Abstract
Microscopic colitis (MC) is a common cause of chronic, non-bloody, watery diarrhoea in older patients. The diagnosis depends on characteristic histological findings. Bile acid malabsorption and autoimmune conditions, including coeliac disease, are more frequently found in patients with MC, but colorectal neoplasia and mortality are not increased. Non-steroidal anti-inflammatory drugs, proton-pump inhibitors, selective serotonin reuptake inhibitors and smoking tobacco confer an increased risk of developing MC. Although a so-called benign disease, which rarely causes serious complications, it does have an impact on the quality of life. Several treatment options exist, but budesonide is the only treatment proven in randomised-controlled trials to be effective and safe for induction and maintenance of remission. This article provides a practical overview for the gastroenterologist looking after patients with MC.
Keywords: microscopic colitis, lymphocytic colitis, collagenous colitis, chronic diarrhoea
Introduction/clinical features
Microscopic colitis (MC) is a chronic inflammatory disease of the colon, detected in up to 19% of patients presenting for colonoscopy for chronic non-bloody diarrhoea.1 2 MC is a collective term for lymphocytic colitis (LC) and collagenous colitis (CC), which have similar clinical features, endoscopic findings, management and response to treatment. For research purposes, and indeed as the understanding of their pathophysiology evolves, they may be treated as distinct entities. For current clinical purposes, however, they are treated simply as MC as demonstrated by the approach in current European and American guidelines.3 4 MC is characterised by chronic or intermittent non-bloody diarrhoea, endoscopically normal or near-normal colonic mucosa and characteristic histological findings. LC is defined histologically by an increased number of intraepithelial lymphocytes, and CC is distinguished by the presence of a thickened subepithelial collagen band.5 The pathogenesis of MC is not yet clear and is beyond the scope of this article. The cause is likely to be multifactorial, incorporating a dysregulated, adaptive mucosal immune response to luminal antigens in predisposed individuals.
Epidemiology
The incidence of MC is similar to that of ulcerative colitis and Crohn’s disease. It affects proportionally more elderly and female patients. A population-based study estimated the incidence at 21.0 cases per 100 000 person-years, with a median age at diagnosis of 65.8 years (range 22.8–92.1) and a female-to-male ratio of 3:1.6
Several risk factors and associations have been identified. MC is associated with autoimmune disorders, most frequently coeliac disease. The prevalence of MC in a cohort of 1009 patients with coeliac disease was 4.4%, which is 45-fold greater than in patients without coeliac disease.7 Bile acid malabsorption (BAM) is also associated and was found in 43% of a cohort of 57 patients with MC.8 A prospective case–control study identified current smoking (OR; 2.4), a history of polyarthritis (OR: 20.8) and the medications lansoprazole (OR: 6.4), low-dose aspirin (OR: 3.8) and beta-blockers (OR: 3.6) to be associated with an increased risk of CC. Similarly, it identified current smoking (OR: 3.8), autoimmune disease (OR: 8.0), omeprazole (OR: 2.7), low-dose aspirin (OR: 4.7) and sertraline (OR: 17.5) with an increased risk of LC. Certain medications are associated with, or may even induce, MC. An attempt has been made to identify those drugs most likely to trigger MC using combined causality and chronological criteria together with the number of published cases. Acarbose, aspirin, lansoprazole, non-steroidal anti-inflammatory drugs (NSAIDs), ranitidine, sertraline and ticlopidine were identified as high likelihood medications.9 Overall, the most evidence for drug-induced MC implicates NSAIDs, proton-pump inhibitors (PPIs) and selective serotonin reuptake inhibitors (SSRIs).
Quality of life
Health-related quality of life is reduced in patients with MC compared with matched controls. However, effective treatment can return quality of life indices back to that of ‘normal’ subjects as measured by Short Inflammatory Bowel Disease Questionnaire scores.10
Symptoms
The ubiquitous symptom of MC is non-bloody, watery diarrhoea. This can have a sudden or insidious onset. Stool frequency varies; commonly patients pass 4–9 stools per day but can often exceed 10 stools per day. Retrospective studies of 199 patients with LC and 163 with CC demonstrated that diarrhoea was present in 96% and 100% of patients, respectively. Weight loss and abdominal pain are present in over 40% of cases. Nocturnal diarrhoea and fatigue are also prominent.11 12
Clinical course
The clinical course and speed of onset of MC is highly variable within the reported literature. This may be a product of variable study design and evolving treatment approaches over the time frame of the body of work. For a proportion of patients, symptoms will resolve spontaneously or after implicated medications are discontinued, some will have single episodes, some a relapsing-remitting course, the remainder will have continuous symptoms. In CC, one cohort experienced a chronic intermittent course in 85% of cases, a continuous course in 13% and a single symptomatic episode in only 2%. In LC, there may be more isolated or self-resolving episodes with one cohort reporting a chronic intermittent course in 30% of case, continuous in 7% and single episodes 63%.11 12
Long-term outcomes and colorectal neoplasia risk
In a 24-patient cohort of CC, aged between 20 years and 82 years old and followed up for 5–16 years postdiagnosis, 42% had chronic or intermittent diarrhoea. Seventeen per cent were asymptomatic. No patients developed colorectal cancer, ulcerative colitis or Crohn’s disease.13 When patients with MC have been compared with the general population and matched controls retrospectively after a follow-up period of up to 12 years, or prospectively to patients investigated for chronic non-bloody diarrhoea, the risk of developing colorectal neoplasia is not increased.2
Evaluation and diagnosis
For a patient’s first presentation with chronic diarrhoea, after a careful history and examination, blood tests including full blood count, urea and electrolytes, C reactive protein, thyroid function tests and coeliac serology are useful as a matter of routine. Stool culture is indicated if infection is suspected or to be excluded, and colonoscopy is indicated if colorectal cancer or an inflammatory bowel disease is suspected. Faecal calprotectin levels are routinely measured during investigation of chronic diarrhoea but are not useful specifically for MC, as detailed below. The British Society of Gastroenterology guidelines provide useful information for the assessment of chronic diarrhoea.
There is clearly overlap between the symptoms of diarrhoea-predominant irritable bowel syndrome and MC. Scoring systems have been proposed to risk stratify patients with respect to MC versus functional diarrhoea, considering the resources, invasiveness and rare, but non-zero, risks associated with colonoscopy.14 Age >50 years, female gender, weight loss, absence of abdominal pain, current smoking, NSAIDs, PPIs, SSRIs, nocturnal diarrhoea and duration of diarrhoea <6 months are all implicated in these systems. These scoring systems, however, have not yet been validated with prospective studies outside their conceptual publications. They largely mirror the known epidemiological risk factors for MC, the presence of which should already predispose clinicians to pursue colonoscopy with biopsies.
If MC is suspected, colonoscopy with biopsies is mandated.
The current European Microscopic Colitis Group consensus statement recommends excluding coeliac disease, BAM and lactose malabsorption during the evaluation of MC.3 Malabsorptive symptoms, iron deficiency or significant weight loss should prompt the careful exclusion of coeliac disease. Associated diseases should especially be sought when therapy for MC and or withdrawal of causative drugs does not ameliorate symptoms.
Faecal calprotectin levels can be elevated in MC, but they are not useful in the diagnosis, exclusion or follow-up of MC.5 Significantly elevated levels, depending on locally determined thresholds, should prompt investigation in patients with otherwise suspected functional bowel disorders, or positive identification of an alternative explanation such as infection or the use of NSAIDs. Analysis of patients with known CC demonstrated that calprotectin was elevated in active CC compared with quiescent CC and controls, but 38% of patients with symptomatically active CC had normal levels, indicating that calprotectin does not have reliable exclusion value when assessing for MC.15 Prospective analysis of calprotectin in patients presenting for colonoscopy with chronic non-bloody diarrhoea did not show an association between a diagnosis of MC and calprotectin levels.1 C reactive protein, erythrocyte sedimentation rate and autoantibody profiles are not helpful in the evaluation of MC.
Diagnosis
In patients with a compatible clinical picture, lower gastrointestinal endoscopy with histological analysis of biopsy samples is required to make the diagnosis of MC. Endoscopy typically reveals grossly normal colonic mucosa, although erythema or oedema may be observed. Reported endoscopic findings in MC have included alteration of the vascular mucosal pattern, nodularity and mucosal tears/lacerations (‘cat-scratch colon’) or cicatricial lesions.16 Figure 1 illustrates such colonoscopic findings.
Figure 1.
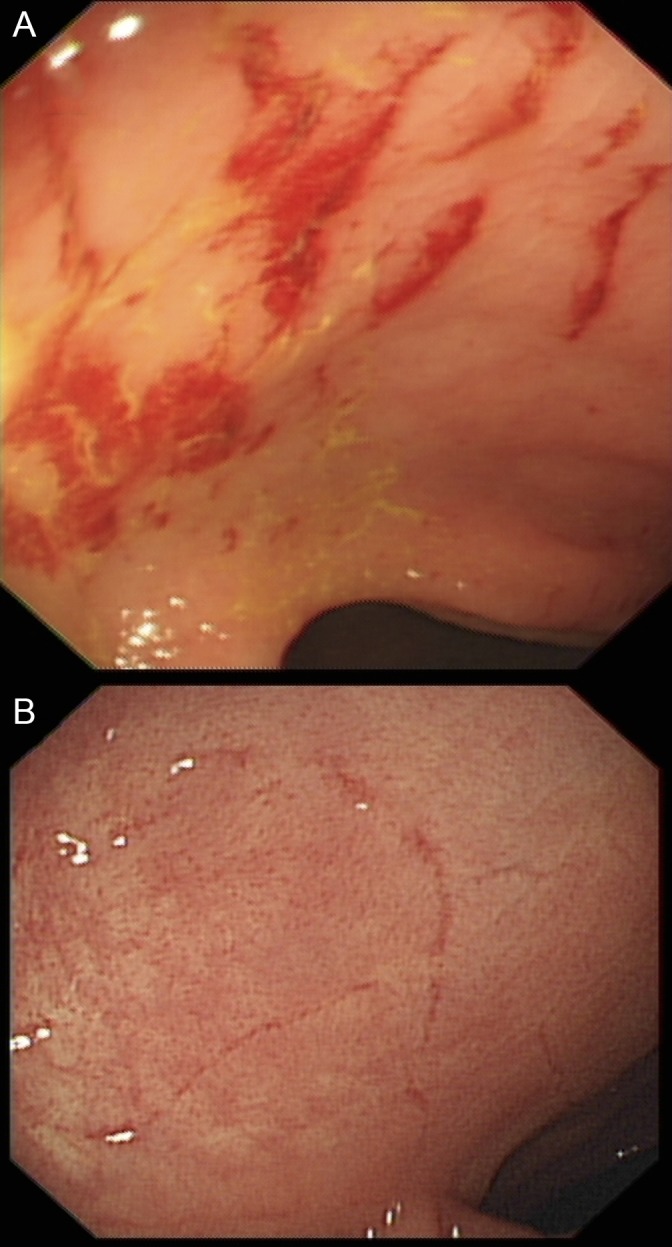
Characteristic colonoscopic findings in microscopic colitis: (A) ‘Cat-scratch colon’: haemorrhagic linear mucosal breaks/tears and (B) cicatricial lesion: fine linear scar-like lesions, possibly the healed remnants of previous cat-scratch type lesions.
MC is the diagnosis made in 19% of patients attending for colonoscopy for chronic non-bloody diarrhoea, making it the most frequent diagnosis in such patients.1 2 The presence of ulcers at colonoscopy suggests an alternative pathology or may be explained by the concurrent use of NSAIDs.
The diagnostic histological finding in CC is a subepithelial collagen band of >10 µm thickness (normal collagen band thickness is approximately 3 µm). There is an increased predominantly mononuclear inflammatory cell infiltrate in the lamina propria, and the surface epithelium can become detached.5 17
The histological diagnosis of LC is defined as >20 intraepithelial lymphocytes per 100 epithelial cells with an increase in the inflammatory infiltrate in the lamina propria but without thickening of the subepithelial collagen band.17 Figure 2 shows the typical histology of CC and LC.
Figure 2.
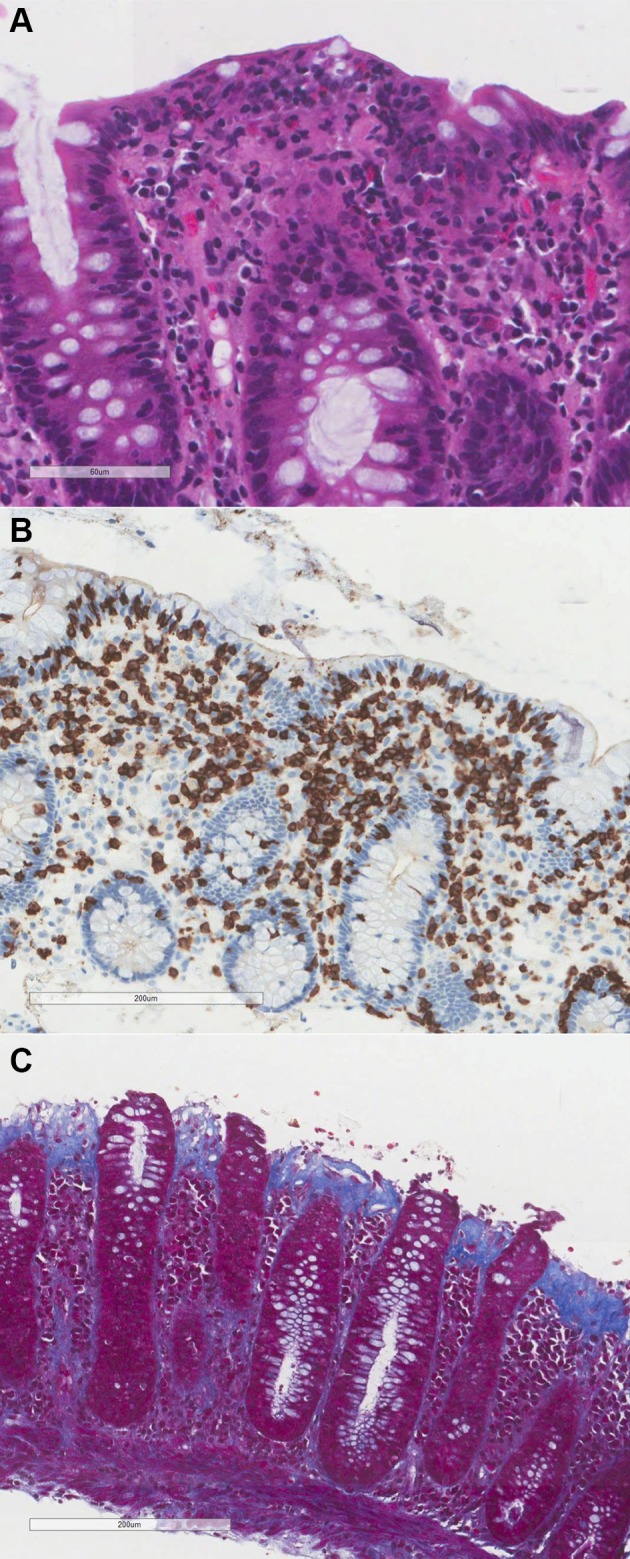
Characteristic histology of lymphocytic colitis and collagenous colitis. (A) Lymphocytic colitis with increased intraepithelial lymphocytes (H&E). (B) CD3 immunohistochemistry demonstrates the increased intraepithelial lymphocytes, stained brown. CD3 antigen is specific to T-lymphocytes. (C) Thickened collagen band and loss of surface epithelium in collagenous colitis (Masson’s trichrome). Masson’s trichrome staining protocol stains the subepithelial collagen band blue.
One should, ideally, obtain biopsies from the ascending, transverse, descending and sigmoid colon, although there is no consensus about the optimum number of biopsies from each segment.5 Studies have demonstrated that omitting segments will lead to missed pathology, and diagnostic histology is more often found in the right colon. Therefore, flexible sigmoidoscopy is insufficient to confidently exclude MC.18
Management
The goal of treatment is to induce remission from symptoms and, in the frequent cases of a relapsing disease course, to maintain remission sufficiently to improve quality of life. The Hjortswang criteria were devised focusing on the effect of symptoms on quality of life and define remission as <3 stools/day with <1 watery stool/day.19 Pragmatically, treatment success will be judged by how satisfied the patient is with their symptomatic response. Proof of histological remission is often described in research literature, but repeat colonoscopy and biopsy is not normally required in clinical practice unless patient progress mandates further evaluation or suggests an alternative diagnosis. Symptomatic and histological remission in MC are usually well correlated.20–22
The first step in management is removing exacerbating factors, that is, smoking and medications. PPIs, SSRIs and NSAIDs are to be avoided; more extensive lists of medications with their likelihood of triggering MC have been formulated.9 There is not yet clear evidence as to the efficacy of withdrawing suspected medications and how it might predictably alter the disease course.
Several pharmacological treatments exist for MC, but budesonide is the mainstay for induction and maintenance of remission in MC. Budesonide is recommended as first-line therapy in both American Gastroenterological Association guidelines and European Microscopic Colitis Group statements.3 4 It is the only treatment studied in randomised controlled trials (RCT). If symptoms are mild, loperamide, cholestyramine, mesalazine or bismuth may be considered, but these are less effective.
Budesonide for induction of remission
An RCT in CC demonstrated that 9 mg oral budesonide once daily for 8 weeks, versus mesalazine or placebo, achieved a clinical remission rate of 80% (n=30) on an intention-to-treat basis with a median time to remission of 7 days. This was statistically significantly better than mesalazine, which was no better than placebo.22 Response in LC is similar; a recent RCT in LC of 9 mg oral budesonide once daily for 8 weeks achieved a clinical remission rate of 79%.20 This study was stopped early after interim analysis showed that budesonide was superior to placebo. Again, mesalazine was not more effective than placebo. Safety analysis suggested budesonide was safe as well as effective. Serum cortisol levels at baseline and after 8 weeks of budesonide treatment were not different.
Budesonide for maintenance of remission after relapse
Patients can have single episodes of MC, especially in drug-induced MC, but more often will suffer relapse after induction therapy is complete. These will require consideration for maintenance therapy. Long-term outcomes of 33 patients with CC who achieved clinical remission with budesonide were assessed over a median follow-up period of 16 months. Sixty-one per cent clinically relapsed, 88% of these relapses occurred within 3 months after cessation of treatment.23
In three RCTs, 4.5–6 mg budesonide daily was superior to placebo to maintain clinical remission and associated health-related quality of life over 6–12 months of treatment. Remission was maintained in 61%–77% of cases.10 24 25 Relapse after discontinuation of 1 year of budesonide treatment was frequent, 82.1% (n=28) suggesting longer term treatment is beneficial.25
Safety of budesonide
Long-term use of conventional corticosteroids, such as prednisolone, is fraught with side effects and complications. Budesonide appears similarly effective but safe for prolonged use possibly due to its extensive presystemic metabolism. One must consider concurrent cytochrome P450 inhibitor use, which could potentially increase systemic exposure.26 Meta-analysis found comparable side effect rates to placebo. Budesonide does not appear to affect endogenous cortisol levels after induction or maintenance therapy for a year, and guidelines do not suggest dose tapering is required.20 25 A large case–control study of corticosteroids and fracture risk showed there was no increased risk with budesonide use (n=91).27 One study suggested cumulative budesonide use in MC was associated with lower hip and spine bone mineral density, with a 2500 mg cumulative dose over 3 years predicting osteopenia.28 Increasing age, female gender and smoking are all associated with MC and osteoporosis. Osteoporosis screening and prevention is recommended in guidelines for any patient with MC requiring maintenance therapy with budesonide. We suggest supplementation of calcium and vitamin D in this cohort and remaining cognizant of side effects including hypertension and steroid-induced diabetes, even if rare.
Relapse and refractory cases
One must always reconsider the diagnosis in the event of persistent symptoms. Medications and smoking status should be carefully reviewed. Coeliac disease, BAM, lactose malabsorption and small intestine bacterial overgrowth should be considered. Loperamide, bismuth and mesalazine can be trialled. Cholestyramine may be especially helpful and can be used concurrently with budesonide. It has been shown to help in MC with coexisting BAM.8 If symptoms are truly refractory and significantly impactful on quality of life, immunomodulating therapy may be required.
Immunomodulating therapy
Data regarding treatment of MC with immunomodulators such as azathioprine, methotrexate and antitumour necrosis factor have been published. One study of budesonide-refractory, budesonide-dependent and budesonide-intolerant patients reported a 43% complete response rate using azathioprine (n=49), 58% with methotrexate (n=35) and 40% with infliximab or adalimumab (n=10). Notably, 35% of patients taking thiopurines had adverse effects resulting in cessation of treatment. Other studies have reported on relative ineffectiveness of azathioprine and methotrexate in MC.29 30 Rigorous controlled studies are required to investigate the safety and efficacy of immunomodulating treatments for MC. Details of surgical intervention such as ileostomy with or without colectomy have been reported, but these are limited to isolated case reports.
Conclusion and our practice
MC is a common cause for chronic non-bloody, watery diarrhoea, especially in elderly women. Diagnosis depends on high-quality colonoscopy and histopathological assessment of biopsies of the left and right colon. Coeliac disease should be excluded at least with antitissue transglutaminase levels and BAM always kept in mind. First-line treatment is 9 mg budesonide once daily for 8 weeks. In the event of relapse, the patient should be reassessed and retreated, aiming to taper to the lowest possible budesonide maintenance dose, for example, 3 mg alternate days. All patients requiring maintenance budesonide should be prescribed calcium and vitamin D supplementation and have bone mineral density checked. One should remain vigilant for corticosteroid side effects and, after a year of therapy, assess whether ongoing treatment is required.
Footnotes
Contributors: TT wrote and revised the manuscript. CP reviewed and revised the manuscript. FC reviewed and revised the manuscript and provided appropriate histology images and advice upon their interpretation. PO reviewed and revised the manuscript and provided appropriate endoscopic images and advice upon their interpretation.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: CP: speaker fees from AbbVie, Avantis, Dr Falk Pharma, Ferring, Hospira, Janssen, Merck, Shire and Takeda; payment for advisory board attendance from Avantis, Dr Falk Pharma, Ferring, Janssen, Hospira (Pfizer), Merck, Napp and Takeda; and support for attendance to other meetings from Avantis, Dr Falk Pharma, Merck, Shire, Hospira, Takeda and Vifor.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Larsson JK, Sjöberg K, Vigren L, et al. Chronic non-bloody diarrhoea: a prospective study in Malmö, Sweden, with focus on microscopic colitis. BMC Res Notes 2014;7:236 10.1186/1756-0500-7-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tontini GE, Pastorelli L, Spina L, et al. Microscopic colitis and colorectal neoplastic lesion rate in chronic nonbloody diarrhea: a prospective, multicenter study. Inflamm Bowel Dis 2014;20:882–91. 10.1097/MIB.0000000000000030 [DOI] [PubMed] [Google Scholar]
- 3. Münch A, Aust D, Bohr J, et al. Microscopic colitis: Current status, present and future challenges: statements of the European Microscopic Colitis Group. J Crohns Colitis 2012;6:932–45. 10.1016/j.crohns.2012.05.014 [DOI] [PubMed] [Google Scholar]
- 4. Nguyen GC, Smalley WE, Vege SS, et al. American gastroenterological association institute guideline on the medical management of microscopic colitis. Gastroenterology 2016;150:242–6. 10.1053/j.gastro.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 5. Langner C, Aust D, Ensari A, et al. Histology of microscopic colitis-review with a practical approach for pathologists. Histopathology 2015;66:613–26. 10.1111/his.12592 [DOI] [PubMed] [Google Scholar]
- 6. Gentile NM, Khanna S, Loftus EV, et al. The epidemiology of microscopic colitis in Olmsted County from 2002 to 2010: a population-based study. Clin Gastroenterol Hepatol 2014;12:838–42. 10.1016/j.cgh.2013.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Green PH, Yang J, Cheng J, et al. An association between microscopic colitis and celiac disease. Clin Gastroenterol Hepatol 2009;7:1210–6. 10.1016/j.cgh.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 8. Fernandez-Bañares F, Esteve M, Salas A, et al. Bile acid malabsorption in microscopic colitis and in previously unexplained functional chronic diarrhea. Dig Dis Sci 2001;46:2231–8. 10.1023/A:1011927302076 [DOI] [PubMed] [Google Scholar]
- 9. Beaugerie L, Pardi DS. Review article: drug-induced microscopic colitis - proposal for a scoring system and review of the literature. Aliment Pharmacol Ther 2005;22:277–84. 10.1111/j.1365-2036.2005.02561.x [DOI] [PubMed] [Google Scholar]
- 10. Miehlke S, Madisch A, Bethke B, et al. Oral budesonide for maintenance treatment of collagenous colitis: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2008;135:1510–6. 10.1053/j.gastro.2008.07.081 [DOI] [PubMed] [Google Scholar]
- 11. Bohr J, Tysk C, Eriksson S, et al. Collagenous colitis: a retrospective study of clinical presentation and treatment in 163 patients. Gut 1996;39:846–51. 10.1136/gut.39.6.846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olesen M, Eriksson S, Bohr J, et al. Lymphocytic colitis: a retrospective clinical study of 199 Swedish patients. Gut 2004;53:536–41. 10.1136/gut.2003.023440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonderup OK, Folkersen BH, Gjersøe P, et al. Collagenous colitis: a long-term follow-up study. Eur J Gastroenterol Hepatol 1999;11:493–5. [PubMed] [Google Scholar]
- 14. Cotter TG, Binder M, Harper EP, et al. Optimization of a scoring system to predict microscopic colitis in a cohort of patients with chronic diarrhea. J Clin Gastroenterol 2017;51:228–34. 10.1097/MCG.0000000000000565 [DOI] [PubMed] [Google Scholar]
- 15. Wildt S, Nordgaard-Lassen I, Bendtsen F, et al. Metabolic and inflammatory faecal markers in collagenous colitis. Eur J Gastroenterol Hepatol 2007;19:567–74. 10.1097/MEG.0b013e328058ed76 [DOI] [PubMed] [Google Scholar]
- 16. Koulaouzidis A, Saeed AA. Distinct colonoscopy findings of microscopic colitis: not so microscopic after all? World J Gastroenterol 2011;17:4157–65. 10.3748/wjg.v17.i37.4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis 2013;7:827–51. 10.1016/j.crohns.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 18. Thijs WJ, van Baarlen J, Kleibeuker JH, et al. Microscopic colitis: prevalence and distribution throughout the colon in patients with chronic diarrhoea. Neth J Med 2005;63:137–40. [PubMed] [Google Scholar]
- 19. Hjortswang H, Tysk C, Bohr J, et al. Defining clinical criteria for clinical remission and disease activity in collagenous colitis. Inflamm Bowel Dis 2009;15:1875–81. 10.1002/ibd.20977 [DOI] [PubMed] [Google Scholar]
- 20. Miehlke S, Aust D, Mihaly E, et al. Efficacy and safety of budesonide, vs mesalazine or placebo, as induction therapy for lymphocytic colitis. Gastroenterology. In Press 2018. 10.1053/j.gastro.2018.08.042 [DOI] [PubMed] [Google Scholar]
- 21. Miehlke S, Madisch A, Karimi D, et al. Budesonide is effective in treating lymphocytic colitis: a randomized double-blind placebo-controlled study. Gastroenterology 2009;136:2092–100. 10.1053/j.gastro.2009.02.078 [DOI] [PubMed] [Google Scholar]
- 22. Miehlke S, Madisch A, Kupcinskas L, et al. Budesonide is more effective than mesalamine or placebo in short-term treatment of collagenous colitis. Gastroenterology 2014;146:1222–30. 10.1053/j.gastro.2014.01.019 [DOI] [PubMed] [Google Scholar]
- 23. Miehlke S, Madisch A, Voss C, et al. Long-term follow-up of collagenous colitis after induction of clinical remission with budesonide. Aliment Pharmacol Ther 2005;22:1115–9. 10.1111/j.1365-2036.2005.02688.x [DOI] [PubMed] [Google Scholar]
- 24. Bonderup OK, Hansen JB, Teglbjaerg PS, et al. Long-term budesonide treatment of collagenous colitis: a randomised, double-blind, placebo-controlled trial. Gut 2009;58:68–72. 10.1136/gut.2008.156513 [DOI] [PubMed] [Google Scholar]
- 25. Münch A, Bohr J, Miehlke S, et al. Low-dose budesonide for maintenance of clinical remission in collagenous colitis: a randomised, placebo-controlled, 12-month trial. Gut 2016;65:47–56. 10.1136/gutjnl-2014-308363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edsbäcker S, Andersson T. Pharmacokinetics of budesonide (Entocort EC) capsules for Crohn’s disease. Clin Pharmacokinet 2004;43:803–21. 10.2165/00003088-200443120-00003 [DOI] [PubMed] [Google Scholar]
- 27. Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with different types of oral corticosteroids and effect of termination of corticosteroids on the risk of fractures. Calcif Tissue Int 2008;82:249–57. 10.1007/s00223-008-9124-7 [DOI] [PubMed] [Google Scholar]
- 28. Wildt S, Munck LK, Becker S, et al. Risk of osteoporosis in microscopic colitis. Postgrad Med 2018;130:348–54. 10.1080/00325481.2018.1441579 [DOI] [PubMed] [Google Scholar]
- 29. Münch A, Bohr J, Vigren L, et al. Lack of effect of methotrexate in budesonide-refractory collagenous colitis. Clin Exp Gastroenterol 2013;6:149–52. 10.2147/CEG.S48201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Münch A, Fernandez-Banares F, Munck LK. Azathioprine and mercaptopurine in the management of patients with chronic, active microscopic colitis. Aliment Pharmacol Ther 2013;37:795–8. 10.1111/apt.12261 [DOI] [PubMed] [Google Scholar]


