Abstract
Botrytis cinerea, a major phytopathogenic fungus, has been reported to infect more than 200 crop species worldwide, and it causes massive losses in yield. The aim of this study was to evaluate the inhibitory abilities and effects of Bacillus amyloliquefaciens RS-25, Bacillus licheniformis MG-4, Bacillus subtilis Z-14, and Bacillus subtilis Pnf-4 and their culture filtrates and extracts against the gray mold caused by B. cinerea on postharvest tomato, strawberry, and grapefruit. The results revealed that the cells of Z-14, culture filtrate of RS-25, and cells of Z-14 showed the strongest biocontrol activity against the gray mold on the strawberry, grape, and tomato fruit, respectively. All the strains produced volatile organic compounds (VOCs), and the VOCs of Pnf-4 displayed the highest inhibition values. Based on headspace solid-phase microextraction in combination with gas chromatography-mass spectrometry, esters accounted for the largest percentage of the VOCs produced by RS-25, MG-4, Z-14, and Pnf-4 (36.80%, 29.58%, 30.78%, and 36.26%, respectively). All the strains showed potent cellulase and protease activities, but no chitinase activity. RS-25, Z-14, and MG-4, but not Pnf-4, grew on chrome azurol S agar, and an orange halo was formed around the colonies. All the strains showed biofilm formation, fruit colonization, and lipopeptide production, which may be the main modes of action of the antagonists against B. cinerea on the fruit. This study provides the basis for developing natural biocontrol agents against the gray mold caused by B. cinerea on postharvest fruit.
Keywords: Bacillus isolate, Botrytis cinerea, gray mold, inhibitory ability, postharvest fruit
Botrytis cinerea is a major phytopathogenic and necrotrophic fungus that causes the gray mold disease (Fekete et al., 2012). The fungus has been reported to infect more than 200 crop species worldwide, such as grapes, stone fruit, berries, and vegetables, and the losses in yield before and after harvest are extensive (Williamson et al., 2007). B. cinerea is not host-specific, and its virulence varies in different plant hosts (Derckel et al., 1999; Mirzaei et al., 2009). Because it has a broad host spectrum and causes significant economic losses, B. cinerea has been considered as the second most important fungal pathogen worldwide (Dean et al., 2012).
In particular, postharvest losses due to fungal infections are more important for highly perishable fresh fruit than for field crops. Generally, postharvest losses are attributed to pathological decomposition due to fungal and bacterial infections; pathophysiological damage during storage due to excessive cooling or lighting or abnormalities in the atmospheric gaseous components; and physical damage, such as mechanical damage (Schnaubelt, 2005).
Synthetic fungicides are commonly used to protect crops, especially fruit, in the postharvest period. However, effective and safe non-fungicides are urgently required to control postharvest pathogens because synthetic fungicide residues are toxic to humans and the environment (Conway et al., 2005; Droby, 2006; Soylu et al., 2010). Therefore, researchers worldwide are trying to develop safe, natural, and biodegradable alternatives to replace synthetic fungicides (Grzegorczyk et al., 2017; Nalinia and Parthasarathi, 2014).
Previous studies have confirmed the efficacy of biocontrol agents against several postharvest phytopathogenic fungi, such as species that belong to the genera Botrytis, Penicillium, and Monilinia (Bautista-Rosales et al., 2013; Mari et al., 2012; Parafati et al., 2015; Platania et al., 2012). Species that belong to the genus Bacillus can form endospores, which ensures the feasibility of commercial products with low storage requirements but a long shelf life (Posada et al., 2016). Thus, Bacillus strains can colonize the surface or wounds of postharvest fruit and produce broad-spectrum antibiotics that suppress a variety of plant pathogens (Stein, 2005). Bacillus subtilis V26 displays high antifungal activity against several fungi, including B. cinerea, and has powerful chitosanase activity (Kilani-Feki et al., 2016). Bacillus atrophaeus CAB-1 displays high inhibitory activity against various fungal pathogens and suppresses cucumber powdery mildew and tomato gray mold. All the compounds extracted from CAB-1 (e.g., C15–C17 fengycin A, a protein, and volatile compounds) also effectively prevented the occurrence of the cucumber powdery mildew caused by Sphaerotheca fuliginea under greenhouse conditions (Zhang et al., 2013). Bacillus cereus S42 screened from Nicotiana glauca plants native to the Tunisian Centre-East demonstrated a strong ability to suppress tomato Fusarium wilt caused by Fusarium oxysporum f. sp. lycopersici and showed proteolytic and chitinolytic activities (Abdallah et al., 2016).
In this study, we have reported the efficacy of four antagonists or their culture filtrates in controlling the gray mold caused by B. cinerea on postharvest fruit and discussed their ability to colonize the wounds on the fruit and synthesize antifungal metabolites.
Materials and Methods
Growth of bacterial isolates and production of culture filtrates
Bacterial isolate RS-25 from jujube fruit, MG-4 from strawberry fruit, Z-14 from wheat rhizosphere soil and Pnf-4 from wheat plant significantly inhibited the growth of various phytopathogenic fungi and were maintained at 4°C on streak-inoculating nutritive agar (NA) slants (Zhang et al., 2017a, 2017b). The strains were cultured overnight at 37°C on NA slants, transferred to Erlenmeyer flasks containing 50 ml of the seed culture medium, and shaken at 220 rpm for 48 h at 37°C. The vegetative cells and supernatant were obtained after centrifugation at 10,000 ×g for 15 min. The cells were suspended in sterile distilled water (SDW) and adjusted to the appropriate concentration (1 × 108 cells/ml), and the supernatant was filtered through a 0.22-μm syringe to obtain the culture filtrate.
Antifungal activities of the Bacillus isolates and their culture filtrates against B. cinerea
Using the dual-culture in vitro assay described by Kilani-Feki et al. (2016), the antifungal activities of the four isolates against B. cinerea were evaluated. A single 5-mm-diameter mycelial disc of the test fungus was placed at the center of potato dextrose agar (PDA) plates. The samples were treated with the bacterial isolates at four symmetrical sites, a distance of 3 cm from the center of the plate, and then allowed to grow for 5 days at 27°C in darkness. The antifungal activity of the culture filtrate was measured using a diffusion plate assay modified from that described by Pretorius et al. (2015). Four evenly spaced wells (7 mm in diameter) were made 2.5 cm from the center of PDA plates containing 40 μg/ml of streptomycin sulfate. A plug of B. cinerea was placed at the center of the plate, and an aliquot (50 μl) of the filtrate or sterile water (control) was added to each well. The plates were incubated at 27°C, and the inhibition zones were measured after clear halos became visible.
Extraction of lipopeptides produced by the antagonists
HCl (6 M) was used to lower the pH of the culture filtrates of the four antagonistic bacteria to 2. The filtrates were maintained overnight at 4°C and centrifuged to isolate the precipitate, which was then washed twice using diluted HCl (pH 2) and extracted twice using methanol (Zhang et al., 2017b). The extract was dried using a rotary vacuum evaporator and resuspended in an equivalent volume of sterile water to detect the biocontrol effects of the extract in controlling the gray mold caused by B. cinerea on postharvest fruit.
Efficacy of the antagonists and their culture filtrate and extracts in controlling the gray mold caused by B. cinerea on postharvest fruit
To evaluate the efficiency of the four antagonist bacteria, RS-25, MG-4, Z-14, and Pnf-4, and their culture filtrates and extracts in controlling the gray mold on the postharvest fruit of tomato, strawberry, and grape, the in vivo assay reported by Panebianco et al. (2014) and Parafati et al. (2015) was used with slight modifications. Tomato (Lycopersicon esculentum Mill. ‘Qianxi’), strawberry (Fragaria × ananassa Duch. ‘Shuangliu’), and grape (Vitis vinifera L. ‘Red Globe’) fruit were obtained from commercial orchards in Baoding, China. In this study, the tomato, strawberry, and grapefruit did not receive any preharvest fungicide treatment, and only healthy and homogenous fruit were selected. Before treatment and inoculation, the fruits were washed with tap water, superficially disinfected for 1 min in 0.1% (v/v) sodium hypochlorite, rinsed with SDW, and air-dried at room temperature.
A sterilized needle was used to make artificial wounds (0.3 cm in depth × 0.5 cm in width) at equatorial areas of the fruit (2 wounds on each fruit). A drop (10 μl; 1 × 106 conidia/ml) of B. cinerea was inoculated in each wound and air-dried for 2 h; then, another drop (10 μl; 1 × 108 cells/ml) of each bacterium (culture filtrate or extract) was added to each wound. The fruits were placed in plastic packaging cartons (5 fruit/carton) and incubated at 25°C and 95% relative humidity for 5 days. Fruits treated with SDW and infected with the pathogen were used as the negative control. Three replicates of 5 fruit each were used per treatment (15 fruit/treatment), and the experiment was repeated thrice. The disease index (DI) and disease reduction (DR) of the gray mold were calculated according to the formulas published by Gong et al. (2017). The degree of disease severity due to inoculation with B. cinerea was calculated as follows: 0 = no lesion; 1 = a few scattered lesions covering < 2% of the fruit surface; 2 = extensive lesions covering > 2% but < 5% of the fruit surface; 3 = extensive lesions covering > 5% but < 25% of the fruit surface, and 4 = extensive lesions covering > 25% of the fruit surface. The DI was calculated using the following formula:
Where i is the disease severity (0–4), and ni is the number of fruit with a severity of i. The DR was calculated using the following formula: DR (%) = 100 × (DI-Bc − DI-test)/DI-Bc. Where Bc is B. cinerea.
In vitro production of volatile organic compounds
A dual-culture method was used to assess the efficacy of volatile organic compounds (VOCs) produced by the antagonists in decreasing the growth of B. cinerea (Gao et al., 2017; Grzegorczyk et al., 2017). Twenty-microliter aliquots of the bacterial suspensions (108 cells/ml) were seeded on NA plates and incubated at 37°C for 24 h. Then, discs (5-mm circular plugs) of actively growing fungal mycelia of B. cinerea were placed at the center of Petri dishes with PDA. Each pathogen plate was covered with a plate containing a bacterial culture incubated for 24 h. Three plates were used for each antagonist, and unseeded PDA plates were used as the negative control. The two plates were superimposed with Parafilm around the edges to prevent the leakage of air and incubated at 25°C. The mycelial diameter was measured after 5 days, and the rate of inhibition of radial mycelial growth was calculated using the equation published by Gao et al. (2018). Three replicates were used for each treatment, and the experiment was repeated thrice. VOCs from the four antagonists were analyzed using solid-phase micro extraction–gas chromatography–mass spectrometry (SPME-GC-MS). For collecting the VOCs, the antagonists were inoculated in NA medium with 25 ml of headspace for 7 days at 28°C. The NA medium without the antagonistic bacteria was used as the control. DVB/CAR/PDMS fiber (Supelco, Inc., Bellafonte, PA, USA) was applied for headspace SPME of the VOCs at 55°C for 40 min. The SPME fiber was immediately inserted into the injection port of the gas chromatography–mass spectrometry (GC-MS; Agilent 7820A-5977E, Santa Clara, CA, USA) for thermal desorption at 250°C for 5 min with a DB-5MS column (30 m × 0.25 mm i.d. × 0.25 μm film thickness; Agilent J&W Scientific, Folsom, CA, USA). The initial oven temperature was 60°C for 2 min, and it was increased to 100°C at a rate of 10°C/min, then increased to 180°C at a rate of 5°C/min, and further increased to 240°C at a rate of 10°C/min and held for 5 min. The mass spectrometer was operated in the electron ionization mode (70 eV). The ion source, quadruple, and GC-MS interface temperatures were set at 230°C, 150°C, and 250°C, respectively. Mass spectrometry (MS) was performed in the full-scan mode with the m/z ranging from 50 to 400 for the analysis of VOCs. The mass spectra data of the volatile compounds were compared with those in the NIST/EPA/NIH Mass Spectrometry Library and Mainlib and/or Replib databases (Gao et al., 2017).
Detection of the in vitro activities of protease, chitinase, and cellulase of the antagonists
The protease activity of the antagonists, indicated by casein degradation, was measured by inoculation on skimmed milk agar with the cross-line method, and the width of each halo was measured as an indicator of the level of protease activity (Yang et al., 2008). The cellulase activity of the antagonists was determined by observing the widths of the clear zones on carboxymethyl cellulose (CMC) agar plates (Ghose, 1987). The chitinase activity of the antagonists was determined by measuring the widths of the clear zones on chitin agar (CA) plates (Roberts and Selitrennikoff, 1988). To measure the activity of the metabolites secreted by the antagonists, four evenly spaced wells (7 mm in diameter) were formed 2.2 cm from the center of the plate by excision, and a 30-μl aliquot of the culture filtrate was added to each well.
Qualitative estimation of siderophore production
The chrome azurol S (CAS) assay was performed to evaluate the effectiveness of the Bacillus antagonists in siderophore production (Kurabachew and Wydra, 2013; Schwyn and Neilands, 1987). After 5 days at 28°C, the siderophore-producing isolates generated orange zones, and the diameter of the clear zone of these colonies was visually rated. Siderophore production in the culture filtrate was measured as described above.
Biofilm formation by the antagonists
The antagonists were cultured in nutritive broth and shaken to midexponential growth; then, 10 μl of the cultured medium was transferred to 1 ml of minimal salts glycerol glutamate (MSgg) medium (Branda et al., 2001) in 24-well polyvinylchloride microtiter plates (Thermo Fisher Scientific, Waltham, MA, USA) and incubated under stationary conditions at 37°C for 4 days (Dietel et al., 2013).
Colonization of the wound sites by the antagonists
The ability of the antagonists to colonize the wounds on the grape, strawberry, and tomato fruit was tested using previously described and partially modified procedures (Cirvilleri et al., 2005; Parafati et al., 2015). A sterile needle was used to make artificial wounds (4 wounds/fruit) on superficially sanitized fruit, and 10 μl aliquots of the bacterial suspensions (1 × 106 cells/ml) were individually inoculated into the wounds; the fruit were incubated for 5 days under the conditions mentioned previously (6 fruit/box). Tissue samples containing the whole wound were cut using a sterile knife after inoculation for 0, 24, 48, 72, 96, and 120 h. The samples were weighed, crushed with a sterilized mortar and pestle, serially diluted, and plated on NA. The bacterial colonies were counted after overnight culture at 37°C to calculate the mean colony forming units (log10 cfu) on the basis of fresh weight. Three replicates of each fruit type were used per treatment (6 fruit per treatment), and the experiment was repeated twice.
Identification of lipopeptides produced by the antagonists
The methanol extracts of the culture filtrates of the four antagonistic bacteria were analyzed using the 5800 matrix-assisted laser desorption ionization–time-of-flight (MALDI-TOF) mass spectrometer (AB SCIEX, Redwood, CA, USA) operated in the positive reflectron mode; α-cyano-4-hydroxycinnamic acid was used as the matrix (prepared by dissolving 5 mg in 1 ml of 50:50 acetonitrile: water containing 0.1% trifluoroacetic acid) (Velho et al., 2011).
Identification of the antagonist isolates
Genomes extracted from the antagonists isolates were analyzed for the 16S rRNA gene sequence by using universal primers, 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1495R (5′-CTACGGCTACCTTGTTACGA-3′), that amplified a 1,400-bp region of the 16S rRNA gene. Phylogenetic analysis was conducted using maximum likelihood in MEGA 5.10 (Tamura et al., 2011). The topology of the phylogenetic tree was evaluated by 1,000 resamplings.
Statistical analysis
The data from the replicates were expressed as mean ± SD values. SPSS version 17.0 software package (SPSS Inc., Chicago, IL, USA) was used to perform the calculations and comparisons of the treatment means for each experiment. Duncan’s test (P ≤ 0.05) and one-way analysis of variance were used to determine whether the means differed significantly.
Results
Activity of the antagonists and their culture filtrates and extracts in controlling the gray mold on the postharvest fruit
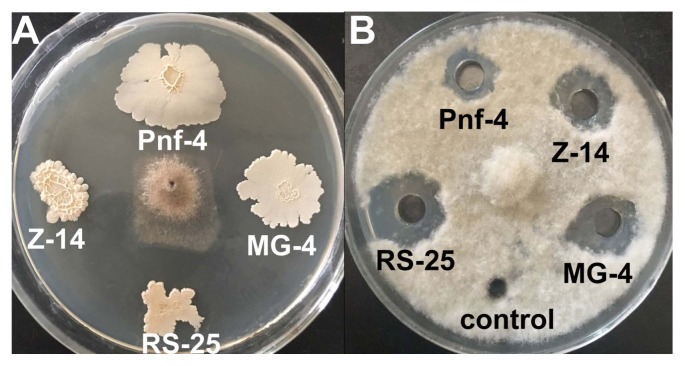
The four isolates, RS-25, MG-4, Z-14, and Pnf-4, and their culture filtrates all demonstrated significant antifungal activities against B. cinerea. The culture filtrate of RS-25 demonstrated the strongest antifungal activity, whereas that of Pnf-4 was the weakest (Fig. 1). The efficacies of RS-25, MG-4, Z-14, and Pnf-4 and their culture filtrates and extracts against the gray mold caused by B. cinerea on the postharvest tomato, strawberry, and grapefruit are reported in Table 1 and Fig. 2. Generally, Z-14, MG-4, and their culture filtrates and extracts were more active against B. cinerea. Pnf-4, RS-25, and their culture filtrates and extracts were relatively ineffective in reducing the DI of the strawberry fruit. The DR of B. cinerea was observed to be the highest (82.14) when the strawberry fruits were treated with the cells of Z-14, whereas the culture filtrate of RS-25 was the most ineffective and yielded the lowest DR value (55.08). The DR values of the tomato fruit treated with Z-14, RS-25, and the culture filtrate and extract of Z-14 were higher (82.62%, 80.46%, 79.25%, and 75.23%, respectively) than those of the tomato fruit treated with Pnf-4, MG-4, and the culture filtrate of RS-25 (68.65%, 67.65%, and 67.29%, respectively). The DR values of the tomato fruit treated with the culture filtrates of Pnf-4 and MG-4 were the lowest (55.27% and 54.03%, respectively). The DR values of the grapefruit treated with MG-4 and RS-25 and culture filtrates of Pnf-4, MG-4, and RS-25 were the highest (84.69%, 86.66%, 83.24%, 86.28%, and 86.81%, respectively), whereas the culture filtrate of Z-14 showed the lowest efficiency in controlling the gray mold (12.20%) on grapefruit.
Fig. 1.
Antifungal activities of the antagonists (A) and their cultural filtrates (B) against Botrytis cinerea.
Table 1.
Efficacy of the vegetable cells, culture filtrates, and crude extracts of the four antagonists in controlling the gray mold caused by Botrytis cinerea on strawberry, grape, and tomato fruit in a greenhouse
| Treatment | Strawberry | Grape | Tomato | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| DI | DR (%) | DI | DR (%) | DI | DR (%) | |
| Pathogen control | 67.03 ± 4.75 a | – | 70.18 ± 5.77 a | – | 62.13 ± 4.61 a | – |
| Vegetable cell of Z-14 | 11.97 ± 1.47 d | 82.14 | 40.40 ± 6.70 d | 42.43 | 10.80 ± 2.57 f | 82.62 |
| Culture filtrate of Z-14 | 14.03 ± 1.43 d | 79.07 | 61.62 ± 4.85 b | 12.20 | 12.89 ± 3.35 ef | 79.25 |
| Crude extract of Z-14 | 19.47 ± 1.90 c | 70.95 | 52.31 ± 4.43 c | 25.46 | 15.39 ± 3.01 def | 75.23 |
| Vegetable cell of Pnf-4 | 25.19 ± 2.92 b | 62.42 | 21.09 ± 5.52 e | 69.95 | 19.48 ± 3.08 cde | 68.65 |
| Culture filtrate of Pnf-4 | 26.71 ± 2.53 b | 60.15 | 11.76 ± 3.13 f | 83.24 | 27.79 ± 4.47 b | 55.27 |
| Crude extract of Pnf-4 | 27.75 ± 1.73 b | 58.60 | 19.69 ± 3.81 e | 71.94 | 25.25 ± 2.58 bc | 59.36 |
| Vegetable cell of MG-4 | 15.42 ± 3.13 cd | 77.00 | 10.75 ± 3.11 f | 84.69 | 20.10 ± 4.36 cd | 67.65 |
| Culture filtrate of MG-4 | 13.75 ± 2.61 d | 79.49 | 9.63 ± 2.93 f | 86.28 | 28.56 ± 4.32 b | 54.03 |
| Crude extract of MG-4 | 19.42 ± 1.95 c | 71.03 | 14.58 ± 2.94 ef | 79.22 | 25.94 ± 4.04 bc | 58.25 |
| Vegetable cell of RS-25 | 26.41 ± 1.61 b | 60.60 | 9.36 ± 2.87 f | 86.66 | 12.14 ± 3.58 f | 80.46 |
| Culture filtrate of RS-25 | 30.11 ± 3.46 b | 55.08 | 9.26 ± 2.38 f | 86.81 | 20.32 ± 4.94 cd | 67.29 |
| Crude extract of RS-25 | 29.15 ± 3.55 b | 56.51 | 17.06 ± 2.08 ef | 75.69 | 24.78 ± 4.39 bc | 60.12 |
Values are mean ± SD of three replicates per treatment (5 fruit each). As determined by Fisher’s protected least significant difference test, the means within a column followed by different letters are significantly different from each other at P = 0.05.
DI, disease index; DR, disease reduction.
Fig. 2.
Efficacy of the vegetable cells, culture filtrates, and crude extracts of the antagonists in controlling the gray mold caused by Botrytis cinerea on postharvest fruit (strawberry, tomato, and grape). VC, vegetable cells; CF, culture filtrate; CE, crude extract.
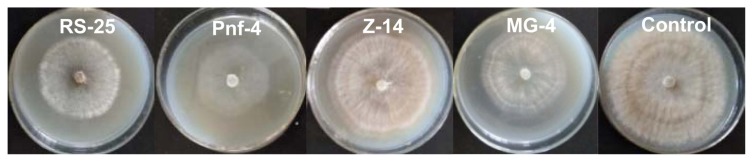
Inhibitory effects of VOCs produced by the antagonists
The variable inhibition efficiency of the VOCs produced by RS-25, MG-4, Z-14, and Pnf-4 against the mycelial growth of B. cinerea is shown in Fig. 3. The highest inhibition values were detected for Pnf-4 against B. cinerea (70.71 ± 3.94%), followed by RS-25 (62.57 ± 3.26%) and MG-4 (53.44 ± 2.71%). In contrast, the VOCs of Z-14 were the most ineffective in reducing the mycelial growth of B. cinerea (24.21 ± 1.85%). Based on the headspace SPME-GC-MS, 109 potential VOCs produced by RS-25, 93 VOCs by MG-4, 92 by Z-14, and 91 by Pnf-4 were identified. The main components of the VOCs were ketone, alcohol, aldehyde, olefin, ester, alkane, and phenol, and esters accounted for the highest percentage in the VOCs produced by RS-25, MG-4, Z-14, and Pnf-4 (36.80%, 29.58%, 30.78%, and 36.26%, respectively) (Table 2).
Fig. 3.
Evaluation of the efficacy of volatile organic compounds produced by the antagonists in reducing the growth of Botrytis cinerea.
Table 2.
Volatile organic compounds (VOCs) produced by the antagonists
| Possible VOCs | RS-25 | MG-4 | Z-14 | Pnf-4 | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| No. | Relative peak area (%) | No. | Relative peak area (%) | No. | Relative peak area (%) | No. | Relative peak area (%) | |
| Ketone | 35 | 15.08 | 21 | 8.75 | 12 | 3.23 | 19 | 5.27 |
| Alcohol | 5 | 5.97 | 11 | 2.88 | 18 | 8.33 | 5 | 0.95 |
| Aldehyde | 5 | 7.02 | 8 | 1.12 | 6 | 5.37 | 1 | 0.94 |
| Olefin | 5 | 1.41 | 3 | 0.50 | 4 | 0.44 | 3 | 0.09 |
| Ester | 18 | 36.80 | 22 | 29.58 | 19 | 30.78 | 29 | 36.26 |
| Alkane | 30 | 29.71 | 17 | 4.32 | 16 | 8.86 | 30 | 16.24 |
| Phenol | 2 | 4.41 | 3 | 5.57 | 1 | 3.20 | 1 | 0.26 |
| Others | 9 | 14.22 | 8 | 2.47 | 16 | 4.94 | 3 | 0.08 |
Enzyme activity and siderophore production assays
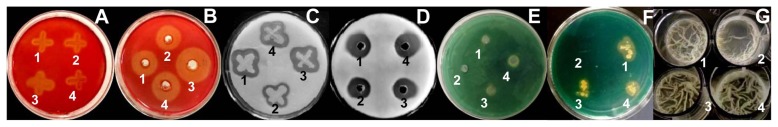
All the tested Bacillus strains and their culture filtrates were able to hydrolyze CMC, and they produced obvious clear zones in the CMC agar plates. The culture filtrate of Z-14 exhibited the strongest cellulase activity; the diameter of the clear zone was 3.4 cm, whereas those of RS-25, Pnf-4, and MG-4 were 2.4 cm, 2.3 cm, and 2.8 cm, respectively (Fig. 4A and B). The four antagonists and their culture filtrates all exhibited potent protease activity, and the hydrolysis zones of RS-25, Z-14, and MG-4 were relatively bigger than that of Pnf-4; this is similar to the protease activity results of the culture filtrates of the antagonists (Fig. 4C and D). The four antagonists and their culture filtrates did not produce clear zones in CA plates, which demonstrated they did not synthesize chitinase. These results are consistent with the findings of Ren et al. (2013), who suggested that Bacillus pumilus JK-SX001 does not secrete chitinase but instead secretes protease and cellulase.
Fig. 4.
Detection of the in vitro activities of cellulase, protease, and chitinase; siderophore production; and biofilm formation by the antagonists and their culture filtrates. (A, C) The in vitro activities of cellulase and protease of the antagonists. (B, D) The in vitro activities of cellulase and protease of the culture filtrate. (E, G) Siderophore production and biofilm formation by the antagonists, respectively. (F) Siderophore production by the culture filtrate; 1–4 represent antagonists RS-25, Pnf-4, Z-14, and MG-4, respectively.
Biofilm formation and colonization of fruit
All four antagonists exhibited the ability to form biofilms on the inert surface of liquid MSgg medium and evolved into whitish aerial structures that appeared to be dry and had a cotton-like texture (Fig. 4G). The population dynamics of RS-25, MG-4, Z-14, and Pnf-4 strains on artificially wounded tomato, strawberry, and grapefruit are shown in Fig. 5. The population trends of the antagonists in the wound tissue of tomato fruit, starting from a similar concentration (log10 cfu/g of tissue = 3.50), remained relatively stable for a period of 24 h; increased for MG-4, Z-14, and Pnf-4 and a few drops of RS-25 up to 72 h; and were similar (log10 cfu/g of tissue = 4.30) after 120 h of incubation (Fig. 5A). The population trend of the antagonists in the wound tissue of strawberry fruit showed fluctuations to a certain extent during 120 h after inoculation; RS-25 showed the highest concentration (log10 cfu/g of tissue = 3.76), whereas MG-4 showed the lowest concentration (log10 cfu/g of tissue = 3.06) (Fig. 5B). The population trend of the antagonists in the wound tissue of grapefruit, starting from similar concentrations, ranged from 3.11 (log10 cfu/g of tissue) for MG-4 to 3.37 (log10 cfu/g of tissue) for Pnf-4; growth appeared to be quite stationary up to 24 h, but it showed variations after that period. The population densities of Z-14 and Pnf-4 increased significantly (log10 cfu/g of tissue = 4.07 and 4.05, respectively), whereas the population density of MG-4 remained relatively stable (log10 cfu/g of tissue = 3.10) and that of RS-25 showed a notable decline (log10 cfu/g of tissue = 2.41) (Fig. 5C). The results prove that the population densities of the four antagonists in the three types of fruit showed different colonization capacities. The colonization abilities of the antagonists were similar in the wounds of the tomato fruit but different in those of the strawberry and grapefruit. The colonization ability of RS-25 was the strongest in the wounds of the strawberry fruit, but weakest in those of the grapefruit.
Fig. 5.
Population dynamics of the antagonists RS-25, Pnf-4, Z-14, and MG-4 in the wound tissue of tomato fruit (A), strawberry fruit (B), and grapefruit (C). Vertical bars indicate the standard error of the mean. cfu, colony forming unit.
Identification of lipopeptides produced by the antagonists
MALDI-TOF mass spectrometry was used to analyze the lipopeptide antibiotics produced by the antagonists. The mass spectra of the crude extract from RS-25 revealed two major clusters with peaks at m/z 1,016.69–1,074.70 Da, which correspond to the isoforms of surfactin B, and peaks at m/z 1,433.88–1,529.88 Da, which correspond to the isoforms of fengycin (Fig. 6A). The mass spectra of the crude extract from Pnf-4 revealed three major clusters with peaks at m/z 1,030.70–1,074.72 Da, which correspond to surfactin B, peaks at m/z 1,079.62–1,125.57 Da, which correspond to iturin A, and peaks at m/z 1,435.87–1,543.88 Da, which correspond to fengycin (Fig. 6B). Surfactin B, iturin A, and fengycin were detected from Z-14 (Fig. 6C), and iturin A and fengycin, from MG-4 (Fig. 6D).
Fig. 6 (A–D).
Mass spectroscopy analysis of the lipopeptides produced by the antagonists.
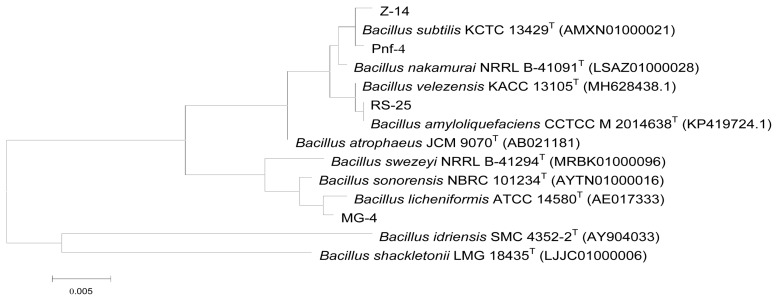
Identification of the antagonists
When compared with the GenBank database, the 16S rRNA sequences of the strains were matched to those of Bacillus spp. The constructed unrooted phylogenetic tree revealed that antagonist Pnf-12 was most similar to B. subtilis, according to the alignment results. Antagonist RS-25 was identified as Bacillus amyloliquefaciens; MG-4, Bacillus licheniformis; and Z-14, B. subtilis (Fig. 7).
Fig. 7.
Unrooted phylogenetic tree based on the 16S rRNA sequences of the antagonists.
Discussion
Previous studies have shown that biological control, such as the application of antagonist microorganisms or their secondary metabolites, is a promising method for decreasing the decay of harvested fruit (Li et al., 2016; Wu et al., 2017). Postharvest treatments with antagonist microorganisms such as Bacillus (Gao et al., 2017; Zhang et al., 2013), Clonostachys rosea (Gong et al., 2017), and yeast strains (Parafati et al., 2015) have been reported to suppress the development of B. cinerea and decay of harvested fruit.
In this study, the main biocontrol modes of action of the four Bacillus antagonists against the in vitro growth of B. cinerea, such as biofilm formation; competition for iron; and production of VOCs, cell-wall-degrading enzymes, and lipopeptide antibiotics, were evaluated on the basis of the mechanisms that play a significant role in the biocontrol activities of antagonistic bacteria. Secondary metabolites play key roles in the antifungal activities of antagonistic bacteria; however, the production of these antagonistic metabolites is greatly affected by the environment (Zhang et al., 2017a). The direct use of antimicrobial substances synthesized by microorganisms as the basis of microbial pesticides can overcome such limitations (Kilani-Feki et al., 2016). Antifungal substances readily decompose and do not pollute the environment (Gu et al., 2017). No significant differences in biocontrol activities were observed between the antagonists and their culture filtrates against the gray mold on the strawberry fruit; however, the crude extract of Z-14 showed lower efficiency than the cells and culture filtrate. The biocontrol efficiency of Z-14, including that of its culture filtrate and extract, was the lowest of the four antagonists on the grapefruit, but the extract of Z-14 showed higher efficiency than the vegetable cells. The vegetable cells of the antagonists demonstrated higher biocontrol activities against the gray mold on the tomato fruit than their culture filtrates and extracts (Table 1, Fig. 2). The different biocontrol activities of the antagonists in the three fruits showed that the effects of the antagonists were influenced by the host fruit.
There is limited information on the antifungal activities of VOCs from microorganisms. Naturally occurring VOCs of endophytic bacteria are regarded as sources of new antifungal agents that are safe for humans and the environment and do not have a harmful influence on the host plants (Gao et al., 2017). Microbial VOCs inhibit the growth of pathogenic fungi; improve plant growth (Park et al., 2015); mediate relationships, interactions, and communications between organisms (Cernava et al., 2015); identify bacterial species; and induce systemic resistance in plants (Zamioudis et al., 2015). GC-MS has revealed that 29 unique VOCs produced by Bacillus velezensis ZSY-1 isolated from Chinese catalpa exhibit significant antifungal activity against plant pathogenic fungi (Gao et al., 2017). VOCs may act in both direct and indirect modes during the interaction between bacterial agents and fungal pathogens (Zhang et al., 2013). The antifungal activity of the VOCs of Z-14 was the weakest of the four antagonists; however, Z-14 displayed the strongest biocontrol effect on the strawberry and tomato fruit. This may indicate that the production of VOCs is not the main mechanism exhibited by Bacillus strains against plant pathogenic fungi.
Aureobasidium pullulans (three strains) and Wickerhamomyces anomalus (four strains) have been tested for extracellular lytic enzyme activity, and all seven strains showed β-1,3-glucanase and protease activities but no chitinase activity (Parafati et al., 2015). Physical damage of the hypha prevents its penetration into the host cell wall. Loss of turgidity and tearing of the pathogen hyphae may both be attributable to cellulase and protease activities. These metabolites may be involved in the control of phytopathogenic fungi through mycoparasitism, which is characterized by colonization of pathogen hyphae (Grzegorczyk et al., 2017). RS-25, Z-14, and MG-4 grew on CAS agar; an orange halo was formed around the colonies, except those of Pnf-4; this is consistent with the results of the culture filtrates of these antagonists (Fig. 3E and F). Generally, siderophores are produced by microorganisms to bind Fe3+ from the environment, transport it to the microbial cell, and make it available for growth (Leong, 1996). Therefore, competition for iron may also be a possible mechanism for controlling phytopathogens (Xue et al., 2013).
Generally, biofilm formation is regarded as a major mechanism of antagonists against plant pathogenic fungi, as biofilms act as physical and chemical barriers (Díaz Herrera et al., 2016; Dietel et al., 2013). Haggag and Timmusk (2008) showed that Paenibacillus polymyxa strains produced biofilms to control the crown root rot disease, which is caused by Aspergillus niger. A previous study has shown that W. anomalus and Metschnikowia pulcherrima strains were able to adhere to polystyrene plates and maintain a high film-forming capacity after 48 h and 72 h of incubation, even after repeated washes (Parafati et al., 2015).
The presence of a high number of antagonists in the wounded tissues would protect the plants or fruit from pathogens because tissue colonization by biocontrol agents is critical for the effective control of phytopathogens (Zeriouh et al., 2014). The antagonists can successfully compete with the phytopathogens for the niches and nutrients, such as organic compounds, that are essential for the reactivation of propagules and/or subsequent proliferation and colonization of tissues (Budiharjo et al., 2014). The colonization capacities of antagonists in tissues may have a key role in disease suppression, even if competition or antibiosis occurs between the antagonists and pathogens (Huang et al., 2013). The four antagonists showed similar colonization abilities but different biocontrol effects against the gray mold in the tomato fruit, which indicates that secondary metabolites may play a major role in the control activity (Table 1, Figs. 2 and 5A). The colonization abilities and control activities of the antagonists did not have a direct correlation in the strawberry and grapefruit. RS-25 displayed the strongest colonization ability and lowest biocontrol effect in the strawberry fruit (Table 1, Figs. 2 and 5B), but the strongest biocontrol effect and lowest colonization ability in the grapefruit (Table 1, Figs. 2 and 5C).
Lipopeptide antibiotics, such as iturins, fengycins, and surfactins, are an important class of antifungal substances synthesized by Bacillus (Moyne et al., 2004). Lipopeptide antibiotics have direct or indirect inhibitory effects on fungal plant pathogens, but they are not toxic to the plant itself (Ongena and Jacques, 2008; Zhang et al., 2017b). MALDI-TOF mass spectrometry can be easily and conveniently used for component identification (Sandrin et al., 2013; Xie et al., 2012). This study showed that MALDI-TOF mass spectrometry could become a new, sensitive, and rapid technique for detecting metabolites. MALDI-TOF mass spectrometry has been used to show that B. amyloliquefaciens FZB42 promotes plant growth, suppresses plant pathogenic organisms in the rhizosphere of plants, and synthetizes and excretes bacillomycin D, fengycin, and surfactin (Koumoutsi et al., 2004). Three types of lipopeptides, surfactin A, iturin A, and fengycin, produced by B. subtilis CMB32 isolated from the soil by Kim et al. (2010) have been reported for their potential to control the anthracnose disease caused by Colletotrichum gloeosporioides. Lipopeptide antibiotics are relatively stable in nature and resistant to acids and bases, which ensure that the lipopeptide antibiotics can exert antagonistic effects over a long period (Fan et al., 2017; Guo et al., 2014). Iturins and fengycins exhibit a wide range of strong inhibitory activities against fungal plant pathogens. Romero et al. (2007) concluded that the iturin and fengycin families of lipopeptides have a major role in the antagonism of B. subtilis towards Podosphaera fusca, which is the main causal agent of cucurbit powdery mildew in Spain. Fengycin-type lipopeptides produced by B. subtilis NCD-2 were the main antifungal compounds that restricted the population of Rhizoctonia solani in the cotton rhizosphere and suppressed cotton damping-off disease (Guo et al., 2014).
In summary, the cells of Z-14, culture filtrate of RS-25, and cells of Z-14 showed the strongest biocontrol activity against the gray mold on the postharvest strawberry, grape, and tomato fruit, respectively. Biofilm formation; strong colonization ability in fruit tissues; and subsequent production of VOCs, cellulase and protease, siderophores, and lipopeptide antibiotics may be the main modes of action of the antagonist bacteria against B. cinerea on the postharvest fruit. This study provides theoretical support and material basis for developing agents against the gray mold caused by B. cinerea on postharvest fruit.
Acknowledgments
We would like to thank the native English-speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript. This work was supported by the Hebei Provincial Natural Science Foundation of China (grant number C2014204027).
References
- Abdallah RAB, Mokni-Tlili S, Nefzi A, Jabnoun-Khiareddine H, Daami-Remadi M. Biocontrol of Fusarium wilt and growth promotion of tomato plants using endophytic bacteria isolated from Nicotiana glauca organs. Biol Control. 2016;97:80–88. doi: 10.1016/j.biocontrol.2016.03.005. [DOI] [Google Scholar]
- Bautista-Rosales PU, Calderon-Santoya M, Servín-Villegas R, Ochoa-Álvarez NA, Ragazzo-Sánchez JA. Action mechanisms of the yeast Meyerozyma caribbica for the control of the phytopathogen Colletotrichum gloesporioides in mangoes. Biol Control. 2013;65:293–301. doi: 10.1016/j.biocontrol.2013.03.010. [DOI] [Google Scholar]
- Branda SS, González-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci U S A. 2001;98:11621–11626. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budiharjo A, Chowdhury SP, Dietel K, Beator B, Dolgova O, Fan B, Bleiss W, Ziegler J, Schmid M, Hartmann A, Borriss R. Transposon mutagenesis of the plant-associated Bacillus amyloliquefaciens ssp. plantarum FZB42 revealed that the nfrA and RBAM17410 genes are involved in plant-microbe-interactions. PLoS ONE. 2014;9:e98267. doi: 10.1371/journal.pone.0098267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernava T, Aschenbrenner IA, Grube M, Liebminger S, Berg G. A novel assay for the detection of bioactive volatiles evaluated by screening of lichen associated bacteria. Front Microbiol. 2015;6:398. doi: 10.3389/fmicb.2015.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirvilleri G, Bonaccorsi A, Scuderi G, Scortichini M. Potential biological control activity and genetic diversity of Pseudomonas syringae pv. syringae strains. J Phytopathol. 2005;153:654–666. doi: 10.1111/j.1439-0434.2005.01033.x. [DOI] [Google Scholar]
- Conway WS, Leverentz B, Janisiewicz WJ, Saftner RA, Camp MJ. Improving biocontrol using antagonist mixtures with heat and/or sodium bicarbonate to control postharvest decay of apple fruit. Postharvest Biol Technol. 2005;36:235–244. doi: 10.1016/j.postharvbio.2005.01.006. [DOI] [Google Scholar]
- Dean R, Van Kan JAL, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, Foster GD. The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol. 2012;13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derckel J-P, Baillieul F, Manteau S, Audran J-C, Haye B, Lambert B, Legendre L. Differential induction of grapevine defences by two strains of Botrytis cinerea. Phytopathology. 1999;89:197–203. doi: 10.1094/PHYTO.1999.89.3.197. [DOI] [PubMed] [Google Scholar]
- Díaz Herrera S, Grossi C, Zawoznik M, Groppa MD. Wheat seeds harbour bacterial endophytes with potential as plant growth promoters and biocontrol agents of Fusarium graminearum. Microbiol Res. 2016;186–187:37–43. doi: 10.1016/j.micres.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Dietel K, Beator B, Budiharjo A, Fan B, Borriss R. Bacterial traits involved in colonization of Arabidopsis thaliana roots by Bacillus amyloliquefaciens FZB42. Plant Pathol J. 2013;29:59–66. doi: 10.5423/PPJ.OA.10.2012.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droby S. Improving quality and safety of fresh fruits and vegetables after harvest by the use of biocontrol agents and natural materials. Acta Hortic. 2006;709:45–51. doi: 10.17660/ActaHortic.2006.709.5. [DOI] [Google Scholar]
- Fan H, Ru J, Zhang Y, Wang Q, Li Y. Fengycin produced by Bacillus subtilis9407 plays a major role in the biocontrol of apple ring rot disease. Microbiol Res. 2017;199:89–97. doi: 10.1016/j.micres.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Fekete É, Fekete E, Irinyi L, Karaffa L, Árnyasi M, Asadollahi M, Sándor E. Genetic diversity of a Botrytis cinerea cryptic species complex in Hungary. Microbiol Res. 2012;167:283–291. doi: 10.1016/j.micres.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Gao H, Li P, Xu X, Zeng Q, Guan W. Research on volatile organic compounds from Bacillus subtilis CF-3: biocontrol effects on fruit fungal pathogens and dynamic changes during fermentation. Front Microbiol. 2018;9:456. doi: 10.3389/fmicb.2018.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Zhang B, Liu H, Han J, Zhang Y. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol Control. 2017;105:27–39. doi: 10.1016/j.biocontrol.2016.11.007. [DOI] [Google Scholar]
- Ghose TK. Measurement of cellulase activities. Pure Appl Chem. 1987;59:257–268. doi: 10.1351/pac198759020257. [DOI] [Google Scholar]
- Gong C, Liu Y, Liu S-Y, Cheng M-Z, Zhang Y, Wang R-H, Chen H-Y, Li J-F, Chen X-L, Wang A-X. Analysis of Clonostachys rosea-induced resistance to grey mould disease and identification of the key proteins induced in tomato fruit. Postharvest Biol Technol. 2017;123:83–93. doi: 10.1016/j.postharvbio.2016.08.004. [DOI] [Google Scholar]
- Grzegorczyk M, Żarowska B, Restuccia C, Cirvilleri G. Postharvest biocontrol ability of killer yeasts against Monilinia fructigena and Monilinia fructicola on stone fruit. Food Microbiol. 2017;61:93–101. doi: 10.1016/j.fm.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Gu K-B, Zhang D-J, Guan C, Xu J-H, Li S-I, Shen G-M, Luo Y-C, Li Y-G. Safe antifungal lipopeptides derived from Bacillus marinus B-9987 against grey mold caused by Botrytis cinerea. J Integr Agric. 2017;16:1999–2008. doi: 10.1016/S2095-3119(16)61616-7. [DOI] [Google Scholar]
- Guo Q, Dong W, Li S, Lu X, Wang P, Zhang X, Wang Y, Ma P. Fengycin produced by Bacillus subtilis NCD-2 plays a major role in biocontrol of cotton seedling damping-off disease. Microbiol Res. 2014;169:533–540. doi: 10.1016/j.micres.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Haggag WM, Timmusk S. Colonization of peanut roots by biofilm-forming Paenibacillus polymyxa initiates biocontrol against crown rot disease. J Appl Microbiol. 2008;104:961–969. doi: 10.1111/j.1365-2672.2007.03611.x. [DOI] [PubMed] [Google Scholar]
- Huang J, Wei Z, Tan S, Mei X, Yin S, Shen Q, Xu Y. The rhizosphere soil of diseased tomato plants as a source for novel microorganisms to control bacterial wilt. Appl Soil Ecol. 2013;72:79–84. doi: 10.1016/j.apsoil.2013.05.017. [DOI] [Google Scholar]
- Kilani-Feki O, Khedher SB, Dammak M, Kamoun A, Jabnoun-Khiareddine H, Daami-Remadi M, Tounsi S. Improvement of antifungal metabolites production by Bacillus subtilis V26 for biocontrol of tomato postharvest disease. Biol Control. 2016;95:73–82. doi: 10.1016/j.biocontrol.2016.01.005. [DOI] [Google Scholar]
- Kim PI, Ryu J, Kim YH, Chi Y-T. Production of biosurfactant lipopeptides Iturin A, Fengycin, and Surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J Microbiol Biotechnol. 2010;20:138–145. [PubMed] [Google Scholar]
- Koumoutsi A, Chen X-H, Henne A, Liesegang H, Hitzeroth G, Franke P, Vater J, Borriss R. Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J Bacteriol. 2004;186:1084–1096. doi: 10.1128/JB.186.4.1084-1096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurabachew H, Wydra K. Characterization of plant growth promoting rhizobacteria and their potential as bioprotectant against tomato bacterial wilt caused by Ralstonia solanacearum. Biol Control. 2013;67:75–83. doi: 10.1016/j.biocontrol.2013.07.004. [DOI] [Google Scholar]
- Leong J. Siderophores: their biochemistry and possible role in the biocontrol of plant pathogens. Annu Rev Phytopathol. 1996;24:187–209. doi: 10.1146/annurev.py.24.090186.001155. [DOI] [Google Scholar]
- Li W, Zhang H, Li P, Apaliya MT, Yang Q, Peng Y, Zhang X. Biocontrol of postharvest green mold of oranges by Hanseniaspora uvarum Y3 in combination with phosphatidylcholine. Biol Control. 2016;103:30–38. doi: 10.1016/j.biocontrol.2016.07.014. [DOI] [Google Scholar]
- Mari M, Martini C, Guidarelli M, Neri F. Postharvest biocontrol of Monilinia laxa, Monilinia fructicola and Monilinia fructigena on stone fruit by two Auerobasidium pullulans strains. Biol Control. 2012;60:132–140. doi: 10.1016/j.biocontrol.2011.10.013. [DOI] [Google Scholar]
- Mirzaei S, Goltapeh EM, Shams-Bakhsh M, Safaie N, Chaichi M. Genetic and phenotypic diversity among Botrytis cinerea isolates in Iran. J Phytopathol. 2009;157:474–482. doi: 10.1111/j.1439-0434.2008.01518.x. [DOI] [Google Scholar]
- Moyne A-L, Cleveland TE, Tuzun S. Molecular characterization and analysis of the operon encoding the antifugal lipopeptide bacillomycin D. FEMS Microbiol Lett. 2004;234:43–49. doi: 10.1111/j.1574-6968.2004.tb09511.x. [DOI] [PubMed] [Google Scholar]
- Nalinia S, Parthasarathi R. Production and characterization of rhamnolipids produced by Serratia rubidaea SNAU02 under solid-state fermentation and its application as biocontrol agent. Bioresour Technol. 2014;173:231–238. doi: 10.1016/j.biortech.2014.09.051. [DOI] [PubMed] [Google Scholar]
- Ongena M, Jacques P. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 2008;16:115–125. doi: 10.1016/j.tim.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Panebianco S, Vitale A, Platania C, Restuccia C, Polizzi G, Cirvilleri G. Postharvest efficacy of resistance inducers for the control of green mold on important Sicilian citrus varieties. J Plant Dis Prot. 2014;121:177–183. doi: 10.1007/BF03356507. [DOI] [Google Scholar]
- Park Y-S, Dutta S, Ann M, Raaijmakers JM, Park K. Promotion of plant growth by Pseudomonas fluorescens strain SS101 via novel volatile organic compounds. Biochem Biophys Res Commun. 2015;461:361–365. doi: 10.1016/j.bbrc.2015.04.039. [DOI] [PubMed] [Google Scholar]
- Parafati L, Vitale A, Restuccia C, Cirvilleri G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015;47:85–92. doi: 10.1016/j.fm.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Platania C, Restuccia C, Muccilli S, Cirvilleri G. Efficacy of killer yeast in the biological control of Penicillium digitatum on Tarocco orange fruits (Citrus sinensis) Food Microbiol. 2012;30:219–225. doi: 10.1016/j.fm.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Posada LF, Ramírez M, Ochoa-Gómez N, Cuellar-Gaviria TZ, Argel-Roldan LE, Ramírez CA, Villegas-Escobar V. Bioprospecting of aerobic endospore-forming bacteria with biotechnological potential for growth promotion of banana plants. Sci Hortic. 2016;212:81–90. doi: 10.1016/j.scienta.2016.09.040. [DOI] [Google Scholar]
- Pretorius D, van Rooyen J, Clarke KG. Enhanced production of antifungal lipopeptides by Bacillus amyloliquefaciens for biocontrol of postharvest disease. New Biotechnol. 2015;32:243–252. doi: 10.1016/j.nbt.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Ren J-H, Li H, Wang Y-F, Ye J-R, Yan A-Q, Wu X-Q. Biocontrol potential of an endophytic Bacillus pumilus JK-SX001 against poplar canker. Biol Control. 2013;67:421–430. doi: 10.1016/j.biocontrol.2013.09.012. [DOI] [Google Scholar]
- Roberts WK, Selitrennikoff C. Plant and bacterial chitinases differ in antifungal activity. J Gen Microbiol. 1988;134:169–176. [Google Scholar]
- Romero D, de Vicente A, Rakotoaly RH, Dufour SE, Veening J-W, Arrebola E, Cazorla FM, Kuipers OP, Paquot M, Pérez-García A. The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol Plant-Microbe Interact. 2007;20:430–440. doi: 10.1094/MPMI-20-4-0430. [DOI] [PubMed] [Google Scholar]
- Sandrin TR, Goldstein JE, Schumaker S. MALDI TOF MS profiling of bacteria at the strain level: a review. Mass Spectrom Rev. 2013;32:188–217. doi: 10.1002/mas.21359. [DOI] [PubMed] [Google Scholar]
- Schnaubelt K. Essential oil therapy according to traditional Chinese medical concepts. Int J Aromather. 2005;15:98–105. doi: 10.1016/j.ijat.2005.03.002. [DOI] [Google Scholar]
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Soylu EM, Kurt Ş, Soylu S. In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int J Food Microbiol. 2010;143:183–189. doi: 10.1016/j.ijfoodmicro.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol. 2005;56:845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA 5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velho RV, Medina LFC, Segalin J, Brandelli A. Production of lipopeptides among Bacillus strains showing growth inhibition of phytopathogenic fungi. Folia Microbiol. 2011;56:297. doi: 10.1007/s12223-011-0056-7. [DOI] [PubMed] [Google Scholar]
- Williamson B, Tudzynski B, Tudzynski P, Van Kan JAL. Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol. 2007;8:561–580. doi: 10.1111/j.1364-3703.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Lin H, Lin Y, Shi J, Xue S, Hung Y-C, Chen Y, Wang H. Effects of biocontrol bacteria Bacillus amyloliquefaciens LY-1 culture broth on quality attributes and storability of harvested litchi fruit. Postharvest Biol Technol. 2017;132:81–87. doi: 10.1016/j.postharvbio.2017.05.021. [DOI] [Google Scholar]
- Xie YL, Xu ZW, Ma LZ, Gao XW. Molecular identification of Bacillus strains isolated from rhizosphere of Betula platyphylla in Qinghai Beishan timberland and its antagonistic activity analysis. Acta Phytophylacica Sin. 2012;39:246–252. [Google Scholar]
- Xue L, Xue Q, Chen Q, Lin C, Shen G, Zhao J. Isolation and evaluation of rhizosphere actinomycetes with potential application for biocontrol of Verticillium wilt of cotton. Crop Prot. 2013;43:231–240. doi: 10.1016/j.cropro.2012.10.002. [DOI] [Google Scholar]
- Yang J-H, Liu H-X, Zhu G-M, Pan Y-L, Xu L-P, Guo J-H. Diversity analysis of antagonists from riceassociated bacteria and their application in biocontrol of rice diseases. J Appl Microbiol. 2008;104:91–104. doi: 10.1111/j.1365-2672.2007.03534.x. [DOI] [PubMed] [Google Scholar]
- Zamioudis C, Korteland J, Van Pelt JA, van Hamersveld M, Dombrowski N, Bai Y, Hanson J, Van Verk MC, Ling H-Q, Schulze-Lefert P, Pieterse CMJ. Rhizobacterial volatiles and photosynthesis-related signals coordinate MYB72 expression in Arabidopsis roots during onset of induced systemic resistance and iron-deficiency responses. Plant J. 2015;84:309–322. doi: 10.1111/tpj.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeriouh H, de Vicente A, Pérez-García A, Romero D. Surfactin triggers biofilm formation of Bacillus subtilis in melon phylloplane and contributes to the biocontrol activity. Environ Microbiol. 2014;16:2196–2211. doi: 10.1111/1462-2920.12271. [DOI] [PubMed] [Google Scholar]
- Zhang D, Gao T, Li H, Lei B, Zhu B. Identification of antifungal substances secreted by Bacillus subtilis Z-14 that suppress Gaeumannomyces graminis vartritici. Biocontrol Sci Technol. 2017a;27:237–251. doi: 10.1080/09583157.2016.1275522. [DOI] [Google Scholar]
- Zhang DD, Guo XJ, Wang YJ, Gao TG, Zhu BC. Novel screening strategy reveals a potent Bacillus antagonist capable of mitigating wheat take-all disease caused by Gaeumannomyces graminis vartritici. Lett Appl Microbiol. 2017b;65:512–519. doi: 10.1111/lam.12809. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li B, Wang Y, Guo Q, Lu X, Li S, Ma P. Lipopeptides, a novel protein, and volatile compounds contribute to the antifungal activity of the biocontrol agent Bacillus atrophaeus CAB-1. Appl Microbiol Biotechnol. 2013;97:9525–9534. doi: 10.1007/s00253-013-5198-x. [DOI] [PubMed] [Google Scholar]