Abstract
Background:
Considered the increasing rate of cardiovascular diseases (CVDs) and a positive relationship between prevalence of CVDs and obesity, the goal of the present study was to investigate the effects of green tea supplement and high-intensity interval training (HIIT) on lipid panel, fibrinogen, and maximal oxygen consumption (VO2max) in overweight women.
Materials and Methods:
In this randomized placebo-controlled clinical trial, 30 overweight women (age range, 20–30 years), were chosen purposefully and randomly divided into three equal groups (green tea, HIIT + green tea, and HIIT + placebo), and they trained HIIT workouts for 10 weeks (40-m maximal shuttle run) and used 500 mg/daily green tea or placebo tablets. Serum levels of low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglyceride (TG), and plasma level of fibrinogen were assessed before and after the intervention in fasting state. To test the hypothesis of the research, Paired t-test, Wilcoxon signed-rank test, analysis of covariance, and Tukey's post hoc tests were used at the significance level of P ≤ 0.05.
Results:
After 10 weeks, TG, LDL, weight, fibrinogen, and body fat percentage decreased in all groups (P ≤ 0.05). Further, HDL (P = 0.012) and VO2max (P = 0.007) significantly increased in HIIT + green tea and HIIT + placebo groups; while in the green tea group, HDL (P = 0.06) and VO2max (P = 0.06) showed no significant difference for within group differences. Average between-group variations of all indicators were statistically significant, and they were more meaningfully pronounced in HIIT + green tea group than the other two groups (P ≤ 0.05).
Conclusion:
Based on the findings, the combination of HIIT and green tea consumption significantly leads to a reduction in weight, body fat percentage, fibrinogen, TG, and LDL while improves VO2max and HDL levels rather than green tea consumption or performing training alone, in overweight women. However, it seems that exercise training has a vital role in the improvement of mentioned variables according to percentage changes.
Keywords: Catechin, fibrinogen, high-intensity interval training, lipid panel, overweight
INTRODUCTION
Risk factors for cardiovascular disease (CVD) are particular habits, circumstances, or conditions that increase a person's risk of developing CVD, including lack of exercise, unhealthy lifestyle, overweight and obesity, diabetes and age.[1] Overweight and obesity may be associated with hyperlipidemia (refers to elevated low-density lipoprotein (LDL), elevated triglyceride (TG) or both) and elevated levels of fibrinogen, all of which increase the risk of CVD affairs.[1,2] In this regard, the results of previous researches showed that only with 1% reduction of LDL, the risk of CVD reduces by 2%.[3] While 1 g/l increase in fibrinogen, may increase the risk of these diseases by 1.8 times.[4] Furthermore, plasma fibrinogen levels have a positive correlation with blood lipids and body mass index (BMI) and a negative correlation with training and maximal oxygen consumption (VO2 max).[5,6] Nowadays, scientific evidence remind us that exercise with different intensity and various schedules has a significant effect on preventing and controlling CVDs.[3] Most of the previous protocols included relatively low to medium intensities; while recent studies, involving high-intensity interval training (HIIT). It has been shown that continuous moderate exercise is more effective on improving physical fitness and cardiovascular health than HIIT;[7] while it takes less time. In this regard, Paoli et al. reported a significant decrease in TG, LDL, and an increase in high-density lipoprotein (HDL) after 12 weeks of HIIT workouts with 75% heart rate (HR) reserved.[8] However, in some studies, there are no reports of changes in these indices after HIIT workouts.[9,10,11,12] Lifestyle modifications such as increasing physical activity and consumption of pharmaceutical supplements, simultaneously as well as natural supplements are the most common solutions to reduce and control diseases.[13] Green tea contains a high percentage of a polyphenol called catechin, and Epigallo Catechin Gallate (EGCG) that is considered as the most notable kind of Catechin, has a high antioxidant and anti-inflammatory properties and helps in reducing risks of CVDs.[13] Maki et al. showed that EGCG existing in green tea reduces fibrinogen and TG, increases metabolism, and regulates HDL, all by reducing LDL oxidation, controlling the proliferation of smooth vascular muscle cells, and absorbing cholesterol.[14] However, no change in lipid panel and fibrinogen after consumption of green tea has been reported elsewhere.[15]
According to the latest report by the WHO, over-weight and obesity are one of the five primary causes of death worldwide. On the other hand, the silent epidemic of overweight and obesity is spreading globally, and compared to 1980, the prevalence of obesity has more than doubled in the world. One of the most important reports on the status of obesity in Iran shows that during the years 1999–2007, the prevalence of obesity in people 25–64 years of age increased from 13.6% to 22.3%.[16] Furthermore, obesity and overweight has been shown to be twice as prevalent in Iranian women as in men.[16] Very few studies have dealt with the combined effect of exercises and green tea consumption on cardiovascular risk indices; in addition, green tea extract or green tea drink or another green tea serving have been explored, thus this study evaluated changes in cardiovascular risk factors, body composition, and maximum aerobic power (VO2max) after 10 weeks of HIIT (field type) with green tea tablets in overweight women.
MATERIALS AND METHODS
Study population
This randomized placebo-controlled study conducted at Birjand University. The study was approved by the Ethics Committee of Birjand University of medical sciences (Iran) (Ir. bums. 1394.312), and the Iranian Registry of Clinical Trials code is (www.irct.ir; IRCT2015121425524N1). We included thirty overweight female students aged between 20 and 30 years, according to MedCalc Statistical Software version 14.8.1 (MedCalc, Ostend, Belgium). This software was used to determine the standard sample size in each group. The sample size was determined based on the main and important variable of the study, “fibrinogen,” based on 80% power, type one error is equal to 5%, and number of samples in each group of 10 people and a total of 30 people were calculated. All the participants were randomly divided into three groups based on simple randomization procedures.
The inclusion criteria were age 20–30 years, BMI ≥25 kg/m2, and having no history of a specific disease, nonuse of medications and antioxidant supplements before the study, no history of physical activity prior the study has begun. Participants were excluded from the study if the following criteria were met: not attending regular exercises during the intervention, taking hormonal tablets, or supplements, traveling, and pregnancy. None of the participants were excluded from the statistical population considering these criteria [Figure 1].
Figure 1.
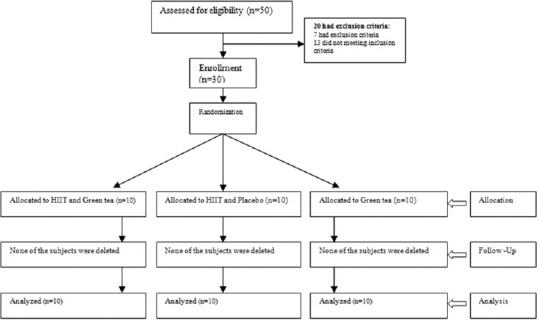
CONSORT flow diagram of the study
Intervention
The training protocol consists of 40-m maximal shuttle run test[12] for 10 weeks and 3 days a week, as shown in Table 1, with 85%–95% of maximum heart rate (HRmax = 220-age), which was controlled by a Polar Xtrainer Plus Watch. During the protocol, all of the participants had the same diet (university restaurant). In addition, they were asked not to take any antioxidant property (black tea, coffee, nonalcoholic beer, fruit juice, or any tablets or drug supplements), and avoid performing severe physical activity. The diet controlled by a questionnaire in the first and last 3 days of the research period. Then, macro, micronutrients, and calories calculated According to Guide to Calculate the Nutritional Value Regimes in Iran, book.[17] There was found that no significant difference between the received calories and the role of macronutrients in the three studied groups, in the first and last weeks.[17] Moreover, the last meal calorie intake was balanced to 500–600 calories for all participants.
Table 1.
High intensity interval exercise protocol
| Weeks | Activity time (s) | Active relaxation time (s) | Intervals | Protocol length of time (min) | Total activity time (main activity, warming-up, and cooling-down) (min) | |
|---|---|---|---|---|---|---|
| 40 m shuttle run protocol | The first and the second | 30 | 30 | 4 | 4 | 24 |
| The third and the fourth | 30 | 30 | 5 | 5 | 25 | |
| The fifth and the sixth | 30 | 30 | 6 | 6 | 26 | |
| The seventh and the eighth | 30 | 30 | 7 | 7 | 27 | |
| The ninth and the tenth | 30 | 30 | 8 | 8 | 28 |
Supplement consumption
Participants in green tea and HIIT+ green tea groups consumed green tea tablets (10 weeks, 3 times/day at morning, noon, and night) 2 h after their meal. In addition, HIIT workouts + placebo group participants had for 10 weeks three tablets a day the same as the two other groups, each containing 500 mg starch powder with similar to green tea tablets. It is worth noting that green tea group had no sport activities in 10 weeks, but the two other groups performed HIIT workouts similarly to each other. Furthermore, to raise precision of the test and make sure of the prescribed consumption dose, we used green tea tablets with a certain amount of catechin in this study. Each 500 mg green tea tablet consisted of 300 mg of Catechin (each 500 mg tablet contained ~294 mg EGCG, 74 mg EGC, 20.7 mg EC, 51.2 mg ECG, and 58.1 mg Caffeine). These tablets were the product of Dineh Iran Pharmaceutical Company with a product code of 1228144011.
Sample collection and biochemical determination
Participants' weight, body fat percentage, VO2max, serum levels of TG, HDL, LDL, and plasma level of fibrinogen assessed 24 h before the beginning of the training program and 48 h after the last training session and intake of the tablets. The participants' weight was assessed by a digital scale (TCM, China) with a precision <100 g and skinfolds were measured using Saehan Medical Skinfold Caliper (SH5020, England) Exerting 10 g/mil according to Pollock three sites methodology and percentage of body fat was extracted from formula.[18] In addition, VO2max was assessed on treadmill (Cosmos T, Cos10199, h-p-150 model) performing Maximum Bruce Test and the following formula:[19] VO2max = (4.38 × Total exercised time)–3.9. The normal range of VO2max for healthy women (20–30 years) is 33–36.9 (ml/kg/min). To analyze the concentration of LDL, TG, and HDL, enzymatic method, using Pars Azmoon diagnostic kit and biochemistry autoanalyzer performed and to gauge plasma fibrinogen, coagulation method, and Mahsa-Yaran company special kit were implemented. Participants asked not to have any strenuous physical activity 48-h before blood sampling [Figure 2]. Five milliliters of blood taken after12 h fasting from brachial vein, and blood samples collected in tubes containing sodium citrate for plasma separation, and in nonanticoagulant tubes for separating serum, they kept at −80°C for future analyzing.
Figure 2.
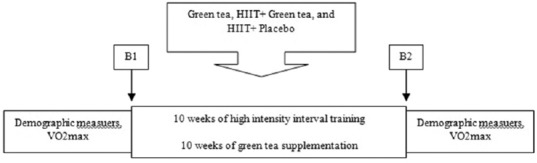
Schematic Illustration of the Experimental Design. B1 = blood sampling pretraining; B2 = blood sampling at posttraining
Statistical analysis
Data were analyzed using SPSS (Statistical Package for the Social Sciences) version 19 software (SPSS Inc., Chicago, IL, USA). The data are expressed as mean ± standard deviation or median (25th, 75th) percentiles. After assessing the normality and nonnormality of data by Shapiro–Wilk test, differences between the baseline means of the three groups in all variables were analyzed using one-way analysis of variance (ANOVA). Within-group analysis assessed by paired t-test for normally distributed data or Wilcoxon signed-rank test for nonnormal distributed data (VO2max and HDL) and between-group differences were examined using analysis of covariance (ANCOVA) through controlling before intervention values; for pairwise comparisons, Tukey post hoc test was used. A two-sided P ≤ 0.05 was considered statistically significant.
RESULTS
Results of one-way ANOVA test show that at baseline, there are no statistically significant differences between groups for demographic characteristics subjects of all three groups [Table 2].
Table 2.
Baseline comparison of demographic characteristics
| Variables | Mean±SD | P (ANOVA) | ||
|---|---|---|---|---|
| Training + green tea (n=10) | Training + placebo (n=10) | Green tea (n=10) | ||
| Age (years) | 22.47±3.32 | 23.58±2.23 | 21.06±2.65 | 0.87 |
| Height (cm) | 160±0.12 | 161.04±0.04 | 160±0.35 | 0.94 |
| BMI (kg/m2) | 27.15±1.47 | 27.32±1.27 | 28.03±1.04 | 0.06 |
BMI=Body mass index; ANOVA=Analysis of variance; SD=Standard deviation
Within-group comparisons showed that the levels of TG (P = 0.001, P = 0.03, P = 0.02 respectively), LDL (P = 0.006, P = 0.01, P = 0.04 respectively), fibrinogen (P = 0.03, P = 0.02, P = 0.04 respectively), body fat percentage (P = 0.001, P = 0.001, P = 0.04, respectively), and weight (P = 0.001, P = 0.03, P = 0.04 respectively) reduce in HIIT + green tea, HIIT + placebo, green tea groups; while, HDL (P = 0.012, P = 0.012 respectively) and VO2max (P = 0.007, P = 0.007 respectively) increased significantly in “HIIT + green tea” and “HIIT + placebo” groups.
Statistical comparison of data by ANCOVA [Table 3] shows a significant difference in mean changes of HDL, LDL, TG, fibrinogen, weight, body fat, and VO2max between three groups (P ≤ 0.05). Based on the results of Tukey post hoc test “HIIT + green tea” group showed a greater reduction rather than the “HIIT + placebo” and “green tea” groups in the indicators TG (P = 0.04, P = 0.001 respectively), LDL (P = 0.03, P = 0.002 respectively), fibrinogen (P = 0.001, P = 0.001 respectively), body fat (P = 0.001, P = 0.001 respectively), and weight (P = 0.001, P = 0.001 respectively). In addition, the diminution in “HIIT + placebo” was more than “green tea” group in TG (P = 0.04), body fat (P = 0.001), and weight (P = 0.047); while in LDL index despite the decrease, this decline was not significant (P = 0.31). Furthermore, increasing HDL (P = 0.01, P = 0.001 respectively) and VO2max (P = 0.02, P = 0.03 respectively) in “HIIT + green tea” group were more than “HIIT + placebo” and “green tea” groups. In addition, the levels of HDL (P = 0.002) and VO2max (P = 0.032) were significantly higher in “HIIT + placebo” rather than the “green tea” group.
Table 3.
Comparison of fat percentage, weight, maximal oxygen consumption, and biochemical mean changes in three studied groups after 10 weeks of green tea administration and high intensity interval training
| Variables | Groups | Measurement time | Within group (P) | Difference in change post-pre (%) | Between group (P) | Groups comparisons | Mean difference±SE | Tukey (P) | |
|---|---|---|---|---|---|---|---|---|---|
| Pretest | Posttest | ||||||||
| TG (mg/dl) | Training + green tea | 140.03±46.67 | 103.38±41.98 | 0.001*** | −26.17 | 0.001† | Training + green tea versus training + placebo | −12.16±1.08 | 0.04* |
| Training + placebo | 119.41±40.53 | 94.92±36.27 | 0.03* | −20.50 | Training + green tea versus green tea | −19.22±1.32 | 0.001* | ||
| Green tea | 135.69±56.07 | 118.26±32.14 | 0.02* | −12.84 | Training + placebo versus green tea | −7.06±0.65 | 0.04* | ||
| LDL (mg/dl) | Training + green tea | 104.12±19.42 | 89.32±18.52 | 0.006** | −14.21 | 0.02† | Training + green tea versus training + placebo | −4.82±0.11 | 0.03* |
| Training + placebo | 99.71±18.16 | 89.73±19.34 | 0.01** | −10.09 | Training + green tea versus green tea | −5.51±0.12 | 0.002* | ||
| Green tea | 113.53±24.36 | 104.24±20.92 | 0.04* | −8.18 | Training + placebo versus green tea | −0.69±0.05 | 0.31 | ||
| HDL (mg/dl)# | Training + green tea | 55 (42.25-60.75) | 63.5 (53.5-6 9.75) | 0.012** | 21.85 | 0.01†† | Training + green tea versus training + placebo | −2.16±0.23 | 0.01* |
| Training + placebo | 46.5 (39-60.25) | 58 (49-68.75) | 0.012** | 19.14 | Training + green tea versus green tea | −6.31±0.12 | 0.001* | ||
| Green tea | 44.5 (39.25-58) | 50 (48.5-62) | 0.06 | 10.66 | Training + placebo versus green tea | −4.15±0.34 | 0.002* | ||
| Fibrinogen (mg/dl) | Training + green tea | 233.50±19.30 | 201.40±20.20 | 0.03* | −13.74 | 0.001† | Training + green tea versus training + placebo | −4.71±0.34 | 0.001* |
| Training + placebo | 240.60±21.30 | 217.40±19.60 | 0.02* | −9.64 | Training + green tea versus green tea | −5.94±0.64 | 0.001* | ||
| Green tea | 238.40±18.90 | 212.20±17.70 | 0.04* | −10.98 | Training + placebo versus green tea | −1.01±0.01 | 0.99 | ||
| Fat percentage (%) | Training + green tea | 34.12±1.80 | 27.12±1.45 | 0.001*** | −20.51 | 0.001† | Training + green tea versus training + placebo | −3.01±0.76 | 0.001* |
| Training + placebo | 33.57±1.39 | 29.57±1.90 | 0.001*** | −11.91 | Training + green tea versus green tea | −5.14±0.35 | 0.001* | ||
| Green tea | 34.28±1.38 | 32.42±1.27 | 0.04* | −5.42 | Training + placebo versus green tea | −2.14±0.05 | 0.001* | ||
| Weight (kg) | Training + green tea | 70.56±6.19 | 65.83±6.27 | 0.001*** | −6.70 | 0.001† | Training + green tea versus training + placebo | −2.42±0.55 | 0.001* |
| Training + placebo | 72.18±3.51 | 69.88±3.79 | 0.03* | −3.18 | Training + green tea versus green tea | −3.22±0.32 | 0.001* | ||
| Green tea | 73.45±8.44 | 71.95±6.37 | 0.04* | −2.04 | Training + placebo versus green tea | −0.80±0.03 | 0.047* | ||
| VO2 max (ml/kg/min)# | Training + green tea | 24 (20.25-27.75) | 29 (25.5-30.75) | 0.007** | 15.91 | 0.001† | Training + green tea versus training + placebo | −2.19±0.43 | 0.02* |
| Training + placebo | 25.5 (18.25-27.75) | 27.5 (20.75-29.75) | 0.007** | 7.62 | Training + green tea versus green tea | −2.16±0.09 | 0.03* | ||
| Green tea | 26.5 (18.25-28) | 28 (20-30) | 0.06 | 7.5 | Training + placebo versus green tea | −0.43±0.05 | 0.032* | ||
*P≤0.05; **P≤0.01; ***P≤0.001significant difference compared to pretest; †P≤0.05 significant difference between three groups. Within group P value: Paired t-test or Wilcoxon signed-ranks test, Between group P value: ANCOVA; #Presented as medians (25th-75th) percentiles and all others were presented as means±SDs. SE=Standard error; VO2 max=Maximal oxygen consumption; TG=Triglyceride; HDL=High-density lipoprotein; LDL=Low-density lipoprotein; ANCOVA=Analysis of covariance; SDs=standard deviations
DISCUSSION
The result of this study showed that 10 weeks of HIIT workouts and green tea supplementary resulted in a significant decrease of LDL, TG, fibrinogen, and a significant increase of HDL. The findings of a research by Paoli et al. showed a significant decrease of LDL, TG, and a significant increase in HDL after 12 weeks of HIIT workouts with 75% reserved HR in 58 subjects, middle-aged men and women. Findings of Biskey showed that the plasma level of fibrinogen and body fat percentage decreased in 16 healthy young men, after 2 weeks of HIIT workouts with 100% of peak power output. Findings of Biskey showed that the plasma level of fibrinogen and body fat percentage decreased in 16 healthy young men, after 2 weeks of HIIT workouts with 100% of peak power output.[20]
Rezaeimanesh et al. reported that 8 weeks of anaerobic interval training in athlete students leads to a significant decrease in plasma fibrinogen concentration.[21] These scholars believed that the reason may be through controlling the production of glycoprotein of the liver after relevant trainings. They also noted that training causes a reduction of inflammation through reducing the production of cytokines in adipose tissue, increased sensitivity to insulin and losing weight. Probably, fibrinogen synthesis reduction in liver cells relates to musculoskeletal system compared to HIIT workouts, that cytokines activities such as interleukin-1 may be reduced.[22] Some researchers also showed that interleukin-1 responses attenuation parallel to improvement in physical fitness level.[23] Hence, it is likely to assume that after doing a regular course of HIIT workouts, production of cytokines such as interleukin-1 will be reduced, which in turn may be influential in reducing fibrinogen derived from liver synthesis.[24] On the other hand, it is obvious that fibrinogen has a direct link with LDL increment and subcutaneous adipose tissue content and an inverse association with HDL. Hence, probably a reduction of LDL and body fat percentage and an increase in HDL as a result of exercise (as seen in subjects of the present study) can be the reasons for the improvement in fibrinogen.[25] Despite mentioned issues, Elmer, reported no significant effect on lipid panel in 12 young men after 8 weeks of HIIT workouts (70%–80% VO2max, 3 sessions/week) and noted that intensity and duration of training are ineffective on these indices. Probably, the contradiction may be due to the type of participants and intensity of the trainings implemented (overweight women and 85%–95% HRmax). We suggested beneficial effects on different types of lipoproteins and fibrinogen has a significant correlation with high intensity of the training,; as well as, more duration and higher intensity of training with a consumption of supplements leads to an improvement in health level.[25] In the present study, we used HIIT as a training intervention, and it seems that its high-intensity nature has led to desirable changes in lipid panel and participant's fibrinogen.
Regarding the positive effect of green tea on lipid panel and fibrinogen, our findings were consistent with other studies that showed a significant improvement in LDL, TG and increase in HDL in diabetic rats after 5 weeks of aerobic exercise + green tea.[26] They believed EpigalloCatechin existing in green tea inhibits mass absorption, cholesterol excretion, total lipid, and LDL serum increase through stimulating LDL receptors activity. In addition, Vinson et al. found significant fibrinogen's decrease in rats with normal diet along with 2 weeks of green tea consumption.[27] In contrast, Nagao et al. did not observe any change in lipid panel and fibrinogen indices after 12 weeks of green tea catechin consumption (690 mg/day) in 35 healthy men.[15] These scholars believed that low dosage of catechin consumed may be the cause of observed results. The contradiction for the above results may relate to different green tea consumption ways (drink vs. tablet) and different kind of participants (healthy vs. overweight women). The short but high intensity of implemented training in the present study takes too much time while the effective strategy for improving the lipid panel and fibrinogen approved in this research. As we explained earlier, training is the main factor of lipid panel improvement and fibrinogen decrease, and green tea supplementary has beneficially affected. The current study showed that green tea along with training can be more effective in improving cardiovascular risk factors. However, in the current study, HDL index in green tea group had no significant change, while in HIIT+ placebo, HDL had a significant increase. The probably reason may be insufficient dosage of green tea. Indeed, HDL unaffected to antioxidant supplementary. Furthermore, we may conclude that training is probably a more effective factor on HDL changes than supplementary consumption alone. Furthermore, we can say that probably little number of samples of green tea group in this study is one of the main factors for the mentioned index to be insignificant. Nevertheless, future studies should consider more samples to analyze the effectiveness of green tea supplementary.
One of the other results of this study was participants' significant weight loss and decreased body fat percentage after 10 weeks of HIIT + green tea. On the other hand, the amount of the weight and body fat percentage lost in green tea + HIIT group was more significant than the other two groups. This finding indicates that the effect of training along with green tea consumption makes getting the most results on weight and body fat percentage possible. Results of some studies were in line with the present study. In this regard, 12 weeks of HIIT workouts in 15 overweight young women resulted in a significant decrease in weight and body fat percentage.[28] Haghighi et al. also analyzed the effect of 10 weeks of aerobic exercise both along with and without green tea (6 g/day dried tea) on body compositions of 20 overweight men. Participants were divided into two groups of HIIT + supplementary and training alone. Results showed that there is no significant difference in amounts of weight and body fat percentage in the two groups' participants.[11] The probable reason behind the contradiction of the aforementioned research results with the current study is consumption ways (drink vs. tablet) and difference in protocol (aerobic exercise with an intensity of 65%–75% HRmax vs. HIIT with 85%–95% HRmax). In this research, weight and body fat percentage decreased significantly after 10 weeks of training and supplementary consumption, and probably this improvement of body composition can lead to a decrease of fibrinogen, LDL, TG and an increase of HDL and VO2max indices in overweight women. In addition, with regards to markedly higher level of HDL and lower levels of LDL, TG, fibrinogen, body fat percentage, and weight in HIIT + green tea group compared to the other two groups, we may consider the role of green tea as a powerful antioxidant supplementary along with HIIT workouts, in controlling obesity, overweight, and emergence of cardiometabolic risk factors. Catechins available in green tea with an inhibitory influence on phospholipase A2 causes a decrease in lipid absorption, and lipogenes is inhibition by controlling synthetic fatty acid gene transcription and acetyl CoA carboxylase, and an increase of fat oxidation, capillary network function, and mitochondrial density and finally leads to an increase in VO2max and a decrease in fat weight.[13,29] On the other hand, it seems that existing catechins in green tea bring about an inhibition of LDL oxidation by copper sulfate, and cholesterol synthesis. The other possible mechanism for LDL decrease relates to the interference of micelles of cholesterol in the digestive system through the formation of insoluble cholesterol, results in cholesterol excretion through feces then the inhibition of cholesterol absorption occurs. On the other hand, the inhibition of LDL oxidation and fibrinogen binding, through the epigallo catechin existing in green tea, leads to decrease its functionality and increase anxiolytic activity in serum, eventually attenuate fibrinogen and improve HDL levels,[29] of course it seems that the combination of supplementary and physical activity, accelerates the result.[30] We suggest further studies with more number of samples and more assessment to conclude whether the positive effects of training with green tea will remain or not. Furthermore, this study lacks a “placebo group” required investigations. In addition, while double-blinding condition was not performed in the present study, it should be considered in future probes.
CONCLUSION
The primary result of the study represents, although consumption of green tea (1500 mg/day) leads to improvement in lipid panel and fibrinogen level of overweight women, it seems that green tea consumption along with a 10-week HIIT has a better effect on these indices, and we can recommend it as a secondary result that nonathlete healthy people can take green tea and perform HIIT workouts to improve heart function, body composition, and increase VO2max health level.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to appreciate all participants who took part in this project and Dineh Iran Company for providing ginger tablets and placebos. The manuscript ethics approval number is ir.bums.1394.312.
REFERENCES
- 1.Siavash M, Naseri M, Rahimi M. Arnebia euchroma ointment can reduce abdominal fat thickness and abdominal circumference of overweight women: A randomized controlled study. J Res Med Sci. 2016;21:63. doi: 10.4103/1735-1995.187347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akil L, Ahmad HA. Relationships between obesity and cardiovascular diseases in four southern states and Colorado. J Health Care Poor Underserved. 2011;22:61–72. doi: 10.1353/hpu.2011.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lira FS, Yamashita AS, Uchida MC, Zanchi NE, Gualano B, Martins E, Jr, et al. Low and moderate, rather than high intensity strength exercise induces benefit regarding plasma lipid profile. Diabetol Metab Syndr. 2010;2:31. doi: 10.1186/1758-5996-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furukawa F, Kazuma K, Kojima M, Kusukawa R. Effects of an off-site walking program on fibrinogen and exercise energy expenditure in women. Asian Nurs Res (Korean Soc Nurs Sci) 2008;2:35–45. doi: 10.1016/S1976-1317(08)60027-4. [DOI] [PubMed] [Google Scholar]
- 5.Freitas RN, Luben R, Wareham NJ, Khaw KT. Relationship between plasma fibrinogen and fiber intake in the EPIC-Norfolk cohort. Eur J Clin Nutr. 2012;66:443–51. doi: 10.1038/ejcn.2011.194. [DOI] [PubMed] [Google Scholar]
- 6.Nazar Ali P, Hanachi P. To investigate the fibrinogen and some of coagulation factors in anaerobic exercise training women. World Appl Sci J. 2011;12:72–5. [Google Scholar]
- 7.Hwang CL, Wu YT, Chou CH. Effect of aerobic interval training on exercise capacity and metabolic risk factors in people with cardiometabolic disorders: A meta-analysis. J Cardiopulm Rehabil Prev. 2011;31:378–85. doi: 10.1097/HCR.0b013e31822f16cb. [DOI] [PubMed] [Google Scholar]
- 8.Paoli A, Pacelli QF, Moro T, Marcolin G, Neri M, Battaglia G, et al. Effects of high-intensity circuit training, low-intensity circuit training and endurance training on blood pressure and lipoproteins in middle-aged overweight men. Lipids Health Dis. 2013;12:131. doi: 10.1186/1476-511X-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugiarto D, Andriati A, Laswati H, Kimura H. Comparison of the increase of both muscle strength and hypertrophy of biceps brachii muscle in strengthening exercise with lowintensity resistance training with and without the application of blood flow restriction and high-intensity resistance training. J Bali Med. 2017;6:251–7. [Google Scholar]
- 10.Elmer D. Effect of 8 Weeks of High-Intensity Interval Training Versus Traditional Endurance Training on the Blood Lipid Profile in Humans. A Thesis of PhD; 2013 [Google Scholar]
- 11.Haghighi AH, Yarahmadi H, Ildarabadi A. The effect of green tea consumption with aerobic exercise on serum adiponectin and ghrelin. Med J Mashad Univ Med Sci. 2015;57:904–12. [Google Scholar]
- 12.Nayebifar S, Afzalpour ME, Kazemi T, Eivary SH, Mogharnasi M. The effect of a 10-week high-intensity interval training and ginger consumption on inflammatory indices contributing to atherosclerosis in overweight women. J Res Med Sci. 2016;21:116. doi: 10.4103/1735-1995.193507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang HY, Yang SC, Chao JC, Chen JR. Beneficial effects of catechin-rich green tea and inulin on the body composition of overweight adults. Br J Nutr. 2012;107:749–54. doi: 10.1017/S0007114511005095. [DOI] [PubMed] [Google Scholar]
- 14.Maki KC, Reeves MS, Farmer M, Yasunaga K, Matsuo N, Katsuragi Y, et al. Green tea catechin consumption enhances exercise-induced abdominal fat loss in overweight and obese adults. J Nutr. 2009;139:264–70. doi: 10.3945/jn.108.098293. [DOI] [PubMed] [Google Scholar]
- 15.Nagao T, Komine Y, Soga S, Meguro S, Hase T, Tanaka Y, et al. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am J Clin Nutr. 2005;81:122–9. doi: 10.1093/ajcn/81.1.122. [DOI] [PubMed] [Google Scholar]
- 16.Khabazkhoob M, Emamian MH, Hashemi H, Shariati M, Fotouhi A. Prevalence of overweight and obesity in the middle-age population: A priority for the health system. Iran J Public Health. 2017;46:827–34. [PMC free article] [PubMed] [Google Scholar]
- 17.Rastmanesh R, Rabie S. Tehran: Dibaj Publication; 2011. A Guide to Calculate the Nutritional Value Regimes in Iran. [Google Scholar]
- 18.Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med Sci Sports Exerc. 1980;12:175–81. [PubMed] [Google Scholar]
- 19.Wilmore JH, Costill DL. 3rd ed. New York: Human Kinetics Publication; 2005. Physiology of Sport and Exercise. [Google Scholar]
- 20.Biskey LM. Effects of High Intensity Interval Training on Emostasis and Fibrinolysis in Healthy Males: Relationship to Sympathetic Nervous System Activation. A Thesis of Master. 2015 [Google Scholar]
- 21.Rezaeimanesh D, Amiri P, Saidian S. The effect of 8-week's anaerobic intermittent exercises on the amount of fibrinogen, CRP and VO2max in student athletes. Procedia Soc Behav Sci. 2011;30:2169–72. [Google Scholar]
- 22.Balagopal P, George D, Sweeten S, Mann KJ, Yarandi H, Mauras N, et al. Response of fractional synthesis rate (FSR) of fibrinogen, concentration of D-dimer and fibrinolytic balance to physical activity-based intervention in obese children. J Thromb Haemost. 2008;6:1296–303. doi: 10.1111/j.1538-7836.2008.03037.x. [DOI] [PubMed] [Google Scholar]
- 23.Alzahrani SH, Ajjan RA. Coagulation and fibrinolysis in diabetes. Diab Vasc Dis Res. 2010;7:260–73. doi: 10.1177/1479164110383723. [DOI] [PubMed] [Google Scholar]
- 24.Jahangard T, Torkaman G, Ghoosheh B, Hedayati M, Dibaj A. The effect of short-term aerobic training on coagulation and fibrinolytic factors in sedentary healthy postmenopausal women. Maturitas. 2009;64:223–7. doi: 10.1016/j.maturitas.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Boutcher SH. High-intensity intermittent exercise and fat loss. J Obes. 2011;2011:868305. doi: 10.1155/2011/868305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hovanloo F, Shahvali Koohshoori Y, Teimoorian M, Saadati M, Fallah Huseini H. The effect of aerobic training combined and green tea (Camellia sinensis L.) extract consumption on blood glucose and lipid profile in streptozotocin induced diabetic rats. J Med Plants. 2014;13:84–92. [Google Scholar]
- 27.Vinson JA, Teufel K, Wu N. Green and black teas inhibit atherosclerosis by lipid, antioxidant, and fibrinolytic mechanisms. J Agric Food Chem. 2004;52:3661–5. doi: 10.1021/jf035255l. [DOI] [PubMed] [Google Scholar]
- 28.Dunn SL. Effects of Exercise and Dietary Intervention on Metabolic Syndrome Markers of Inactive Premenopausal Women. A Thesis of PhD. 2009 [Google Scholar]
- 29.Hodgson AB, Randell RK, Boon N, Garczarek U, Mela DJ, Jeukendrup AE, et al. Metabolic response to green tea extract during rest and moderate-intensity exercise. J Nutr Biochem. 2013;24:325–34. doi: 10.1016/j.jnutbio.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Afzalpour ME, Ghasemi E, Zarban AM. Effects of an intensive resistance training session and green tea supplementation on malondialdehyde and total thiol in non-athletes women. Zahedan J Res Med Sci. 2014;16:59–63. [Google Scholar]


