This mechanism can be thought of as a variation on that of UST, which inhibits signalling via interleukin 12 as well as interleukin 23. There are currently several agents proceeding through the various phases of clinical trial development. Risankizumab is the closest to market and will be followed by mirikizumab and guselkumab. All three are also being used/studied for the treatment of other idiopathic inflammatory conditions such as psoriasis and psoriatic arthritis.
Finally, there are new formulations of existing mechanisms that may offer practical benefits for patients and healthcare providers alike. Phase III trials are currently running to investigate the efficacy of subcutaneously administered VDZ. Initial reports demonstrate efficacy and this route of administration would offer clear benefits both in terms of patient convenience and healthcare utilisation costs. Another novel route of administration is the possibility to administer anti-TNF therapy orally, using protease-resistant antibodies or a plant cell encapsulated anti-TNF fusion protein, which serve as protection from the gastric environment. Phase II trials are underway in both UC and CD and other than ease of administration this route also offers the possibility to target therapy at the mucosal level, minimising systemic exposure and immunogenicity.
Conclusion
Monoclonal antibodies have revolutionised IBD treatment and a great deal has been learnt about how to maximise their benefit. We now understand that their optimal use requires careful management and a clear understanding of their pharmacokinetic and pharmacodynamic properties. As experience grows with newer mechanisms, we will no doubt be able to personalise their use more accurately and better appreciate their position in treatment algorithms. This field will continue to expand and as the number of agents grows, so too will the number of effective treatments we have to offer patients with IBD.
Significance of this study.
Clinicians should consider giving an additional week 10 vedolizumab infusion in patients with Crohn’s disease (CD) with incomplete response at week 6.
In patients with ulcerative colitis (UC) or CD with loss of response to standard 8-weekly vedolizumab dosing, escalation to 4-weekly infusions should be considered.
Patients commencing ustekinumab should be reassessed just prior to week 16 to decide on maintenance dosing frequency (8 or 12 weekly). Ideally, this should include objective evaluation of change in clinical disease activity, using paired Harvey-Bradhaw Index scores and biochemical markers of disease activity (C reactive protien and/or faecal calprotectin (FC)).
Where uncertainty exists regarding ustekinumab dosing frequency, there is rationale to opt for 8-weekly dosing as this appears likely to result in higher rates of endoscopic response and healing than 12 weekly.
Ustekinumab and vedolizumab are both currently understood to have minimal immunogenicity and there currently exists little (if any) evidence for the need to use them in combination with conventional immunosuppressants.
Footnotes
AGT and GC contributed equally.
Twitter: @SamaanMark
Contributors: AGT, GC, PMI and MAS were responsible for planning the content and structure of the article. AGT, GC and MAS drafted the manuscript, which PMI and MAS critically reviewed and revised.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: PMI: Advisory fees: Abbvie, Warner Chilcott, Takeda, MSD, Vifor Pharma, Pharmacosmos, Topivert, Genentech, Hospira, Samsung Bioepis; Lecture fees: Abbvie, Warner Chilcott, Ferring, Falk Pharma, Takeda, MSD, Johnson and Johnson, Shire. Financial support for research: MSD, Takeda. MAS: Advisory fees: Hospira, Takeda, Janssen; Lecture fees: Hospira, Takeda, MSD, Janssen, Falk.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Dulai PS, Singh S, Jiang X, et al. . The real-world effectiveness and safety of vedolizumab for moderate-severe crohn’s disease: results from the US victory consortium. Am J Gastroenterol 2016;111:1147–55. 10.1038/ajg.2016.236 [DOI] [PubMed] [Google Scholar]
- 2. Narula N, Peerani F, Meserve J, et al. . Vedolizumab for ulcerative colitis: treatment outcomes from the VICTORY Consortium. Am J Gastroenterol 2018;113:1345–54. 10.1038/s41395-018-0162-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feagan BG, Rutgeerts P, Sands BE, et al. . Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. 10.1056/NEJMoa1215734 [DOI] [PubMed] [Google Scholar]
- 4. Sandborn WJ, Feagan BG, Rutgeerts P, et al. . Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–21. 10.1056/NEJMoa1215739 [DOI] [PubMed] [Google Scholar]
- 5. Samaan MA, Pavlidis P, Johnston E, et al. . Vedolizumab: early experience and medium-term outcomes from two UK tertiary IBD centres. Frontline Gastroenterol 2017;8:196–202. 10.1136/flgastro-2016-100720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sands BE, Feagan BG, Rutgeerts P, et al. . Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014;147:618–27. 10.1053/j.gastro.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 7. Peyrin-Biroulet L, Danese S, Argollo M, et al. . Loss of response to vedolizumab and ability of dose intensification to restore response in patients with crohn’s disease or ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018. 10.1016/j.cgh.2018.06.026 [DOI] [PubMed] [Google Scholar]
- 8. Sierra Morales M, Birdi S, Samaan MA, et al. . P673 Vedolizumab dose escalation as a way of recapturing response in patients with inflammatory bowel disease. Journal of Crohn’s and Colitis 2018;12:S451–2. 10.1093/ecco-jcc/jjx180.800 [DOI] [Google Scholar]
- 9. Ward MG, Sparrow MP, Roblin X. Therapeutic drug monitoring of vedolizumab in inflammatory bowel disease: current data and future directions. Therap Adv Gastroenterol 2018;11:17 10.1177/1756284818772786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ungar B, Kopylov U, Yavzori M, et al. . Association of vedolizumab level, anti-drug antibodies, and α4β7 occupancy with response in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018;16:697–705. 10.1016/j.cgh.2017.11.050 [DOI] [PubMed] [Google Scholar]
- 11. Bian S, Dreesen E, Tang HT, et al. . Antibodies toward vedolizumab appear from the first infusion onward and disappear over time. Inflamm Bowel Dis 2017;23:2202–8. 10.1097/MIB.0000000000001255 [DOI] [PubMed] [Google Scholar]
- 12. Rosario M, French JL, Dirks NL, et al. . Exposure-efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or crohn’s disease. J Crohns Colitis 2017;11:921–9. 10.1093/ecco-jcc/jjx021 [DOI] [PubMed] [Google Scholar]
- 13. Christensen B, Gibson PR, Micic D, et al. . Safety and efficacy of combination treatment with calcineurin inhibitors and vedolizumab in patients with refractory inflammatory bowel disease. Clin Gastroenterol Hepatol 2018. 10.1016/j.cgh.2018.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yacoub W, Williet N, Pouillon L, et al. . Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther 2018;47:906–12. 10.1111/apt.14548 [DOI] [PubMed] [Google Scholar]
- 15. Feagan BG, Sandborn WJ, Gasink C, et al. . Ustekinumab as induction and maintenance therapy for crohn’s disease. N Engl J Med 2016;375:1946–60. 10.1056/NEJMoa1602773 [DOI] [PubMed] [Google Scholar]
- 16. Ma C, Fedorak RN, Kaplan GG, et al. . Clinical, endoscopic and radiographic outcomes with ustekinumab in medically-refractory Crohn’s disease: real world experience from a multicentre cohort. Aliment Pharmacol Ther 2017;45:1232–43. 10.1111/apt.14016 [DOI] [PubMed] [Google Scholar]
- 17. Ustekinumab summary of product characteristics. https://www.medicines.org.uk/emc/product/4413/smpc#INDICATIONS (Accessed Nov 2018).
- 18. Sands B, Gasink C, Jacobstein D, et al. . Efficacy & safety of dose adjustment & delayed response to ustekinumab in moderate–severe crohn’s disease patients: results from im-uniti maintenance study. Gut 2017;66:A23. [Google Scholar]
- 19. Rutgeerts P, Gasink C, Chan D, et al. . Efficacy of Ustekinumab for induction and maintenance of endoscopic healing in patients with crohn’s disease. United European Gastroenterology Journal 2016;2. [Google Scholar]
- 20. Adedokun OJ, Xu Z, Gasink C, et al. . Pharmacokinetics and exposure response relationships of ustekinumab in patients with crohn’s disease. Gastroenterology 2018;154:1660–71. 10.1053/j.gastro.2018.01.043 [DOI] [PubMed] [Google Scholar]
- 21. Ma C, Fedorak RN, Kaplan GG, et al. . Long-term maintenance of clinical, endoscopic, and radiographic response to ustekinumab in moderate-to-severe crohn’s disease: real-world experience from a multicenter cohort study. Inflamm Bowel Dis 2017;23:833–9. 10.1097/MIB.0000000000001074 [DOI] [PubMed] [Google Scholar]
- 22. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. . Selecting therapeutic targets in inflammatory bowel disease (stride): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–38. 10.1038/ajg.2015.233 [DOI] [PubMed] [Google Scholar]
- 23. de Lange T, Moum BA, Tholfsen JK, et al. . Standardization and quality of endoscopy text reports in ulcerative colitis. Endoscopy 2003;35:835–40. 10.1055/s-2003-42619 [DOI] [PubMed] [Google Scholar]
- 24. Devlin SM, Melmed GY, Irving PM, et al. . Recommendations for quality colonoscopy reporting for patients with inflammatory bowel disease: results from a RAND appropriateness panel. Inflamm Bowel Dis 2016;22:1418–24. 10.1097/MIB.0000000000000764 [DOI] [PubMed] [Google Scholar]
- 25. Singh S, Fumery M, Sandborn WJ, et al. . Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn’s disease. Aliment Pharmacol Ther 2018;48:394–409. 10.1111/apt.14852 [DOI] [PubMed] [Google Scholar]
- 26. Singh S, Fumery M, Sandborn WJ, et al. . Systematic review with network meta-analysis: first- and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment Pharmacol Ther 2018;47:162–75. 10.1111/apt.14422 [DOI] [PubMed] [Google Scholar]
- 27. Bohm M, Sagi SV, Fischer M, et al. . OP025 Comparative effectiveness of vedolizumab and tumour necrosis factor-antagonist therapy in Crohn’s disease: a multicentre consortium propensity score-matched analysis. Journal of Crohn’s and Colitis 2018;12:S018 10.1093/ecco-jcc/jjx180.024 [DOI] [Google Scholar]
- 28. Faleck D, Shashi P, Meserve J, et al. . OP026 Comparative effectiveness of vedolizumab and TNF-antagonist therapy in ulcerative colitis: a multicentre consortium propensity score-matched analysis. Journal of Crohn’s and Colitis 2018;12:S019 10.1093/ecco-jcc/jjx180.025 [DOI] [Google Scholar]
- 29. Mitrev N, Vande Casteele N, Seow CH, et al. . Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2017;46:1037–53. 10.1111/apt.14368 [DOI] [PubMed] [Google Scholar]
- 30. Feuerstein JD, Nguyen GC, Kupfer SS, et al. . American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017;153:827–34. 10.1053/j.gastro.2017.07.032 [DOI] [PubMed] [Google Scholar]
- 31. Kochhar G, Parikh M, Chaudrey K, et al. . PD-002 Mucosal healing with vedolizumab in ulcerative colitis and crohn’s disease: outcomes from the VICTORY consortium. Inflammatory Bowel Diseases 2017;23:S6. [Google Scholar]
- 32. Loftus EV, Colombel JF, Feagan BG, et al. . Long-term efficacy of vedolizumab for ulcerative colitis. J Crohns Colitis 2017;11:400–11. 10.1093/ecco-jcc/jjw177 [DOI] [PubMed] [Google Scholar]



