Keywords: nerve regeneration, Alzheimer's disease, amyloid-ß1–42, dentate gyrus, channelrhodopsin-2, glutamate receptors, memory, neuroinflammation, novel object recognition, immunohistochemistry, western blot assay, neural regeneration
Abstract
Optogenetics is a combination of optics and genetics technology that can be used to activate or inhibit specific cells in tissues. It has been used to treat Parkinson’s disease, epilepsy and neurological diseases, but rarely Alzheimer’s disease. Adeno-associated virus carrying the CaMK promoter driving the optogenetic channelrhodopsin-2 (CHR2) gene (or without the CHR2 gene, as control) was injected into the bilateral dentate gyri, followed by repeated intrahippocampal injections of soluble low-molecular-weight amyloid-β1–42 peptide (Aβ1–42). Subsequently, the region was stimulated with a 473 nm laser (1–3 ms, 10 Hz, 5 minutes). The novel object recognition test was conducted to test memory function in mice. Immunohistochemical staining was performed to analyze the numbers of NeuN and synapsin Ia/b-positive cells in the hippocampus. Western blot assay was carried out to analyze the expression levels of glial fibrillary acidic protein, NeuN, synapsin Ia/b, metabotropic glutamate receptor-1a (mGluR-1a), mGluR-5, N-methyl-D-aspartate receptor subunit NR1, glutamate receptor 2, interleukin-1β, interleukin-6 and interleukin-10. Optogenetic stimulation improved working and short-term memory in mice with Alzheimer’s disease. This neuroprotective effect was associated with increased expression of NR1, glutamate receptor 2 and mGluR-5 in the hippocampus, and decreased expression of glial fibrillary acidic protein and interleukin-6. Our results show that optogenetics can be used to regulate the neuronal-glial network to ameliorate memory functions in mice with Alzheimer’s disease. The study was approved by the Animal Resources Committee of Jinan University, China (approval No. LL-KT-2011134) on February 28, 2011.
Chinese Library Classification No. R456; R338.64; R741.05
Introduction
Alzheimer’s disease (AD) is the most common progressive neurodegenerative disease that results in dementia. Substantial synaptic and neuronal losses are observed in the early stage of AD (Gómez-Isla et al., 1996; Scheff et al., 2006; Ono et al., 2009; Crews and Masliah, 2010). Soluble low-molecular-weight amyloid-β1–42 peptide (Aβ1–42) has been shown to be the main neurotoxic culprit (Lambert et al., 1998; Lesné et al., 2006). Memory deficits and neurotoxicity have been shown to be directly caused by Aβ1–42 by Brouillette and colleagues using a novel animal model (Brouillette et al., 2012). They demonstrated that repeated intrahippocampal injection of soluble Aβ1–42 causes Aβ deposition, tau hyperphosphorylation and neuronal loss, which are the key histopathological changes in AD.
Glutamate is the main excitatory neurotransmitter in the brain, and is involved in regulating functions such as learning and memory (Ordaz et al., 2017). Dysregulated glutamatergic signaling has been implicated in neuronal and synaptic loss in AD (Piani et al., 1991, 1992; Albasanz et al., 2005). Glutamate has two main receptors: ionotropic and metabotropic glutamate receptors (iGluR and mGluR). Both types of receptors are involved in AD pathogenesis. The PrP(c)/Aβ1–42 peptide complex may contribute to AD pathogenesis via mGluR5 and N-methyl-D-aspartate receptor (NMDAR) (Hamilton et al., 2015). NMDAR is involved in Aβ-induced synaptic loss in cultured hippocampal slices from arcAβ transgenic mice (Terry et al., 1991; McLean et al., 1999; Shankar et al., 2007).
Optogenetics combines both optical and genetic techniques to activate or inhibit activity or expression in specific cells in vitro and in vivo (Deisseroth, 2015). It has been used to investigate neural network activity in behaviours and sleep/wakefulness, as well as in therapies for Parkinson’s disease, epilepsy and other neurological diseases. However, very few studies have reported on the use of optogenetics in AD.
In this study, we used an adeno-associated virus (AAV) carrying the Ca2+/calmodulin-dependent protein kinase (CaMK) promoter (Aravanis et al., 2007), optogenetic channelrhodopsin-2 (CHR2) and fluorescein-mCherry (AAV5-CaMK-CHR2-mCherry) for expression in glutamatergic neurons. Light stimulation of cells carrying AAV5-CaMK-CHR2-mCherry results in the activation of glutamatergic neurons. In the present study, we used this method to investigate the therapeutic potential of optogenetic neuromodulation in a mouse model of AD.
Materials and Methods
Animals
A total of 36 female C57BL/6 mice, 8 months old, were bought from Guangdong Medical Laboratory Animal Center, China (license No. SCXK(Yue)2008-0002). All experiments were performed following the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 1985), with the approval of the Animal Resources Committee, Jinan University, China (approval No. LL-KT-2011134) on February 28, 2011. The mice were housed in groups of 10 per cage (470 mm × 350 mm × 200 mm), with free access to food and water, under a 12/12-hour light/dark cycle, with lights on from 7:00 a.m. to 7:00 p.m., in a room with controlled temperature (20 ± 2°C) and humidity (55 ± 5%).
Experimental procedures
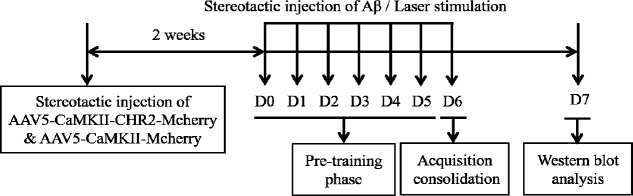
A flow chart of the experimental protocol is shown in Figure 1. Mice were randomly divided into the following three groups: Aβ group (n = 12), Aβ-non-CHR2 group (n = 12) and Aβ-CHR2 group (n = 12). AAV5-CaMK-CHR2-mCherry and AAV5-CaMK-mCherry were injected bilaterally into the dentate gyrus of the hippocampus. Then, 14 days later, 0.2 μg soluble low-molecular-weight Aβ1–42 was injected into the same site. Afterwards, light stimulation was performed for 5 minutes with an optical fiber at the same site in the dentate gyrus. Aβ1–42 injection and light stimulation were performed from day 0 to day 6. The object recognition task was conducted from day 0 to day 7 after Aβ injection (n = 8). Thereafter, the mice were sacrificed for western blot assay (n = 6) or immunohistochemical staining (n = 6).
Figure 1.
Flow chart of the experimental protocol.
AAV: Adeno-associated virus; CaMK: Ca2+/calmodulin-dependent protein kinase; CHR2: channelrhodopsin-2; Aβ: amyloid-β; D: day(s).
Aβ preparation
Aβ1–42 peptide solution was prepared as described before (Kuperstein et al., 2010; Brouillette et al., 2012). In short, Aβ1–42 peptide (A9810, Sigma-Aldrich, Shanghai, China) was dissolved in 99% hexafluoroisopropanol (Sigma-Aldrich) at 1 mg/mL. A gentle stream of N2 gas was used for evaporation. The peptide film was dissolved to a 1 mg/mL final concentration in dimethyl sulfoxide (Sigma-Aldrich), and then eluted with 50 mM Tris/1 mM EDTA buffer (pH 7.5) on a 5 mL HiTrap desalting column (GE Healthcare). A BCA protein assay kit (Pierce, Rockford, IL, USA) was used to measure the Aβ1–42 concentration. The Aβ1–42 peptide solution was kept on ice until the experiments started, and the maximum lag time was 30 minutes.
AAV infection and optical fiber implant
All surgical procedures were performed stereotactically. Avertin (500 mg/kg; Jiangsu Changjili New Energy Technology Co., Ltd., Yixing, China) was used as anesthetic. Bilateral cannulae (328OPD-2.8/Spc, plus a removable dummy wire; Plastics One) were implanted into the hippocampal dentate gyrus ((from the bregma) 2.2 mm anteroposterior, ± 1.4 mm mediolateral, 2.1 mm dorsoventral) (Paxinos and Watson, 2005).
AAV5-CaMK-CHR2-mCherry (1 × 1013 genome copies/mL) and AAV5-CaMK-mCherry (1 × 1013 genome copies/mL) were injected with a glass micropipette connected to a 10 μL Hamilton microsyringe (701LT; Hamilton, Beijing, China) fixed to a microelectrode holder (MPH6S; WPI, Shanghai, China). The injection speed was set at 100 nL/min for 10 minutes, for a total volume of 1 μL, which was controlled by a microsyringe pump (UMP3; WPI) and a controller (Micro4; WPI). After the injection, the needle was kept in place for an additional 5 minutes and then withdrawn slowly.
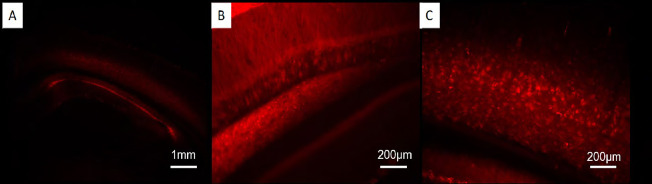
Histology showed that 14 days after injection, AAV5-CaMK-CHR2-mCherry and AAV5-CaMK-mCherry were expressed adequately (Figure 2). Bilateral cannulae were screwed into the skull around the dentate gyrus of the hippocampus. Aβ1–42 peptide solution (0.2 μg/μL) was injected into the same site of the dentate gyrus as described above. A patchcord optical fiber (200 mm core diameter; Doric Lenses, Quebec, Canada) was implanted at the site of Aβ1–42 injection. Optical stimulation was generated by a laser (473 nm, 1–3 ms, 10 Hz) (Changchun New Industries, Changchun, China) for 5 minutes. The mice were left on the heating pad until they fully recovered from anesthesia.
Figure 2.
Fluorescence staining in mice injected with AAV5-CaMKII-CHR2-mCherry.
Labeled cells are stained red when carrying AAV5-CaMK-CHR2-mCherry or AAV5-CaMK-mCherry. (A) Hippocampus (original magnification, 40×); (B) CA1 region (original magnification, 200×); (C) dentate gyrus (original magnification, 200×). AAV: Adeno-associated virus; CaMK: Ca2+/calmodulin-dependent protein kinase; CHR2: channelrhodopsin-2.
Novel object recognition test
The novel object recognition test was conducted as described previously (Fortress et al., 2013; Ruby et al., 2013). In the pre-training phase, the mice were given one habituation session for 6 days (day 0 to day 5 after Aβ injection), in which they were allowed 5 minutes to explore the empty space without objects. In the training (acquisition) phase, on day 6 after Aβ injection, the mice were placed in the testing zone facing the center of the other side, in which two identical objects (A1 and A2) were each placed 100 mm from the neighboring wall. The mice were allowed to explore the objects for 3 minutes, and the time exploring each object was measured. Afterwards, the mice were returned to their home cage. In the test (consolidation) phase, working, short-term and long-term memory were assessed. The same procedures were repeated 5 minutes, 2 hours or 1 day after the training stage. The mice were placed again in the testing zone, in which two objects were placed in the same location: one object (A1) was the same as before, and the other object was novel (B). In the training phase, the memory index was calculated using the following formula: memory index (%) = (exploring time for object A2 / total exploring time for object A1 and object A2) × 100%. In the test phase, memory index (%) = (exploring time for novel object B / total exploring time for object A1 and object B) × 100% (Leger et al., 2013). M0, M1, M2 and M3 represent the memory index in the training stage, and 5 minutes, 2 hours and 1 day after the training stage (in the testing phase), respectively.
Immunohistochemistry
Immunohistochemical analysis (Fachim et al., 2016) was performed using paraffin brain sections. The mice were anesthetized by intraperitoneal injection of sodium pentobarbital, and the brains were collected after cardiac perfusion. A graded ethanol series, 70–100%, was used for dehydration. Sections were blocked, and then incubated in 0.3% Triton X-100/phosphate-buffered saline with primary antibody overnight at 4°C. Primary antibodies included monoclonal mouse anti-NeuN (1:500; Cat# MAB377; Millipore, Darmstadt, Germany) and monoclonal mouse anti-synapsin Ia/b(A-1) (1:100; Cat# sc-398849; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Goat anti-mouse IgG (H&L) (1:2000; Cat# ab7067; Abcam, Shanghai, China) was incubated at ambient temperature in the dark for 1 hour. Using a light microscope (DMI 3000 B; Leica, Buffalo Grove, IL, USA), images were taken of the CA1, CA3, dentate gyrus and subventricular zone. The number of immunostained cells was counted using ImageJ software (NIH, Bethesda, MD, USA) in a double blind manner, and expressed as a percentage of the Aβ group.
Western blot assay
Western blot assay was performed as previously described (Brouillette et al., 2012). In brief, hippocampal tissue, extending 2 mm on one side of the injection site, was immediately dissected on ice and homogenized in cold radioimmunoprecipitation assay solution. Samples were gently agitated for 45 minutes at 4°C, and then centrifuged at 11,500 ×g for 20 minutes at 4°C. The total protein content in the supernatant was determined with a BCA kit.
Using SDS-NuPAGE Novex 4–12% Bis-Tris gels (Invitrogen), 30 µg of total protein was separated by electrophoresis and transferred to Hybond-ECL membranes (GE Healthcare) by electroblotting. Membranes were incubated in 5% fat-free milk containing blotting buffer for 1 hour at ambient temperature, and then with the primary antibody at 4°C overnight. Primary antibodies included the following: monoclonal mouse anti-glial fibrillary acidic protein (GFAP) (1:500; Cat# MAB3402; Millipore), monoclonal mouse anti-NeuN (1:100; Cat# MAB377; Millipore), monoclonal mouse anti-synapsin Ia/b (A-1) (1:100; Cat# sc-398849; Santa Cruz Biotechnology), polyclonal goat anti-mGluR-1a (C-20) (1:200; Cat# sc-47128; Santa Cruz Biotechnology), polyclonal goat anti-mGluR-5 (N-14) (1:200; Cat# sc-47147; Santa Cruz Biotechnology), polyclonal goat anti-NMDAR1 (C-20) (1:200; Cat# sc-1467; Santa Cruz Biotechnology), polyclonal rabbit anti-GluR-2 (1:4000; Cat# AB1768-I; Millipore), polyclonal goat anti-interleukin (IL)-1β (M-20) (1:200; Cat# sc-1251; Santa Cruz Biotechnology), polyclonal goat anti-IL-6 (M-19) (1:200; Cat# sc-1265; Santa Cruz Biotechnology), and monoclonal mouse anti-IL-10 (A-2) (1:100; Cat# sc-365858; Santa Cruz Biotechnology). Expression was normalized to β-actin (1:5000; Cat# ab6276; Abcam) as the loading control. The membranes were washed and then incubated with horseradish peroxidase-labeled goat anti-mouse, goat anti-rabbit or horse anti-goat IgG secondary antibody (1:500; Cat# BA-9200, PI-1000, PI-9500; Vector Laboratories, Burlingame, CA, USA) for 1 hour. After washing, an enhanced chemiluminescence kit (GE Healthcare, Chicago, IL, USA) and the Image Reader LAS-3000 system (Fujifilm; Tokyo, Japan) were used for visualization. Quantitative densitometric analysis was performed using ImageJ software (NIH, Bethesda, MD, USA). Results were expressed as a percentage of the Aβ group.
Statistical analysis
Data are shown as the mean ± SEM. A significance level of P < 0.05 was set for two-tailed, one-way and two-way analysis of variance using Prism 6 (GraphPad, San Diego, CA, USA).
Results
Effect of AAV5-CaMK-CHR2-mCherry on memory function in mice
Using the novel object recognition test, working and short- and long-term memory were assessed during the test phase (consolidation) 5 minutes, 2 hours and 1 day after the training stage (M1, M2 and M3, respectively). Figure 3 shows the effect of stimulating CaMK-CHR2-expressing neurons in the dentate gyrus of the bilateral hippocampi on memory functions. M1 and M2 were significantly higher in the Aβ-CHR2 group than in the Aβ-non-CHR2 and Aβ groups (F = 53.93, P < 0.001 for M1; F = 18.31, P < 0.001 for M2). There were no differences in M3 among the three groups (F = 2.002, P > 0.05 for M3), suggesting that working and short-term memories were rescued by the treatment. There were no significant differences among M0, M1, M2 and M3 in the Aβ-non-CHR2 and Aβ groups (F = 1.099, P > 0.05, for the Aβ1–42 group; F = 1.524, P > 0.05, for the Aβ-non-CHR2 group). However, in the Aβ-CHR2 group, compared with M0, M1 and M2 were increased significantly (F = 25.12, P < 0.0001). Furthermore, there was no significantly difference between M0 and M3 (P > 0.05), implying that CHR2 did not affect long-term memory.
Figure 3.
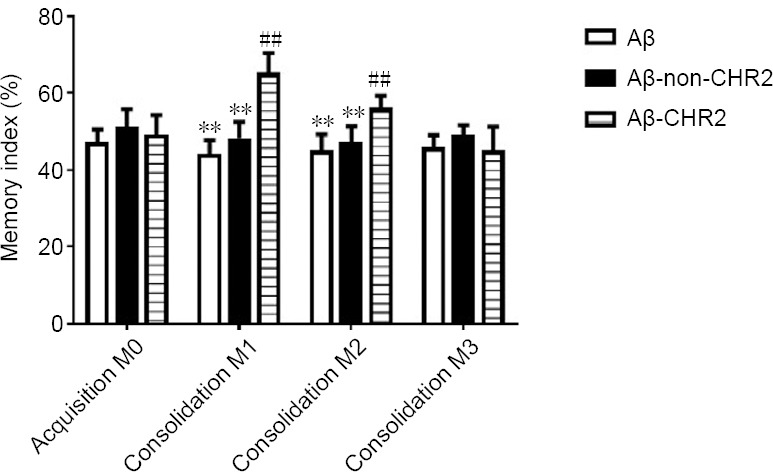
Effect of AAV5-CaMKII-CHR2-mCherry on memory in mice.
Using the novel object recognition test, working, and short-term and long-term memories were assessed with the memory index during the test phase (consolidation), 5 minutes, 2 hours and 24 hours after the training phase (M1, M2 or M3). Data are expressed as the mean ± SEM (n = 8). **P < 0.001, vs. Aβ-CHR2 group; ##P < 0.001, vs. M0 (one-way analysis of variance). Aβ: Repeated Aβ1–42 injection into the bilateral dentate gyrus; Aβ-non-CHR2: Repeated Aβ1–42 injection into the bilateral dentate gyrus together with AAV5-CaMK-mCherry injection and light stimulation; Aβ-CHR2: repeated Aβ1–42 injection into the bilateral dentate gyrus together with AAV5-CaMK-CHR2-mCherry injection and light stimulation. Memory index (%) = (exploring time for novel object B/total exploring time for object A1 and object B) × 100%. AAV: Adeno-associated virus; CaMK: Ca2+/calmodulin-dependent protein kinase; CHR2: channelrhodopsin-2.
Effect of AAV5-CaMK-CHR2-mCherry on neuronal cells and synaptic vesicles in the hippocampus
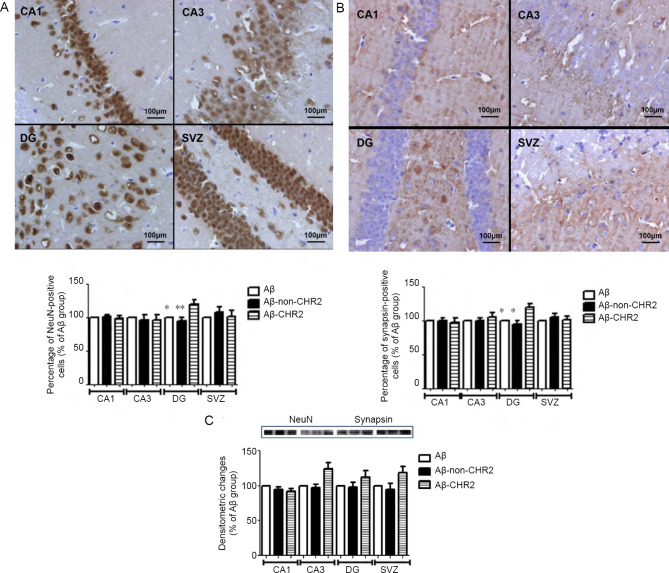
Immunohistochemistry revealed expression of NeuN and synapsin in the CA1, CA3, dentate gyrus and subventricular zone (Figure 4A & B). Compared with the Aβ-non-CHR2 and Aβ groups, the percentages of NeuN-positive and synapsin-positive cells were increased in the dentate gyrus in the Aβ-CHR2 group (F = 29.89, P < 0.01 for NeuN; F = 67.22, P < 0.01 for synapsin). There were no significant differences in the percentage of NeuN or synapsin-positive cells in the CA1, CA3 or subventricular zone among the Aβ-CHR2, Aβ-non-CHR2 and Aβ groups (P > 0.05).
Figure 4.
Effect of AAV5-CaMKII-CHR2-mCherry on neuronal cells and synaptic vesicles in the hippocampus of mice.
(A, B) Effect of CHR2 on NeuN (A) and synapsin (B) in the CA1, CA3, DG and SVZ (original magnification, 400×): Expression of NeuN in the CA1, CA3, DG and SVZ is demonstrated. Scale bars: 100 μm. (C) Quantification of neuronal cells and synaptic vesicles in the hippocampus was assessed by measuring the protein expression levels of NeuN (46 kDa) and synapsin (86 kDa) using western blot assay. Data are expressed as a percentage of the Aβ group and are shown as the mean ± SEM (n = 6). *P < 0.05, **P < 0.01, vs. Aβ-CHR2 group (one-way analysis of variance). Aβ: Mice with repeated Aβ1–42 injection into the bilateral DG; Aβ-non-CHR2: mice with repeated Aβ1–42 injection into the bilateral DG together with AAV5-CaMK-mCherry injection and light stimulation; Aβ-CHR2: mice with repeated Aβ1–42 injection into the bilateral DG together with AAV5-CaMK-CHR2-mCherry injection and light stimulation; DG: dentate gyrus; NeuN: neuronal nuclei; SVZ: subventricular zone; AAV: adeno-associated virus; CaMK: Ca2+/calmodulin-dependent protein kinase; CHR2: channelrhodopsin-2.
Neurons and synaptic vesicles in the hippocampus were assessed by measuring protein levels of NeuN and synapsin by western blot assay (Figure 4C). There was no significant difference in NeuN or synapsin expression among the Aβ-CHR2, Aβ-non-CHR2 and Aβ groups (F = 0.553, P > 0.05 for NeuN; F = 1.156, P > 0.05 for synapsin). Neither AAV5-CaMK-CHR2-mCherry nor AAV5-CaMK-mCherry affected neuronal cells or synaptic vesicles in the hippocampus.
Effect of AAV5-CaMK-CHR2-mCherry on glutamate receptors in the hippocampus of mice
Glutamate receptors can be divided into two groups: iGluR and mGluR (Reiner and Levitz, 2018). AAV5-CaMK-CHR2-mCherry had no effect on mGluR1; there was no significant difference in mGluR1 expression among the Aβ-CHR2, Aβ-non-CHR2 and Aβ groups (F = 1.521, P > 0.05; Figure 5). mGluR5, NMDAR1 and GluR2 expression levels were increased in the Aβ-CHR2 group compared with the Aβ-non-CHR2 and Aβ groups (F = 6.723, P < 0.05 for mGluR5; F = 4.234, P < 0.05 for NMDAR1; F = 4.714, P < 0.05 for GluR2) (Figure 5).
Figure 5.
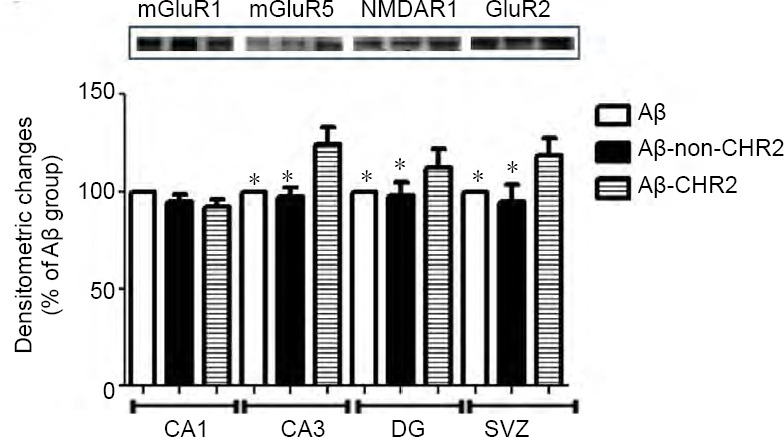
Effect of AAV5-CaMKII-CHR2-mCherry on glutamate receptors in the hippocampus of mice.
Glutamate receptors, including mGluR1 (145 kDa), mGluR5 (145 kDa), NMDAR1 (115 kDa) and GluR2 (110 kDa), were measured with western blot assay. Data are expressed as a percentage of the Aβ group and are shown as the mean ± SEM (n = 6). *P < 0.05, vs. Aβ-CHR2 group (one-way analysis of variance). Aβ: Repeated Aβ1–42 injection into the bilateral dentate gyrus; Aβ-non-CHR2: repeated Aβ1–42 injection into the bilateral dentate gyrus together with AAV5-CaMK-mCherry injection and light stimulation; Aβ-CHR2: repeated Aβ1–42 injection into the bilateral dentate gyrus together with AAV5-CaMK-CHR2-mCherry injection and light stimulation. mGluR1: Metabotropic glutamate receptor 1; mGluR5: metabotropic glutamate receptor 5; NMDA: N-methyl-D-aspartic acid receptor; AAV: adeno-associated virus; CaMK: Ca2+/calmodulin-dependent protein kinase; CHR2: channelrhodopsin-2.
Effect of AAV5-CaMK-CHR2-mCherry on neuroinflammation in the hippocampus
GFAP is a cell-specific biomarker of astrocytes that is associated with neuroinflammation (Yang and Wang, 2015). Compared with the Aβ-non-CHR2 and Aβ groups, AAV5-CaMK-CHR2-mCherry markedly inhibited GFAP expression in the Aβ-CHR2 group (F = 14.03, P < 0.01; Figure 6). Interleukins are a group of cytokines on which the function of the immune system depends greatly (Brocker et al., 2010). IL-6 expression was decreased in the Aβ-CHR2 group compared with the Aβ-non-CHR2 and Aβ groups (F = 48.05, P < 0.01). No significant differences in IL-1β or IL-10 expression were observed among the three groups (F = 0.665, P > 0.05 for IL-1β; F = 0.296, P > 0.05 for IL-10; Figure 6).
Figure 6.
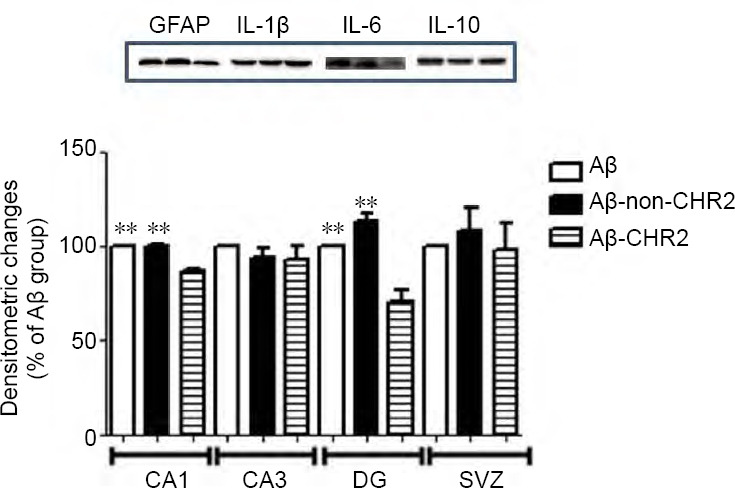
Effect of AAV5-CaMKII-CHR2-mCherry on neuroinflammation in the hippocampus of mice.
Neuroinflammation was measured by the amount of GFAP (50 kDa), IL-1β (29 kDa), IL-6 (29 kDa) and IL-10 (20 kDa). Data are expressed as a percentage of the Aβ group and are shown as the mean ± SEM (n = 6). **P < 0.01, vs. Aβ-CHR2 group (one-way analysis of variance). Aβ: Repeated Aβ1–42 injection into the bilateral dentate gyrus; Aβ-non-CHR2: repeated Aβ1–42 injection into the bilateral dentate gyrus together with AAV5-CaMK-mCherry injection and light stimulation; Aβ-CHR2: repeated Aβ1–42 injection into the bilateral dentate gyrus together with AAV5-CaMK-CHR2-mCherry injection and light stimulation; GFAP: glial fibrillary acidic protein; IL: interleukin; AAV: adeno-associated virus; CaMK: Ca2+/calmodulin-dependent protein kinase; CHR2: channelrhodopsin-2.
Discussion
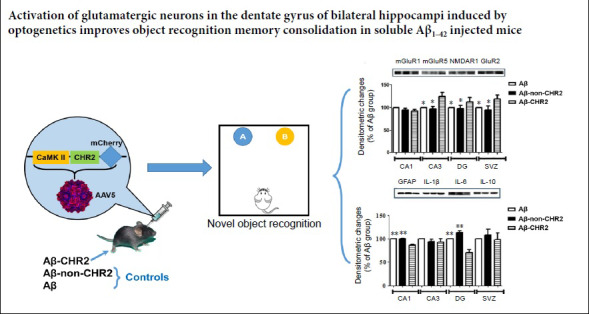
In the present study, we investigated the effect of stimulating CaMK-CHR2-expressing neurons in the dentate gyrus of the bilateral hippocampus on memory function. The main findings are as follows: (1) optogenetics improved working memory and short-term memory in the test phase. (2) Optogenetics upregulated GluR2, NMDAR1 and mGluR5, and inhibited neuroinflammation, thereby decreasing neuronal and synaptic loss in the dentate gyrus caused by Aβ1–42.
The incidence of AD is increasing. However, current therapeutic strategies for AD have limited efficacy (Alzheimer’s Association, 2013). Individuals with AD have difficulty recognizing objects because of semantic memory deficits (Laatu et al., 2003). In the present study, stimulation of CaMK-CHR2-expressing neurons in the dentate gyrus of the hippocampus improved object recognition memory consolidation in Aβ1–42-injected mice. The novel object recognition test was first proposed by Ennaceur and Delacour (1988) as a method to evaluate the ability of mice to recognize a novel object in familiar surroundings. In 2004, the method was further developed by Hammond et al. (2004) for testing object recognition memory and the influence of amnesic medicine on exploratory activity. This simple reliable test provides information on working, short-term and long-term memory. A higher memory index for the novel object in the testing stage implies a better ability for familiar object recognition. Aβ mice and Aβ-non-CHR2 mice spent < 50% of the total time exploring the novel object 5 minutes, 2 hours and 24 hours after the training phase, while Aβ-CHR2 mice spent a significantly greater proportion of time exploring the novel object 5 minutes and 2 hours after the training phase, implying that working memory and short-term memory were improved by AAV5-CaMK-CHR2-mCherry expression combined with light stimulation. Thus, CHR2 activation improved memory consolidation. It is worth noting that the memory index in the testing phase, 24 hours after the training phase, was markedly decreased compared with 5 minutes after the training phase in the Aβ-CHR2 group. The substantial elapsed time may be the reason why there was no effect of optogenetics on long-term memory.
Optogenetics combines both optical and genetic techniques to activate or inhibit the activity of specific cells or tissues, in vitro as well as in vivo (Deisseroth, 2011). Optogenetics can be used to selectively control neuronal activity with a temporal precision of a millisecond, and can be applied to examine the effect of neuronal activity on behavior (Gradinaru et al., 2009; Tye et al., 2011). Huff et al. (2013) demonstrated that activation of ChR2(E123A)-expressing neurons in the basolateral amygdala using trains of 40-Hz light pulses consolidated retention, whereas inhibition of ArchT-expressing neurons in the basolateral amygdala for 15 minutes impaired retention. Van den Oever et al. (2013) found that optical stimulation of pyramidal cells did not influence recent memory, but facilitated extinction of remote memory. Furthermore, while inhibition of pyramidal cells affected the recall of recent cocaine memory, it did not affect recall of remote memory. Their study was the first to demonstrate that working and short-term memories can be rescued by optogenetics in a model of AD. Pathophysiologically, because of the severe loss of synapses and neurons, the AD brain shrinks dramatically (Dubois et al., 2014), which is considered a main cause of memory impairment (Masliah et al., 2001; Monacelli et al., 2003; Pai and Jacobs, 2004).
In the present study, working and short-term memories were rescued by optogenetics. Additionally, expression levels of NeuN and synapsin in the dentate gyrus in the Aβ-CHR2 group were increased compared with the Aβ-non-CHR2 and Aβ groups. These changes might underlie the improvement in working and short-term memories in the Aβ-CHR2 group. Two key characteristics of optogenetics are accuracy of timing and location. Therefore, the neuroprotective effect in the dentate gyrus, the core area of CHR2 expression, did not extend to other areas. As a result, when synaptic and neuronal losses were measured with western blot assay, there were no significant differences in hippocampal area among the three groups.
Optogenetics is used to manipulate the activity of neurons (Deisseroth, 2015; Rosen et al., 2015; Lux et al., 2017). ChR2 is a light-activated cation channel engineered for stable membrane expression (Boyden et al., 2005). Under blue light (470 nm), ChR2-expressing neurons are depolarized by a strong and ultrafast current sufficient to induce single or multiple action potentials (Boyden et al., 2005). AAV5-CaMKII-CHR2-mCherry is expressed in glutamatergic neurons and can be activated by laser (473 nm, 1–3 ms, 10 Hz), which results in the release of glutamate. Glutamate is the main excitatory neurotransmitter in the brain, and is involved in regulating functions such as learning and memory. The role of glutamate in memory loss and neurodegeneration in AD is still unclear in humans. However, cholinergic dysfunction is strongly associated with memory loss (Esposito et al., 2013), which may in turn perturb glutamatergic transmission in AD (Esposito et al., 2013). Therefore, activation of glutamatergic neurons and the release of glutamate may be one of the mechanisms contributing to the improved memory consolidation produced by optogenetic stimulation.
Another mechanism contributing to the improved memory consolidation by optogenetics may be activation of GluR2, NMDAR1 and mGluR5 by AAV5-CaMKII-CHR2-mCherry. The two families of glutamate receptors-iGluR and mGluR-are located in the plasmalemma of neurons. Although ChR2 is a photo-sensitive cation channel, and action potentials are induced in ChR2-expressing neurons under light stimulation, it is unknown which glutamate receptors are activated after light stimulation. mGluR5 is a metabotropic receptor (Esposito et al., 2013) that is mainly expressed in CA3 pyramidal neurons, the dentate gyrus granule cell layer, as well as the lateral hippocampus (Ferraguti et al., 1998). Therefore, implantation of AAV5-CaMKII-CHR2-mCherry in the dentate gyrus of the bilateral hippocampus activated mGluR5 in the present study. A major role for group 1 mGluRs has been suggested in several types of hippocampal synaptic plasticity, such as slow-onset and long-term potentiation, chemical depression, and protein expression-dependent and -independent forms of long-term depression (Naie and Manahan-Vaughan, 2005; Moult et al., 2006). mGlu5 positive allosteric modulators strengthen both long-term potentiation and long-term depression in the hippocampus, and strengthen spatial learning in the water maze (Ayala et al., 2009). These studies support our current findings that optogenetics improves memory consolidation via activation of mGluR5.
GluR2 and NMDAR1 are ionotropic receptors that are activated in ChR2-expressing neurons exposed to 470 nm blue light. NMDAR has a critical role in long-term potentiation and long-term depression (Esposito et al., 2013). Moreover, NMDAR is involved in the Aβ-induced perturbation in synaptic plasticity (Birnbaum et al., 2015; Lei et al., 2016). Aβ may downregulate the levels of GluR1 and GluR2 on the cell surface and alter AMPA receptor activity, thereby disrupting synaptic activity and contributing to the decline in cognitive function in AD (Hsieh et al., 2006; Liu et al., 2010).
Aβ1–42 injection upregulates pro-inflammatory cytokines, such as IL-1β and IL-6, and GFAP in the brain, which are associated with astrocytosis (Meunier et al., 2015). Neuroinflammation is a major cause of neuronal and synaptic loss in AD (Doost Mohammadpour et al., 2015).
In the present study, inhibition of neuroinflammation was demonstrated by decreased GFAP and IL-6 immunostaining after optogenetic activation. Glutamate released from activated glutamatergic neurons is taken up by glial cells, which convert it into glutamine for uptake by presynaptic terminals (Esposito et al., 2013). Glutamate uptake by astrocytes is influenced by adjacent cells and by diffusable factors, including glutamate itself (Vermeiren et al., 2006). For example, glutamate uptake is affected by inflammatory changes. In the present study, activation of glutamatergic neurons resulted in the release of glutamate and the activation of glutamate receptors, including GluR2, NMDAR1 and mGluR5. This suppressed neuroinflammation and decreased GFAP and IL-6 expression, thereby protecting against neuronal and synaptic losses after Aβ1–42 injection in the dentate gyrus. Further study is needed to clarify how activation of glutamate receptors impacts glutamate homeostasis and attenuates neuroinflammation.
In conclusion, the activation of glutamatergic neurons in the bilateral dentate gyri of the hippocampus, induced by optogenetic stimulation, improves object recognition memory consolidation in soluble Aβ1–42-injected mice. The laser-induced activation of CHR2 regulates glutamate receptors, inhibits neuroinflammation, and reduces neuronal and synaptic losses caused by Aβ1–42 injection.
The neuronal-glial network is considered a promising intervention target for AD; however, progress has been limited. In the present study, the optogenetic technique improved memory in the mouse model of AD by modulating the neuronal-glial network, and this effect was likely mediated, at least in part, by activation of glutamate receptors. However, the technique is invasive and neuronal damage could not be avoided during the surgical procedures. Therefore, there is an urgent need to improve optogenetic techniques before clinical application.
Footnotes
Conflicts of interest: None declared.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81171191 (to LYZ); the Shenzhen Special Fund Project on Strategic Emerging Industry Development of China, No. JCYJ20160422170522075 (to LYZ); the Shenzhen Healthcare Research Project of China, No. 201601015 (to LYZ). Funders had no involvement in the study design; data collection, analysis, and interpretation; paper writing; or decision to submit the paper for publication.
Institutional review board statement: The study was approved by Animal Resources Committee of Jinan University, China (approval No. LL-KT-2011134) on February 28, 2011.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81171191 (to LYZ); the Shenzhen Special Fund Project on Strategic Emerging Industry Development of China, No. JCYJ20160422170522075 (to LYZ); the Shenzhen Healthcare Research Project of China, No. 201601015 (to LYZ).
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Patel B, Wysong S, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Albasanz JL, Dalfo E, Ferrer I, Martin M. Impaired metabotropic glutamate receptor/phospholipase C signaling pathway in the cerebral cortex in Alzheimer’s disease and dementia with Lewy bodies correlates with stage of Alzheimer’s-disease-related changes. Neurobiol Dis. 2005;20:685–693. doi: 10.1016/j.nbd.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- 4.Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, Lindsley CW, Olive MF, Conn PJ. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birnbaum JH, Bali J, Rajendran L, Nitsch RM, Tackenberg C. Calcium flux-independent NMDA receptor activity is required for Abeta oligomer-induced synaptic loss. Cell Death Dis. 2015;6:e1791. doi: 10.1038/cddis.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond- timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 7.Brocker C, Thompson D, Matsumoto A, Nebert DW, Vasiliou V. Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum Genomics. 2010;5:30–55. doi: 10.1186/1479-7364-5-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouillette J, Caillierez R, Zommer N, Alves-Pires C, Benilova I, Blum D, De Strooper B, Buee L. Neurotoxicity and memory deficits induced by soluble low-molecular-weight amyloid-beta1-42 oligomers are revealed in vivo by using a novel animal model. J Neurosci. 2012;32:7852–7861. doi: 10.1523/JNEUROSCI.5901-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crews L, Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum Mol Genet. 2010;19:R12–20. doi: 10.1093/hmg/ddq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deisseroth K. Optogenetics. Nat Methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci. 2015;18:1213–1225. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doost Mohammadpour J, Hosseinmardi N, Janahmadi M, Fathollahi Y, Motamedi F, Rohampour K. Non-selective NSAIDs improve the amyloid-beta-mediated suppression of memory and synaptic plasticity. Pharmacol Biochem Behav. 2015;132:33–41. doi: 10.1016/j.pbb.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert MO, Jicha GA, Nordberg A, Pasquier F, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 14.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 15.Esposito Z, Belli L, Toniolo S, Sancesario G, Bianconi C, Martorana A. Amyloid beta, glutamate, excitotoxicity in Alzheimer's disease: are we on the right track? CNS Neurosci Ther. 2013;19:549–555. doi: 10.1111/cns.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fachim HA, Pereira AC, Iyomasa-Pilon MM, Rosa ML. Differential expression of AMPA subunits induced by NMDA intrahippocampal injection in rats. Front Neurosci. 2016;10:32. doi: 10.3389/fnins.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferraguti F, Conquet F, Corti C, Grandes P, Kuhn R, Knopfel T. Immunohistochemical localization of the mGluR1beta metabotropic glutamate receptor in the adult rodent forebrain: evidence for a differential distribution of mGluR1 splice variants. J Comp Neurol. 1998;400:391–407. [PubMed] [Google Scholar]
- 18.Fortress AM, Schram SL, Tuscher JJ, Frick KM. Canonical Wnt signaling is necessary for object recognition memory consolidation. J Neurosci. 2013;33:12619–12626. doi: 10.1523/JNEUROSCI.0659-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gómez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton A, Zamponi GW, Ferguson SS. Glutamate receptors function as scaffolds for the regulation of beta-amyloid and cellular prion protein signaling complexes. Molecular brain. 2015;8:18. doi: 10.1186/s13041-015-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huff ML, Miller RL, Deisseroth K, Moorman DE, LaLumiere RT. Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proc Natl Acad Sci U S A. 2013;110:3597–3602. doi: 10.1073/pnas.1219593110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuperstein I, Broersen K, Benilova I, Rozenski J, Jonckheere W, Debulpaep M, Vandersteen A, Segers-Nolten I, Van Der Werf K, Subramaniam V, Braeken D, Callewaert G, Bartic C, D’Hooge R, Martins IC, Rousseau F, Schymkowitz J, De Strooper B. Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 2010;29:3408–3420. doi: 10.1038/emboj.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laatu S, Revonsuo A, Jäykkä H, Portin R, Rinne JO. Visual object recognition in early Alzheimer’s disease: deficits in semantic processing. Acta Neurol Scand. 2003;108:82–89. doi: 10.1034/j.1600-0404.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 27.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T. Object recognition test in mice. Nat Protoc. 2013;8:2531–2537. doi: 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
- 29.Lei M, Xu H, Li Z, Wang Z, O’Malley TT, Zhang D, Walsh DM, Xu P, Selkoe DJ, Li S. Soluble Abeta oligomers impair hippocampal LTP by disrupting glutamatergic/GABAergic balance. Neurobiol Dis. 2016;85:111–121. doi: 10.1016/j.nbd.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 31.Liu SJ, Gasperini R, Foa L, Small DH. Amyloid-beta decreases cell-surface AMPA receptors by increasing intracellular calcium and phosphorylation of GluR2. J Alzheimers Dis. 2010;21:655–666. doi: 10.3233/JAD-2010-091654. [DOI] [PubMed] [Google Scholar]
- 32.Lux V, Masseck OA, Herlitze S, Sauvage MM. Optogenetic destabilization of the memory trace in CA1: insights into reconsolidation and retrieval processes. Cereb Cortex. 2017;27:841–851. doi: 10.1093/cercor/bhv282. [DOI] [PubMed] [Google Scholar]
- 33.Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr, Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology. 2001;56:127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- 34.McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 35.Meunier J, Borjini N, Gillis C, Villard V, Maurice T. Brain toxicity and inflammation induced in vivo in mice by the amyloid-beta forty-two inducer aftin-4, a roscovitine derivative. J Alzheimers Dis. 2015;44:507–524. doi: 10.3233/JAD-140711. [DOI] [PubMed] [Google Scholar]
- 36.Monacelli AM, Cushman LA, Kavcic V, Duffy CJ. Spatial disorientation in Alzheimer’s disease: the remembrance of things passed. Neurology. 2003;61:1491–1497. doi: 10.1212/wnl.61.11.1491. [DOI] [PubMed] [Google Scholar]
- 37.Moult PR, Gladding CM, Sanderson TM, Fitzjohn SM, Bashir ZI, Molnar E, Collingridge GL. Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. J Neurosci. 2006;26:2544–2554. doi: 10.1523/JNEUROSCI.4322-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naie K, Manahan-Vaughan D. Pharmacological antagonism of metabotropic glutamate receptor 1 regulates long-term potentiation and spatial reference memory in the dentate gyrus of freely moving rats via N-methyl-D-aspartate and metabotropic glutamate receptor-dependent mechanisms. Eur J Neurosci. 2005;21:411–421. doi: 10.1111/j.1460-9568.2005.03864.x. [DOI] [PubMed] [Google Scholar]
- 39.Ono K, Condron MM, Teplow DB. Structure-neurotoxicity relationships of amyloid beta-protein oligomers. Proc Natl Acad Sci U S A. 2009;106:14745–14750. doi: 10.1073/pnas.0905127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ordaz JD, Wu W, Xu XM. Optogenetics and its application in neural degeneration and regeneration. Neural Regen Res. 2017;12:1197–1209. doi: 10.4103/1673-5374.213532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pai MC, Jacobs WJ. Topographical disorientation in community-residing patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2004;19:250–255. doi: 10.1002/gps.1081. [DOI] [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. London: Academic Press; [DOI] [PubMed] [Google Scholar]
- 43.Piani D, Frei K, Do KQ, Cuenod M, Fontana A. Murine brain macrophages induced NMDA receptor mediated neurotoxicity in vitro by secreting glutamate. Neurosci Lett. 1991;133:159–162. doi: 10.1016/0304-3940(91)90559-c. [DOI] [PubMed] [Google Scholar]
- 44.Piani D, Spranger M, Frei K, Schaffner A, Fontana A. Macrophage-induced cytotoxicity of N-methyl-D-aspartate receptor positive neurons involves excitatory amino acids rather than reactive oxygen intermediates and cytokines. Eur J Immunol. 1992;22:2429–2436. doi: 10.1002/eji.1830220936. [DOI] [PubMed] [Google Scholar]
- 45.Reiner A, Levitz J. Glutamatergic signaling in the central nervous system: ionotropic and metabotropic receptors in concert. Neuron. 2018;98:1080–1098. doi: 10.1016/j.neuron.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosen ZB, Cheung S, Siegelbaum SA. Midbrain dopamine neurons bidirectionally regulate CA3-CA1 synaptic drive. Nat Neurosci. 2015;18:1763–1771. doi: 10.1038/nn.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruby NF, Fernandez F, Garrett A, Klima J, Zhang P, Sapolsky R, Heller HC. Spatial memory and long-term object recognition are impaired by circadian arrhythmia and restored by the GABAAAntagonist pentylenetetrazole. PLoS One. 2013;8:e72433. doi: 10.1371/journal.pone.0072433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 51.Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van den Oever MC, Rotaru DC, Heinsbroek JA, Gouwenberg Y, Deisseroth K, Stuber GD, Mansvelder HD, Smit AB. Ventromedial prefrontal cortex pyramidal cells have a temporal dynamic role in recall and extinction of cocaine-associated memory. J Neurosci. 2013;33:18225–18233. doi: 10.1523/JNEUROSCI.2412-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vermeiren C, Hemptinne I, Vanhoutte N, Tilleux S, Maloteaux JM, Hermans E. Loss of metabotropic glutamate receptor-mediated regulation of glutamate transport in chemically activated astrocytes in a rat model of amyotrophic lateral sclerosis. J Neurochem. 2006;96:719–731. doi: 10.1111/j.1471-4159.2005.03577.x. [DOI] [PubMed] [Google Scholar]
- 54.Yang Z, Wang KK. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015;38:364–374. doi: 10.1016/j.tins.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]