Abstract
Non-alcoholic fatty liver disease (NAFLD) is a globally prevalent health problem, associated in its more severe forms with increased liver-related and cardiovascular-related morbidity and mortality. We established a multidisciplinary metabolic hepatology clinic in 2014 and have analysed the clinical data to evaluate the effectiveness of this service.
Patients with NAFLD (n=165) who had attended two or more appointments were included. Prespecified clinical data were collected prospectively at clinic appointments and analysed retrospectively. Interventions offered included lifestyle advice, signposting to weight loss services and pharmacological treatment of diabetes and cardiovascular risk factors.
Median follow-up was 13 months (range: 2–34). 59% (n=97) of patients had type 2 diabetes mellitus (T2DM). 53% (n=87) underwent liver biopsy of whom 18% (n=16) had cirrhosis. Median alanine aminotransferase (ALT) reduced by 11 IU/L (p<0.0001), median weight reduced by 3.3 kg (p=0.0005). There were significant reductions in HbA1c, total cholesterol and liver stiffness. Specifically, in patients with T2DM, HbA1c decreased by 4 mmol/mol (p=0.01) with significant reductions in ALT, weight and total cholesterol. Relative cardiovascular risk assessed by the QRISK3 score reduced in the whole cohort and in those with T2DM. Health economic modelling suggested the clinic intervention among those patients with poorly controlled T2DM was cost-effective.
In conclusion, a multidisciplinary approach to the management of patients with NAFLD in this observational cohort study was associated with improvements in liver-related and cardio-metabolic related health parameters and with evidence of cost-effectiveness in patients with poorly controlled T2DM.
Keywords: fatty liver, economic evaluation, diabetes mellitus, non-alcoholic steatohepatitis
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a globally prevalent public health burden. Estimates indicate that between 20% and 30% of many populations are affected and the prevalence is expected to rise with increasing prevalence of obesity and type 2 diabetes mellitus (T2DM).1
NAFLD is considered a disease spectrum from hepatic steatosis, which may be accompanied by histologically defined inflammation (non-alcoholic steatohepatitis, NASH), with or without liver fibrosis (typically classified as mild fibrosis (stages: F0–F1), significant fibrosis (F2) or advanced fibrosis (stages: F3–F4) with F4 fibrosis being a diagnosis of cirrhosis).2 Liver cirrhosis confers a significantly increased risk of hepatic decompensation and development of hepatocellular carcinoma. The presence of advanced fibrosis predicts reduced transplant-free survival, increased liver-related mortality and all-cause mortality.3 4 Nevertheless, the principal causes of morbidity and mortality from NAFLD are from cardiovascular and metabolic complications.3 4 Furthermore, T2DM is the strongest risk factor for NAFLD disease progression.5 Those with both NAFLD and T2DM have accelerated liver disease progression and more prevalent and severe complications of diabetes.6 7 Studies have suggested that even significant fibrosis is associated with increased mortality.8
NAFLD should thus be considered a cardio-metabolic disease as well as a liver disease with implications for risk stratification and for management. Accordingly, appropriate disease staging at presentation will identify those at high risk of adverse outcomes who would benefit from more intensive management. As there are no currently licenced treatments specifically for NAFLD, management focuses on modification of risk factors for NAFLD progression and cardiovascular disease within a multidisciplinary framework.9–11
This study aimed to evaluate the impact of a metabolic hepatology clinic that adopts a multidisciplinary, holistic approach to treat NAFLD and uses a number of markers of liver and cardio-metabolic health to assess its performance. These included thorough assessment of surrogate markers of liver injury, diabetes control and cardiovascular risk. Furthermore, this study evaluated the effects of the clinic on quality adjusted life expectancy (QALE), and estimated the cost-effectiveness of the approach.
Methods
Intervention: a multidisciplinary metabolic hepatology clinic
The Oxford University Hospitals National Health Service (NHS) Foundation Trust (OUH) delivers a weekly secondary/tertiary multidisciplinary metabolic hepatology clinic, managing patients from across Oxfordshire, UK, and the surrounding regions. Referrals to the clinic are received from both primary care and secondary care settings. OUH works closely with the Oxfordshire Clinical Commissioning Group and has produced guidelines12 to assist primary care professionals to investigate and refer patients. These guidelines assist general practitioners to risk stratify patients and help ensure that those at high risk of advanced liver disease are referred to and managed in secondary care (this pathway was updated in November 2017 after the period covered by the analysis in this paper). From January 2015 to November 2017, local guidance recommended that patients with suspected NAFLD in primary care first underwent risk-stratification with the NAFLD fibrosis score (NFS) with referral of those patients with indeterminate or high-risk scores to the metabolic hepatology clinic. However, referrals without risk stratification were not refused and risk stratification was performed in clinic. If the diagnosis was unclear, patients were referred to the general hepatology clinic. Risk stratification would then be performed by NFS, Fib-4 score and/or FibroScan and subsequent appointments scheduled in the metabolic hepatology clinic if appropriate.
The clinic is jointly led by hepatologists and diabetologists/metabolic physicians. The aim of the clinic is to improve liver-related and cardiovascular health in patients with NAFLD/NASH. A range of lifestyle and medical interventions are employed. Emphasis is given to weight management and the achievement of meaningful weight loss in those who are overweight or obese via dietary and lifestyle modifications. Medications are used to aid reduction of cardiovascular risk, for example, the use of antihypertensive and statin therapy, as well as to improve diabetes control where appropriate. For the control of diabetes, therapies that are weight-neutral or encourage weight loss such as SGLT2 inhibitors and GLP-1 agonists are favoured.13–16 These newer diabetes therapies are further beneficial as they also reduce cardiovascular risk.17–19
The clinic is supported by specialist nurses performing transient elastography (FibroScan) and anthropometrics immediately prior to the medical consultation and by specialist practitioners via the Here for Health service. This is a special service at OUH that bridges the link between the acute hospital setting and currently available community services. It provides a range of health and well-being advice including weight management (diet, lifestyle and exercise), but also smoking cessation, alcohol reduction and signposting to mental health services (www.ouh.nhs.uk/patient-guide/here-for-health/default.aspx). Blood testing, imaging, liver biopsy and screening for hepatocellular carcinoma in patients with established cirrhosis are performed where clinically appropriate and in accordance with relevant clinical guidelines. Patients with mild disease, either on non-invasive markers (low-risk NFS or Fib-4, FibroScan <8 kPa without adjustable metabolic complications, or liver biopsy F≤2 in absence of metabolic complications) are typically discharged from clinic. Exceptions include those with complex metabolic comorbidities, florid NASH in young people and others at the clinician’s discretion. Local guidance since November 2017 recommends repeat non-invasive risk stratification in patients with mild disease in 3 years to look for evidence of progression.
Study analysis
A retrospective analysis of all patients who were managed through the metabolic hepatology clinic at OUH between inception in March 2014 until May 2017 was performed. Patients were included in the analysis if they had (1) attended the clinic at least twice, (2) had an alanine aminotransferase (ALT) level recorded at baseline and their latest clinic visit and (3) had weight recorded at baseline. Clinical data from all patients attending since clinic inception were collected on a clinic proforma and subsequently recorded on a centrally held, secure, departmental clinical spreadsheet for audit purposes.
Diagnosis of NAFLD was made according to the National Institute for Health and Care Excellence (NICE)20 and other guidance; either radiologically (liver ultrasound or liver MRI), histologically on liver biopsy, or on the basis of persistently elevated liver enzymes (elevated ALT and/or aspartate aminotransferase (AST) levels (>40 IU/L) in the context of features of the metabolic syndrome and where other causes of liver pathology had been excluded. Patients with known hepatic comorbidity, patients who did not have a diagnosis of NAFLD, those who had type 1 diabetes and those who had previously undergone or who underwent bariatric surgery during the follow-up period were excluded from the analysis (online supplementary figure 1).
flgastro-2018-101155supp001.pdf (548KB, pdf)
Patients underwent assessments of liver and cardio-metabolic health using routine non-invasive tools including measurement of serum ALT, AST and liver stiffness measurement by transient elastography (FibroScan), a validated surrogate marker of liver fibrosis.21 Calculation of the Fib-4 score22 provided additional non-invasive assessment of risk of advanced liver fibrosis. Liver biopsy, still the reference standard for diagnosis and assessment of disease severity, was performed where clinically indicated.
Cardio-metabolic assessment included measurement of weight, body mass index (BMI), alcohol intake, blood pressure, lipids and diabetes control (HbA1c). All patients were asked to complete a qualitative 7-day food diary in advance of clinic attendance. Cardio-metabolic risk factors were defined using standard measures employed in clinical practice: hypertension if blood pressure was ≥140/90 mm Hg, or if the patient was taking antihypertensive medication; type 2 diabetes according to WHO criteria (HbA1c≥48 mmol/mol), if the diagnosis was already established at baseline or if patients were taking anti-diabetic medication; dyslipidaemia according to local thresholds (triglycerides>1.7 mmol/L and/or high-density lipoprotein (HDL) cholesterol <1.0 mmol/L (male),<1.3 mmol/L (female) and obesity if BMI was >30 kg/m2. All patients had 10-year cardiovascular risk assessment (absolute and relative risk) calculated retrospectively using the QRISK3-2017 score23 and each patient’s current drug history was recorded at clinic visits. For patients with T2DM, data on prescribed medical therapies were collated from clinic letters and cross-referenced with written clinic proformas.
The primary outcome was change in ALT level between baseline and latest clinic visit. Secondary outcomes were change in weight, glycated haemoglobin (HbA1c), AST, lipid profile (total cholesterol, HDL, triglycerides), systolic blood pressure, transient elastography, Fib-4 score, NFS and QRISK3 cardiovascular risk score. Patients with a missing variable value at baseline and/or latest clinic visit were not included for paired analysis of that variable. A subgroup analysis was performed on patients with (1) T2DM at baseline and (2) poorly controlled T2DM at baseline, defined as baseline HbA1c>58 mmol/mol, who also had a latest clinic HbA1c measurement.
Economic analysis and assessment of QALE
The UK Prospective Diabetes Study (UKPDS) Outcomes Model (V.2.0, UKPDS-OM2), a lifetime simulation model for patients with T2DM which has been extensively validated, was used to model and predict changes in QALE as well as the potential cost-effectiveness of our approach.24 UKPDS-OM2 was first applied to all patients with T2DM and subsequently in those patients with poorly controlled T2DM at baseline (HbA1c>58 mmol/mol). The model, incorporates phenotypic data and changes to various modifiable cardio-metabolic risk factors over time. A standard, accepted modelling approach was adopted to compare outcomes in the changes observed in those attending the clinic to outcomes in the absence of these changes (see online supplementary materials for in-depth description).
Statistical analysis
Statistical analysis was performed using GraphPad Prism (V.7.0). Data were non-parametrically distributed as assessed by the D'Agostino-Pearson omnibus test. Continuous variables are quoted as median (range), and categorical variables as numbers and percentages. Non-parametric tests were used to assess for statistical significance (Wilcoxon signed rank test for paired baseline and latest visits, Mann-Whitney U and χ2 tests for comparing between the T2DM and non-T2DM subgroups) and the threshold for significance was set at the 5%. Endpoints were set as baseline to latest follow-up and response.
Results
Baseline characteristics
165 patients with NAFLD were followed from baseline until their latest clinic visit. Baseline characteristics are described in table 1. Median interval between baseline and latest follow-up clinic visit was 13.3 months (range 2–34), with a median of two follow-up visits (range 1–5).
Table 1.
Baseline characteristics of the total cohort and by T2DM status
| Baseline characteristic, median (range) or number (%) | Total cohort | T2DM subgroup | Non-T2DM subgroup | |||
| n=165 | n=97 | n=68 | ||||
| Demographic | ||||||
| Age, years | 53 | (16–79) | 57 | (29–79) | 47.5 | (16–69) |
| Sex, male | 106 | (64.2%) | 62 | (63.9%) | 44 | (64.7%) |
| Caucasian ethnicity | 132 | (80.0%) | 78 | (80.4%) | 54 | (79.4%) |
| Metabolic | ||||||
| HbA1c, mmol/mol* | 46 | (25–124) | 59 | (35–124) | 36.5 | (25–46) |
| Total cholesterol, mmol/L†† | 4.4 | (2.4–9.0) | 4.0 | (2.4–7.7) | 4.9 | (2.7–9.0) |
| HDL, mmol/L†† | 1.0 | (0.5–2.4) | 1.0 | (0.5–1.7) | 1.0 | (0.8–2.4) |
| Triglyceride, mmol/L† | 1.93 | (0.52–17.01) | 2.13 | (0.52–10.71) | 1.72 | (0.68–17.01) |
| Systolic blood pressure, mm Hg‡ | 140 | (105–190) | 140 | (105–189) | 138 | (108–190) |
| Metabolic syndrome (MetS) | ||||||
| BMI, kg/m2 | 33.3 | (23.9–72.1) | 34.0 | (23.9–52.6) | 32.5 | (24.2–72.1) |
| Obesity (BMI>30 kg/m2) | 120 | (72.7%) | 71 | (73.2%) | 49 | (72.1%) |
| Hypertension | 93 | (56.4%) | 72 | (74.2%) | 21 | (30.9%) |
| Dyslipidaemia | 105 | (63.6%) | 71 | (73.2%) | 34 | (50.0%) |
| Number of MetS components, /4 | 3 | (0–4) | 3 | (1–4) | 2 | (0–3) |
| Lifestyle | ||||||
| Alcohol, units/week | 0 | (0–21) | 0 | (0–21) | 0 | (0–20) |
| Current smoker | 10 | (6.1%) | 6 | (6.2%) | 4 | (5.9%) |
| Ex-smoker | 53 | (32.1%) | 35 | (36.1%) | 18 | (26.5%) |
| Liver | ||||||
| ALT, IU/L | 52 | (12–215) | 50 | (12–200) | 54 | (12–215) |
| Abnormal ALT (>40 IU/L) | 111 | (67.3%) | 60 | (61.9%) | 51 | (75.0%) |
| Transient elastrography, kPa§ | 9.2 | (3.5–75.0) | 10.1 | (4.3–75.0) | 8.2 | (3.5–53.3) |
| Biopsy characteristics | ||||||
| Number (%) | 87 | (52.7%) | 54 | (55.7%) | 33 | (48.5%) |
| NAS, /8¶ | 5 | (2–8) | 5 | (2–8) | 5 | (2–7) |
| NASH¶ | 57 | (72.1%) | 36 | (73.5%) | 21 | (70.0%) |
| Fibrosis stage, (F0–F4)** | 2 | (0–4) | 3 | (0–4) | 2 | (1–4) |
| F0 (n, %) | 1 | (1.2%) | 1 | (1.9%) | 0 | (0.0%) |
| F1 (n, %) | 15 | (17.2%) | 6 | (11.1%) | 9 | (27.3%) |
| F2 (n, %) | 34 | (39.1%) | 20 | (37.0%) | 14 | (42.4%) |
| F3 (bridging fibrosis) (n, %) | 21 | (24.1%) | 14 | (25.9%) | 7 | (21.2%) |
| F4 (cirrhosis) (n, %) | 16 | (18.4%) | 13 | (24.1%) | 3 | (9.1%) |
Biopsy characteristics provided for available data.
*Number of patients with baseline measurement, n=136, 88, 48 (of total cohort, T2DM subgroup, non-T2DM subgroup, respectively).
†N=100, 59, 41.
‡N=137, 82, 55.
§N=133, 73, 60.
¶NAS (NAFLD activity score) (/8); NASH defined as NAS>5; number of patients with measurement per group (percentage with NASH): n=79, 49, 30 in each column group.
**Fibrosis stage (Brunt’s scale) (F0–F4); cirrhosis defined as fibrosis stage F4 or historical diagnosis in the case of one missing biopsy score. Number of patients with measurement per group (percentage): n=86, 53, 33.
††n=113, 68, 45.
ALT, alanine aminotransferase; BMI, body mass index; HDL, high-density lipoprotein; NAS, NAFLD activity scores; NASH, non-alcoholic steatohepatitis; T2DM, type 2 diabetes mellitus.
Patients reported taking a median of four prescription medications (range 0–17) at baseline. Oral hypoglycaemic agents were the most commonly prescribed medication with 52% taking >1 agent, followed by anti-hypertensive therapy (51%) and statin therapy (42%).
87 patients (53%) had a liver biopsy prior to or during the follow-up period (table 2). There was no significant relationship between T2DM status and histological NAFLD activity scores (NAS) (p=0.75). Median NAS in patients with T2DM and without T2DM were both five. There was also no significant relationship between T2DM status and fibrosis stage (p=0.19) although the majority of patients with biopsy-proven cirrhosis in the cohort had T2DM (13/16, 81%).
Table 2.
Change in liver and cardio-metabolic health parameters from baseline to latest visit
| Measure (median) | Total cohort | T2DM subgroup | ||||||||
| N | Base | Latest | Δ | P value | N | Base | Latest | Δ | P value | |
| Liver function test | ||||||||||
| ALT, IU/L | 165 | 52 (12–215) | 41 (11–240) | −11 | < 0.0001 | 97 | 50 (12–200) | 40 (11–125) | −10.0 | < 0.0001 |
| AST, IU/L | 65 | 40 (15–171) | 33 (14–105) | −7 | 0.011 | 35 | 35 (16–171) | 31 (14–63) | −4.0 | 0.13 |
| Weight | ||||||||||
| Weight, kg | 159 | 97.3 (55.0–206.0) | 94.0 (53.9–182.2) | −3.3 | 0.0005 | 94 | 96.8 (55.0–154.6) | 94.6 (53.9–180.5) | −2.2 | 0.0030 |
| Metabolic | ||||||||||
| HbA1c, mmol/mol | 112 | 49 (25–124) | 47 (22–110) | −1.5 | 0.0045 | 84 | 59 (35–124) | 55 (33–110) | −4.0 | 0.011 |
| Total cholesterol, mmol/L | 76 | 4.7 (2.4–9.0) | 4.0 (1.9–8.1) | −0.7 | 0.0023 | 48 | 4.1 (2.4–7.7) | 3.9 (1.9–8.1) | −0.20 | 0.0071 |
| HDL, mmol/L | 76 | 1.0 (0.5–2.4) | 1.0 (0.6–1.9) | 0.0 | 0.75 | 48 | 1.0 (0.5–1.7) | 1.0 (0.6–1.9) | 0.00 | 0.85 |
| Triglyceride, mmol/L | 47 | 2.1 (0.67–17.0) | 1.9 (0.6–9.0) | −0.25 | 0.28 | 29 | 2.1 (0.7–7.8) | 2.0 (0.6–9.0) | −0.12 | 0.36 |
| Systolic blood pressure, mm Hg | 125 | 140 (105–189) | 135 (102–193) | −5 | 0.24 | 74 | 141 (105–189) | 136 (102–193) | −5 | 0.76 |
| Liver | ||||||||||
| Fib-4 score | 62 | 1.1 (0.2–7.3) | 1.2 (0.2–9.3) | 0.05 | 0.71 | 35 | 1.5 (0.4–7.3) | 1.2 (0.4–9.3) | −0.28 | 0.94 |
| NFS | 59 | −1.0 (−6.5 to +5.8) | −0.69 (−6.0 to +3.9) | 0.32 | 0.0012 | 33 | 0.02 (−3.9 to −3.0) |
−0.3 (−4.7 to −3.1 | −0.33 | 0.089 |
| Transient elastography, kPa | 73 | 9.1 (3.5–75) | 7.8 (3.2–57) | −1.3 | 0.0097 | 39 | 9.7 (4.3–75) | 8.4 (4.4–57) | −1.3 | 0.067 |
| Cardiovascular Disease (QRISK3) | ||||||||||
| Absolute risk, % | 159 | 12.5 (0.1–60.9) | 12.7 (0.1–52.6) | 0.2 | 0.17 | 94 | 18.3 (1.7–61.0) | 19.3 (1.9–53.0) | 1.0 | 0.13 |
| Relative risk | 159 | 2.1 (0.7–11.5) | 2.0 (0.8–12.9) | −0.1 | 0.0001 | 94 | 2.7 (1.1–11.5) | 2.6 (1.1–12.9) | −0.15 | 0.0006 |
Wilcoxon signed rank test between baseline and latest; bold p value represents statistical significance.
Δ, difference between median at baseline (base) and latest visit; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Fib-4, Fibrosis-4; HDL, high-density lipoprotein; N, number of patients with paired data; NFS, NAFLD fibrosis score; T2DM, type 2 diabetes mellitus.
Changes to liver and cardio-metabolic health: baseline to latest clinic visit in all patients
At latest follow-up, median ALT had reduced significantly by 11 IU/L and median AST reduced significantly by 7 IU/L (table 2 and figure 1). Median liver transient elastography also reduced significantly by 1.3 kPa. For Fib-4 and NFS, there were no changes in the proportion of patients switching between categories (risk of presence or absence of advanced liver fibrosis or indeterminate score) (Fib-4: p=0.8, NFS: p=0.7).
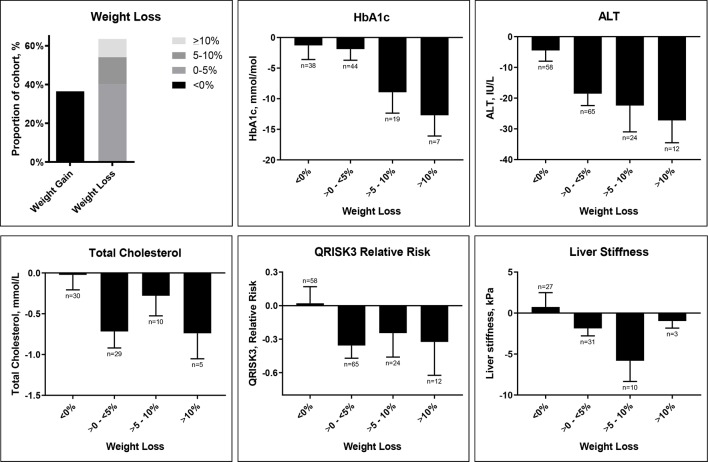
Figure 1.
Change in weight and change in cardio-metabolic and liver health stratified by weight loss: baseline to follow-up. ALT, alanine aminotransferase.
Analysis of weight changes revealed that the majority of patients lost weight. The distribution of weight loss is shown in figure 1. Figure 1 also summarises changes in cardio-metabolic and liver parameters stratified by weight loss. In this retrospective analysis of clinical data, even modest weight loss of <5% was associated with improvement in these parameters and suggested that the magnitude of improvement was greater with increased weight loss.
QRISK3 10-year cardiovascular relative risk reduced significantly by 0.1 (5%). There was no significant change in QRISK3 10-year absolute risk from baseline to latest visit, though when follow-up age, a component of this algorithm, was corrected to ‘age-match’ (remain the same) to baseline, this reduced significantly by 0.8 (6%).
Additional analyses of subjects by fibrosis stage (early fibrosis and advanced fibrosis) and by weight response (weight gain or weight loss) were also performed to gain an insight into improvements in liver and metabolic health. These results are summarised in online supplementary tables 1 and 2.
Changes to liver and cardio-metabolic health in patients with T2DM
T2DM is strongly associated with NAFLD progression,5 and therefore a subgroup analysis of patients with T2DM was performed (tables 1 and 2).
At baseline, patients with T2DM were older than patients without T2DM (p<0.0001), and as expected, had higher baseline HbA1c (p<0.0001) (table 1). Liver stiffness was also significantly higher at baseline in those with T2DM (p=0.011). There was a significant relationship between the presence of T2DM and total number of metabolic syndrome components (p<0.0001). Those with T2DM had lower total cholesterol at baseline than those without (p=0.0011) (table 1). Statin use at baseline was higher in those with T2DM (65%) compared with those without T2DM (42%).
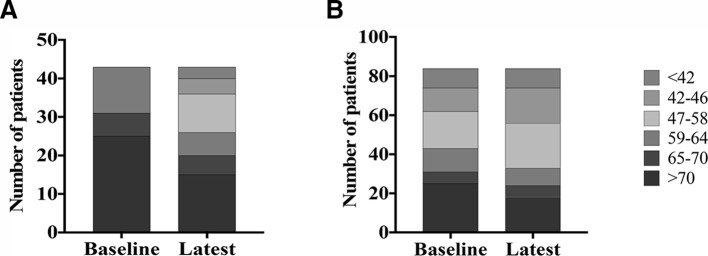
Median HbA1c reduced significantly by 4 mmol/mol between baseline to last follow-up in those patients with T2DM (table 2). Improvement was most marked in patients who had poorly controlled T2DM at baseline (HbA1c>58 mmol/mol, n=43), reducing by 14 mmol/mol (online supplementary table 3). There were also significant improvements in categories of glycaemic control for those with poorly controlled T2DM subgroup (p=0.0006) (figure 2). Weight also decreased in patients with T2DM (table 2).
Figure 2.
Change in HbA1c in patients with type 2 diabetes mellitus (T2DM): baseline to follow-up. (A) Patients with poorly controlled T2DM (HbA1c>58 mmol/mol) at baseline. (B) All patients with T2DM at baseline.
At baseline, 85 (88%) of patients with T2DM were already prescribed oral glucose lowering therapy, 26% were prescribed insulin therapy and 5% were prescribed GLP-1 therapy. 21% of patients with T2DM started GLP-1 therapy over the duration of the study and 20% of patients who were on insulin therapy at baseline had this discontinued. In those who had insulin therapy discontinued (n=5), median HbA1c reduced by 13 mmol/mol from this point to latest follow-up. 15% of patients were commenced on statin therapy. Net changes to medications in patients with T2DM are summarised in online supplementary figure 2.
Changes in QALE and economic analysis in patients with T2DM
For all patients with T2DM, the UKPDS-OM2 model estimated no significant difference in mean life expectancy in the intervention group (clinic attendees) compared with the reference group (baseline measures carried forward) (online supplementary table 4). In those with poorly controlled T2DM, the intervention group had a significantly increased mean life expectancy of 24 days (95% CI 2 to 50 days) compared with the reference group. Similarly, mean QALE was significantly increased by 29 days (95% CI 6 to 55 days) suggesting that patients with poorly controlled T2DM managed through our multidisciplinary clinic approach had significant improvements in both overall and quality adjusted life expectancy.
UKPDS-OM2 was also used to evaluate cost-effectiveness in patients with poorly controlled T2DM. Lifetime (total) costs were £30.0 k in the clinic group and £29.5 k in the reference group who did not attend the clinic, a mean difference of £0.5 k. Lifetime quality-adjusted life years (QALYs) were 11 years in the clinic group and 10.9 years in the reference group, a difference of 0.1 years (29 days). The resulting incremental cost-effectiveness ratio (cost per QALY was £6.1 k (95% CI £0.3 k to £59.3 k) with 91% of model bootstraps runs falling below a cost per QALY threshold of £20 000.
Discussion
Recent UK and European guidelines,20 25 advise that management of NAFLD should be multifaceted and patients should be managed using a multidisciplinary approach.9 10 Nevertheless, there is still a paucity of best practice data describing how such services should be shaped and delivered as well as objective evaluations of the impacts of a multidisciplinary approach on patient outcomes.
Here, we have evaluated our experience of managing a cohort of patients with NAFLD using a dedicated multidisciplinary metabolic hepatology clinic. We describe significant improvements in both clinical and surrogate markers of liver and cardio-metabolic health over a 13-month median follow-up period. This is an observational study of real-life clinical data making it applicable to clinical practice. There was, however, no control arm and the interventions are not uniform for the cohort given the personalised nature of the clinical approach, so causality cannot be inferred. Moreover, such data may be confounded by selection bias and regression to the mean. Nevertheless, it forms a useful benchmark of current clinical practice to which other services or interventions may be compared.
Improvements in markers of liver health
We observed a 14% reduction in liver stiffness in patients attending the metabolic hepatology clinic, measured at point of care and an associated 21% improvement in serum ALT, an insensitive marker of NASH, though one which is still commonly used by healthcare professionals.26 27 This improvement in ALT is similar to that observed in patients with NAFLD managed through a similar multidisciplinary approach9 and in lifestyle intervention trials.28 ALT has also been highlighted as a non-invasive surrogate marker of response in clinical trials in NAFLD/NASH.29 No other current non-invasive biomarkers, serological or non-serological, have been validated to monitor changes in liver disease severity or to predict liver-related health outcomes, mainly due to poor sensitivity to detect small changes in liver fibrosis.5 In this cohort, there was no significant improvement in the Fib-4 score. There were also no significant changes in the numbers of patients predicted to have advanced liver fibrosis between baseline and follow-up using non-invasive scores, though the relevance or utility of these findings is not known.
Weight loss and glycaemic control
Both UK NICE30 and American Diabetes Association (ADA)31 guidance highlights the importance of ‘personalised’ or ‘individualised’ care when managing patients’ diabetes taking into account comorbidities and include guidance on weight management. In particular, the more recent ADA guidance outlines that clinicians should consider the weight and cardiovascular risk effects of glucose lowering therapies when escalating diabetes therapy which is particularly important as some newer agents such as GLP-1 agonists and SGLT2 inhibitors have been shown to have beneficial effects on either or both of weight and cardiovascular risk.
In the UK, GLP-1 therapy has a licence for use in patients with T2DM, and NICE guidance30 outlines that this therapy is recommended in patients with poorly controlled T2DM who are obese, owing to improvements to glycaemic control and promotion of weight loss. Our analysis indicates a reduction in prescriptions for diabetes medications, such as insulin, that are associated with weight gain and an increase in prescriptions for diabetes medications that are weight neutral or associated with weight loss and those which potentially confer cardiovascular protection.
These finding were complemented by overall improvement in glycaemic control for all patients with T2DM, with improvement most marked in those with poorly controlled T2DM. These findings on improvement in glycaemic control in a real-life clinic setting are consistent with previously published studies investigating the impact of diet and or exercise in patients with T2DM. For example, in the Early Activity in Diabetes (Early ACTID) trial in patients with recently diagnosed T2DM who had good glycaemic control at baseline (mean baseline HbA1c 49–50 mmol/mol), HbA1c improved by around 3.3 mmol/mol at 6 and 12 months in those provided with ‘dietary support’ (dietary consultation every 3 months with monthly nurse support), compared with a control group provided with ‘usual care’ (initial dietary consultation and follow-up every 6 months—which is often the standard model and frequency of care for patients managed in primary care).32 Additionally, in this study there was no difference in improvement in HbA1c between the ‘dietary support’ group and a ‘diet plus activity’ group (as per diet group, plus 30 min brisk walking five times per week) at either six or 12 months. Previous studies have however shown that a combination of diet and exercise is effective in improving glycaemic control by around 11 mmol/mol at 6 and 12 months.33 Similarly, in another study, patients with T2DM either provided with specifically tailored food for 1 year or with monthly individual dietary counselling advice saw an approximate 11 mmol/mol reduction in HbA1c over 12 months while a control group receiving standard diabetes care monthly via appointment with a doctor or nurse saw no change in HbA1c over 12 months.34 35 Improvement in glycaemic control in the scale observed in our study are accepted as likely to improve both diabetes-related and liver-related complications.
Overall, patients achieved a median 3.4% weight loss, though there was considerable variation in response. 23% of patients (37/159 with both baseline and latest weight measurements recorded) lost at least 5% of their baseline weight by latest visit. Previous studies have demonstrated an association between weight loss and improvements in NAFLD, with a reduction in excess of 3%–5% thought to be required for improvement of hepatic steatosis and/or NASH.36 37 Stratification of markers of liver and cardio-metabolic health by weight response demonstrated that improvements to these markers appear to correlate with degree of weight loss. Our data also suggests that even modest weight loss is associated with improvements in markers of liver and cardio-metabolic health. Given the relatively short duration of follow-up, the dynamics of weight loss and any subsequent weight gain were not analysed in this study but would comprise an important follow-up analysis.
Patients often struggle to lose and maintain weight loss without structured support, but there is evidence that commercial weight loss programmes have significant benefit38 with weight loss of >5% reported, which is of a magnitude that will confer benefit to patients with NAFLD. Liraglutide at a higher dose of 3 mg daily is also licenced for use in patients with obesity without diabetes, but its use in this context is not yet supported through NICE guidance within the NHS.
Finally, our simulation of the lifetime costs and outcomes of the clinic intervention in patients with poorly controlled T2DM indicated that it had a high probability of being cost-effective at the thresholds used by NICE, with an incremental cost per QALY of £6.1 k. While there was no dedicated control group available, a standardised modelling approach was adopted. As such, these findings provide useful additional information and have importance due to the high and growing burden of NAFLD and the need for health authorities to have both clinically effective and cost-effective strategies at their disposal when working within confined financial envelopes.
Conclusions
While considerable effort is going into the development of novel therapies that target the hepatic manifestations of NAFLD/NASH, data from this study indicate that significant improvements in surrogate markers of liver and cardio-metabolic disease can be achieved through effective implementation of existing risk-stratification and treatment strategies. Furthermore, given that NAFLD is both a liver and a cardio-metabolic disease, we have shown that adopting a multidisciplinary approach to its management—involving not only hepatologists, diabetologists and metabolic physicians, but also allied health professionals including diet and lifestyle experts—is a clinically effective and potentially cost-effective way to manage these complex patients.
Significant of this study.
What is already known about this subject?
Non-alcoholic fatty liver disease (NAFLD) is a globally prevalent public health burden with the principal causes of morbidity and mortality being from cardiovascular complications as well as liver disease and cancer. A multidisciplinary approach to the management of patients with NAFLD is advocated but there is a paucity of best practice data describing how such services should be shaped and delivered.
What are the new findings?
We have evaluated our experience of managing a cohort of patients with NAFLD using a dedicated multidisciplinary approach that is jointly led by hepatologists and diabetologists in a teaching hospital setting with significant input from allied health professionals including diet and lifestyle experts. We describe significant improvements in both clinical and surrogate markers of liver and cardio-metabolic health over a 13-month median follow-up period. These include improvements in liver chemistry and hepatic elastography as well as improvements in weight and glycaemic (diabetes) control.
How might it impact on clinical practice in the foreseeable future?
This study forms a useful benchmark of current clinical practice to which other services or interventions may in future be compared. In addition, it may help healthcare providers and health policy makers to refine or design and implement healthcare services related to NAFLD, diabetes and metabolic syndrome.
Acknowledgments
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Contributors: JFC, AM and JT planned and designed the study and take overall responsibility for the content. JFC and JT jointly lead the metabolic hepatology clinical service at OUH NHS Trust described in the manuscript and along with AM, MP, JDR and MA provide clinical care to subjects attending this service. AM, KM, MA, TM and AS collated the clinical data analysed. AM and KM undertook the primary data analysis. AM, KM, RH and AG designed and undertook the health economic analysis. AM, KM, JT and JFC wrote the manuscript which all authors subsequently reviewed and contributed to.
Funding: This work was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC), by the Medical Research Council (programme grant to JWT) and AM was supported by a Novo Nordisk Clinical Research Fellowship run in partnership with the University of Oxford.
Competing interests: JFC and JT have received consultancy fees from Novo Nordisk. JFC has received speaker fees from Intercept Pharmaceuticals.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274–85. 10.1111/j.1365-2036.2011.04724.x [DOI] [PubMed] [Google Scholar]
- 2. Brunt EM, Janney CG, Bisceglie AM, et al. . Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterology 1999;94:2467–74. 10.1111/j.1572-0241.1999.01377.x [DOI] [PubMed] [Google Scholar]
- 3. Angulo P, Kleiner DE, Dam-Larsen S, et al. . Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389–97. 10.1053/j.gastro.2015.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ekstedt M, Hagström H, Nasr P, et al. . Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547–54. 10.1002/hep.27368 [DOI] [PubMed] [Google Scholar]
- 5. McPherson S, Hardy T, Henderson E, et al. . Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. Journal of Hepatology 2015;62:1148–55. 10.1016/j.jhep.2014.11.034 [DOI] [PubMed] [Google Scholar]
- 6. Yan L-hui, Mu B, Guan Y, et al. . Assessment of the relationship between non-alcoholic fatty liver disease and diabetic complications. J Diabetes Investig 2016;7:889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Targher G, Bertolini L, Padovani R, et al. . Increased prevalence of cardiovascular disease in type 2 diabetic patients with non-alcoholic fatty liver disease. Diabet Med 2006;23:403–9. 10.1111/j.1464-5491.2006.01817.x [DOI] [PubMed] [Google Scholar]
- 8. Dulai PS, Singh S, Patel J, et al. . Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 2017;65:1557–65. 10.1002/hep.29085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cobbold JFL, Raveendran S, Peake CM, et al. . Piloting a multidisciplinary clinic for the management of non-alcoholic fatty liver disease: initial 5-year experience. Frontline Gastroenterol 2013;4:263–9. 10.1136/flgastro-2013-100319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Townsend SA, Newsome PN. The role of a dedicated non-alcoholic fatty liver disease clinic in 2016. Dig Dis 2017;35:371–6. 10.1159/000456589 [DOI] [PubMed] [Google Scholar]
- 11. Chalasani N, Younossi Z, Lavine JE, et al. . The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the study of liver diseases. Hepatology 2018;67:328–57. 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 12. Oxford University Hospitals NHS Foundation Trust & Oxfordshire Clinical Commissioning Group Investigating and Referring Incidental Findings of Abnormal Liver Tests [Internet]. v3.5 - 17/01/2018, 2018. Available: https://clinox.info/clinical-support/local-pathways-and-guidelines/Clinical Guidelines/Hepatology Referral Guidelines.pdf [Accessed cited 2019 Jan 8].
- 13. Armstrong MJ, Gaunt P, Aithal GP, et al. . Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (lean): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. The Lancet 2016;387:679–90. 10.1016/S0140-6736(15)00803-X [DOI] [PubMed] [Google Scholar]
- 14. Townsend SA, Newsome PN. Review article: new treatments in non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2017;46:494–507. 10.1111/apt.14210 [DOI] [PubMed] [Google Scholar]
- 15. Ohki T, Isogawa A, Toda N, et al. . Effectiveness of ipragliflozin, a sodium-glucose co-transporter 2 inhibitor, as a second-line treatment for non-alcoholic fatty liver disease patients with type 2 diabetes mellitus who do not respond to incretin-based therapies including glucagon-like peptide-1 analogs and dipeptidyl peptidase-4 inhibitors. Clin Drug Investig 2016;36:313–9. 10.1007/s40261-016-0383-1 [DOI] [PubMed] [Google Scholar]
- 16. Kuchay MS, Krishan S, Mishra SK, et al. . Effect of Empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT trial). Diabetes Care 2018;41:1801–8. 10.2337/dc18-0165 [DOI] [PubMed] [Google Scholar]
- 17. Marso SP, Bain SC, Consoli A, et al. . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. Massachusetts Medical Society 2016;375:1834–44. [DOI] [PubMed] [Google Scholar]
- 18. Zinman B, Wanner C, Lachin JM, et al. . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 19. Neal B, Perkovic V, Mahaffey KW, et al. . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–57. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 20. Non-alcoholic fatty liver disease (NAFLD): assessment and management. National Institute for Health and Care Excellence (NICE) guideline [NG49, 2016. [PubMed] [Google Scholar]
- 21. Hsu C, Caussy C, Imajo K, et al. . Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol 2018. (pii: S1542-3565(18)30613-X). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sterling RK, Lissen E, Clumeck N, et al. . Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. 10.1002/hep.21178 [DOI] [PubMed] [Google Scholar]
- 23. ClinRisk Ltd QRISK®3-2018 Risk Calculator [Internet]. Available: https://qrisk.org/three/ [Accessed cited 2018 Nov 12].
- 24. Diabetes Trials Unit, OCDEM U of O UKPDS Outcomes Model [Internet]. Available: https://www.dtu.ox.ac.uk/outcomesmodel/ [Accessed cited 2018 Nov 12].
- 25. European association for the study of the liver (EASL), European association for the study of diabetes (EASD), European association for the study of Obesity (EASO). EASL–EASD–EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. [DOI] [PubMed] [Google Scholar]
- 26. Marjot T, Sbardella E, Moolla A, et al. . Prevalence and severity of non-alcoholic fatty liver disease are underestimated in clinical practice: impact of a dedicated screening approach at a large university teaching hospital. Diabet. Med. 2018;35:89–98. 10.1111/dme.13540 [DOI] [PubMed] [Google Scholar]
- 27. Portillo-Sanchez P, Bril F, Maximos M, et al. . High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. The Journal of Clinical Endocrinology & Metabolism 2015;100:2231–8. 10.1210/jc.2015-1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Katsagoni CN, Georgoulis M, Papatheodoridis GV, et al. . Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: a meta-analysis. Metabolism 2017;68:119–32. 10.1016/j.metabol.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 29. Arsik I, Frediani J, Frezza D, et al. . Alanine aminotransferase as a monitoring biomarker in children with nonalcoholic fatty liver disease: a secondary analysis using tonic trial data. Children 2018;5 10.3390/children5060064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Type 2 diabetes in adults: management. National Institute for Health and Care Excellence (NICE) guideline [NG28]. NICE 2015. [PubMed] [Google Scholar]
- 31. American diabetes association AD. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in Diabetes-2018. Diabetes Care. American Diabetes Association 2018;41(Suppl 1):S73–85. [DOI] [PubMed] [Google Scholar]
- 32. Andrews RC, Cooper AR, Montgomery AA, et al. . Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the early ACTID randomised controlled trial. The Lancet 2011;378:129–39. 10.1016/S0140-6736(11)60442-X [DOI] [PubMed] [Google Scholar]
- 33. Nield L, Moore H, Hooper L, et al. . Dietary advice for treatment of type 2 diabetes mellitus in adults. Cochrane Database Syst Rev 2007;20 10.1002/14651858.CD004097.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Imai S, Kozai H, Matsuda M, et al. . Intervention with delivery of diabetic meals improves glycemic control in patients with type 2 diabetes mellitus. J Clin Biochem Nutr 2008;42:59–63. 10.3164/jcbn.2008010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mitchell LJ, Ball LE, Ross LJ, et al. . Effectiveness of dietetic consultations in primary health care: a systematic review of randomized controlled trials. J Acad Nutr Diet 2017;117:1941–62. 10.1016/j.jand.2017.06.364 [DOI] [PubMed] [Google Scholar]
- 36. Weiß J, Rau M, Geier A. Non-alcoholic fatty liver disease. Dtsch Arztebl Int 2014;111:447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. . Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149:367–78. 10.1053/j.gastro.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 38. Jebb SA, Ahern AL, Olson AD, et al. . Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. The Lancet 2011;378:1485–92. 10.1016/S0140-6736(11)61344-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
flgastro-2018-101155supp001.pdf (548KB, pdf)