Abstract
A new role has emerged for progesterone after discovering its potent actions away from reproduction in both the central and the peripheral nervous system. The aim of the present report is to discuss progesterone’s mechanisms of action involved in myelination, remyelination and neuroinflammation. The pivotal role of the classic progesterone receptor is described and evidence is compiled about progesterone’s direct effects on oligodendrocyte linage and its indirect effects on oligodendrocyte precursor cell differentiation by decreasing the neuroinflammatory environment.
Keywords: progesterone, progesterone receptor, oligodendrocyte differentiation, myelination, remyelination, neuroinflammation
Introduction
Progesterone is a well-known sex hormone that plays a pivotal role in reproductive tissues. However, a new role has emerged for the steroid after discovering its potent actions away from reproduction in both the central nervous system (CNS) and the peripheral nervous system (PNS) (Schumacher et al., 2012). Progesterone effects beyond reproduction include myelin formation, neuroprotection, anti-inflammatory actions, neurogenesis regulation, preservation of mitochondrial function and regulation of mood, memory and cognition (Brinton, 2013; De Nicola et al., 2018). The aim of the present report is to discuss progesterone mechanisms of action involved in myelination, remyelination and neuroinflammation. We not only reviewed the preponderant role of progesterone receptor (PR) in the mentioned process but also described the pro-myelinating actions of its reduced derivative tetrahidroprogesterone (THP), a potent agonist of the GABAA receptor. Finally, attention is drawn to the fact that progesterone actions could be direct on oligodendrocyte linage or alternatively indirect through the downregulation of the inflammatory process.
It is important to establish the differences between myelination and remyelination. While myelination addresses the formation of myelin during development, remyelination is related to myelin repair in the adult nervous system. In the CNS mature oligodendrocytes in both processes derive from the differentiation of oligodendrocyte precursor cells (OPC), but adult OPC are indeed distinct from the perinatal ones (Fancy et al., 2011). For example, the proliferation rate of perinatal OPC is higher than the one found in adult OPC (Fancy et al., 2011). Another important difference between myelination and remyelination is referred to the signals that drive the process. Myelination is mainly driven by axons signals while remyelination is induced by neuroinflammation (for review see Fancy et al., 2011).
Effect of Progesterone on Myelination and Remyelination in the Peripheral Nervous System
Progesterone pro-myelinating effects were first demonstrated 20 years ago in the PNS and are summarized in Table 1. In this regard, in co-cultures of Schwann cells and dorsal root ganglia neurons progesterone accelerates myelination and enhances the rate of myelin synthesis (Chan et al., 1998). Progesterone also stimulates myelination in the regenerating sciatic nerve after a cryolesion (Schumacher et al., 2012) and increases both the number of Schwann cells and the g-ratio of myelinated fibers during regeneration of the facial nerve (Chavez-Delgado et al., 2005).
Table 1.
Effects of progesterone and THP on myelination, remyelination and neuroinflammation.
| Steroid | Experimental model | Studied effects | Accions | Reference |
|---|---|---|---|---|
| Prog | Cultures of Schwann cells and DRG neurons | Myelination in PNS | Accelerates myelination and enhances the rate of myelin synthesis | Chan et al., 1998 |
| Prog | Lesion of facial nerve | Remyelination in PNS | Increases the number of Schwann cells and the g-ratio | Chavez et al., 2005; Sclumacher et al., 2012 |
| Prog, DHP, THP | Aged mice | Remyelination in PNS | Reverses morphological myelin abnormalities | Azcoitia et al., 2003 |
| Prog, DHP, THP | Diabetic mice | Remyelination in PNS | Reverses morphological myelin abnormalitie | Melcangi et al., 2011 |
| Prog, DHP, THP | Cultures of Schwann cells and adult sciatic nerve | Myelination in PNS | Increases P0 and PMP22 | Guennoun el al., 2001; Mercier et al., 2001; Magnaghi et al., 2007 |
| THP | Floating spheres cultures | Myelination in CNS | Increases OPP proliferation | Gago et al., 2004 |
| Prog | Cerebellar organotypic cultures | Myelination in CNS | Increases OPC proliferation, the number of mature oligodendrocytes and MBP expression | Ghoumari et al., 2003, 2005 |
| THP | Cerebellar organotypic cultures | Myelination in CNS | Increases MBP expression | Ghoumari et al., 2003, 2005 |
| Prog | Ethidium bromide-induced demyelination | Remyelination in CNS | Increases the number of remyelinated axons | Ibanez et al., 2004 |
| Prog | Spinal cord injury | Remyelination and neuroinflammation in CNS | Increases OPC differentiation, myelin protein expression and OPC survival. Decreases the number of astrocytes and microglial cells and pro-inflammatory cytokines. Promotes anti-inflammatory microglia | Labombarda et al., 2009, 2011; Jure et al., 2018 |
| Prog Nestorone | Lysolecithin-induced demyelination in cerebellar organotypic cultures | Remyelination in CNS | Increases MBP expression and OPC migration | Hussain et al., 2011 |
| Prog Nestorone | Cuprizone-induced demyelination | Remyelination and neuroinflammation in CNS | Increases myelin protein expression and the number of oligodendrocytes. Decreases the number of microglial and astrocytes | El-Etr et al., 2015 |
| THP | Trangenic mice of Alzheimer’s disease | Remyelination in CNS | Promotes the regeneration of white matter | Brinton et al., 2013 |
| Prog THP | EAE model | Remyelination and neuroinflammation in CNS | Increases remyelination; Decreases pro-inflammatory cytokines and microglial cells. | Garay et al., 2012; Noorbakhsh et al., 2014 |
| Prog | Cuprizone-induced demyelination | Neuroinflammation in CNS | Promotes the switch from pro-inflamatory to anti-inflammatory microglia | Aryanpour et al., 2017 |
Prog: Progesterone; DHP: dihidroprogesterone; THP: tetrahidroprogesterone; DRG: dorsal root ganglia; CNS: central nervous system; PNS: peripheral nervous system; EAE: experimental autoimmune encephalomyelitis; OPC: oligodendrocyte precursor cells; MBP: myelin-basic protein; OPP: oligodendrocyte pre-progenitors; P0: glycoprotein zero; PMP22: peripheral myelin protein 22.
In the nervous system progesterone can be converted into dihidroprogesterone (DHP) by the enzyme 5α-reductase and subsequently DHP can be reduced to THP by the enzyme 3α-hydroxysteroid oxidoreductase (Figure 1) (Schumacher et al., 2012; Melcangi and Panzica, 2014). These conversions contribute to the pleiotropic mechanisms of progesterone since DHP interacts with the PR and THP is a potent positive modulator of the GABAA receptor (Lambert et al., 2001). In the PNS, both Schwann cells and dorsal root ganglia neurons express not only the PR and GABAA receptor but also the mentioned enzymes, which metabolize progesterone into THP (Melcangi et al., 2011). As demonstrated in several experimental models, progesterone and its derivatives reverse the frequency of myelin morphological abnormalities in peripheral nerves during aging (Azcoitia et al., 2003) and diabetic disease (Melcangi et al., 2011).
Figure 1.
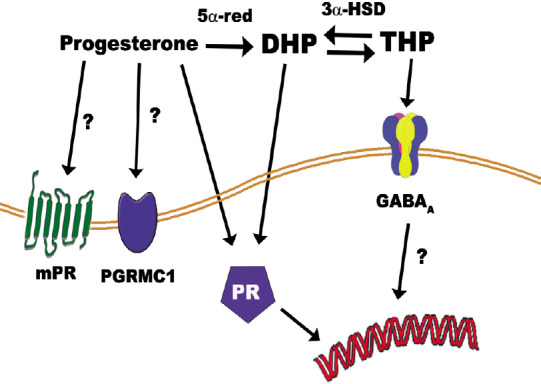
Progesterone pleiotropic effects are mediated via the progesterone receptor (PR), progesterone membrane receptor (mPR), and the membrane-associated protein progesterone receptor-membrane component-1 (PGRMC1).
Progesterone can be reduced to dihidroprogesterone (DHP) by the enzyme 5α-reductase (5α-red). DHP can also activate PR. DHP is subsequently converted into tetrahidroprogesterone (THP) by the enzyme 3α-hydroxysteroid oxidoreductase (3α-HSD). THP activates the GABAA receptor.
Progesterone and its reduced metabolites promote the synthesis of glycoprotein zero (P0) and the peripheral myelin protein 22 (PMP22) by stimulating the expression of key transcription factors in the myelination program of Schwann cells such as Krox-20, FosB, Sox 10 and Krox 24 (Guennoun et al., 2001; Mercier et al., 2001). Progesterone, DHP and THP increase Po and PMP22 expression in the adult sciatic nerve and in Schwann cell cultures (Melcangi et al., 2011) using different mechanisms of action (Magnaghi et al., 2001). On the one hand, progesterone, DHP and THP enhance mRNA levels of P0 involving PR as the potent PR-antagonist RU-486 abolishes P0 up-regulation (Magnaghi et al., 2001). Since the enzyme 3α-hydroxysteroid oxidoreductase is bi-directional (Rupprecht et al., 1996) the action of THP (which does not bind PR) may be due to its retro-conversion into DHP. On the other hand, neither progesterone nor DHP increase the mRNA levels of PMP22 (Magnaghi et al., 2001). Only THP stimulating the GABAA receptor is able to modify PMP22 expression. Altogether, these results indicate that both PR and GABAA receptor are involved in the regulation of PNS myelination.
Involvement of Progesterone in Developmental Myelination in the Central Nervous System
Progesterone effects on myelin formation in the PNS are also described in the CNS. Table 1 shows progesterone actions on the CNS. Schumacher’s group was the first to describe progesterone actions on developmental myelination in the oligodendroglial linage (Gago et al., 2001, 2004) and organotypic cultures (Ghoumari et al., 2003, 2005). In this regard, progesterone and THP are synthetized by oligodendrocyte pre-progenitors (OPP) during their differentiation into mature oligodendrocytes and increase their proliferation in cultures obtained from floating spheres (Gago et al., 2001; 2004). Progesterone increases the proliferation of OPP through its conversion to THP, while THP enhances their proliferation activating the GABAA receptor (Gago et al., 2004). These progenitors release GABA, which is involved in their proliferation, generating an autocrine mechanism of propagation. In this respect, THP and GABA in neural progenitor cells depolarize the plasma membrane and open the voltage-dependent L-type Ca2+ channel. Calcium influx leads to a downstream signaling pathway via Ca2+-dependent kinase which activates the cyclic AMP-responsive element-binding protein 1 and genes that regulate the cell cycle (Wang et al., 2005).
In cerebellar organotypic cultures, while progesterone increases OPC proliferation, the number of mature oligodendrocytes and myelin-basic protein (MBP) expression, THP only enhances MBP synthesis (Ghoumari et al., 2003; 2005). In OPC, which are more differentiated than OPP, progesterone and THP enhance MBP levels involving mainly PR and to a lesser extent the GABAA receptor (Ghoumari et al., 2003). PR plays a key role in OPC as no progesterone promyelinating effects have been observed in PR knockout mice (Ghoumari et al., 2003; 2005). These results suggest that THP modulates OPP proliferation but as cells become more and more differentiated progesterone may drive this process via PR.
Effect of Progesterone on Remyelination in the Central Nervous System
The prolonged administration of progesterone enhances the number of remyelinated axons in a model of toxin-induced demyelination in the brain of aging rats (Ibanez et al., 2004). In the mentioned model, the rate of remyelination is age-dependent and the process is efficient only in young rats. In this scenario, progesterone does not modify the remyelination process of young animals but exerts pro-myelinating effects on aged rats, where remyelination occurs with sub-optimal efficiency (Ibanez et al., 2004).
De Nicola’s group was the first to demonstrate that progesterone indeed differentiates proliferating OPC into mature oligodendrocyte after spinal cord injury (SCI) (Labombarda et al., 2006, 2009; Jure et al., 2019). Prolonged progesterone administration during 21 days after SCI increases the number of mature oligodendrocytes and enhances the expression of both mRNA and protein of MBP and proteolipid protein (Labombarda et al., 2009). During the acute phase after SCI, progesterone treatment enhances OPC survival and stimulates mitotic OPC to differentiate into mature oligodendrocytes after 3 weeks of treatment (Labombarda et al., 2009, 2015).
The differentiation program is a regulated process which involves the finely-tuned interplay of transcription factors and epigenetic modifiers (Huang et al., 2013). This program involves the synchronization of transcriptional inhibitors downregulation with the upregulation of transcriptional activators (Liu and Casaccia, 2010). The downregulation of the transcriptional inhibitors opens a window of opportunity for the activators to enhance and drive remyelination. It has been recently published that after SCI both the transcriptional inhibitors of the differentiation program and the transcriptional activators are down-regulated (Jure et al., 2019). Thus OPC missed the window of opportunity to differentiate after SCI. However, after progesterone treatment, the number of OPC which express the transcriptional activators of the differentiation program are up-regulated and the process is stimulated (Jure et al., 2019). Specifically, the expression of mRNA levels of the key activators of the differentiation program such as Olig2, Nkx2.2, Sox10 and Mash1 is enhanced after progesterone administration (Jure et al., 2019). The mentioned transcription factors are well-known players in OPC commitment to mature oligodendrocytes (Huang et al., 2013; Mitew et al., 2014).
Additionally, a recent report using MRI technology has demonstrated that progesterone reduces secondary damage and preserves white matter volume after spinal cord contusion (Garcia-Ovejero et al., 2014). They have shown that progesterone treatment increases the number of mature oligodendrocytes. After the CatWalk gait analysis, they found that the steroid improves locomotor outcome after SCI (Garcia-Ovejero et al., 2014).
Several publications have supported these results showing progesterone remyelintation properties. Progesterone and nestorone (a potent PR agonist) increase MBP expression after lysolecithin-induced demyelination in cerebellar slices (Hussain et al., 2011) and enhance remyelination after cuprizone treatment in corpus callosum and cerebral cortex (El-Etr et al., 2015). Remyelinating progesterone and nestorone actions are mediated by PR since the use of PRKO transgenic mice inhibits progesterone effect in the mentioned models (Hussain et al., 2011; El-Etr et al., 2015). Notably, in cerebellar organotypic cultures Nestorone enhances not only the maturation of OPC but also the migration of these progenitors towards demyelinating axons after lysolecithin application (Hussain et al., 2011). On the contrary, medroxyprogesterone acetate (MPA) has no effect neither on MBP immunoreactivity nor on the replenishment of oligodendrocytes in the mentioned demyelinated cerebellar culture (Hussain et al., 2011). It is known that glucocorticoids are not involved in the remyelination of cerebellar slices (Hussain et al., 2011). As a consequence, MPA remyelinating failure may be due to binding not only to PR but also to GR.
Finally, with regard to the effects of THP on remyelination in the CNS, Brinton (2013) has demonstrated that the steroid enhances myelin protein expression and promotes regeneration of white matter in a transgenic mice model of Alzheimer’s disease. On the other hand, in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS), progesterone and THP attenuate neurological deficits and increase remyelination (Yu et al., 2010; Garay et al., 2012). An outstanding study reported a significant decrease in the rate of relapses in MS patients during pregnancy and a significant increase during the first three months post-partum by comparison to the relapse rate observed during the year prior to pregnancy (Vukusic et al., 2009). Based on these results, a clinical trial began in 2009 (POPART´MUS – Reference number NTC00127075) using a synthetic progestin (nomegestrol acetate) to prevent post-partum relapses in women with MS. The POPART´MUS study is still ongoing.
Possible Mechanisms for the Pro-Myelinating Effect of Progesterone
The precise mechanism in myelin formation in the CNS is controversial. As already mentioned PR is involved in developmental myelination (Ghoumari et al., 2003, 2005) and remyelination (Hussain et al., 2011; El-Etr et al., 2015; Labombarda et al., 2015). However, it is currently unknown whether progesterone stimulates OPC differentiation directly and/or indirectly by the regulation of astrocytes, neurons and microglial cells. Indeed, PR has been detected in the spinal cord not only in neurons but also in glial cells (Labombarda et al., 2000).
Regarding direct progesterone actions on the oligodendrocyte linage, PR expression in oligodendrocytes remains to be clarified. Several reports have determined the expression of PR in oligodendrocyte cultures and in the white matter of the spinal cord by immunohistochemistry (Jung-Testas et al., 1991; Labombarda et al., 2000). However, these publications do not demonstrate exactly in which type of cell PR is expressed, since a double labeling experiment with an antibody against PR and antibody against a cell-specific marker of oligodendrocyte linage has not been performed (Jung-Testas et al., 1991; Labombarda et al., 2000). Concerning progesterone effects in vitro, the steroid is synthetized by OPC during the differentiation program in neurosphere-derived cultures (Gago et al., 2001) and increases the number of MBP-positive oligodendrocytes in mixed glial cell cultures (Schumacher et al., 2012). However, the intervention of other glial cells should not be ruled out since both cultures are not extremely pure and contain a small percentage of astrocytes (Gago et al., 2001). Experiments showing PR expression in OPC should be performed in the future since there are no reports in the literature that demonstrate it convincingly.
Astroglial and microglial cells regulate adult OPC differentiation in several pathological situations. In this regard, after lesions to the spinal cord, astrocytes and microglia cells react and produce proinflammatory mediators, oxygen free radicals and neurotoxic levels of nitric oxide (Sofroniew, 2009; Zhou et al., 2014). These proinflammatory mediators produce a feed-forward mechanism that propagates secondary injury and inflammation. Neuroinflammation contributes to white matter demyelination, oligodendrocyte loss and lack of OPC differentiation reported in SCI (Bracchi-Ricard et al., 2013). In mixed glial cultures, activated microglial cells release TNF-α which induces the apoptosis of OPC via the TNFR1 expressed in these cells. The same authors describe that astrocytes participate in promoting TNF-α toxicity to OPC in a contact-dependent manner (Kim et al., 2011).
Recent evidence has shown that progesterone decreases the number of astrocytes and microglial cells after SCI (Labombarda et al., 2011, 2015). Moreover, the steroid down-regulates the mRNA expression of interleukin-1β, tumor necrosis factor α, interleukin-6, inducible nitric oxide synthase, cyclooxygenase-2 (Labombarda et al., 2015) and increases the expression of transforming growth factor β, an anti-inflammatory and potent OPC differentiating factor (Palazuelos et al., 2014; Jure et al., 2019). Noteworthy, progesterone increases the number of transforming growth factorβ-expressing astrocytes and microglial cells (Jure et al., 2019). Based on the fact that anti-inflammatory microglial cells drive OPC differentiation (Miron and Franklin, 2014) the change of microglia phenotype caused by progesterone could create a pro-differentiating environment for OPC. These results are in agreement with another report which indicates that progesterone therapy induces a switch in microglia phenotype from pro-inflammatory to anti-inflammatory and suppresses NLRP3 inflammasome in cuprizone-induced demyelination mice (Aryanpour et al., 2017). These immunomodulatory effects evoke progesterone actions during pregnancy, in which a change occurs from the Th1 pro-inflammatory to a Th2 anti-inflammatory response (De Leon-Nava et al., 2009; Szekeres-Bartho et al., 2009). Other publications have supported these findings showing that progesterone has anti-inflammatory effects along with the aforementioned remyelinating actions in different animal models such as EAE (Yates et al., 2010; Garay et al., 2012) and chemical-induced demyelination ones (El-Etr et al., 2015). Table 1 compiles the action of progesterone on neuroinflammation.
In accordance with the promyelinating actions, progesterone anti-inflammatory effects also depend on PR. It has been recently published, that PR via NF-κB downregulates the expression of interleukin-1β, tumor necrosis factorα and interleukin-6 mRNA after SCI (Labombarda et al., 2015). The modulation of astrocytes and microglial cells also requires PR, since their number remains elevated after progesterone treatment in spinal cord injured PRKO mice (Labombarda et al., 2015). Interestingly, PR is not expressed in surveillance microglial cells (Sierra et al., 2008). Thus, progesterone effects on microglial cells might be due to astrocyte and neuron modulation. Since progesterone stimulates growth factor production via PR-signaling in astrocytes, these cells become an interesting target of progesterone (Lacroix-Fralish et al., 2006; Chesik and De Keyser, 2010). On the other hand, neurons could also be involved in progesterone actions because they express PR (Labombarda et al., 2000). However, after SCI the level of PR expression is downregulated in motoneurons (Labombarda et al., 2003). One more interesting possibility is that microglial cells up-regulate PR expression after CNS injury or other demyelinating diseases. This alternative should be explored in future experiments. Since peripheral macrophages express PR and progesterone modulates their function via a PR-dependent mechanism (Khan et al., 2005; Jones et al., 2008) another possibility arises. Progesterone could exert anti-inflammatory actions by inhibiting the infiltrated macrophages which invade the tissue after SCI, EAE induction and chemical-demyelination (Garay et al., 2012; El-Etr et al., 2015; Labombarda et al., 2015). In the case of lysolecithin-induced demyelination in cerebellar organotypic cultures, the perivascular macrophages could invade the tissue and mediate progesterone actions (Hussain et al., 2011).
Based on experimental evidence, progesterone effects on neuroinflammation and oligodendrocyte linage involve a PR-dependent mechanism (Ghoumari et al., 2003, 2005; Hussain et al., 2011; El-Etr et al., 2015; Labombarda et al., 2015). However, the involvement of other progesterone signaling mechanisms cannot be totally excluded. In fact, progesterone interacts with several G-couple- membrane receptors and the PR membrane component 1 (Figure 1). In addition, as already mentioned, progesterone is reduced to THP (GABAA modulator), which enhances the proliferation of oligodendrocyte pre-progenitors (Gago et al., 2004) and MBP synthesis (Ghoumari et al., 2003) (Figure 1).
Progesterone could exert myelinating and remyelinating effects by two possible mechanisms. On the one hand, it acts directly on the oligodendrocyte linage (if PR expression is definitely confirmed). Further experiments in vitro with extremely pure OPC cultures are needed to confirm this possibility. On the other hand, it acts indirectly via the inhibition of neuroinflammation by modulation of astroctyes, neurons and probably infiltrated macrophages.
Conclusion
Progesterone and THP seem to be good candidates to treat demyelinating diseases as both steroids enhance remyelination. Whether progesterone pro-myelinating effects are acting directly on oligodendrocyte linage or only mediated by the regulation of the neuroinflammatory process still remains unclear. Concerning the mechanism of action, how it is possible that progesterone antinflammatory actions are mediated by PR when microglial cells do not express this receptor. On the other hand, OPC and challenged microglial express the mPRα receptor (Labombarda et al., 2010). This non-canonical PR may also play a role in myelinating cells and neuroinflammation. However, how PR and the GABAA receptor interaction contribute to OPC differentiation and myelin synthesis is still unknown. The downstream pathways of both receptors might cross talk to each other and regulate oligodendrogenesis. THP via GABAA receptor could contribute to the early differentiation of OPP, when GABAA receptor activates Ca2+-dependent kinases (Brinton, 2013), while progesterone via PR might enhance late differentiation. Further experiments should be performed to elucidate these assumptions. Lastly, it needs to be established if progesterone mechanisms of action in myelination are different from those involved in remyelination? Elucidation of multiple progesterone mechanisms remains an exciting challenge. During the last few years more groups have studied the effects of progesterone on myelination, remyelination and neuroinflammation. In the future new data related to progesterone effects on oligodendrogenesis is expected, designing novel therapeutic strategies based on progesterone pro-myelinating actions for treating demyelinating diseases.
Additional file: Open peer review report 1 (97.2KB, pdf) .
Footnotes
Conflicts of interest: We declare no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Una FitzGerald, National University of Ireland Galway, Ireland.
P-Reviewer: FitzGerald U; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Aryanpour R, Pasbakhsh P, Zibara K, Namjoo Z, Beigi Boroujeni F, Shahbeigi S, Kashani IR, Beyer C, Zendehdel A. Progesterone therapy induces an M1 to M2 switch in microglia phenotype and suppresses NLRP3 inflammasome in a cuprizone-induced demyelination mouse model. Int Immunopharmacol. 2017;51:131–139. doi: 10.1016/j.intimp.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Azcoitia I, Leonelli E, Magnaghi V, Veiga S, Garcia-Segura LM, Melcangi RC. Progesterone and its derivatives dihydroprogesterone and tetrahydroprogesterone reduce myelin fiber morphological abnormalities and myelin fiber loss in the sciatic nerve of aged rats. Neurobiol Aging. 2003;24:853–860. doi: 10.1016/s0197-4580(02)00234-8. [DOI] [PubMed] [Google Scholar]
- 3.Bracchi-Ricard V, Lambertsen KL, Ricard J, Nathanson L, Karmally S, Johnstone J, Ellman DG, Frydel B, McTigue DM, Bethea JR. Inhibition of astroglial NF-kappaB enhances oligodendrogenesis following spinal cord injury. J Neuroinflammation. 2013;10:92. doi: 10.1186/1742-2094-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinton RD. Neurosteroids as regenerative agents in the brain: therapeutic implications. Nat Rev Endocrinol. 2013;9:241–250. doi: 10.1038/nrendo.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan JR, Phillips LJ, 2nd, Glaser M. Glucocorticoids and progestins signal the initiation and enhance the rate of myelin formation. Proc Natl Acad Sci U S A. 1998;95:10459–10464. doi: 10.1073/pnas.95.18.10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavez-Delgado ME, Gomez-Pinedo U, Feria-Velasco A, Huerta-Viera M, Castaneda SC, Toral FA, Parducz A, Anda SL, Mora-Galindo J, Garcia-Estrada J. Ultrastructural analysis of guided nerve regeneration using progesterone- and pregnenolone-loaded chitosan prostheses. J Biomed Mater Res B Appl Biomater. 2005;74:589–600. doi: 10.1002/jbm.b.30243. [DOI] [PubMed] [Google Scholar]
- 7.Chesik D, De Keyser J. Progesterone and dexamethasone differentially regulate the IGF-system in glial cells. Neurosci Lett. 2010;468:178–182. doi: 10.1016/j.neulet.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 8.De Leon-Nava MA, Nava K, Soldevila G, Lopez-Griego L, Chavez-Rios JR, Vargas-Villavicencio JA, Morales-Montor J. Immune sexual dimorphism: effect of gonadal steroids on the expression of cytokines sex steroid receptors and lymphocyte proliferation. J Steroid Biochem Mol Biol. 2009;113:57–64. doi: 10.1016/j.jsbmb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 9.De Nicola AF, Garay LI, Meyer M, Guennoun R, Sitruk-Ware R, Schumacher M, Gonzalez Deniselle MC. Neurosteroidogenesis and progesterone anti-inflammatory/neuroprotective effects. J Neuroendocrinol. 2018 doi: 10.1111/jne.12502. doi:101111/jne12502. [DOI] [PubMed] [Google Scholar]
- 10.El-Etr M, Rame M, Boucher C, Ghoumari AM, Kumar N, Liere P, Pianos A, Schumacher M, Sitruk-Ware R. Progesterone and nestorone promote myelin regeneration in chronic demyelinating lesions of corpus callosum and cerebral cortex. Glia. 2015;63:104–117. doi: 10.1002/glia.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fancy SP, Chan JR, Baranzini SE, Franklin RJ, Rowitch DH. Myelin regeneration: a recapitulation of development? Annu Rev Neurosci. 2011;34:21–43. doi: 10.1146/annurev-neuro-061010-113629. [DOI] [PubMed] [Google Scholar]
- 12.Gago N, Akwa Y, Sananes N, Guennoun R, Baulieu EE, El-Etr M, Schumacher M. Progesterone and the oligodendroglial lineage: stage-dependent biosynthesis and metabolism. Glia. 2001;36:295–308. doi: 10.1002/glia.1117. [DOI] [PubMed] [Google Scholar]
- 13.Gago N, El-Etr M, Sananes N, Cadepond F, Samuel D, Avellana-Adalid V, Baron-Van Evercooren A, Schumacher M. 3alpha,5alpha-Tetrahydroprogesterone (allopregnanolone) and gamma-aminobutyric acid: autocrine/paracrine interactions in the control of neonatal PSA-NCAM+ progenitor proliferation. J Neurosci Res. 2004;78:770–783. doi: 10.1002/jnr.20348. [DOI] [PubMed] [Google Scholar]
- 14.Garay LI, Gonzalez Deniselle MC, Brocca ME, Lima A, Roig P, De Nicola AF. Progesterone down-regulates spinal cord inflammatory mediators and increases myelination in experimental autoimmune encephalomyelitis. Neuroscience. 2012;226:40–50. doi: 10.1016/j.neuroscience.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 15.Ghoumari AM, Baulieu EE, Schumacher M. Progesterone increases oligodendroglial cell proliferation in rat cerebellar slice cultures. Neuroscience. 2005;135:47–58. doi: 10.1016/j.neuroscience.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Ghoumari AM, Ibanez C, El-Etr M, Leclerc P, Eychenne B, O’Malley BW, Baulieu EE, Schumacher M. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem. 2003;86:848–859. doi: 10.1046/j.1471-4159.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- 17.Guennoun R, Benmessahel Y, Delespierre B, Gouezou M, Rajkowski KM, Baulieu EE, Schumacher M. Progesterone stimulates Krox-20 gene expression in Schwann cells. Brain Res Mol Brain Res. 2001;90:75–82. doi: 10.1016/s0169-328x(01)00094-8. [DOI] [PubMed] [Google Scholar]
- 18.Huang H, Zhao XF, Zheng K, Qiu M. Regulation of the timing of oligodendrocyte differentiation: mechanisms and perspectives. Neurosci Bull. 2013;29:155–164. doi: 10.1007/s12264-013-1314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussain R, El-Etr M, Gaci O, Rakotomamonjy J, Macklin WB, Kumar N, Sitruk-Ware R, Schumacher M, Ghoumari AM. Progesterone and Nestorone facilitate axon remyelination: a role for progesterone receptors. Endocrinology. 2011;152:3820–3831. doi: 10.1210/en.2011-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibanez C, Shields SA, El-Etr M, Baulieu EE, Schumacher M, Franklin RJ. Systemic progesterone administration results in a partial reversal of the age-associated decline in CNS remyelination following toxin-induced demyelination in male rats. Neuropathol Appl Neurobiol. 2004;30:80–89. doi: 10.1046/j.0305-1846.2003.00515.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones LA, Anthony JP, Henriquez FL, Lyons RE, Nickdel MB, Carter KC, Alexander J, Roberts CW. Toll-like receptor-4-mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors. Immunology. 2008;125:59–69. doi: 10.1111/j.1365-2567.2008.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung-Testas I, Renoir JM, Gasc JM, Baulieu EE. Estrogen-inducible progesterone receptor in primary cultures of rat glial cells. Exp Cell Res. 1991;193:12–19. doi: 10.1016/0014-4827(91)90532-y. [DOI] [PubMed] [Google Scholar]
- 23.Jure I, De Nicola AF, Labombarda F. Progesterone effects on oligodendrocyte differentiation in injured spinal cord. Brain Res. 2019;1708:36–46. doi: 10.1016/j.brainres.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Matsuyama T, Ishimaru T. Estrogen and progesterone receptor expression in macrophages and regulation of hepatocyte growth factor by ovarian steroids in women with endometriosis. Hum Reprod. 2005;20:2004–2013. doi: 10.1093/humrep/deh897. [DOI] [PubMed] [Google Scholar]
- 25.Labombarda F, Guennoun R, Gonzalez S, Roig P, Lima A, Schumacher M, De Nicola AF. Immunocytochemical evidence for a progesterone receptor in neurons and glial cells of the rat spinal cord. Neurosci Lett. 2000;288:29–32. doi: 10.1016/s0304-3940(00)01191-5. [DOI] [PubMed] [Google Scholar]
- 26.Labombarda F, Gonzalez SL, Deniselle MC, Vinson GP, Schumacher M, De Nicola AF, Guennoun R. Effects of injury and progesterone treatment on progesterone receptor and progesterone binding protein 25-Dx expression in the rat spinal cord. J Neurochem. 2003;87:902–913. doi: 10.1046/j.1471-4159.2003.02055.x. [DOI] [PubMed] [Google Scholar]
- 27.Labombarda F, Gonzalez S, Gonzalez Deniselle MC, Garay L, Guennoun R, Schumacher M, De Nicola AF. Progesterone increases the expression of myelin basic protein and the number of cells showing NG2 immunostaining in the lesioned spinal cord. J Neurotrauma. 2006;23:181–192. doi: 10.1089/neu.2006.23.181. [DOI] [PubMed] [Google Scholar]
- 28.Labombarda F, Gonzalez SL, Lima A, Roig P, Guennoun R, Schumacher M, de Nicola AF. Effects of progesterone on oligodendrocyte progenitors, oligodendrocyte transcription factors, and myelin proteins following spinal cord injury. Glia. 2009;57:884–897. doi: 10.1002/glia.20814. [DOI] [PubMed] [Google Scholar]
- 29.Labombarda F, Gonzalez S, Lima A, Roig P, Guennoun R, Schumacher M, De Nicola AF. Progesterone attenuates astro- and microgliosis and enhances oligodendrocyte differentiation following spinal cord injury. Exp Neurol. 2011;231:135–146. doi: 10.1016/j.expneurol.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Labombarda F, Jure I, Gonzalez S, Lima A, Roig P, Guennoun R, Schumacher M, De Nicola AF. A functional progesterone receptor is required for immunomodulation reduction of reactive gliosis and survival of oligodendrocyte precursors in the injured spinal cord. J Steroid Biochem Mol Biol. 2015;154:274–284. doi: 10.1016/j.jsbmb.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Labombarda F, Meffre D, Delespierre B, Krivokapic-Blondiaux S, Chastre A, Thomas P, Pang Y, Lydon JP, Gonzalez SL, De Nicola AF, Schumacher M, Guennoun R. Membrane progesterone receptors localization in the mouse spinal cord. Neuroscience. 2010;166:94–106. doi: 10.1016/j.neuroscience.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Lacroix-Fralish ML, Tawfik VL, Nutile-McMenemy N, Harris BT, Deleo JA. Differential regulation of neuregulin 1 expression by progesterone in astrocytes and neurons. Neuron Glia Biol. 2006;2:227–234. doi: 10.1017/S1740925X07000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambert JJ, Belelli D, Harney SC, Peters JA, Frenguelli BG. Modulation of native and recombinant GABA(A) receptors by endogenous and synthetic neuroactive steroids. Brain Res Brain Res Rev. 2001;37:68–80. doi: 10.1016/s0165-0173(01)00124-2. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Casaccia P. Epigenetic regulation of oligodendrocyte identity. Trends Neurosci. 2010;33:193–201. doi: 10.1016/j.tins.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magnaghi V, Cavarretta I, Galbiati M, Martini L, Melcangi RC. Neuroactive steroids and peripheral myelin proteins. Brain Res Brain Res Rev. 2001;37:360–371. doi: 10.1016/s0165-0173(01)00140-0. [DOI] [PubMed] [Google Scholar]
- 36.Melcangi RC, Panzica GC. Allopregnanolone: state of the art. Prog Neurobiol. 2014;113:1–5. doi: 10.1016/j.pneurobio.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Melcangi RC, Magnaghi V, Cavarretta I, Zucchi I, Bovolin P, D’Urso D, Martini L. Progesterone derivatives are able to influence peripheral myelin protein 22 and P0 gene expression: possible mechanisms of action. J Neurosci Res. 1999;56:349–357. doi: 10.1002/(SICI)1097-4547(19990515)56:4<349::AID-JNR3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 38.Melcangi RC, Giatti S, Pesaresi M, Calabrese D, Mitro N, Caruso D, Garcia-Segura LM. Role of neuroactive steroids in the peripheral nervous system. Front Endocrinol (Lausanne) 2011;2:104. doi: 10.3389/fendo.2011.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercier G, Turque N, Schumacher M. Early activation of transcription factor expression in Schwann cells by progesterone. Brain Res Mol Brain Res. 2001;97:137–148. doi: 10.1016/s0169-328x(01)00311-4. [DOI] [PubMed] [Google Scholar]
- 40.Miron VE, Franklin RJ. Macrophages and CNS remyelination. J Neurochem. 2014;130:165–171. doi: 10.1111/jnc.12705. [DOI] [PubMed] [Google Scholar]
- 41.Palazuelos J, Klingener M, Aguirre A. TGFbeta signaling regulates the timing of CNS myelination by modulating oligodendrocyte progenitor cell cycle exit through SMAD3/4/FoxO1/Sp1. J Neurosci. 2014;34:7917–7930. doi: 10.1523/JNEUROSCI.0363-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rupprecht R, Hauser CA, Trapp T, Holsboer F. Neurosteroids: molecular mechanisms of action and psychopharmacological significance. J Steroid Biochem Mol Biol. 1996;56:163–168. doi: 10.1016/0960-0760(95)00233-2. [DOI] [PubMed] [Google Scholar]
- 43.Schumacher M, Hussain R, Gago N, Oudinet JP, Mattern C, Ghoumari AM. Progesterone synthesis in the nervous system: implications for myelination and myelin repair. Front Neurosci. 2012;6:10. doi: 10.3389/fnins.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56:659–674. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- 45.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szekeres-Bartho J, Halasz M, Palkovics T. Progesterone in pregnancy; receptor-ligand interaction and signaling pathways. J Reprod Immunol. 2009;83:60–64. doi: 10.1016/j.jri.2009.06.262. [DOI] [PubMed] [Google Scholar]
- 47.Vukusic S, Ionescu I, El-Etr M, Schumacher M, Baulieu EE, Cornu C, Confavreux C. Prevention of Post-Partum Relapses with P, Estradiol in Multiple Sclerosis Study G (2009) The prevention of post-partum relapses with progestin and estradiol in multiple sclerosis (POPART'MUS) trial: rationale, objectives and state of advancement. J Neurol Sci. 286:114–118. doi: 10.1016/j.jns.2009.08.056. [DOI] [PubMed] [Google Scholar]
- 48.Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005;25:4706–4718. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yates MA, Li Y, Chlebeck P, Proctor T, Vandenbark AA, Offner H. Progesterone treatment reduces disease severity and increases IL-10 in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;220:136–139. doi: 10.1016/j.jneuroim.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu HJ, Fei J, Chen XS, Cai QY, Liu HL, Liu GD, Yao ZX. Progesterone attenuates neurological behavioral deficits of experimental autoimmune encephalomyelitis through remyelination with nucleus-sublocalized Olig1 protein. Neurosci Lett. 2010;476:42–45. doi: 10.1016/j.neulet.2010.03.079. [DOI] [PubMed] [Google Scholar]
- 51.Zhou X, He X, Ren Y. Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen Res. 2014;9:1787–1795. doi: 10.4103/1673-5374.143423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


