Abstract
Neural plasticity in the adult central nervous system involves the adaptation of myelination, including the formation of novel myelin sheaths by adult-born oligodendrocytes. Yet, mature oligodendrocytes slowly but constantly turn over their pre-existing myelin sheaths, thereby establishing an equilibrium of replenishment and degradation that may also be subject to adaptation with consequences for nerve conduction velocity. In this short review we highlight selected approaches to the normal turnover of adult myelin in vivo, from injecting radioactive precursors of myelin constituents in the 1960s to current strategies involving isotope labeling and tamoxifen-induced gene targeting.
Keywords: oligodendrocyte, myelin plasticity and turnover, degradation, tamoxifen, metabolic labeling, isotope labeling, neural plasticity, myelinoid bodies, central nervous system
Introduction
Myelination of axons by oligodendrocytes facilitates rapid and precise nerve impulse propagation in the central nervous system (CNS) of jawed vertebrates (Hartline and Colman, 2007; Nave and Werner, 2014; Weil et al., 2018). The regular structure of the myelin sheath in healthy conditions and its abnormalities in myelin-related disorders are commonly approached by electron microscopy (Möbius et al., 2016). However, the two-dimensional character of electron micrographs provides static images, necessitating careful quantification when assessing changes of myelination during normal development and aging. Indeed, myelination of axonal segments is adapted throughout life (Bergles and Richardson, 2015; Baraban et al., 2016), and it is now widely recognized that the addition of new myelin sheaths by adult-born oligodendrocytes represents a mechanism of plasticity in the mature CNS (O’Rourke et al., 2014; Sampaio-Baptista and Johansen-Berg, 2017). Yet, the maintenance of pre-existing myelin sheaths by mature oligodendrocytes involves slow but constant replenishment and degradation of myelin constituents and thus life-long turnover of myelin sheaths. This was emphasized when the fallout of nuclear bomb tests in the late 1950s and the early 1960s was utilized as a labeling pulse (Yeung et al., 2014). Indeed, measuring the carbon isotope 14C in human post-mortem brains allowed calculating the age of oligodendroglial cell bodies and of myelin. Importantly, in the analyzed white matter tract (the corpus callosum), nearly all oligodendrocytes were born before the age of 5 years; only a small proportion was replaced throughout life. Conversely, myelin was entirely replenished during life, indicating a much faster turnover compared to that of the myelinating oligodendrocytes themselves (Yeung et al., 2014). Together, this work has shown that existing oligodendrocytes remodel their myelin sheaths in adult humans in vivo; however, the approach did not allow quantifying a particular turnover rate.
The Medline database was electronically searched for articles from 1954 to 2019 using the following keywords: oligodendrocyte; neural plasticity; myelin turnover; myelin degradation; tamoxifen; Cre-ERT2, metabolic labeling; isotope labeling; myelinoid bodies.
Radioactive Labeling
In first attempts to approach myelin turnover directly in the 1960s (Hildebrand et al., 1993), radioactively labeled precursors of lipids or proteins were injected into young or adult animals, which were sacrificed at various times after injection to measure the radioactivity in myelin-enriched brain fractions. Plotting the remaining radioactivity against time allowed calculating protein half-lives. However, the reported half-lives of myelin lipids and proteins differed widely across various studies depending on the age at the time of injection and the type of precursor used. Additionally, interpretations at first were difficult owing to the reutilization of myelin constituents over the course of long-term experiments and poorly developed protocols for myelin purification (Hildebrand et al., 1993). Notwithstanding that the morphological equivalent of the measured half-lives initially remained speculative, these studies established for the first time that myelin membranes are indeed subject to turnover in the adult brain (Smith, 1968).
Upon the establishment of more reliable myelin purification protocols, proteolipid protein (PLP) and myelin basic protein (MBP) appeared more stable compared to cyclic nucleotide phosphodiesterase (CNP; previously referred to as Wolfgram protein) (Fischer and Morell, 1974). Considering that CNP is a marker of non-compact myelin while PLP and MBP are major constituents of compact myelin, this result implies that non-compacted myelin is turned over faster than compact myelin. Indeed, the non-compacted cytosolic channels through myelin, including the paranodal myelin sub-compartment and the adaxonal myelin layer, differ from compacted myelin membranes not only in their turnover rate but also with respect to protein composition, ultrastructure and metabolic activity (Nave and Werner, 2014).
Metabolic Labeling
More recently, techniques became available that enable systematic measurement of the lifetimes of numerous individual proteins in vivo. Here, metabolic labeling by a dietary labeling pulse using amino acids harboring the stable isotopes 15N or 13C is followed by a period of feeding unlabeled chow and subsequent mass spectrometric analysis (Price et al., 2010; Toyama et al., 2013; Fornasiero et al., 2018). When calculating protein lifetimes from the ratio of labeled and unlabeled amino acids in tryptic peptides, myelin proteins were consistently among the proteins with the longest average lifetimes in rodent brains, in agreement with their localization in a structure that is turned over slowly. Generalized, the lifetime of myelin proteins is approximately as long as that of nuclear histones (Price et al., 2010; Toyama et al., 2013). Yet, considering that the postnatal period of most active developmental myelination involves substantial remodeling of oligodendroglial processes and myelin sheaths (Hines et al., 2015), the measured protein half-lives may not accurately reflect the turnover of myelin in adults if the dietary pulse was applied during juvenile development (Toyama et al., 2013). Importantly, the lifetime of proteins that localize to compact myelin (CLDN11, PLP, MBP, and MOBP) was about twice as long as that of those residing in non-compact myelin (CNP, MAG) (Fornasiero et al., 2018), confirming the previous studies utilizing radioactive precursors (Fischer and Morell, 1974). However, measurements by metabolic labeling so far probably underestimated the lifetimes of myelin in the adult CNS because the analysis of total brains does not allow discriminating between proteins present in the cell bodies of oligodendrocytes from those that have been incorporated into myelin sheaths, which most likely undergo turnover at different rates. Yet, this limitation can easily be overcome in future approaches if purified myelin rather than total brain is assessed.
Tamoxifen-Induced Gene Targeting
Conditional gene targeting in experimental mice enables the tamoxifen-induced deletion of myelin-related genes after developmental myelination has ceased (Leone et al., 2003). This experimental system allows assessing the abundance of a given protein in myelin purified at various timepoints after terminating its replenishment. Notably, if the tamoxifen-induced deletion of genes for crucial transcription factors or signal-transducing proteins interferes with the maintenance of myelin per se (Koenning et al., 2012; Ishii et al., 2014), the resulting severe demyelination prevents conclusions about the normal turnover of myelin in healthy states. However, tamoxifen-induced deletion of several other myelin-related genes does not cause considerable demyelination. For example, the abundance of the myelin septin filaments that stabilize the adaxonal non-compact myelin subcompartment was approximately halved in myelin purified from Sept8flox/flox; PlpCreERT2 mice 4 weeks after tamoxifen injection into adult mice (Patzig et al., 2016). Conversely, the abundance of the major constituent of compact myelin, PLP, was reduced by half in myelin purified from Plpflox/flox; PlpCreERT2 mice (Lüders et al., 2019) not until 6 months after tamoxifen injection (Figure 1). Together, this confirms that in adult mice the turnover of compact myelin is much slower compared to that of non-compact myelin. We note that owing to the experimental design the continued differentiation of oligodendrocyte precursor cells generates a number of adult-born myelinating oligodendrocytes with newly-formed myelin sheaths that contain septins or PLP, respectively. Yet, we believe that this approach reflects comparatively well the half-life of non-compacted and compact myelin membranes in adult mice.
Figure 1.
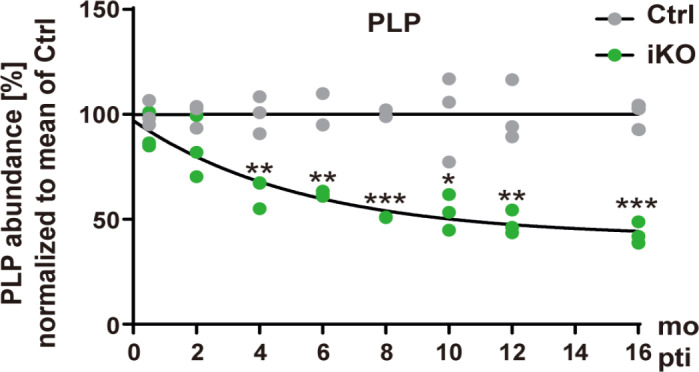
Turnover of proteolipid protein (PLP) in central nervous system myelin.
Quantitative immunoblot was performed to detect the most abundant structural myelin protein, PLP, in myelin biochemically purified from the brains of Plpflox/Y; PlpCreERT2 (iKO) and Plpflox/Y (Ctrl) mice at the indicated time points after tamoxifen injection (pti). The graph shows the abundance of PLP in myelin compared to the mean of the age-matched Ctrl. Note that the abundance of PLP in iKO myelin is halved about 6 months (mo) pti, probably reflecting the turnover of the compact layers of central nervous system myelin. Figure taken from Lüders et al. (2019) with permission.
Degradation of Myelin Membranes
How is myelin degraded upon its turnover? The existence of myelin turnover implies that new membranes replace old membranes, which then must be removed. Degradation of membranes may principally take place via exocytic or endocytic processes. Indeed, previous observations support the existence of exocytotic pathways. Importantly, myelin fragments termed myelinoid bodies are frequently observed in proximity to but seemingly detached from myelin sheaths, at least in pathological states and in aged brains (Hildebrand et al., 1993). Although the mechanisms are not fully understood, it is commonly assumed that myelinoid bodies are mainly cleared via lysosomal degradation in microglia (Safaiyan et al., 2016). Interestingly, the increasing amount of degraded myelin causes the formation of lysosomal inclusions, which might result in microglial dysfunction in the aged brain (Safaiyan et al., 2016). Notably, astrocytes in the brain and microvascular endothelial cells in the spinal cord can also engulf myelin debris (Ponath et al., 2017; Zhou et al., 2019). Engulfment and lysosomal degradation of myelin debris by microglia, astrocytes or endothelial cells may represent an early response to myelin pathology that subsequently induces recruitment of macrophages and lymphocytes.
Outlook
Taken together, recent technical developments allow assessing the turnover of myelin in vivo. Notwithstanding that these approaches may still over- or undere-stimate the precise rate of myelin turnover to some degree, it appears feasible to apply these tools to test if the rate of myelin turnover differs between thick and thin sheaths, gray and white matter, upon aging or in myelin-related disorders that involve degeneration and/or regeneration of myelin sheaths. Notably, it is also possible to experimentally stimulate the net growth of myelin membranes by pre-existing oligodendrocytes in adult mice beyond what is required for normal myelin turnover (Goebbels et al., 2010; Gibson et al., 2014), possibly involving local protein translation of the transcript pool enriched in myelin sheaths (Thakurela et al., 2016). We speculate that modulating the rate of myelin replenishment and degradation may serve to optimize myelin sheath thickness and thus the speed and precision of nerve impulse propagation (Arancibia-Carcamo et al., 2017), thereby representing a possible mechanism of neuroplasticity. Toward a full understanding of myelin turnover in adults, it will also be relevant to consider genetic tools to entirely inhibit the replenishment or degradation of myelin membranes.
Footnotes
Conflicts of interest: We declare no conflicts of interest.
Financial support: This work was supported by the Deutsche Forschungsgemeinschaft (DFG, WE2720/2-2 and WE2720/4-1, both to HBW).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the Deutsche Forschungsgemeinschaft (DFG, WE2720/2-2 and WE2720/4-1, both to HBW).
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Arancibia-Carcamo IL, Ford MC, Cossell L, Ishida K, Tohyama K, Attwell D. Node of Ranvier length as a potential regulator of myelinated axon conduction speed. Elife. 2017 doi: 10.7554/eLife.23329. doi:107554/eLife23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baraban M, Mensch S, Lyons DA. Adaptive myelination from fish to man. Brain Res. 2016;1641:149–161. doi: 10.1016/j.brainres.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergles DE, Richardson WD. Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol. 2015;8:a020453. doi: 10.1101/cshperspect.a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer CA, Morell P. Turnover of proteins in myelin and myelin-like material of mouse brain. Brain Res. 1974;74:51–65. doi: 10.1016/0006-8993(74)90111-5. [DOI] [PubMed] [Google Scholar]
- 5.Fornasiero EF, Mandad S, Wildhagen H, Alevra M, Rammner B, Keihani S, Opazo F, Urban I, Ischebeck T, Sakib MS, Fard MK, Kirli K, Centeno TP, Vidal RO, Rahman RU, Benito E, Fischer A, Dennerlein S, Rehling P, Feussner I, et al. Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nat Commun. 2018;9:4230. doi: 10.1038/s41467-018-06519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Mobius W, Liu X, Lappe-Siefke C, Rossner MJ, Groszer M, Suter U, Frahm J, Boretius S, Nave KA. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J Neurosci. 2010;30:8953–8964. doi: 10.1523/JNEUROSCI.0219-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol. 2007;17:R29–35. doi: 10.1016/j.cub.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 9.Hildebrand C, Remahl S, Persson H, Bjartmar C. Myelinated nerve fibres in the CNS. Prog Neurobiol. 1993;40:319–384. doi: 10.1016/0301-0082(93)90015-k. [DOI] [PubMed] [Google Scholar]
- 10.Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B. Neuronal activity biases axon selection for myelination in vivo. Nat Neurosci. 2015;18:683–689. doi: 10.1038/nn.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii A, Furusho M, Dupree JL, Bansal R. Role of ERK1/2 MAPK signaling in the maintenance of myelin and axonal integrity in the adult CNS. J Neurosci. 2014;34:16031–16045. doi: 10.1523/JNEUROSCI.3360-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koenning M, Jackson S, Hay CM, Faux C, Kilpatrick TJ, Willingham M, Emery B. Myelin gene regulatory factor is required for maintenance of myelin and mature oligodendrocyte identity in the adult CNS. J Neurosci. 2012;32:12528–12542. doi: 10.1523/JNEUROSCI.1069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leone DP, Genoud S, Atanasoski S, Grausenburger R, Berger P, Metzger D, Macklin WB, Chambon P, Suter U. Tamoxifen-inducible glia-specific Cre mice for somatic mutagenesis in oligodendrocytes and Schwann cells. Mol Cell Neurosci. 2003;22:430–440. doi: 10.1016/s1044-7431(03)00029-0. [DOI] [PubMed] [Google Scholar]
- 14.Lüders KA, Nessler S, Kusch K, Patzig J, Jung RB, Möbius W, Nave KA, Werner HB. Maintenance of high proteolipid protein level in adult central nervous system myelin is required to preserve the integrity of myelin and axons. Glia. 2019;67:634–649. doi: 10.1002/glia.23549. [DOI] [PubMed] [Google Scholar]
- 15.Möbius W, Nave KA, Werner HB. Electron microscopy of myelin: Structure preservation by high-pressure freezing. Brain Res. 2016;1641:92–100. doi: 10.1016/j.brainres.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 16.Nave KA, Werner HB. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- 17.O’Rourke M, Gasperini R, Young KM. Adult myelination: wrapping up neuronal plasticity. Neural Regen Res. 2014;9:1261–1264. doi: 10.4103/1673-5374.137571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patzig J, Erwig MS, Tenzer S, Kusch K, Dibaj P, Möbius W, Goebbels S, Schaeren-Wiemers N, Nave KA, Werner HB. Septin/anillin filaments scaffold central nervous system myelin to accelerate nerve conduction. 2016 doi: 10.7554/eLife.17119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponath G, Ramanan S, Mubarak M, Housley W, Lee S, Sahinkaya FR, Vortmeyer A, Raine CS, Pitt D. Myelin phagocytosis by astrocytes after myelin damage promotes lesion pathology. Brain. 2017;140:399–413. doi: 10.1093/brain/aww298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price JC, Guan S, Burlingame A, Prusiner SB, Ghaemmaghami S. Analysis of proteome dynamics in the mouse brain. Proc Natl Acad Sci U S A. 2010;107:14508–14513. doi: 10.1073/pnas.1006551107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, Edinger AL, Jung S, Rossner MJ, Simons M. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci. 2016;19:995–998. doi: 10.1038/nn.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sampaio-Baptista C, Johansen-Berg H. White matter plasticity in the adult brain. Neuron. 2017;96:1239–1251. doi: 10.1016/j.neuron.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith ME. The turnover of myelin in the adult rat. Biochim Biophys Acta. 1968;164:285–293. doi: 10.1016/0005-2760(68)90154-9. [DOI] [PubMed] [Google Scholar]
- 24.Thakurela S, Garding A, Jung RB, Muller C, Goebbels S, White R, Werner HB, Tiwari VK. The transcriptome of mouse central nervous system myelin. Sci Rep. 2016;6:25828. doi: 10.1038/srep25828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toyama BH, Savas JN, Park SK, Harris MS, Ingolia NT, Yates JR, 3rd, Hetzer MW. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 2013;154:971–982. doi: 10.1016/j.cell.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weil MT, Heibeck S, Topperwien M, Tom Dieck S, Ruhwedel T, Salditt T, Rodicio MC, Morgan JR, Nave KA, Möbius W, Werner HB. Axonal ensheathment in the nervous system of lamprey: implications for the evolution of myelinating glia. J Neurosci. 2018;38:6586–6596. doi: 10.1523/JNEUROSCI.1034-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeung MS, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, Druid H, Frisen J. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159:766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Zhou T, Zheng Y, Sun L, Badea SR, Jin Y, Liu Y, Rolfe AJ, Sun H, Wang X, Cheng Z, Huang Z, Zhao N, Sun X, Li J, Fan J, Lee C, Megraw TL, Wu W, Wang G, Ren Y. Microvascular endothelial cells engulf myelin debris and promote macrophage recruitment and fibrosis after neural injury. Nat Neurosci. 2019;22:421–435. doi: 10.1038/s41593-018-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


