Neurotrophins (NTs) are implicated in the maintenance and survival of the peripheral and central nervous systems and mediate several forms of synaptic plasticity. Members of the family include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), NT3, and NT4. NTs were first identified as survival factors for developing neurons, but are pleiotropic molecules that can exert a variety of functions, including the regulation of neuronal differentiation, axonal and dendritic growth, and synaptic plasticity (Bothwell, 2014). NTs interact with two distinct types of receptors: the common p75 neurotrophin receptor, which belongs to the tumor necrosis factor receptor superfamily of death receptors and the Trk receptor tyrosine kinase family. Trks contain an extracellular domain at which the NT binds, a single transmembrane domain, and an intracellular domain with tyrosine kinase activity. Three different Trks have been identified during the vertebrate evolution: TrkA, TrkB, and TrkC. NGF is the preferred ligand for TrkA, BDNF, and NT4/5 are preferred for TrkB, and NT3 for TrkC.
In bony fish, the same NGF ancestor gene gave origins by duplication to NGF and NT6. The latter is a neurotrophin not known from other vertebrates. NT6 was described in platyfish (Xiphophorus maculatus, Xiphophorus helleri and in Nothobranchius furzeri. Its ortholog was reported in zebrafish (Danio rerio) and carp (Cyprinus carpio) as NT7 (Leggieri et al., 2019). Another duplication was seen for TrkB and TrkC genes: TrkB1–TrkB2 and TrkC1–TrkC2 paralogs have been identified in both zebrafish and pufferfish genomes (Lucini et al., 2018).
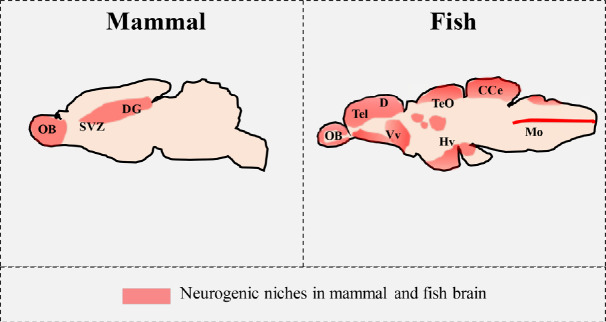
The expression pattern of NTs and their receptors in neurogenic stem niches of adult mammalian brain: In recent years, NTs and their receptors have emerged as important regulators of adult neurogenesis. In adult mammalian brain only two neurogenic zones are present: the subventricular zone adjacent to the lateral ventricle, and the dentate gyrus of the hippocampus (Figure 1).
Figure 1.
Schematic comparison of distribution of neurogenic niches in the brain of adult mammals and fish.
CCe: Cerebellar corpus; D: dorsal telencephalic area; DG: dendate gyrus; Hv: ventral zone of periventricular hypothalamus; Mo: medulla oblongata; OB: olfactory bulb; SVZ: subvenventricular zone; Tel: telencephalon; TeO: optic tectum; Vv: ventral nucleus of ventral telencephalic area.
The expression of NTs by local cells in the rodent subventricular zone niche is low, although some ligands such as BDNF can be detected by immunohistochemistry (Galvao et al., 2008). In addition, among several NTs expressed in type B-cells of the choroid plexus, NT4 was the most abundant and NT3 is also released by choroid plexus capillaries and by endothelial cells (Delgado et al., 2014). In primates, NGF and BDNF are expressed in subventricular zone by astrocytes while NT3 is found in ependymal cells (Tonchev, 2011). BDNF is mainly detected in the olfactory bulbs and rostral migratory stream, where BDNF is synthesized by the endothelial cells of blood vessels that outline the migratory stream for new neurons (Snapyan et al., 2009).
The expression pattern of NTs and their receptors and the neurogenic stem niches of adult zebrafish brain: Adult zebrafish brain possess intense adult neurogenesis, mainly localized in 16 neurogenic niches, often lining ventricula (Figure 1) (Grandel et al., 2006). Among these stem niches, BDNF expressing neurons were seen in the periventricular grey zone of optic tect and near the diencephalic ventricle (Cacialli et al., 2016). Also in other teleost fish, European eel and Nothobranchius furzeri (D’Angelo et al., 2014) BDNF mRNA was reported in the periventricular areas of diencephalon and mesencephalon. NGF mRNA and protein were detected in adult zebrafish brain in neurons dorsally and ventrally lining the telencephalic ventricle and along the ventral zone of periventricular hypothalamus. Positive cells to aromatase (glial cell marker) and proliferative nuclear antigen, as expected distributed along telencephalic and diencephalic ventricles, were very closely intermingled with NGF-positive neurons (Cacialli et al., 2019). TrkA (NGF receptor) and TrkB (BDNF receptor) were immunohistochemically detected in the midline of cerebellar corpus (Gatta et al., 2016). This site is a cerebellar staminal niche, correspondent to a protrusion of granular cell layer, where progenitor cells are interspersed with radial glia. These progenitors differentiate into Bergmann glia‐like cells and granule cell precursors. These latter enter the granular cell layer by two ways: along radial glial fibers at the midline, and ventro-laterally through the molecular layer. The presence of TrkA and TrkB receptors in this structure might indicate the sensitiveness of this area of proliferation to neurotrophin action. Finally, preliminary data indicate the presence of TrkA mRNA in other two neurogenic regions: lining of the dorsal telencephalon and periventricular gray zone of optic tectum (unpublished data).
The role of NTs and their receptors in regenerative processes: The high regenerative properties of the zebrafish brain can be correlated to the continuous neurogenesis present in the numerous stem niches. Profound traumatic brain injury on zebrafish encephalon is fast repaired with full recovery of locomotion and normal behavior. BDNF mRNA, after traumatic brain injury on telencephalon of adult zebrafish, increased not only in mature, but also in newborn neurons of the lesioned area. This increase of BDNF in neurons of the lesion was generally maintained up to 15 days post-injury (Cacialli et al., 2018). The use of a selective non-competitive small molecule antagonist of the BDNF receptor TrkB (ANA-12), exerted a reduction of proliferative nuclear antigen in zebrafish brain after injury (Anand and Mondal, 2018). All these data suggest that BDNF-TrkB signaling might be involved in the regeneration response. Furthermore, levels of NGF and its receptor TrkA, according to preliminary unpublished data obtained by qPCR, increase after 2 week post-injury, later than BDNF-TrkB pathway. Thus, it can be assumed that regenerative processes could be timely regulated by different NTs/Trks pathways.
Conclusion: The presence of NGF and BDNF and their Trks receptors in stem niches zones, although not present in proliferative cells and/or early differentiating neurons, suggests the involvement of these neurotrophic factors in adult neurogenesis. Furthermore, regenerative processes after injury also seem to be regulated by their timely alternate influence. More functional studies generating knock-out zebrafish are clearly needed to fully understand the link between neurotrophic pathways and other factors in neurogenic niches.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Anand SK, Mondal AC. Cellular and molecular attributes of neural stem cell niches in adult zebrafish brain. Dev Neurobiol. 2017;77:1188–1205. doi: 10.1002/dneu.22508. [DOI] [PubMed] [Google Scholar]
- 2.Cacialli P, D’Angelo L, Kah O, Coumailleau P, Gueguen MM, Pellegrini E, Lucini C. Neuronal expression of brain derived neurotrophic factor in the injured telencephalon of adult zebrafish. J Comp Neurol. 2018;526:569–582. doi: 10.1002/cne.24352. [DOI] [PubMed] [Google Scholar]
- 3.Cacialli P, Gatta C, D’Angelo L, Leggieri A, Palladino A, de Girolamo P, Pellegrini E, Lucini C. Nerve growth factor is expressed and stored in central neurons of adult zebrafish. J Anat. 2019 doi: 10.1111/joa.12986. doi:101111/joa12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cacialli P, Gueguen MM, Coumailleau P, D'Angelo L, Kah O, Lucini C, Pellegrini E. BDNF expression in larval and adult zebrafish brain: distribution and cell identification. PLoS One. 2016;11:e0158057. doi: 10.1371/journal.pone.0158057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Angelo L, De Girolamo P, Lucini C, Terzibasi ET, Baumgart M, Castaldo L, Cellerino A. Brain-derived neurotrophic factor: mRNA expression and protein distribution in the brain of the teleost Nothobranchius furzeri. J Comp Neurol. 2014;522:1004–1030. doi: 10.1002/cne.23457. [DOI] [PubMed] [Google Scholar]
- 6.Delgado AC, Ferrón SR, Vicente D, Porlan E, Perez-Villalba A, Trujillo CM, D’Ocón P, Fariñas I. Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron. 2014;83:572–585. doi: 10.1016/j.neuron.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Galvão RP, Garcia-Verdugo JM, Alvarez-Buylla A. Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J Neurosci. 2008;28:13368–13383. doi: 10.1523/JNEUROSCI.2918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gatta C, Altamura G, Avallone L, Castaldo L, Corteggio A, D’Angelo L, de Girolamo P, Lucini C. Neurotrophins and their Trk-receptors in the cerebellum of zebrafish. J Morphol. 2016;277:725–736. doi: 10.1002/jmor.20530. [DOI] [PubMed] [Google Scholar]
- 9.Grandel H, Kaslin J, Ganz J, Wenzel I, Brand M. Neural stem cells and neurogenesis in the adult zebrafish brain: Origin, proliferation dynamics, migration and cell fate. Dev Biol. 2006;295:263–277. doi: 10.1016/j.ydbio.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 10.Leggieri A, Attanasio C, Palladino A, Cellerino A, Lucini C, Paolucci M, Terzibasi Tozzini E, de Girolamo P, D’Angelo L. Identification and expression of neurotrophin-6 in the brain of nothobranchius furzeri: one more piece in neurotrophin research. J Clin Med. 2019 doi: 10.3390/jcm8050595. doi:103390/jcm8050595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucini C, D’Angelo L, Cacialli P, Palladino A, de Girolamo P. BDNF, brain, and regeneration: insights from zebrafish. Int J Mol Sci. 2018 doi: 10.3390/ijms19103155. doi: 10.3390/ijms19103155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Götz M, Barker PA, Parent A, Saghatelyan A. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]