Abstract
Spinal cord injury is associated with chronic sensorimotor deficits due to the interruption of ascending and descending tracts between the brain and spinal cord. Functional recovery after anatomically complete spinal cord injury is limited due to the lack of long-distance axonal regeneration of severed fibers in the adult central nervous system. Most spinal cord injuries in humans, however, are anatomically incomplete. Although restorative treatment options for spinal cord injury remain currently limited, research from experimental models of spinal cord injury have revealed a tremendous capability for both spontaneous and treatment-induced plasticity of the corticospinal system that supports functional recovery. We review recent advances in the understanding of corticospinal circuit plasticity after spinal cord injury and concentrate mainly on the hindlimb motor cortex, its corticospinal projections, and the role of spinal mechanisms that support locomotor recovery. First, we discuss plasticity that occurs at the level of motor cortex and the reorganization of cortical movement representations. Next, we explore downstream plasticity in corticospinal projections. We then review the role of spinal mechanisms in locomotor recovery. We conclude with a perspective on harnessing neuroplasticity with therapeutic interventions to promote functional recovery.
Keywords: spinal cord injury, motor cortex, motor map, corticospinal tract, neuroplasticity, functional recovery, animal models, forelimb, hindlimb, locomotion
Introduction
Spinal cord injury (SCI) is a devastating neurological condition associated with significant morbidity and chronic disturbances in motor, sensory, and autonomic function. The prevalence of SCI has been increasing globally over the past decades and ranges of 236–1298 affected individuals per million (Khorasanizadeh et al., 2019) with an estimated 768,737 new cases annually worldwide (Kumar et al., 2018). Despite an increasing need to develop effective repair strategies after SCI, restorative treatment options remain limited (Chen and Levi, 2017). The functional consequences of SCI result from the disruption of ascending and descending fibers within the spinal cord to supraspinal structures. Deficits in the voluntary control of movement after SCI result, in part, from the interruption of motor cortex projections that retract from the injury site. Although long-distance axonal regeneration of severed fibers is limited in the adult central nervous system, extensive neuronal reorganization and plasticity in spared circuitry has been demonstrated in experimental SCI models throughout the neuraxis that is associated with functional recovery (Fouad and Tse, 2008; Moxon et al., 2014; Filli and Schwab, 2015). A better understanding of the neuroplastic changes that occur after SCI and how they can be promoted is warranted to develop therapeutic repair strategies and rehabilitation paradigms.
In this article, we review recent developments in the understanding of corticospinal plasticity and functional recovery that occurs after SCI and mainly focus on plasticity occurring at the level of the hindlimb motor cortex, its corticospinal projections, and the role of spinal mechanisms supporting spontaneous locomotor recovery that have recently been revealed in experimental SCI models. We begin with a discussion on plasticity that occurs at the level of the motor cortex and the reorganization of cortical movement representations. Next, we consider how motor cortex reorganization is supported by downstream plasticity in corticospinal projections and indirect relays to the spinal cord. We then review the role of spinal mechanisms in supporting locomotor recovery. We conclude with a perspective on harnessing neuroplasticity with therapeutic interventions to promote functional recovery.
Search Strategy and Selection Criteria
We have performed a PubMed literature search of articles with search terms including “spinal cord”, “injury”, “lesion”, “hemisection”, “contusion”, “motor cortex”, “motor map”, “intracortical”, “stimulation”, “corticospinal tract”, “locomotion”, “behaviour”, “movement”, “neurotechnology”, “modulation”, “training”, and “plasticity”. Selection criteria included recent articles (2009–2019) on corticospinal plasticity and functional recovery after SCI mainly focusing on plasticity occurring in the hindlimb motor cortex, corticospinal projections, and the role of spinal mechanisms supporting spontaneous locomotor recovery. In addition, we also include important articles on the subject matter published earlier (< 2009), as well as a pertinent selection involving forelimb function where appropriate.
Motor Cortex Plasticity and Functional Recovery after Spinal Cord Injury
The motor cortex contains a topographic representational map of body movements (motor map) that can be revealed by stimulation, typically performed with transcranial magnetic stimulation (TMS) in humans (Hallett, 2007) and intracortical microstimulation in animal models (Asanuma and Sakata, 1967). Motor mapping studies have provided a rich source of information on motor cortex organization and plasticity during motor learning as well as during functional recovery after SCI. Importantly, motor map plasticity appears to be a conserved trait among mammals and is observed following motor learning in humans (Pascual-Leone et al., 1995), non-human primates (Nudo et al., 1996) and rats (Kleim et al., 1998). Motor map plasticity during motor learning is reflected by a proportional increase in the cortical area devoted to learned task. In humans for example, skilled learning of a piano exercise increases the representation of the fingers (Pascual-Leone et al., 1995); while in rats, learning of a skilled reaching task increases the representation of the wrist and digits (Kleim et al., 1998). Motor map plasticity during motor learning is thought to reflect the acquisition of a skilled behavior as the repetition of unskilled tasks that do not require learning do not lead to cortical reorganization in either primates (Plautz et al., 2000) or rats (Kleim et al., 2004). The underlying mechanisms supporting motor map plasticity after motor learning are numerous (Papale and Hooks, 2018) and involve changes in protein synthesis (Kleim et al., 2003), dendritic remodeling (Xu et al., 2009), synapse formation (Kleim et al., 2002) and synaptic efficacy (Monfils and Teskey, 2004) that can alter the synaptic weighting between pyramidal neurons via potentiation and depotentiation of cortical horizontal fiber connections (Hess and Donoghue, 1994, 1996; Rioult-Pedotti et al., 2000) to change representational boundaries.
Reductions in motor cortex excitability are routinely observed in SCI with TMS, reflected by an increase in both the threshold and latency to evoke movement in the contralateral upper and lower limbs (Davey et al., 1998; Smith et al., 2000; Roy et al., 2011). Motor maps are also reorganized in humans with SCI at both the cervical (Levy et al., 1990; Freund et al., 2011) and thoracic (Topka et al., 1991) levels, where the representations of more impaired movements are reduced or abolished and the representations of less impaired movements are expanded and shifted. Motor map reorganization, however, is not consistently observed after SCI in humans as revealed by a recent TMS study in which the representation of wrist movements were appreciably normal following an incomplete cervical injury despite only liminal activation of the wrist under voluntary control (Cortes et al., 2016). Although the underlying cause of the functional paralysis in the previous study is unknown, the authors speculate that it may involve a learned disuse of the affected wrist chronically after injury (Cortes et al., 2016). To further examine the extent of motor cortex plasticity after SCI and its role in functional recovery, experimental animal models allowing for the precise targeting of spinal lesions are especially valuable.
Following a complete thoracic spinal transection that paralyses the hindlimbs in rats, motor maps for movements below the level of injury are chronically abolished and a reorganization of movement representation rostral to the lesion is observed (Oza and Giszter, 2014, 2015; Manohar et al., 2017). In one study, adult rats were subjected to a complete thoracic transection and received either daily unassisted treadmill locomotion training, robot-assisted treadmill training that provided body weight support and trunk stability, or no locomotor training for up to 5 weeks after injury (Oza and Giszter, 2014). In all groups hindlimb motor maps were abolished but there was a significant caudal expansion of adjacent trunk representation into former hindlimb cortical territory and a rostral expansion of the trunk into forelimb cortex, resulting in an increased overlap in evoked trunk and forelimb movements. Further, the expansion of trunk representation was significantly larger in both training groups compared to the spontaneous plasticity in untrained transected rats. Despite cortical plasticity after treadmill training, there was no improvement of hindlimb locomotor function. In comparison to injury occurring in adulthood, there is an augmented potential for motor recovery after injury occurring during development (Friel et al., 2012). In a follow-up study (Oza and Giszter, 2015), neonatal rats were subjected to a complete thoracic spinal transection and received unassisted treadmill training for 8–10 months after injury at which point separate groups received an additional 4–5 weeks of training period with either continued unassisted training or robot-assisted training. The robot-assisted training group exhibited significantly improved hindlimb locomotor stepping compared to the unassisted training group. Moreover, in robot-assisted rats there was a significant caudal shifting of trunk representation leading to a decreased overlap of forelimb and trunk representation indicating that the training associated with locomotor improvement reversed the cortical plasticity observed spontaneously after injury.
The ability to partially recover hindlimb locomotor function also occurs after a complete thoracic spinal transection in adult rats (Manohar et al., 2017). Rats received 12 weeks of pharmacological therapy using serotonergic agonists (quipazine and 8-OHDPAT) combined with locomotor rehabilitation consisting of either passive hindlimb exercise or active weight-supported treadmill training. A therapy control group received sham drug infusion and no rehabilitative training. Rats receiving pharmacological therapy combined with active treadmill training exhibited significant improvement in the ability to generate both weight-supported and unsupported hindlimb stepping compared to rats receiving pharmacotherapy with passive hindlimb training. Therapy control rats did not show any improvement in locomotor function. Importantly, the functional recovery in rats receiving pharmacological therapy combined with active treadmill training related to a significant expansion of motor cortex trunk representation in the former cortical territory occupied by the deefferented hindlimb. Subsequent electrolytic lesion of the reorganized motor cortex was then shown to reverse functional recovery, indicating that cortical plasticity was actively supporting the locomotor improvement from therapy (Manohar et al., 2017).
In contrast to complete spinal transection which abolishes all cortical representations of movement below the level of the injury, spontaneous motor map reorganization of movements caudal to the injury are observed after incomplete SCI with partial sparing of descending motor tracts to the spinal cord. After a cervical hemisection in the rat that disrupts all ascending and descending tracts on one side only, the forelimb on the side of the lesion partially recovers spontaneously during the first month, despite persistent deficits in distal movements (Martinez et al., 2010). While the overall size of the motor map of the affected forelimb in the contralesional motor cortex of these rats is drastically reduced after injury, the representation of shoulder and elbow movements are significantly increased relative to the wrist and digits and consistent with recovery (Martinez et al., 2010). Further, rehabilitative training of the affected forelimb after a unilateral dorsal funiculus lesion at the cervical level has been shown to potentiate functional recovery and is paralleled by an expansion of wrist representation in the contralesional motor cortex (Girgis et al., 2007). In rats that do not receive rehabilitative training after unilateral cervical SCI, persistent deficits in the affected forelimb along with an expansion of adjacent head and neck representation into the cortical territory formerly occupied by the de-efferented forelimb are observed for up to 5 months after injury (Tandon et al., 2008).
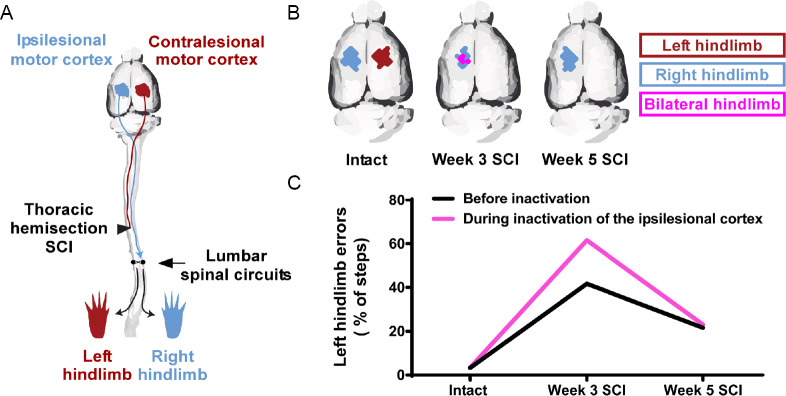
Motor map plasticity associated with functional hindlimb locomotor recovery has also been observed after incomplete SCI. Although injury that servers the crossed dorsal corticospinal tract abolishes hindlimb motor maps in the de-efferented contralesional hemisphere for up to 5 weeks in rats (Fouad et al., 2001; Bareyre et al., 2004; Frost et al., 2015; Manohar et al., 2017), we have recently shown that there is a compensatory spontaneous reorganization in the uninjured ipsilesional motor cortex that retains its corticospinal connectivity to the spinal cord after unilateral SCI (Brown and Martinez, 2018). By using a thoracic hemisection model in the rat that initially induces paralysis in one hindlimb, we observed significant recovery of locomotor performance over the first three weeks. Motor mapping in the intact state and at weekly intervals after hemisection in separate groups of rats revealed time-dependent changes in motor cortex organization, characterized by a chronic abolishment of hindlimb motor representation in the contralesional motor cortex and the development of a transient bilateral hindlimb representation in the ipsilesional motor cortex at 3 weeks after hemisection, when significant locomotor recovery occurred. To determine whether motor map reorganization was functionally related to spontaneous locomotor recovery, we next reversibly inactivated either the ipsilesional or contralesional hindlimb motor cortex using a cortical cooling approach during skilled locomotion in a within-group design at weekly intervals over the first five weeks after hemisection. Reversible inactivation of the ipsilesional, but not the contralesional motor cortex, during skilled locomotion at 3 weeks after hemisection reinstated deficits in both hindlimbs. This finding indicates that cortical plasticity is functionally supporting locomotor recovery of the affected hindlimb (Figure 1).
Figure 1.
Motor cortex plasticity is involved in spontaneous locomotor recovery after spinal cord injury (SCI).
(A) Thoracic hemisection SCI on the left side in the rat severs the crossed corticospinal tract from the contralesional motor cortex to the left hindlimb, but spares the crossed corticospinal projection from the ipsilesional motor cortex to the right hindlimb. The lumbosacral spinal circuits are located below the lesion. (B) In the intact state, intracortical microstimulation of either motor cortex elicits movement in the contralateral hindlimb. After SCI, stimulation of the contralesional motor cortex no longer elicits hindlimb movement for up to 5 weeks. Three weeks after SCI, stimulation of the ipsilesional motor cortex, that retains access to lumbosacral spinal circuits, elicits movement in both hindlimbs. This time point coincides with significant locomotor recovery of the affected (left) hindlimb (Brown and Martinez, 2018). Five weeks after SCI, stimulation of the ipsilesional motor cortex no longer elicits bilateral hindlimb movements. (C) Reversible inactivation of the ipsilesional motor cortex during skilled locomotion on a horizontal rung-ladder 3 weeks after SCI reinstated deficits in the affected hindlimb, but not in the intact state or 5 weeks after SCI. These findings indicate that after SCI, the ipsilesional motor cortex spontaneously gains a novel functional access to the affected hindlimb during the motor recovery process. Data presented are from a representational rat (Brown and Martinez, 2018).
Following a unilateral pyramidotomy in the rat, plasticity in the uninjured motor cortex associated with functional recovery of the forelimb has also recently been demonstrated (Wen et al., 2018). Rats received a unilateral neonatal pyramidotomy causing significant impairment in the contralateral forelimb and were reared to adulthood. Intracortical microstimulation of the uninjured motor cortex evoked movement in both forelimbs while stimulation of the de-efferented motor cortex did not evoked responses from either forelimb. Intriguingly, pharmacological inactivation of either motor cortex impaired the affected forelimb in injured rats during a skilled reaching task while only impairing the contralateral forelimb in uninjured rats. Cortical reorganization following unilateral cortical contusion is also observed in the rat where intracortical microstimulation of the uninjured motor cortex similarly evokes bilateral movement of the forelimbs (Axelson et al., 2013). Thus, following unilateral corticospinal injury both forelimb (Axelson et al., 2013; Wen et al., 2018) and hindlimb (Brown and Martinez, 2018) motor cortex in the uninjured hemisphere can reorganize to evoke bilateral limb movement to intracortical microstimulation.
In summary, there are three generalized findings on motor cortex plasticity observed after experimental SCI. First, following a complete SCI cortical movement representations rostral to the injury can expand in size to occupy the cortical territory of lost representation caudal to the injury. Second, following an incomplete SCI with partial sparing of corticospinal connectivity, movement representations of the affected limb are reduced in size but can expand with rehabilitative training. Third, following unilateral corticospinal injury there can be spontaneous plasticity in the uninjured the motor cortex to gain a movement representation of the injured limb. The underlying structural and synaptic mechanisms supporting motor cortex and movement representation plasticity after SCI are numerous and have been recently comprehensively reviewed (Fink and Cafferty, 2016; Serradj et al., 2017). Although transection injuries are useful for investigating neural plasticity in spared and damaged pathways associated with functional recovery after SCI and have provided invaluable insight towards motor cortex plasticity that occurs spontaneously to foster locomotor recovery, they are less commonly observed clinically and differences in neuroplasticity observed between experimental animal models and human SCI have been recently reviewed (Filipp et al., 2019).
Corticospinal Plasticity Supporting Functional Recovery after Spinal Cord Injury
The corticospinal tract is the major descending pathway for the control of voluntary movement ubiquitous in all mammals and prominently developed in primates (Nudo and Frost, 2007). Corticospinal circuitry is predominantly conserved between mammals, but there are some differences across species in fiber origins, projection patterns, and spinal terminations (Kuypers, 1981; Nudo and Masterton, 1988). Corticospinal fibers originate from layer V pyramidal neurons in the frontal and anterior parietal lobes and terminate diffusely in the spinal grey matter in primates. In humans, the primary, premotor, and supplementary motor cortices give rise to roughly 80% of corticospinal fibers with the remainder of innervation originating from cingulate motor and parietal somatosensory regions (Kuypers, 1981). The corticospinal tract projects mainly in the contralateral dorsolateral funiculus in primates, with minor ipsilateral components in the dorsolateral and ventromedial funiculi (Kuypers, 1981). In rats, the corticospinal tract originates from layer V pyramidal neurons in the sensorimotor cortex (Miller, 1987) and projects primarily in the base of the contralateral dorsomedial funiculus with minor components in the contralateral dorsolateral and ipsilateral ventromedial funiculi (Armand, 1982; Brosamle and Schwab, 1997; Anderson et al., 2009). Corticospinal axon terminals synapse in all mammals indirectly with lower motoneurons via spinal interneurons, while in primates there are additional monosynaptic cortico-motoneuronal connections (Lemon, 2008). In addition to primary spinal terminations, pyramidal neurons also send widespread collateral fibers to the rubrospinal, tectospinal, vestibulospinal and reticulospinal descending motor systems as well as to the cortex, striatum, thalamus, and dorsal column sensory nuclei (Canedo, 1997).
After SCI, functional recovery can be mediated by corticospinal plasticity that contributes to cortical reorganization. Following large spinal lesions at the thoracic level that disrupt the dorsal corticospinal tracts bilaterally in the rat, compensatory sprouting of axotomized corticospinal fibers from the hindlimb motor cortex is observed in the cervical spinal cord (Fouad et al., 2001; Ghosh et al., 2010). Intracortical microstimulation reveals an expansion in the representation of forelimb, whisker, and trunk movements into the de-efferented hindlimb motor cortex (Fouad et al., 2001). Intriguingly, retrograde tracing of the newly sprouted cervical corticospinal axon collaterals also reveals the emergence of a new forelimb corticospinal projection from the rostral part of the former hindlimb cortex that is responsive to subcutaneous stimulation of the forelimb and expansion of the forelimb somatosensory representation (Ghosh et al., 2010).
Strengthening of the minor uncrossed corticospinal tract components may also contribute to functional recovery after SCI. The ipsilateral corticospinal tract can functionally compensate for acute dysfunction in contralateral corticospinal connectivity in humans (Strens et al., 2003). In the rat, both unilateral pyramidotomy and electrical stimulation of the corticospinal tract at the medullary level separately strengthen ipsilateral corticospinal connections and the effect is enhanced with combined injury and stimulation (Brus-Ramer et al., 2007; Wen et al., 2018). Moreover, electrical stimulation of the motor cortex in the uninjured hemisphere after unilateral pyramidotomy promotes the recovery of skilled locomotion in rats through ipsilateral projections (Carmel et al., 2014). Following a dorsal column SCI that ablates the major dorsomedial corticospinal tracts in rats and mice bilaterally, the minor dorsolateral corticospinal tract component remains intact and can provide an alternative route for corticospinal input to caudal spinal segments (Steward et al., 2004). In mice submitted to a bilateral dorsal column transection at the cervical level, a re-emergence of forelimb and hindlimb motor maps within both motor cortices has been observed during behavioral recovery (Hilton et al., 2016). Selective chemogenetic silencing of the spared dorsolateral corticospinal pathway was subsequently found to abrogate spontaneous recovery, indicating that it was supporting functional recovery.
Unilateral SCI is also associated with spontaneous and extensive collateral sprouting of uninjured contralateral corticospinal fibers. Corticospinal collaterals can cross the spinal midline to innervate the de-efferented hemicord in both primates (Rosenzweig et al., 2009) and rats (Ghosh et al., 2009). Sprouting of intact corticospinal fibers may underlie functional recovery of the affected limb and lead to the development of a bilateral limb representation in the ipsilesional motor cortex (Brown and Martinez, 2018; Wen et al., 2018). Pharmacological inactivation of the motor cortex (van den Brand et al., 2012) or a secondary lesion rostral to the injury (Hollis et al., 2016) have both confirmed the functional role of corticospinal collateral sprouting after SCI.
Bilateral movements evoked by intracortical microstimulation depend on constitutive activity in the contralateral cortex (Brus-Ramer et al., 2009). Brain imaging and reversible pharmacological inactivation of the motor cortex in primates following unilateral cervical corticospinal transection reveals that the recovery of finger dexterity involves the motor cortex bilaterally during the initial recovery stage but transitions to the contralesional motor cortex during chronic recovery time points (Nishimura et al., 2007). In rats, a similar re-emergence of hindlimb cortical representation is observed in the contralateral motor cortex during the late recovery stage (12 weeks) after thoracic hemisection that is due to a sprouting of transected corticospinal fibers onto propriospinal interneurons that, in turn, arborize on lumbar motor neurons to bypass the lesion and reinstate descending cortical input (Bareyre et al., 2004). Such rewiring of corticospinal connectivity through propriospinal relays can be extensive and even allow for the spontaneous recovery of hindlimb stepping in mice after spatially and temporally staggered spinal hemisections that effectively interrupt all long-descending supraspinal pathways (Courtine et al., 2008). An additional pathway for restoring corticospinal input after SCI involves the sprouting of corticospinal collaterals to brainstem reticulospinal nuclei (Zorner et al., 2014) that can mediate functional recovery by providing bilateral access to spinal circuitry caudal to the lesion via cortico-reticulo-propriospinal relays (Filli et al., 2014; Asboth et al., 2018).
Thus, there is plasticity occurring through multiple mechanisms and at multiple levels to reinstate descending cortical input to the spinal cord after SCI. In the next section, we discuss how the spinal circuits below the injury participate in locomotor recovery.
Spinal Circuit Plasticity and Functional Recovery after Spinal Cord Injury
The lumbosacral spinal cord contains neural circuits that can generate lower limb locomotion in a variety of species from primitive protovertebrates to man (Dimitrijevic et al., 1998; Nadeau et al., 2010). These spinal circuits are remarkably plastic and previous work has established that they remodel in a functionally meaningful manner following a partial or total loss of descending inputs. After a complete SCI at the thoracic level in cats and rats, non-voluntary hindlimb locomotion can be re-expressed by pharmacological (Forssberg and Grillner, 1973; Antri et al., 2005; Fong et al., 2005), electrical (Ichiyama et al., 2005; Barthélemy et al., 2007) and/or afferent input stimulation (Barbeau and Rossignol, 1987; Ichiyama et al., 2008). These studies demonstrate that, when isolated from brain inputs, excitatory inputs facilitate re-expression of spinal locomotion. A critical question is whether spinal mechanisms participate to the recovery of locomotion after incomplete SCI, in which supraspinal structures remain partially connected to spinal locomotor circuits. In this scenario the main challenge is to isolate the spinal circuits from its main sources of modulatory inputs.
A dual lesion paradigm has been developed (Barrière et al., 2008; Martinez and Rossignol, 2013). A unilateral hemisection of the cord is first performed at T10, inducing an initial paresis of the hindlimb on the side of the lesion and an asymmetrical gait pattern (Martinez et al., 2011, 2012b). To evaluate whether such lesion induces spinal changes, a complete spinal lesion is then performed below the first one at T13 to isolate the spinal circuits from its supraspinal influences (Figure 2). When isolated from brain inputs, the spinal locomotor circuitry of most cats could express a locomotor pattern as soon as tested (24 hours) (Martinez et al., 2011, 2012b), a skill that usually develops only after several weeks of treadmill training when a single complete spinal injury is performed (Barbeau and Rossignol, 1987). Specifically, 6/11 cats (55%) expressed a bilateral hindlimb locomotion, 3/11 expressed a unilateral pattern on the side of the previous hemisection and 2/11 were not able to walk. By quantifying specific locomotor parameters that are mainly controlled by the spinal circuits, including step cycle structure, we found that some changes observed after hemisection were retained after spinalization (Martinez et al., 2012b). This work demonstrates that a spinal hemisection imprints spinal locomotor circuits, affecting their state of excitability and reshaping spinal circuits within a short period of time.
Figure 2.
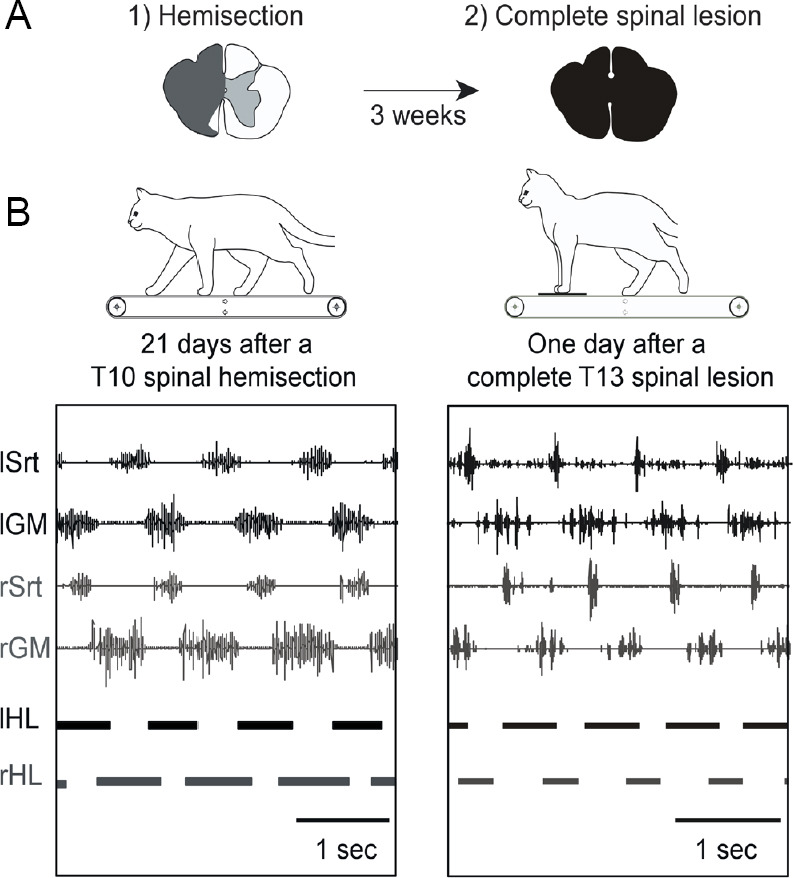
Episodes of treadmill locomotion at 0.4 m/s displayed by a cat 3 weeks after a spinal hemisection at T10 and 24 hours after a second complete spinal lesion at T13.
(A) Three weeks after hemisection, hindlimb locomotion was already re-expressed and well-organized. (B) After the subsequent complete spinal section isolating spinal circuits from all supraspinal inputs, the cat could express hindlimb locomotion as soon as tested (24 hours). The locomotor pattern was asymmetrical and consisted mainly in a better capacity to walk with the hindlimb previously impacted by the hemisection. For instance, the phase of support on the side of the previous hemisection was longer than on the other side (horizontal bars below the EMG traces). Schematic drawings above each panel represent the extent of the hemisection (in grey) and of the subsequent complete spinalization (in black). Top traces of EMGs recordings are shown. Duty cycles (horizontal bars) below the EMGs illustrate the support periods. Srt: Sartorius; GM: median gastrocnemius; HL: hindlimb; sec: second; EMG: electromyography; l: left; r: right.
Because the dual lesion paradigm does not eliminate sensory inputs that may modulate the spinal locomotor output, we next investigated the nature of spinal changes produced by a T10 hemisection using a preparation where the activity of spinal circuits can be examined in relative isolation from brain and sensory inputs (Gossard et al., 2015). This preparation consists of recording the locomotor output generated by the spinal cord using bilateral electroneurogram recordings after decerebration and curarization. We showed that, acutely after hemisection, there was weak or no rhythmic activity in the hindlimb on the side of the hemisection. However, 3 weeks after hemisection, a much stronger and organized rhythmic locomotor pattern characterized by an alternation between flexors and extensors was re-expressed on that side. In contrast to the hemisected side, rhythmic activity on the contralateral side decreased. The hemisection thus induces an asymmetrical reorganization of spinal locomotor circuits that most likely participates in the recovery of the paretic hindlimb after hemisection.
Considering the role of locomotor training in triggering spinal locomotion after complete SCI (Barbeau and Rossignol, 1987), we next tested the impact of treadmill training over locomotor recovery and spinal plasticity. Cats were submitted to a spinal hemisection at T10 and were either trained to walk 30 minutes per day for 3 weeks or allowed to recover in their cage (Martinez et al., 2013). Locomotor training had a beneficial impact over recovery and restored a symmetrical gait pattern. Furthermore, 24 hours after removing brain inputs by the spinalization, the spinal circuits were capable of generating a bilateral and symmetrical pattern of hindlimb locomotion in 100% of the trained cats. This contrasts with the results obtained in untrained cats that were able to generate a bilateral, but asymmetrical pattern of locomotion in 60% of cases. This study demonstrates that locomotor training after hemisection increases spinal excitability and re-establishes a left/right balance within spinal circuits.
The mechanisms responsible for modifying the spinal cord below the lesion remain elusive. On one hand, because about half of untrained hemispinal cats receiving minimal sensory feedback may walk after spinalization (Martinez et al., 2011), this suggests a role for supraspinal mechanisms in sublesional plasticity. On the other hand, given the role of locomotor training in reshaping spinal circuits (Martinez et al., 2012a, 2013), one possible mechanism relates to changes that may occur in locomotion-related sensory feedback. Most likely, dynamic changes in both descending and ascending pathways are at play to shape new input-output functions of the spinal circuits.
Conclusion
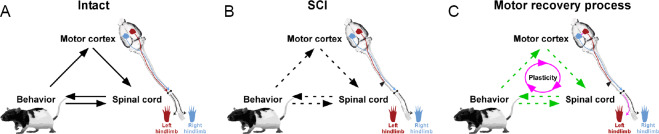
This review outlines how plasticity at the level of the motor cortex, in its descending connectivity to the spinal cord, and also in the spinal cord itself can participate in motor recovery after SCI. Plasticity occurs spontaneously in animal models and can be promoted by a variety of training (Girgis et al., 2007; Martinez et al., 2012b, 2013), molecular (Lee et al., 2010; Hollis et al., 2016), regenerative cell therapy (Assinck et al., 2017; Mukhamedshina et al., 2017) and stimulation (Brus-Ramer et al., 2007; Carmel et al., 2010; Carmel and Martin, 2014; Shah and Lavrov, 2017) approaches. Currently, there is intensive investigation to identify effective targets and approaches to aid functional recovery after SCI. In our perspective, it is important to consider the dynamic and reciprocal interactions between the motor cortex, spinal cord, and motor output that are occurring over the recovery process from a systems-level approach (Figure 3).
Figure 3.
Schematic representation of the interactions between the motor cortex, spinal cord, and behavior during the motor recovery process after an incomplete spinal cord injury (SCI).
(A) In the intact state, behavioral output (movement) is generated by the spinal cord which, in turn, is regulated by the motor cortex. Feedback (sensory afferents) is provided to the spinal cord and motor cortex to adjust the desired output response of the system. (B) After SCI, behavioral output is impaired due to disruptions in both its generation and feedback control. (C) During the recovery process, spontaneous plasticity can occur at multiple levels of the neuraxis, and be further promoted by therapeutic interventions to re-establish connectivity within the system. Circuit interactions are depicted indicating the directionality of communication (solid black arrows). Disruptions in communicative flow in the system occur after SCI (dashed black arrows). Strengthening of systems interactions (green dashed arrows) can be mediated through plasticity (purple arrows).
For example, one may ask “What is the mechanism behind motor map plasticity and functional recovery?” There very likely may be multiple answers including changes in motor cortex structure and function, corticospinal projection pathways, spinal excitability and organization, and afferent input at the cortical and spinal levels. Thus, treatment strategies, including a combinatorial approach at these multiple levels of the nervous system to promote plasticity, show great promise (Weishaupt et al., 2013; Wahl et al., 2014; Foffani et al., 2016). Additionally, targeted neurotechnology approaches have recently been applied in rats (Wenger et al., 2016; Bonizzato and Martinez, 2018; Bonizzato et al., 2018), and primates (Capogrosso et al., 2016) to promote locomotor recovery. In humans, selective spinal cord stimulation using real-time triggering capabilities coinciding with intended movement can not only re-establish locomotor control after paralysis, but also shows a neuroplastic effect to maintain locomotor control in the absence of stimulation (Wagner et al., 2018).
Additional file: Open peer review reports 1 (109.4KB, pdf) –3 (107.6KB, pdf) .
Footnotes
Conflicts of interest: We declare no conflicts of interest.
Financial support: This work was partially supported by the Canadian Institutes for Health Research (CIHR; MOP-142288 to MM); MM was supported by a salary award from Fonds de Recherche Québec Santé (FRQS) and ARB was supported by a fellowship from FRQS.
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Hisham Sharif, City University of NY School of Medicine, USA; Waleed M. Renno, Kuwait University, Kuwait; Albert A. Rizvanov, Kazan Federal University, Russia.
Funding: This work was partially supported by the Canadian Institutes for Health Research (CIHR; MOP-142288 to MM); MM was supported by a salary award from Fonds de Recherche Québec Santé (FRQS) and ARB was supported by a fellowship from FRQS.
P-Reviewers: Sharif H, Renno WM, Rizvanov AA; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Anderson KD, Sharp KG, Steward O. Bilateral cervical contusion spinal cord injury in rats. Exp Neurol. 2009;220:9–22. doi: 10.1016/j.expneurol.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antri M, Barthe JY, Mouffle C, Orsal D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci Lett. 2005;384:162–167. doi: 10.1016/j.neulet.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 3.Armand J. The origin course and terminations of corticospinal fibers in various mammals. Prog Brain Res. 1982;57:329–360. doi: 10.1016/S0079-6123(08)64136-9. [DOI] [PubMed] [Google Scholar]
- 4.Asanuma H, Sakata H. Functional organization of a cortical efferent system examined with focal depth stimulation in cats. J Neurophysiol. 1967;30:35–54. [Google Scholar]
- 5.Asboth L, Friedli L, Beauparlant J, Martinez-Gonzalez C, Anil S, Rey E, Baud L, Pidpruzhnykova G, Anderson MA, Shkorbatova P, Batti L, Pages S, Kreider J, Schneider BL, Barraud Q, Courtine G. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat Neurosci. 2018;21:576–588. doi: 10.1038/s41593-018-0093-5. [DOI] [PubMed] [Google Scholar]
- 6.Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20:637–647. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 7.Axelson HW, Winkler T, Flygt J, Djupsjo A, Hanell A, Marklund N. Plasticity of the contralateral motor cortex following focal traumatic brain injury in the rat. Restor Neurol Neurosci. 2013;31:73–85. doi: 10.3233/RNN-2012-120242. [DOI] [PubMed] [Google Scholar]
- 8.Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- 9.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 10.Barrière G, H. L, Provencher J, Rossignol S. Prominent role of the spinal central pattern generator in the recovery of locomotion after partial spinal cord injuries. J Neurosci. 2008;28:3976–3987. doi: 10.1523/JNEUROSCI.5692-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barthélemy D, Leblond H, Rossignol S. Characteristics and mechanisms of locomotion induced by intraspinal microstimulation and dorsal root stimulation in spinal cats. J Neurophysiol. 2007;97:1986–2000. doi: 10.1152/jn.00818.2006. [DOI] [PubMed] [Google Scholar]
- 12.Bonizzato M, Martinez M. SfN 48th annual meeting. San Diego: 2018. Intracortical neuroprosthesis fosters locomotor recovery after spinal cord injury. [Google Scholar]
- 13.Bonizzato M, Pidpruzhnykova G, DiGiovanna J, Shkorbatova P, Pavlova N, Micera S, Courtine G. Brain-controlled modulation of spinal circuits improves recovery from spinal cord injury. Nat Commun. 2018;9:3015. doi: 10.1038/s41467-018-05282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brosamle C, Schwab ME. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J Comp Neurol. 1997;386:293–303. doi: 10.1002/(sici)1096-9861(19970922)386:2<293::aid-cne9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Brown AR, Martinez M. Ipsilesional motor cortex plasticity participates in spontaneous hindlimb recovery after lateral hemisection of the thoracic spinal cord in the rat. J Neurosci. 2018;38:9977–9988. doi: 10.1523/JNEUROSCI.1062-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brus-Ramer M, Carmel JB, Martin JH. Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. J Neurosci. 2009;29:6196–6206. doi: 10.1523/JNEUROSCI.5852-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brus-Ramer M, Carmel JB, Chakrabarty S, Martin JH. Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. J Neurosci. 2007;27:13793–13801. doi: 10.1523/JNEUROSCI.3489-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canedo A. Primary motor cortex influences on the descending and ascending systems. Prog Neurobiol. 1997;51:287–335. doi: 10.1016/s0301-0082(96)00058-5. [DOI] [PubMed] [Google Scholar]
- 19.Capogrosso M, Milekovic T, Borton D, Wagner F, Moraud EM, Mignardot JB, Buse N, Gandar J, Barraud Q, Xing D, Rey E, Duis S, Jianzhong Y, Ko WK, Li Q, Detemple P, Denison T, Micera S, Bezard E, Bloch J, et al. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature. 2016;539:284–288. doi: 10.1038/nature20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmel JB, Martin JH. Motor cortex electrical stimulation augments sprouting of the corticospinal tract and promotes recovery of motor function. Front Integr Neurosci. 2014;8:51. doi: 10.3389/fnint.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmel JB, Kimura H, Martin JH. Electrical stimulation of motor cortex in the uninjured hemisphere after chronic unilateral injury promotes recovery of skilled locomotion through ipsilateral control. J Neurosci. 2014;34:462–466. doi: 10.1523/JNEUROSCI.3315-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmel JB, Berrol LJ, Brus-Ramer M, Martin JH. Chronic electrical stimulation of the intact corticospinal system after unilateral injury restores skilled locomotor control and promotes spinal axon outgrowth. J Neurosci. 2010;30:10918–10926. doi: 10.1523/JNEUROSCI.1435-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S, Levi AD. Restorative treatments for spinal cord injury. Neurosurg Clin N Am. 2017;28:63–71. doi: 10.1016/j.nec.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Cortes M, Thickbroom GW, Elder J, Rykman A, Valls-Sole J, Pascual-Leone A, Edwards DJ. The corticomotor projection to liminally-contractable forearm muscles in chronic spinal cord injury: a transcranial magnetic stimulation study. Spinal Cord. 2016;55:362. doi: 10.1038/sc.2016.161. [DOI] [PubMed] [Google Scholar]
- 25.Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davey NJ, Smith HC, Wells E, Maskill DW, Savic G, Ellaway PH, Frankel HL. Responses of thenar muscles to transcranial magnetic stimulation of the motor cortex in patients with incomplete spinal cord injury. J Neurol Neurosurg Psychiatry. 1998;65:80. doi: 10.1136/jnnp.65.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimitrijevic MR, Gerasimenko Y, Pinter MM. Evidence for a spinal central pattern generator in humans. Ann N Y Acad Sci. 1998;860:360–376. doi: 10.1111/j.1749-6632.1998.tb09062.x. [DOI] [PubMed] [Google Scholar]
- 28.Filipp ME, Travis BJ, Henry SS, Idzikowski EC, Magnuson SA, Loh MY, Hellenbrand DJ, Hanna AS. Differences in neuroplasticity after spinal cord injury in varying animal models and humans. Neural Regen Res. 2019;14:7–19. doi: 10.4103/1673-5374.243694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filli L, Schwab ME. Structural and functional reorganization of propriospinal connections promotes functional recovery after spinal cord injury. Neural Regen Res. 2015;10:509–513. doi: 10.4103/1673-5374.155425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filli L, Engmann AK, Zorner B, Weinmann O, Moraitis T, Gullo M, Kasper H, Schneider R, Schwab ME. Bridging the gap: a reticulo-propriospinal detour bypassing an incomplete spinal cord injury. J Neurosci. 2014;34:13399–13410. doi: 10.1523/JNEUROSCI.0701-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fink KL, Cafferty WBJ. Reorganization of intact descending motor circuits to replace lost connections after injury. Neurotherapeutics. 2016;13:370–381. doi: 10.1007/s13311-016-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foffani G, Shumsky J, Knudsen EB, Ganzer PD, Moxon KA. Interactive effects between exercise and serotonergic pharmacotherapy on cortical reorganization after spinal cord injury. Neurorehabil Neural Repair. 2016;30:479–489. doi: 10.1177/1545968315600523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, Edgerton VR. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J Neurosci. 2005;25:11738–11747. doi: 10.1523/JNEUROSCI.1523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forssberg H, Grillner S. The locomotion of the acute spinal cat injected with clonidine i.v. Brain Res. 1973;50:184–186. doi: 10.1016/0006-8993(73)90606-9. [DOI] [PubMed] [Google Scholar]
- 35.Fouad K, Tse A. Adaptive changes in the injured spinal cord and their role in promoting functional recovery. Neurol Res. 2008;30:17–27. doi: 10.1179/016164107X251781. [DOI] [PubMed] [Google Scholar]
- 36.Fouad K, Pedersen V, Schwab ME, Brosamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol. 2001;11:1766–1770. doi: 10.1016/s0960-9822(01)00535-8. [DOI] [PubMed] [Google Scholar]
- 37.Freund P, Rothwell J, Craggs M, Thompson AJ, Bestmann S. Corticomotor representation to a human forearm muscle changes following cervical spinal cord injury. Eur J Neurosci. 2011;34:1839–1846. doi: 10.1111/j.1460-9568.2011.07895.x. [DOI] [PubMed] [Google Scholar]
- 38.Friel K, Chakrabarty S, Kuo H-C, Martin J. Using motor behavior during an early critical period to restore skilled limb movement after damage to the corticospinal system during development. J Neurosci. 2012;32:9265–9276. doi: 10.1523/JNEUROSCI.1198-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frost SB, Dunham CL, Barbay S, Krizsan-Agbas D, Winter MK, Guggenmos DJ, Nudo RJ. Output properties of the cortical hindlimb motor area in spinal cord-injured rats. J Neurotrauma. 2015;32:1666–1673. doi: 10.1089/neu.2015.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh A, Sydekum E, Haiss F, Peduzzi S, Zorner B, Schneider R, Baltes C, Rudin M, Weber B, Schwab ME. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J Neurosci. 2009;29:12210–12219. doi: 10.1523/JNEUROSCI.1828-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh A, Haiss F, Sydekum E, Schneider R, Gullo M, Wyss MT, Mueggler T, Baltes C, Rudin M, Weber B, Schwab ME. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat Neurosci. 2010;13:97–104. doi: 10.1038/nn.2448. [DOI] [PubMed] [Google Scholar]
- 42.Girgis J, Merrett D, Kirkland S, Metz GA, Verge V, Fouad K. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain. 2007;130:2993–3003. doi: 10.1093/brain/awm245. [DOI] [PubMed] [Google Scholar]
- 43.Gossard J-P, Delivet-Mongrain H, Martinez M, Kundu A, Escalona M, Rossignol S. Plastic changes in lumbar locomotor networks after a partial spinal cord injury in cats. J Neurosci. 2015;35:9446–9455. doi: 10.1523/JNEUROSCI.4502-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 45.Hess G, Donoghue JP. Long-term potentiation of horizontal connections provides a mechanism to reorganize cortical motor maps. J Neurophysiol. 1994;71:2543–2547. doi: 10.1152/jn.1994.71.6.2543. [DOI] [PubMed] [Google Scholar]
- 46.Hess G, Donoghue JP. Long-term depression of horizontal connections in rat motor cortex. Eur J Neurosci. 1996;8:658–665. doi: 10.1111/j.1460-9568.1996.tb01251.x. [DOI] [PubMed] [Google Scholar]
- 47.Hilton BJ, Anenberg E, Harrison TC, Boyd JD, Murphy TH, Tetzlaff W. Re-Establishment of cortical motor output maps and spontaneous functional recovery via spared dorsolaterally projecting corticospinal neurons after dorsal column spinal cord injury in adult mice. J Neurosci. 2016;36:4080–4092. doi: 10.1523/JNEUROSCI.3386-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hollis ER, Ishiko N, Yu T, Lu CC, Haimovich A, Tolentino K, Richman A, Tury A, Wang SH, Pessian M, Jo E, Kolodkin A, Zou Y. Ryk controls remapping of motor cortex during functional recovery after spinal cord injury. Nat Neurosci. 2016;19:697–705. doi: 10.1038/nn.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett. 2005;383:339–344. doi: 10.1016/j.neulet.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 50.Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, Van den Brand R, Lazrov IA, Zhong H, Roy RR, Edgerton VR. Step Training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci. 2008;28:7370–7375. doi: 10.1523/JNEUROSCI.1881-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khorasanizadeh M, Yousefifard M, Eskian M, Lu Y, Chalangari M, Harrop JS, Jazayeri SB, Seyedpour S, Khodaei B, Hosseini M, Rahimi-Movaghar V. Neurological recovery following traumatic spinal cord injury: a systematic review and meta-analysis. J Neurosurg Spine. 2019:1–17. doi: 10.3171/2018.10.SPINE18802. [DOI] [PubMed] [Google Scholar]
- 52.Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- 53.Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, Nudo RJ. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- 55.Kleim JA, Bruneau R, Calder K, Pocock D, VandenBerg PM, MacDonald E, Monfils MH, Sutherland RJ, Nader K. Functional organization of adult motor cortex is dependent upon continued protein synthesis. Neuron. 2003;40:167–176. doi: 10.1016/s0896-6273(03)00592-0. [DOI] [PubMed] [Google Scholar]
- 56.Kumar R, Lim J, Mekary RA, Rattani A, Dewan MC, Sharif SY, Osorio-Fonseca E, Park KB. Traumatic spinal injury: global epidemiology and worldwide volume. World Neurosurg. 2018;113:e345–363. doi: 10.1016/j.wneu.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 57.Kuypers HGJM. Handbook of Physiology. Section 1: The Nervous System. II. Bethesda: American Physiology Society; 1981. Anatomy of the Descending Pathways. Motor control, part 1 (Brooks VB, ed) [Google Scholar]
- 58.Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 60.Levy WJ, Amassian VE, Traad M, Cadwell J. Focal magnetic coil stimulation reveals motor cortical system reorganized in humans after traumatic quadriplegia. Brain Res. 1990;510:130–134. doi: 10.1016/0006-8993(90)90738-w. [DOI] [PubMed] [Google Scholar]
- 61.Manohar A, Foffani G, Ganzer PD, Bethea JR, Moxon KA. Cortex-dependent recovery of unassisted hindlimb locomotion after complete spinal cord injury in adult rats. eLife. 2017;6:e23532. doi: 10.7554/eLife.23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinez M, Delivet-Mongrain H, Leblond H, Rossignol S. Recovery of hindlimb locomotion after incomplete spinal cord injury in the cat involves spontaneous compensatory changes within the spinal locomotor circuitry. J Neurophysiol. 2011;106:1969–1984. doi: 10.1152/jn.00368.2011. [DOI] [PubMed] [Google Scholar]
- 63.Martinez M, Delivet-Mongrain H, Leblond H, Rossignol S. Effect of locomotor training in completely spinalized cats previously submitted to a spinal hemisection. J Neurosci. 2012a;32:10961–10970. doi: 10.1523/JNEUROSCI.1578-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinez M, Delivet-Mongrain H, Leblond H, Rossignol S. Incomplete spinal cord injury promotes durable functional changes within the spinal locomotor circuitry. J Neurophysiol. 2012b;108:124–134. doi: 10.1152/jn.00073.2012. [DOI] [PubMed] [Google Scholar]
- 65.Martinez M, Delivet-Mongrain H, Rossignol S. Treadmill training promotes spinal changes leading to locomotor recovery after partial spinal cord injury in cats. J Neurophysiol. 2013;109:2909–2922. doi: 10.1152/jn.01044.2012. [DOI] [PubMed] [Google Scholar]
- 66.Martinez M, Delcour M, Russier M, Zennou-Azogui Y, Xerri C, Coq JO, Brezun JM. Differential tactile and motor recovery and cortical map alteration after C4-C5 spinal hemisection. Exp Neurol. 2010;221:186–197. doi: 10.1016/j.expneurol.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 67.Martinez M, Rossignol S. A dual spinal cord lesion paradigm to study spinal locomotor plasticity in the cat. Ann N Y Acad Sci. 2013;1279:127–134. doi: 10.1111/j.1749-6632.2012.06823.x. [DOI] [PubMed] [Google Scholar]
- 68.Miller MW. The origin of corticospinal projection neurons in rat. Exp Brain Res. 1987;67:339–351. doi: 10.1007/BF00248554. [DOI] [PubMed] [Google Scholar]
- 69.Monfils MH, Teskey GC. Skilled-learning-induced potentiation in rat sensorimotor cortex: a transient form of behavioural long-term potentiation. Neuroscience. 2004;125:329–336. doi: 10.1016/j.neuroscience.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 70.Moxon KA, Oliviero A, Aguilar J, Foffani G. Cortical reorganization after spinal cord injury: always for good? Neuroscience. 2014;283:78–94. doi: 10.1016/j.neuroscience.2014.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mukhamedshina YO, Gilazieva ZE, Arkhipova SS, Galieva LR, Garanina EE, Shulman AA, Yafarova GG, Chelyshev YA, Shamsutdinova NV, Rizvanov AA. Electrophysiological, morphological, and ultrastructural features of the injured spinal cord tissue after transplantation of human umbilical cord blood mononuclear cells genetically modified with the VEGF and GDNF genes. Neural Plast. 2017;2017:9857918. doi: 10.1155/2017/9857918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nadeau S, Jacquemin G, Fournier C, Lamarre Y, Rossignol S. Spontaneous motor rhythms of the back and legs in a patient with a complete spinal cord transection. Neurorehabil Neural Repair. 2010;24:377–383. doi: 10.1177/1545968309349945. [DOI] [PubMed] [Google Scholar]
- 73.Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science. 2007;318:1150–1155. doi: 10.1126/science.1147243. [DOI] [PubMed] [Google Scholar]
- 74.Nudo R, Frost SB. The Evolution of Motor Cortex and Motor Systems. In: Kaas JH, Striedter GF, Bullock TH, Preuss TM, Rubenstein J, Krubitzer LA, editors. Evolution of nervous systems. Oxford: Academic Press; pp. 373–395. [Google Scholar]
- 75.Nudo RJ, Masterton RB. Descending pathways to the spinal cord: a comparative study of 22 mammals. J Comp Neurol. 1988;277:53–79. doi: 10.1002/cne.902770105. [DOI] [PubMed] [Google Scholar]
- 76.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oza CS, Giszter SF. Plasticity and alterations of trunk motor cortex following spinal cord injury and non-stepping robot and treadmill training. Exp Neurol. 2014;256:57–69. doi: 10.1016/j.expneurol.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oza CS, Giszter SF. Trunk robot rehabilitation training with active stepping reorganizes and enriches trunk motor cortex representations in spinal transected rats. J Neurosci. 2015;35:7174–7189. doi: 10.1523/JNEUROSCI.4366-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Papale AE, Hooks BM. Circuit changes in motor cortex during motor skill learning. Neuroscience. 2018;368:283–297. doi: 10.1016/j.neuroscience.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. 1995;74:1037–1045. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- 81.Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 2000;74:27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- 82.Rioult-Pedotti M-S, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 83.Rosenzweig ES, Brock JH, Culbertson MD, Lu P, Moseanko R, Edgerton VR, Havton LA, Tuszynski MH. Extensive spinal decussation and bilateral termination of cervical corticospinal projections in rhesus monkeys. J Comp Neurol. 2009;513:151–163. doi: 10.1002/cne.21940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roy FD, Zewdie ET, Gorassini MA. Short-interval intracortical inhibition with incomplete spinal cord injury. Clin Neurophysiol. 2011;122:1387–1395. doi: 10.1016/j.clinph.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 85.Serradj N, Agger SF, Hollis ER. Corticospinal circuit plasticity in motor rehabilitation from spinal cord injury. Neurosci Lett. 2017;652:94–104. doi: 10.1016/j.neulet.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 86.Shah PK, Lavrov I. Spinal epidural stimulation strategies: clinical implications of locomotor studies in spinal rats. Neuroscientist. 2017;23:664–680. doi: 10.1177/1073858417699554. [DOI] [PubMed] [Google Scholar]
- 87.Smith HC, Savic G, Frankel HL, Ellaway PH, Maskill DW, Jamous MA, Davey NJ. Corticospinal function studied over time following incomplete spinal cord injury. Spinal Cord. 2000;38:292. doi: 10.1038/sj.sc.3100994. [DOI] [PubMed] [Google Scholar]
- 88.Steward O, Zheng B, Ho C, Anderson K, Tessier-Lavigne M. The dorsolateral corticospinal tract in mice: An alternative route for corticospinal input to caudal segments following dorsal column lesions. J Comp Neurol. 2004;472:463–477. doi: 10.1002/cne.20090. [DOI] [PubMed] [Google Scholar]
- 89.Strens LHA, Fogelson N, Shanahan P, Rothwell JC, Brown P. The ipsilateral human motor cortex can functionally compensate for acute contralateral motor cortex dysfunction. Curr Biol. 2003;13:1201–1205. doi: 10.1016/s0960-9822(03)00453-6. [DOI] [PubMed] [Google Scholar]
- 90.Tandon S, Kambi N, Jain N. Overlapping representations of the neck and whiskers in the rat motor cortex revealed by mapping at different anaesthetic depths. Eur J Neurosci. 2008;27:228–237. doi: 10.1111/j.1460-9568.2007.05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Topka H, Cohen LG, Cole RA, Hallett M. Reorganization of corticospinal pathways following spinal cord injury. Neurology. 1991;41:1276. doi: 10.1212/wnl.41.8.1276. [DOI] [PubMed] [Google Scholar]
- 92.van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S, Dominici N, Micera S, Musienko P, Courtine G. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- 93.Wagner FB, Mignardot JB, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, Rowald A, Seáñez I, Caban M, Pirondini E, Vat M, McCracken LA, Heimgartner R, Fodor I, Watrin A, Seguin P, Paoles E, Van Den Keybus K, Eberle G, Schurch B, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563:65–71. doi: 10.1038/s41586-018-0649-2. [DOI] [PubMed] [Google Scholar]
- 94.Wahl AS, Omlor W, Rubio JC, Chen JL, Zheng H, Schröter A, Gullo M, Weinmann O, Kobayashi K, Helmchen F, Ommer B, Schwab ME. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science. 2014;344:1250. doi: 10.1126/science.1253050. [DOI] [PubMed] [Google Scholar]
- 95.Weishaupt N, Li S, Di Pardo A, Sipione S, Fouad K. Synergistic effects of BDNF and rehabilitative training on recovery after cervical spinal cord injury. Behav Brain Res. 2013;239:31–42. doi: 10.1016/j.bbr.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 96.Wen TC, Lall S, Pagnotta C, Markward J, Gupta D, Ratnadurai-Giridharan S, Bucci J, Greenwald L, Klugman M, Hill NJ, Carmel JB. Plasticity in one hemisphere, control from two: adaptation in descending motor pathways after unilateral corticospinal injury in neonatal rats. Front Neural Circuit. 2018;12:28. doi: 10.3389/fncir.2018.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wenger N, Moraud EM, Gandar J, Musienko P, Capogrosso M, Baud L, Le Goff CG, Barraud Q, Pavlova N, Dominici N, Minev IR, Asboth L, Hirsch A, Duis S, Kreider J, Mortera A, Haverbeck O, Kraus S, Schmitz F, DiGiovanna J, et al. Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat Med. 2016;22:138–145. doi: 10.1038/nm.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zorner B, Bachmann LC, Filli L, Kapitza S, Gullo M, Bolliger M, Starkey ML, Rothlisberger M, Gonzenbach RR, Schwab ME. Chasing central nervous system plasticity: the brainstem’s contribution to locomotor recovery in rats with spinal cord injury. Brain. 2014;137:1716–1732. doi: 10.1093/brain/awu078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.