Abstract
OBJECTIVE:
To assess whether dietary fat intake influences Parkinson’s disease risk.
DATA SOURCES:
We systematically surveyed the Embase and PubMed databases, reviewing manuscripts published prior to October 2018. The following terms were used: (“Paralysis agitans” OR “Parkinson disease” OR “Parkinson” OR “Parkinson’s” OR “Parkinson’s disease”) AND (“fat” OR “dietary fat” OR “dietary fat intake”).
DATA SELECTION:
Included studies were those with both dietary fat intake and Parkinson’s disease risk as exposure factors. The Newcastle-Ottawa Scale was adapted to investigate the quality of included studies. Stata V12.0 software was used for statistical analysis.
OUTCOME MEASURES:
The primary outcomes included the relationship between high total energy intake, high total fat intake, and Parkinson’s disease risk. The secondary outcomes included the relationship between different kinds of fatty acids and Parkinson’s disease risk.
RESULTS:
Nine articles met the inclusion criteria and were incorporated into this meta-analysis. Four studies scored 7 and the other five studies scored 9 on the Newcastle-Ottawa Scale, meaning that all studies were of high quality. Meta-analysis results showed that high total energy intake was associated with an increased risk of Parkinson’s disease (P = 0.000, odds ratio (OR) = 1.49, 95% confidence interval (CI): 1.26–1.75); in contrast, high total fat intake was not associated with Parkinson’s disease risk (P = 0.123, OR = 1.07, 95% CI: 0.91–1.25). Subgroup analysis revealed that polyunsaturated fatty acid intake (P = 0.010, OR = 1.03, 95% CI: 0.88–1.20) reduced the risk of Parkinson’s disease, while arachidonic acid (P = 0.026, OR = 1.15, 95% CI: 0.97–1.37) and cholesterol (P = 0.002, OR = 1.09, 95% CI: 0.92–1.29) both increased the risk of Parkinson’s disease. Subgroup analysis also demonstrated that, although the results were not significant, consumption of n-3 polyunsaturated fatty acids (P = 0.071, OR = 0.88, 95% CI: 0.73–1.05), α-linolenic acid (P = 0.06, OR = 0.86, 95% CI: 0.72–1.02), and the n-3 to n-6 ratio (P = 0.458, OR = 0.89, 95% CI: 0.75–1.06) were all linked with a trend toward reduced Parkinson’s disease risk. Monounsaturated fatty acid (P = 0.450, OR = 1.06, 95% CI: 0.91–1.23), n-6 polyunsaturated fatty acids (P = 0.100, OR = 1.15, 95% CI: 0.96–1.36) and linoleic acid (P = 0.053, OR = 1.11, 95% CI: 0.94–1.32) intakes were associated with a non-significant trend toward higher PD risk. Saturated fatty acid (P = 0.619, OR = 1.01, 95% CI: 0.87–1.18) intake was not associated with Parkinson’s disease.
CONCLUSION:
Dietary fat intake affects Parkinson’s disease risk, although this depends on the fatty acid subtype. Higher intake of polyunsaturated fatty acids may reduce the risk of Parkinson’s disease, while higher cholesterol and arachidonic acid intakes may elevate Parkinson’s disease risk. However, further studies and evidence are needed to validate any link between dietary fat intake and Parkinson’s disease.
Keywords: nerve regeneration, dietary fat, Parkinson's disease risk, meta-analysis, total energy intake, polyunsaturated fatty acids, arachidonic acid, cholesterol, a-linolenic acid, linoleic acid, n-3/n-6 polyunsaturated fatty acid intake ratio, monounsaturated fatty acids, neural regeneration
Chinese Library Classification No. R459.3; R741
Introduction
Parkinson’s disease (PD) is a neurodegenerative disease that is progressive and has a high incidence rate, with characteristic substantia nigra dopaminergic neuron depletion that gives rise to striatal dopamine depletion (Zecca et al., 2004; Sampaio et al., 2017; Martinez et al., 2018; Qu et al., 2019). PD development is influenced by both environmental and genetic mechanisms (Di Monte et al., 2002; Ma et al., 2015a, b, c; Liu et al., 2018), and it is a multi-factorial disease that arises from a combination of family history, age, ethnicity, occupation, and diet (Chaturvedi et al., 1995; Logroscino et al., 1998; Taylor et al., 1999; Kirkey et al., 2001; Priyadarshi et al., 2001; Zorzon et al., 2002; Li et al., 2005). There is evidence that, rather than a single disease, PD is actually a set of individual illnesses with a similar presentation (Dick et al., 2007). Although the mechanisms of PD development and progression are incompletely understood, inflammation, oxidative stress, and impaired mitochondrial function are all known to contribute to this disease (Jenner, 2003; Wullner and Klockgether, 2003; Schapira, 2007; Wang et al., 2017). Oxidative damage readily impacts the brain, because it requires substantial oxygen and iron availability (Noseworthy and Bray, 1998).
Dietary fat intake refers to the sum of fats from the various foods we eat every day, including simple lipids, complex lipids, terpenoids and steroids and their derivatives, derived lipids, and binding lipids. As well as providing energy to organisms, different fats have specific functions. Fatty acids are essential for brain function, and studies in rats have demonstrated that brains are dependent on dietary fatty acid intake (Ikemoto et al., 2001; Bowen and Clandinin, 2002; Hashimoto et al., 2002; Levant et al., 2007). Epidemiological evidence also suggests that dietary fat consumption may be linked with PD risk, but research results have to date been inconsistent (Hellenbrand et al., 1996; Noseworthy and Bray, 1998; Schatzkin et al., 2001; Chen et al., 2003; de Lau et al., 2005; Gao et al., 2007; Powers et al., 2009; Miyake et al., 2010; Kyrozis et al., 2013; Kamel et al., 2014). In recent years, increasing numbers of PD animal models and epidemiological investigations have shown that polyunsaturated fatty acids (PUFAs) play an important role in cell membrane sequencing, gene transcription, cell signal transduction, and protease activation of glial and neuronal cells, thereby influencing PD progress (Logroscino et al., 1996; Akbar and Kim, 2002; Akbar et al., 2005; Calon et al., 2005). Furthermore, in a PD autopsy report, docosahexaenoic acid levels were markedly decreased in the substantia nigra pars compacta and frontal cortex lipid raft (Dalfo et al., 2005; Fabelo et al., 2011).
Daily dietary fat intake may influence PD development, but further exploration of this association is needed. In the present meta-analysis, we conducted a systematic review with the aim of summarizing the available evidence regarding links between fat consumption and PD risk.
A systematic review was performed using the Embase and PubMed database, and relevant observational studies assessing the link between lipid or dietary fat content and PD risk were identified. Reference review of identified papers was also used to identify additional relevant publications. Only human studies were considered, and all studies were published in English.
The Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines were followed to design, implement, analyze, and report the results of this meta-analysis. The MOOSE guidelines were described in JAMA (Stroup et al., 2000) and propose a common methodology for meta-analyses.
Data and Methods
Search strategy
Embase and PubMed were searched for the following terms: (“Paralysis agitans” OR “Parkinson disease” OR “Parkinson” OR “Parkinson’s” OR “Parkinson’s disease”) AND (“fat” OR “dietary fat” OR “dietary fat intake”). Manuscripts published prior to October 2018 were reviewed. Two authors (YQ and XC) independently conducted the literature search.
Selection criteria
A total of 343 references were screened, using the following inclusion criteria: (1) the study could be defined as epidemiological, including case-control, nested case-control, cohort, and prospective studies; (2) dietary fat intake was the exposure of interest; (3) PD risk was the outcome of interest; (4) the study reported the odds ratio (OR) or relative risk (RR) and 95% confidence interval (CI), or the reported data were sufficient to be able to calculate these.
Articles that did not involve humans or that were not original, such as reviews, editorials, meta-analyses, or commentaries, were excluded. We also excluded studies of other exposures or endpoints.
Data extraction
Two authors (YQ and XC) independently collected detailed information from each identified article, with any discrepancies being resolved via discussion with the third author (MMX). The following data were extracted: (1) basic information: authors, year of publication, study population, age, sex, sample size, diagnoses, and case number; (2) study characteristics: study name and design, study location, follow-up duration; (3) variables adjusted during analysis; (4) outcome assessment method; (5) risk estimates and corresponding 95% CIs. If multiple multivariate-adjusted models were used for risk extraction, we extracted the confound-adjusted OR estimate.
Data regarding dietary intake in non-overlapping individuals were derived from questionnaires, which had high heterogeneity (I2 = 75.9%).
Quality assessment
The Newcastle-Ottawa Scale (Cota et al., 2013) was adapted to investigate the quality of included studies. Case-control and cohort studies were investigated separately (Additional Tables 1 and 2). A total score of 0–3, 4–6, or 7–9 indicated a study of low, intermediate, or high quality, respectively.
Additional Table 1.
Newcastle-Ottawa Scale Assessment of Case-Control Studies
| Articale | Selection | Combarability | Exposure | Scores | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case Definition | Representativeness of the cases | Selection of controls | Definition of controls | Ascertainment of exposure | Same determination method adoption | Non-response rate | ||||||||||||||||
| Requires some independent validation | Record linkage | No description | All eligible cases with outcome of interest over a defined period of time | Not satisfying requirements or not stated | Community controls | Hospital controls | No description | No history of Parkinson’s disease | No mention of source | Intakes of fat | Other controlled factors | Reliable record | Structured survey | Written self-report or medical record | No description | Yes | No | Identication | No description | Unidentication and have no expalnation | ||
| Logroscino et al. (1996) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | ||||||||||||||
| Hellenbrand et al. (1996) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 9 | ||||||||||||
| Powers et al. (2009) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 9 | ||||||||||||
| Miyake et al. (2010) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 9 | ||||||||||||
| Kamel et al. (2015) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | ||||||||||||||
Additional Table 2.
Newcastle-Ottawa Scale Assessment of Cohort Studies
| Articale | Selection | Combarability | Outcome | Scores | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | |||||||||||||||||||
| Truly represent the average fat intake of the community | Basiclly represent the average fat intake of the community | Special population | No description | The same community from the exposed alignment | Different from the source of the exposure alignment | No description | Reliable record | Record linkage | Written self-report | No description | Yes | No | Intakes of fat | Other controlled factors | Independent or blind assessment | Record linkage | Self-report | No description | Yes | No | 100% follow up | >70% follow up and described of loss of follow-up | <70% follow up and not described the loss of follow-up | No description | ||
| Chen et al. (2002) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | ||||||||||||||||||
| Akyrozis et al. (2013) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 9 | ||||||||||||||||
| Dong et al. (2014) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 9 | ||||||||||||||||
| Gao et al. (2008) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | ||||||||||||||||||
Both authors (YQ and XC) independently used this scale to establish the quality of each study (Fang et al., 2015).
Outcome measures
The primary outcomes included high total energy intake, high total fat intake, and sex. The secondary outcomes included different kinds of fatty acids.
Statistical analysis
The OR and corresponding 95% CI were used as risk estimates for studies that satisfied the inclusion criteria. Dietary fat intake was determined based on Etminan’s classification, as follows: high fat intake was within the 4th quartile or 5th quintile, while moderate fat intake was within the 2nd, 3rd, or 4th quintile or the 2nd and 3rd quartile. A random-effects (Der Simonian and Laird) model was used to pool these OR s, with the model combining heterogeneity within and between studies. The RR values of the four cohort analyses were converted to the corresponding OR values (Zhang et al., 1998). The values used in statistical analyses were all OR values.
Subgroup analysis was carried out to investigate significant differences in ORs, and whether results were influenced by residual confounding factors adjusted for sex, geographical location, numbers of participants, follow-up duration, and study quality.
The I2 statistic was used as a measure of heterogeneity of the included studies, with I2 values of 25%, 50%, or 75% respectively indicating low, intermediate, or high heterogeneity.
Both a funnel plot and Egger’s test were used to assess the potential for publication bias. Studies that were identified as having a high risk of bias were subjected to both Egger’s and Begg’s tests. P < 0.05 was considered to indicate significant publication bias. Two-tailed statistical tests were performed using Stata 12.0 software (Stata Corporation, College Station, TX, USA), with P < 0.05 as the significance threshold.
Results
Search results
We retrieved 259 PubMed articles and 173 Embase articles, all of which were published before October 2018 (Figure 1). Nine articles (four cohort studies and five case–control studies) met the inclusion criteria and were incorporated into this meta-analysis.
Figure 1.
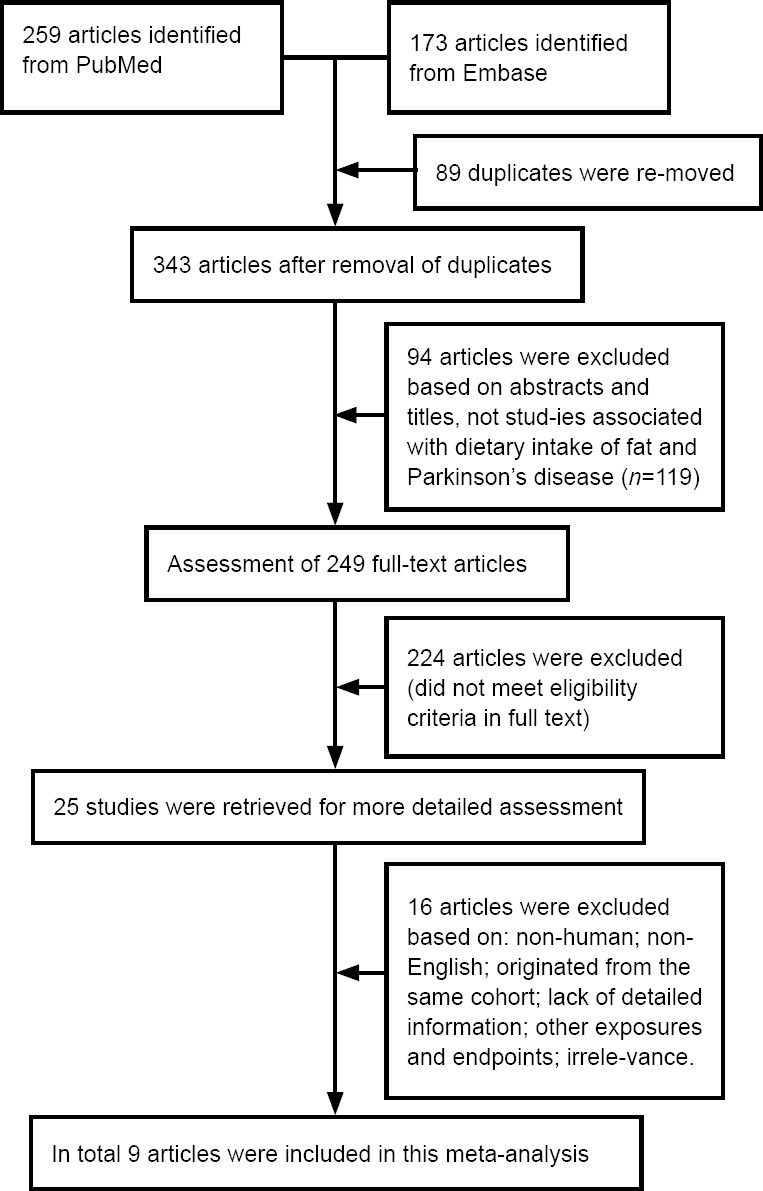
Flow chart depicting the literature search and selection strategy.
Study characteristics
Basic parameters of the included studies are summarized in Tables 1 and 2. A total of 778,571 participants were included in the nine studies, including 5751 PD cases. There was a range of follow-up durations from 2–14 years. Four studies (Chen et al., 2003; Gao et al., 2007; Kyrozis et al., 2013; Dong et al., 2014) were cohort studies, while five studies (Hellenbrand et al., 1996; Logroscino et al., 1996; Powers et al., 2009; Miyake et al., 2010; Kamel et al., 2014) were case-control studies. Four studies scored 7 on the Newcastle-Ottawa Scale, and the other five studies scored 9, meaning that all studies were of high quality (Additional Tables 1 and 2).
Table 1.
Characteristics of the included studies (n = 9) regarding the association between dietary fats intake and Parkinson’s disease
| Author | Type of study | Study design | Location | No. of participants (case/control) | Gender | Ages for cases and controls (range or mean ± SD, years) | Clinical diagnostic criteria | Exposure assessment |
|---|---|---|---|---|---|---|---|---|
| Hellenbrand et al. (1996) | NA | Case-control study | German | 342/342 | Male/ female | 56.2±6.7/56.1±6.9 | UK Parkinson’s Disease Society Brain Bank clinical diagnostic criteria | FFQ |
| Logroscino et al. (1996) | Community study | Case-control study | United States | 110/287 | Male/ female | < 70, 70–80, > 80 | Published criteria; DSM-III-R; the Hoehn and Yahr scale; direct interview | A semiquantitative food-frequency questionnaire |
| Chen et al. (2002) | HPFS NHS | Retrospective cohort study | United States | 51529 (394 cases)* | Male/ female | 40–75 | NA | FFQ, disease history, life style |
| Gao et al. (2008) | HPFS NHS | Retrospective cohort study | United States | 131368 (508 cases)* | Male/ female | 40–75 | NA | FFQ |
| Powers et al. (2009) | SMMSE | Case-control study | United States | 420/560 | Male/ female | 29–88 | NA | FFQ |
| Miyake et al. (2010) | NA | Case-control study | Japan | 249/368 | Male/ female | NA | UK Parkinson’s Disease Society Brain Bank clinical diagnostic criteria | DHQ |
| Akyrozis et al. (2013) | EPIC-Greece | Retrospective cohort study | Greece | 26173 (120 cases)* | Male/ female | 20–86 | UK Parkinson’s Disease Society Brain Bank clinical diagnostic criteria | A questionnaire |
| Dong et al. (2014) | NIH-AARP Diet and Health Study | Prospective cohort study | United States | 566398 (3519 cases)* | Male/ female | 50–71 | NA | FFQ, questions on demographics and life style |
| Kamel et al. (2015) | AHS, FAME, NIH | Case-control study | United States | 89/336 | Male/ female | 68/69 | NA | DHQ |
*Cohort study (participants/Parkinson’s disease onset). DHQ: Self-administered, semi-quantitative, comprehensive, diet history questionnaire; FFQ: the Willett food frequency questionnaire; NA: not available; HPFS: the Health Professionals Follow-up Study; NHS: the Nurses’ Health Study; DSM-III-R: Diagnostic and Statistical Manual of Mental Disorders; AHS: Agricultureral Health Study; FAME: the Farming and Movement Evaluation
Table 2.
Characteristics of included studies
| Study | Case ascertainment | Comparison | Multivariates controlled | NOS score |
|---|---|---|---|---|
| Hellenbrand et al. (1996) | Attending neurologists were asked to verify inclusion and exclusion criteria according to the UK Parkinson’s Disease Society Brain Bank clinical diagnostic criteria | Energy intake, carbohydrate intake, monosaccharide intake, disaccharide intake, polysaccharide intake; individual amino acid intake, total protein intake; antioxidant vitamin intake, ascorbic acid intake, beta-carotene intake, alpha-tocopherol intake; thiamine intake, various B vitamins intake; niacin intake | Age, sex, body mass index, smoking status, disease duration; education | 9 |
| Logroscino et al. (1996) | Confirmed by three experienced neurologists according to published criteria | Calories, fat intake | Age, sex, education, ethnic | 7 |
| Chen et al. (2002) | Confirmed by neurologists | Food groups low to high, man vs. woman | Age, lengths of follow-up, body mass index, smoking status, energy intake, caffeine intake, physical activity, alcohol consumption | 7 |
| Gao et al. (2008) | Identified by biennial self-reported questionnaires | Prudent dietary pattern, western dietary pattern | Age, weight, height, smoking status, physical activity, body mass index, use of nonsteroidal anti-inflammatory drugs, total energy intake, caffeine intake, alcohol intake, urate index and iron intake | 7 |
| Powers et al. (2009) | Confirmed by medical records | The lowest and highest quartiles of iron; low SatFat, low Fe vs. high SatFat, high Fe vs. low SatFat, high Fe; low cholest, low Fe vs. high cholest, high Fe vs. low cholest, high Fe | Age, gender, smoking, ethnicity, education | 9 |
| Miyake et al. (2010) | NA | Arachidonic acid intake, cholesterol intake, total fat intake, individual fat intake | Sex, age, region of residence, pack-years of smoking, years of education, intake of vitamin E, iron, alcohol and body mass index | 9 |
| Akyrozis et al. (2013) | Diagnosed according to UKBB-based questionnaire | Dairy total intake, milk intake, Yoghurt intake, cheese intake; fat total intake; individual fat intake | Age, gender, marital status, farm occupation, height, weight, body mass index, physical activity, energy intake, alcohol intake, smoking status, caffeinated coffee, tea consumption, years of education | 9 |
| Dong et al. (2014) | Confirmed by self-reported, completed a diagnostic questionnaire and provide a copy of the patient’s medical records. | Total fat intake, individual fat intake, total energy intake, protein intake, carbohydrate, cholesterol, | Age, gender, race/ethnicity caffeine intake, total energy intake, smoking status, diabetes, and self-reported health status | 9 |
| Kamel et al. (2015) | Confirmed by movement disorder specialists or a corresponding dates | High fat no pesticide; high fat yes pesticide; low fat no pesticide; low fat yes pesticide | Age, gender, state, smoking, total energy intake | 7 |
NA: Not available; NOS: Newcastle-Ottawa Scale
Meta-analysis results
Primary outcomes
To assess the link between different factors of interest and the exposure assessments, we performed separate analyses. The pooled OR for PD in those with a high total energy intake was 1.49 (P = 0.000, OR = 1.49, 95% CI: 1.26–1.75), while it was 1.07 (P =0.123, OR = 1.07, 95% CI: 0.91–1.25) in those with high total fat intake, and 1.02 (P = 0.005, OR = 1.02, 95% CI: 0.79–1.30) in men. The overall pooled OR was 1.21 (P = 0.000, OR = 1.21, 95% CI: 1.09–1.34; Figure 2). However, fat included many subtypes, and different food sources may have different amounts of fat subtypes, which may have led to the high heterogeneity observed in these results (I2 = 75.9%), so we carried out subgroup analyses and a sensitivity analysis simultaneously.
Figure 2.
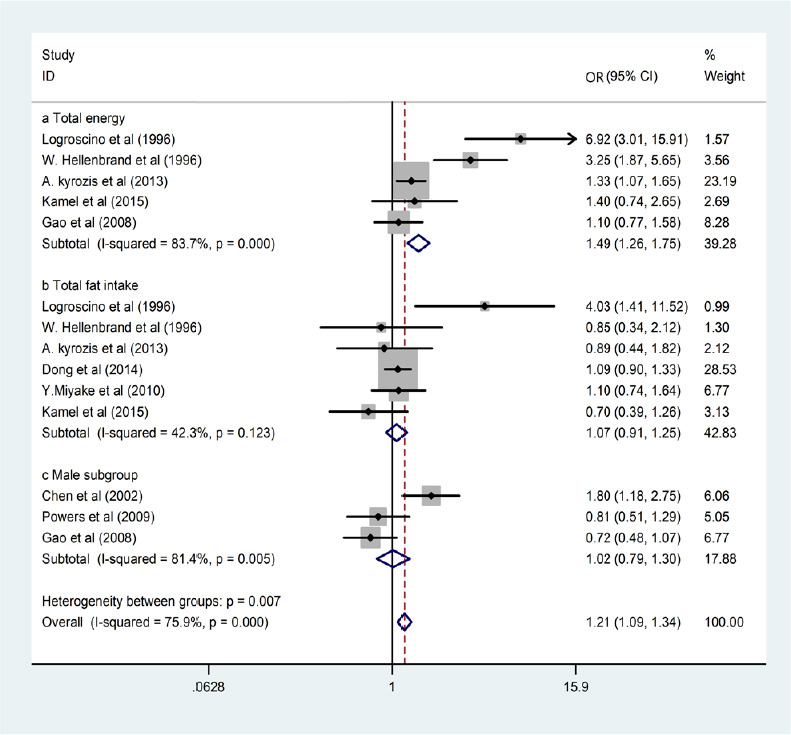
Forest plots of total energy intake, total fat intake, and male subgroup associations with Parkinson’s disease (PD) risk.
High total energy intake and sex were both linked with elevated PD risk, while total fat intake was not associated with PD risk.
Subgroup analyses
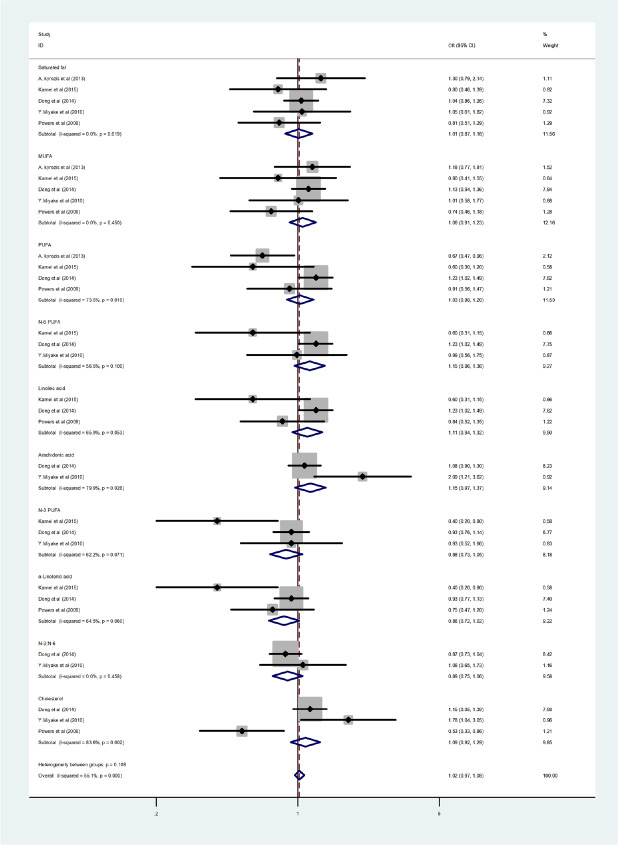
The subgroup analyses were conducted based on fat subtypes (PUFA, arachidonic acid, cholesterol, n-3 PUFA, n-6 PUFA, α-linolenic acid, linoleic acid, monounsaturated fatty acid [MUFA], saturated fatty acids, and n-3 to n-6 PUFA ratio) to further explore the source of heterogeneity. An association was found between high PUFA intake and reduced PD risk (P = 0.010, OR = 1.03, 95% CI: 0.88–1.20). In contrast, arachidonic acid (P = 0.026, OR = 1.15, 95% CI: 0.97–1.37) and cholesterol (P = 0.002, OR = 1.09, 95% CI: 0.92–1.29) intakes were linked with an elevated PD risk. Moreover, although the results were not significant, consumption of n-3 PUFA (P = 0.071, OR = 0.88, 95% CI: 0.73–1.05), α-linolenic acid (P = 0.06, OR = 0.86, 95% CI: 0.72–1.02), and the n-3 to n-6 PUFA ratio (P = 0.458, OR = 0.89, 95% CI: 0.75–1.06) were all linked with a non-significant trend toward reduced PD risk, while MUFA (P = 0.450, OR = 1.06, 95% CI: 0.91–1.23), linoleic acid (P = 0.053, OR = 1.11, 95% CI: 0.94–1.32), and n-6 PUFA (P = 0.100, OR = 1.15, 95% CI: 0.96–1.36) intakes were associated with a non-significant trend toward higher PD risk. Saturated fatty acid intake (P = 0.619, OR = 1.01, 95% CI: 0.87–1.18) was not associated with PD (Figure 3).
Figure 3.
Forest plots of saturated fatty acids, monounsaturated fatty acid (MUFA), high polyunsaturated fatty acids (PUFA), arachidonic acid, n-3 PUFA, α-linolenic acid, n-6 PUFA, linoleic acid, the ratio of n-3 to n-6 PUFA, and cholesterol intake associations with Parkinson’s disease (PD).
There was a consistent link between PUFA consumption and lower PD risk, while higher cholesterol and arachidonic acid intakes were linked with elevated PD risk. Although the results were not significant, consumption of n-3 PUFA, α-linolenic acid, and the n-3 to n-6 PUFA ratio were all linked with a trend toward reduced PD risk, while MUFA, linoleic acid, and n-6 PUFA intakes were associated with a trend toward higher PD risk. Saturated fatty acid intake was not associated with PD.
Publication bias
We did not detect publication bias for studies of either high total energy intake and PD risk or high total fat intake and PD risk, based on a fully adjusted model (P = 0.114). There are two articles that seem farther outside the funnel, possibly caused by the high heterogeneity of both articles. These studies were not excluded, however, because they met the inclusion criteria and Egger’s test gave P > 0.05 (Figure 4).
Figure 4.
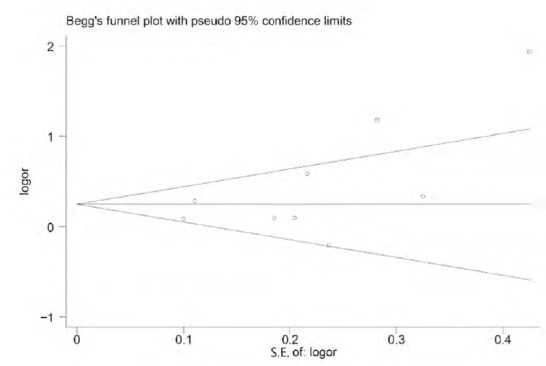
Publication bias measured by a funnel plot and Egger’s test (P = 0.114).
Two articles are farther outside the funnel; they may have only represented a trend.
Discussion
High dietary fat intake and Parkinson’s disease risk
We found that high total energy intake was linked with elevated PD risk, whereas total fat intake was not. However, we revealed an association between high PFUA and reduced PD risk; in contrast, arachidonic acid and cholesterol intakes were linked with an elevated PD risk. Although the results were not significant, consumption of n-3 PUFA, α-linolenic acid, and the n-3 to n-6 PUFA ratio was all linked with a trend toward reduced PD risk, while MUFA, linoleic acid, n-6 PUFA intakes were associated with a trend toward higher PD risk. Saturated fatty acids were not associated with PD.
Elevated PD risk may result from the consumption of dietary fat, because of its effects involving increased oxidative stress and neuroinflammation, which potentially exacerbate neurotoxin-induced dopaminergic neuron loss. PUFAs are primarily found in the SN2 position of phosphoglycerates in neural cell membranes where, in response to lipid peroxidation, they can give rise to oxygen free radicals (Choi et al., 2005; Shchepinov et al., 2011; Bousquet et al., 2012). PUFAs are also necessary for appropriate glial cell membrane formation, and can further regulate the generation of inflammatory cytokines and prostaglandins (Laye, 2010). Dietary n-3 and n-6 α-linoleic acids are used to synthesize PUFA in cell membranes, and can also give rise to long-chain PUFA via desaturation and elongation (Youdim et al., 2000). In particular, n-3 PUFAs play anti-inflammatory roles, while n-6 PUFAs serve as inflammatory prostaglandin precursors (Dong et al., 2014). Arachidonic acid, one of the major types of PUFA present in the brain, is one of several key types of n-6 PUFAs (Porter et al., 1995; Simopoulos, 1999; Hadders-Algra, 2008), and linoleic acid is also a subtype of n-6 PUFA. In the present study, PUFAs were linked with a decreased risk of PD, in contrast to the expected increased risk, and this result suggests that the n-3/n-6 ratio might be an important factor when assessing PD development risk. If intake of n-3 is greater than n-6 intake, the risk of PD may be reduced. Although our study revealed that there was no significant relationship between n-3/n-6 PUFA ratio and PD risk, there was a non-significant trend toward reduced risk of PD when the n-3/n-6 PUFA ratio was higher.
The brain contains the most cholesterol of any organ, and it is capable of synthesizing cholesterol (Noguchi et al., 2014). However, few studies have reported that cholesterol-rich diets drive neurotoxin-induced dopaminergic neuron loss (Choi et al., 2005; Bousquet et al., 2012). Elevated cholesterol levels can contribute to oxidative stress (Pappolla et al., 2002; Thirumangalakudi et al., 2008; Prasanthi et al., 2010) and neuroinflammation (Thirumangalakudi et al., 2008; Ullrich et al., 2010; Pirchl et al., 2012). In addition, high levels of cholesterol can cause mitochondrial dysfunction and influence α-synuclein aggregation (Bar-On et al., 2008). Therefore, cholesterol may be a risk factor for neurodegenerative disease in general (Vance, 2012; Martin et al., 2014), which is consistent with our results.
There are many factors that affect the results of our analysis. Some come from the original literature, and were possibly caused by defects in research design. Of the reviewed references, only Kamel et al. (2014) provided evidence that α-linolenic acid and linoleic acid intakes decreased PD risk. Others reported that a moderately reduced PD risk was not associated with α-linolenic acid or linoleic acid intake (Porter et al., 1995; Youdim et al., 2000; Ikemoto et al., 2001; Levant et al., 2007; Hadders-Algra, 2008; Laye, 2010; Shchepinov et al., 2011). In addition, only Dong et al. (2014) provided evidence for a positive relationship between dietary PUFA intake and PD risk. Some PUFA are associated with specific functions of the human body, and although N-3 must be obtained from the diet, other fatty acids can be synthesized in the body; thus, we cannot rule out the effects of self-synthesized fatty acids on our results. Moreover, exposure assessments in the included references were all obtained via different questionnaires, such as diet history questionnaires and food frequency questionnaires. This may have led to variation in survey accuracy, because dietary consumption does not necessarily translate to biological nutritional status.
Limitations
This study has certain limitations. First, a more careful analysis of other dietary PUFA fats is needed to confirm the protective PUFA concentrations that are necessary to reduce PD risk, and to confirm the adverse results of eating other types of fats. Second, we did not pool vitamins or other types of nutrition in this study, and therefore potentially overlooked their roles as antioxidants in protecting against PD. Third, the food sources of each fat were not considered, which may have led to the high heterogeneity that we found. Fourth, we did not consider the contributions of regionalism and dietary customs, which also may have influenced our results.
Conclusions and future directions
This meta-analysis revealed that higher energy intake is linked with elevated PD risk. We also demonstrated that high PUFA was associated with reduced PD risk; in contrast, arachidonic acid and cholesterol intakes were linked with an elevated risk of PD. Although the results were not significant, consumption of n-3 PUFA, α-linolenic acid, and the n-3/n-6 PUFA ratio were all linked with a trend toward reduced PD risk, while MUFA, linoleic acid, and n-6 PUFA intakes were associated with a trend toward higher PD risk. Saturated fatty acids were not associated with PD risk.
Further research is necessary to confirm the link between dietary fat and PD risk, and other nutritional antioxidants such as vitamins should also be considered in this context. New studies should focus on the dietary sources of each fat (such as the intake of the various PUFAs, and the n-3/n-6 intake ratio), as well as how regional dietary variations may influence these outcomes, to avoid high heterogeneity.
Additional files:
Additional Table 1: Newcastle-Ottawa Scale Assessment of Case-Control Studies.
Additional Table 2: Newcastle-Ottawa Scale Assessment of Cohort Studies.
Additional file 1: Open peer review report 1 (89KB, pdf) .
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was financially supported by the National Natural Science Foundation of China, No. 31200868 (to XC). The funding source had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Reporting Statement: This study followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Anupom Borah, Assam University, India.
P-Reviewer: Borah A; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Gardner B, Yajima W, Qiu Y, Song LP; T-Editor: Jia YChinese
Funding: This study was supported by the National Natural Science Foundation of China, No. 31200868 (to XC).
References
- 1.Akbar M, Kim HY. Protective effects of docosahexaenoic acid in staurosporine-induced apoptosis: involvement of phosphatidylinositol-3 kinase pathway. J Neurochem. 2002;82:655–665. doi: 10.1046/j.1471-4159.2002.01015.x. [DOI] [PubMed] [Google Scholar]
- 2.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci U S A. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar-On P, Crews L, Koob AO, Mizuno H, Adame A, Spencer B, Masliah E. Statins reduce neuronal alpha-synuclein aggregation in in vitro models of Parkinson’s disease. J Neurochem. 2008;105:1656–1667. doi: 10.1111/j.1471-4159.2008.05254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bousquet M, St-Amour I, Vandal M, Julien P, Cicchetti F, Calon F. High-fat diet exacerbates MPTP-induced dopaminergic degeneration in mice. Neurobiol Dis. 2012;45:529–538. doi: 10.1016/j.nbd.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Bowen RA, Clandinin MT. Dietary low linolenic acid compared with docosahexaenoic acid alter synaptic plasma membrane phospholipid fatty acid composition and sodium-potassium ATPase kinetics in developing rats. J Neurochem. 2002;83:764–774. doi: 10.1046/j.1471-4159.2002.01156.x. [DOI] [PubMed] [Google Scholar]
- 6.Calon F, Lim GP, Morihara T, Yang F, Ubeda O, Salem N, Jr, Frautschy SA, Cole GM. Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer’s disease. Eur J Neurosci. 2005;22:617–626. doi: 10.1111/j.1460-9568.2005.04253.x. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi S, Ostbye T, Stoessl AJ, Merskey H, Hachinski V. Environmental exposures in elderly Canadians with Parkinson’s disease. Can J Neurol Sci. 1995;22:232–234. doi: 10.1017/s0317167100039901. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Zhang SM, Hernan MA, Willett WC, Ascherio A. Dietary intakes of fat and risk of Parkinson’s disease. Am J Epidemiol. 2003;157:1007–1014. doi: 10.1093/aje/kwg073. [DOI] [PubMed] [Google Scholar]
- 9.Choi JY, Jang EH, Park CS, Kang JH. Enhanced susceptibility to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in high-fat diet-induced obesity. Free Radic Biol Med. 2005;38:806–816. doi: 10.1016/j.freeradbiomed.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Cota GF, de Sousa MR, Fereguetti TO, Rabello A. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis. 2013;7:e2195. doi: 10.1371/journal.pntd.0002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalfo E, Portero-Otin M, Ayala V, Martinez A, Pamplona R, Feorer I. Evidence of oxidative stress in the neocortex in incidental Lewy body disease. J Neuropathol Exp Neurol. 2005;64:816–830. doi: 10.1097/01.jnen.0000179050.54522.5a. [DOI] [PubMed] [Google Scholar]
- 12.de Lau LM, Bornebroek M, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Dietary fatty acids and the risk of Parkinson disease: the Rotterdam study. Neurology. 2005;64:2040–2045. doi: 10.1212/01.WNL.0000166038.67153.9F. [DOI] [PubMed] [Google Scholar]
- 13.Di Monte DA, Lavasani M, Manning-Bog AB. Environmental factors in Parkinson’s disease. Neurotoxicology. 2002;23:487–502. doi: 10.1016/s0161-813x(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 14.Dick FD, De Palma G, Ahmadi A, Scott NW, Prescott GJ, Bennett J, Semple S, Dick S, Counsell C, Mozzoni P, Haites N, Wettinger SB, Mutti A, Otelea M, Seaton A, Soderkvist P, Felice A Geoparkinson study. Environmental risk factors for Parkinson’s disease and parkinsonism: the Geoparkinson study. Occup Environ Med. 2007;64:666–672. doi: 10.1136/oem.2006.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong J, Beard JD, Umbach DM, Park Y, Huang X, Blair A, Kamel F, Chen H. Dietary fat intake and risk for Parkinson’s disease. Mov Disord. 2014;29:1623–1630. doi: 10.1002/mds.26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabelo N, Martin V, Santpere G, Marin R, ToORent L, FeORer I, Diaz M. Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease. Mol Med. 2011;17:1107–1118. doi: 10.2119/molmed.2011.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang X, An P, Wang H, Wang X, Shen X, Li X, Min J, Liu S, Wang F. Dietary intake of heme iron and risk of cardiovascular disease: a dose-response meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis. 2015;25:24–35. doi: 10.1016/j.numecd.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Gao X, Chen H, Fung TT, Logroscino G, Schwarzschild MA, Hu FB, Ascherio A. Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr. 2007;86:1486–1494. doi: 10.1093/ajcn/86.5.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadders-Algra M. Prenatal long-chain polyunsaturated fatty acid status: the importance of a balanced intake of docosahexaenoic acid and arachidonic acid. J Perinat Med. 2008;36:101–109. doi: 10.1515/JPM.2008.029. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto M, Hossain S, Shimada T, Sugioka K, Yamasaki H, Fujii Y, Ishibashi Y, Oka J, Shido O. Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J Neurochem. 2002;81:1084–1091. doi: 10.1046/j.1471-4159.2002.00905.x. [DOI] [PubMed] [Google Scholar]
- 21.Hellenbrand W, Boeing H, Robra BP, Seidler A, Vieregge P, Nischan P, Joerg J, Oertel WH, Schneider E, Ulm G. Diet and Parkinson's disease. II: a possible role for the past intake of specific nutrients. Results from a self-administered food-frequency questionnaire in a case-control study. Neurology. 47:644–650. doi: 10.1212/wnl.47.3.644. [DOI] [PubMed] [Google Scholar]
- 22.Ikemoto A, Ohishi M, Sato Y, Hata N, Misawa Y, Fujii Y, Okuyama H. Reversibility of n-3 fatty acid deficiency-induced alterations of learning behavior in the rat: level of n-6 fatty acids as another critical factor. J Lipid Res. 2001;42:1655–1663. [PubMed] [Google Scholar]
- 23.Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;3(53 Suppl):S26–36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 24.Kamel F, Goldman SM, Umbach DM, Chen H, Richardson G, Barber MR, Meng C, MaORas C, Korell M, Kasten M, Hoppin JA, Comyns K, Chade A, Blair A, Bhudhikanok GS, Webster Ross G, William Langston J, Sandler DP, Tanner CM. Dietary fat intake, pesticide use, and Parkinson's disease. Parkinsonism Relat Disord. 2014;20:82–87. doi: 10.1016/j.parkreldis.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirkey KL, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Gorell JM. Occupational categories at risk for Parkinson’s disease. Am J Ind Med. 2001;39:564–571. doi: 10.1002/ajim.1055. [DOI] [PubMed] [Google Scholar]
- 26.Kyrozis A, Ghika A, Stathopoulos P, Vassilopoulos D, Trichopoulos D, Trichopoulou A. Dietary and lifestyle variables in relation to incidence of Parkinson’s disease in Greece. Eur J Epidemiol. 2013;28:67–77. doi: 10.1007/s10654-012-9760-0. [DOI] [PubMed] [Google Scholar]
- 27.Laye S. Polyunsaturated fatty acids, neuroinflammation and well being. Prostaglandins Leukot Essent Fatty Acids. 2010;82:295–303. doi: 10.1016/j.plefa.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Levant B, Ozias MK, Carlson SE. Specific brain regions of female rats are differentially depleted of docosahexaenoic acid by reproductive activity and an (n-3) fatty acid-deficient diet. J Nutr. 2007;137:130–134. doi: 10.1093/jn/137.1.130. [DOI] [PubMed] [Google Scholar]
- 29.Li AA, Mink PJ, McIntosh LJ, Teta MJ, Finley B. Evaluation of epidemiologic and animal data associating pesticides with Parkinson’s disease. J Occup Environ Med. 2005;47:1059–1087. doi: 10.1097/01.jom.0000174294.58575.3e. [DOI] [PubMed] [Google Scholar]
- 30.Liu Q, Xu Y, Wan W, Ma Z. An unexpected improvement in spatial learning and memory ability in alpha-synuclein A53T transgenic mice. J Neural Transm (Vienna) 2018;125:203–210. doi: 10.1007/s00702-017-1819-3. [DOI] [PubMed] [Google Scholar]
- 31.Logroscino G, Marder K, Cote L, Tang MX, Shea S, Mayeux R. Dietary lipids and antioxidants in Parkinson's disease: a population-based, case-control study. Ann Neurol. 1996;39:89–94. doi: 10.1002/ana.410390113. [DOI] [PubMed] [Google Scholar]
- 32.Logroscino G, Marder K, Graziano J, Freyer G, Slavkovich V, Lojacono N, Cote L, Mayeux R. Dietary iron, animal fats, and risk of Parkinson's disease. Mov Disord. 1998;13:13–16. [PubMed] [Google Scholar]
- 33.Ma ZG, Xu J, Liu TW. Quantitative assessment of the association between fibroblast growth factor 20 rs1721100 C/G polymorphism and the risk of sporadic Parkinson’s diseases: a meta-analysis. Neurol Sci. 2015;36:47–51. doi: 10.1007/s10072-014-1884-4. [DOI] [PubMed] [Google Scholar]
- 34.Ma ZG, Liu TW, Bo YL. HLA-DRA rs3129882 A/G polymorphism was not a risk factor for Parkinson’s disease in Chinese-based populations: a meta-analysis. Int J Neurosci. 2015;125:241–246. doi: 10.3109/00207454.2014.926349. [DOI] [PubMed] [Google Scholar]
- 35.Ma ZG, He F, Xu J. Quantitative assessment of the association between GAK rs1564282 C/T polymorphism and the risk of Parkinson’s disease. J Clin Neurosci. 2015;22:1077–1080. doi: 10.1016/j.jocn.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Martin MG, Pfrieger F, Dotti CG. Cholesterol in brain disease: sometimes determinant and frequently implicated. EMBO Rep. 2014;15:1036–1052. doi: 10.15252/embr.201439225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyake Y, Sasaki S, Tanaka K, Fukushima W, Kiyohara C, Tsuboi Y, Yamada T, Oeda T, Miki T, Kawamura N, Sakae N, Fukuyama H, Hirota Y, Nagai M Fukuoka Kinki Parkinson's Disease Study G. Dietary fat intake and risk of Parkinson's disease: a case-control study in Japan. J Neurol Sci. 2010;288:117–122. doi: 10.1016/j.jns.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Martinez B, Peplow PV. Neuroprotection by immunomodulatory agents in animal models of Parkinson’s disease. Neural Regen Res. 2018;13:1493–1506. doi: 10.4103/1673-5374.237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noguchi N, Saito Y, Urano Y. Diverse functions of 24(S)-hydroxycholesterol in the brain. Biochem Biophys Res Commun. 2014;446:692–696. doi: 10.1016/j.bbrc.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Noseworthy MD, Bray TM. Effect of oxidative stress on brain damage detected by MRI and in vivo 31P-NMR. Free Radic Biol Med. 1998;24:942–951. doi: 10.1016/s0891-5849(97)00383-3. [DOI] [PubMed] [Google Scholar]
- 41.Pappolla MA, Smith MA, Bryant-Thomas T, Bazan N, Petanceska S, PeORy G, Thal LJ, Sano M, Refolo LM. Cholesterol, oxidative stress, and Alzheimer's disease: expanding the horizons of pathogenesis. Free Radic Biol Med. 2002;33:173–181. doi: 10.1016/s0891-5849(02)00841-9. [DOI] [PubMed] [Google Scholar]
- 42.Pirchl M, Ullrich C, Sperner-Unterweger B, Humpel C. Homocysteine has anti-inflammatory properties in a hypercholesterolemic rat model in vivo. Mol Cell Neurosci. 2012;49:456–463. doi: 10.1016/j.mcn.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 44.Powers KM, Smith-Weller T, Franklin GM, Longstreth WT, Jr, Swanson PD, Checkoway H. Dietary fats, cholesterol and iron as risk factors for Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:47–52. doi: 10.1016/j.parkreldis.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasanthi JR, Dasari B, Marwarha G, Larson T, Chen X, Geiger JD, Ghribi O. Caffeine protects against oxidative stress and Alzheimer’s disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radic Biol Med. 2010;49:1212–1220. doi: 10.1016/j.freeradbiomed.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and Parkinson’s disease: a metaanalysis. Environ Res. 2001;86:122–127. doi: 10.1006/enrs.2001.4264. [DOI] [PubMed] [Google Scholar]
- 47.Sampaio TB, Savall AS, Gutierrez MEZ, Pinton S. Neurotrophic factors in Alzheimer’s and Parkinson’s diseases: implications for pathogenesis and therapy. Neural Regen Res. 2017;12:549–557. doi: 10.4103/1673-5374.205084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schapira AH. Mitochondrial dysfunction in Parkinson’s disease. Cell Death Differ. 2007;14:1261–1266. doi: 10.1038/sj.cdd.4402160. [DOI] [PubMed] [Google Scholar]
- 49.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, Hurwitz PE, Coyle L, Schussler N, Michaud DS, Freedman LS, Brown CC, Midthune D, Kipnis V. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154:1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 50.Shchepinov MS, Chou VP, Pollock E, Langston JW, Cantor CR, Molinari RJ, Manning-Bog AB. Isotopic reinforcement of essential polyunsaturated fatty acids diminishes nigrostriatal degeneration in a mouse model of Parkinson’s disease. Toxicol Lett. 2011;207:97–103. doi: 10.1016/j.toxlet.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 51.Simopoulos AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr. 1999;70:560S–569S. doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- 52.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 53.Taylor CA, Saint-Hilaire MH, Cupples LA, Thomas CA, Burchard AE, Feldman RG, Myers RH. Environmental, medical, and family history risk factors for Parkinson's disease: a New England-based case control study. Am J Med Genet. 1999;88:742–749. [PubMed] [Google Scholar]
- 54.Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MS, Bhat NR. High cholesterol-induced neuroinflammation and amyloid precursor protein processing coORelate with loss of working memory in mice. J Neurochem. 2008;106:475–485. doi: 10.1111/j.1471-4159.2008.05415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ullrich C, Pirchl M, Humpel C. Hypercholesterolemia in rats impairs the cholinergic system and leads to memory deficits. Mol Cell Neurosci. 2010;45:408–417. doi: 10.1016/j.mcn.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vance JE. Dysregulation of cholesterol balance in the brain: contribution to neurodegenerative diseases. Dis Model Mech. 2012;5:746–755. doi: 10.1242/dmm.010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang QM, Xu YY, Liu S, Ma ZG. Isradipine attenuates MPTP-induced dopamine neuron degeneration by inhibiting up-regulation of L-type calcium channels and iron accumulation in the substantia nigra of mice. Oncotarget. 2017;8:47284–47295. doi: 10.18632/oncotarget.17618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wullner U, Klockgether T. Inflammation in Parkinson’s disease. J Neurol. 2003;1(250 Suppl):I35–I38. doi: 10.1007/s00415-003-1107-x. [DOI] [PubMed] [Google Scholar]
- 59.Youdim KA, Martin A, Joseph JA. Essential fatty acids and the brain: possible health implications. Int J Dev Neurosci. 2000;18:383–399. doi: 10.1016/s0736-5748(00)00013-7. [DOI] [PubMed] [Google Scholar]
- 60.Zecca L, Youdim MB, Riederer P, Connor JR, Crichton OR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 61.Zhang J, Yu KF. What’s the relative risk? (1998) A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 62.Zorzon M, Capus L, Pellegrino A, Cazzato G, Zivadinov R. Familial and environmental risk factors in Parkinson’s disease: a case-control study in north-east Italy. Acta Neurol Scand. 2002;105:77–82. doi: 10.1034/j.1600-0404.2002.1o040.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.