ABSTRACT
Objectives
The present manuscript aims to critically detail the physiologic process of socket healing, in the absence or presence of grafting materials or platelet concentrates, addressing the associated molecular and cellular events that culminate in the restoration of the lost tissue architecture and functionality.
Material and Methods
An electronic search in the National Library of Medicine database MEDLINE through its online site PubMed and Web of Science from inception until May 2019 was conducted to identify articles concerning physiologic process of socket healing, in the absence or presence of grafting materials or platelet concentrates. The search was restricted to English language articles without time restriction. Additionally, a hand search was carried out in oral surgery, periodontology and dental implants related journals.
Results
In total, 122 literature sources were obtained and reviewed. The detailed biological events, at the molecular and cellular level, that occur in the alveolus after tooth extraction and socket healing process modulated by grafting materials or autologous platelet concentrates were presented as two entities.
Conclusions
Tooth extraction initiates a convoluted set of orderly biological events in the alveolus, aiming wound closure and socket healing. The healing process comprises a wide range of events, regulated by the interplay of cytokines, chemokines and growth factors that determine cellular recruitment, proliferation and differentiation in the healing milieu, in a space- and time-dependent choreographic interplay. Additionally, the healing process may further be modulated by the implantation of grafting materials or autologous platelet concentrates within the tooth socket, aiming to enhance the regenerative outcome.
Keywords: alveolar bone grafting, bone remodeling, tooth extraction, tooth socket, platelet-rich plasma, platelet-rich fibrin
INTRODUCTION
The surgical extraction of teeth is one of the most common dental procedures and the subsequent process of socket healing became a fundamental topic of research and discussion, both within the frame of biomedical and clinical-based dental sciences. The continuous demand for improved aesthetics and functionality have led to the need of preserving and maintaining an adequate tissue volume, of both soft and hard tissues, in order to achieve a successful and long-lasting implant-based oral rehabilitation. Notwithstanding, healing is an intricate process that runs from a timely organized interplay of distinct biological systems, involving a plethora of molecules and cells throughout distinct phases [1].
The presence or absence of teeth influences alveolar socket remodelling. After tooth extraction, a series of orderly biological events occurs in the alveolus, which results in socket healing [2]. Healing comprises a wide range of processes including vascular alterations; inflammatory activation; migration, proliferation and differentiation of distinct cell populations; extracellular matrix production and maturation; bone formation, modelling and remodelling, culminating in the restoration of the lost tissues [1]. This is achieved through space- and time-dependent choreographic interplay of cytokines, chemokines and growth factors, activation of signalling pathways with modulation of genes’ and transcription factors’ expression, that determine cellular fate within the healing milieu [3].
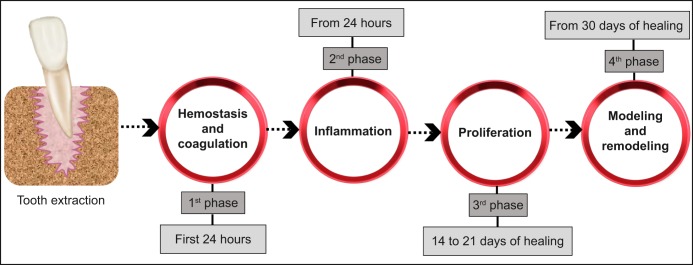
In the recent literature, the process of socket healing was studied in humans, as well as in distinct experimental animal models, as mice, rats, dogs, and monkeys (Table 1) through histological, histochemical and radiographic methods [1,4-6]. Despite the similarities regarding the attained biological events and sequence of healing, these studies revealed differences in the healing time scale [4]. Furthermore, the authors used different nomenclatures for the described processes and events, as well as different staging systems and time points for the characterization of the healing process [1,5,6]. Notwithstanding, there is a generalized consensus on the occurrence of fundamental biologic events that develop continuously, despite being arbitrarily staged into distinct phases, as a mean to address and discuss the physiological processes taking place in the socket and surrounding tissues [7]. These can be summed up, closely mirroring the classic stages of the wound healing process described into a sequence of four time-dependent phases (Figure 1):
Table 1.
Studies that evaluated the process of socket healing with different type of model and different time of healing
| Study |
Year of publication |
Model |
Time of healing |
|---|---|---|---|
| Vieira et al. [3] | 2015 | Mice | 0 - 21 days |
| Smith [4] | 1974 | Rat | 1 - 20 days |
| Cardaropoli et al. [5] | 2003 | Dog | 1 - 80 days |
| Scala et al. [6] | 2014 | Monkey | 4 - 180 days |
| Amler et al. [10] | 1969 | Human | 2 - 32 days |
| Trombelli et al. [14] | 2008 | Human | 2 - 24 weeks |
| Lin et al. [33] | 2011 | Rat | 3 - 14 days |
| Hsieh et al. [43] | 1994 | Rat | 5 - 14 days |
| Devlin and Sloan [44] | 2002 | Human | 2 weeks |
| Lindhe et al. [51] | 2012 | Human | Varying periods of time |
| Araújo and Lindhe [52] | 2005 | Dog | 1 - 8 weeks |
Figure 1.
Biological events occurring in the socket after tooth extraction described into a sequence of four time-dependent phases.
Coagulation and haemostasis, which immediately follows the teeth extraction;
Inflammation, that is initiated shortly thereafter;
Proliferation, initiated in the subsequent days and incorporating the majority of the healing process;
Modelling and remodelling, aiming to restore the lost architecture and functionality, and lasting for several months [8].
The purpose of the present study was to detail the biological events of the socket healing process after tooth extraction, and further provide a comprehensive overview of the molecular and cellular aspects that occur through the healing, in the absence or the presence of graft materials and autologous platelet concentrates, aiming improved tissue healing.
MATERIAL AND METHODS
Protocol and registration
The protocol for this study was registered prospectively in the PROSPERO, an international prospective register of systematic reviews.
The protocol can be accessed at:
http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42019132277.
Registration number: CRD42019132277.
The reporting of this study followed, the guidelines of PRISMA statement [9].
Focus question
Focused question 1: What are the detailed biological events, at the molecular and cellular level, that occur in the alveolus after tooth extraction?
Focused question 2: How is the socket healing process modulated by grafting materials or autologous platelet concentrates?
Information sources
The search strategy was based on electronic database examination. A search was implemented on the National Library of Medicine database MEDLINE through its online site PubMed and Web of Science, and was conducted from their inception until May 2019, to identify articles concerning the physiologic process of socket healing.
Search
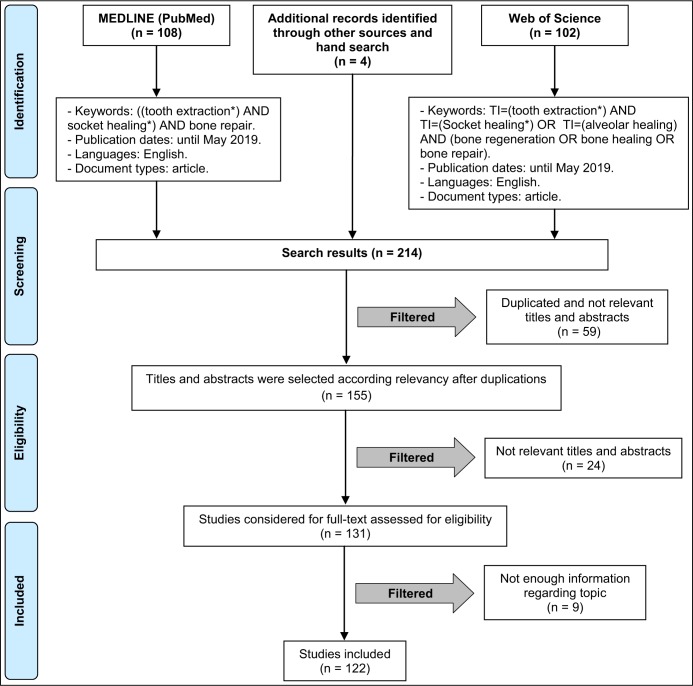
The following terms and their combinations were used for the search: "tooth extraction AND socket healing OR alveolar healing AND bone repair OR bone regeneration OR bone healing". Details of the electronic search strategy are presented in Figure 2. Additionally, a hand search was carried out in oral surgery, periodontology and dental implants related journals, including "Journal of Oral and Maxillofacial Implants", "Clinical Oral Implants Research", "European Journal of Oral Implantology", "Journal of Oral and Maxillofacial Surgery", "Journal of Clinical Periodontology", "Journal of Periodontology", "International Journal of Oral and Maxillofacial Surgery", "The International Journal of Periodontics and Restorative Dentistry" and "Journal of Oral & Maxillofacial Research". An extensive hand-search was also performed encompassing the bibliographies of the included papers and other narrative and systematic reviews. The extracted data were copied into EndNote X9 software (Thomson Reuters, New York, NY, USA).
Figure 2.
Flowchart of article selection procedure.
Selection of studies
The articles, at any stage (abstract or full-text assessment) were independently reviewed by 4 of the authors (P.S.G, L.P, P.D. and L.M) to confirm each study’s eligibility. Any discrepancies between reviewers were resolved by discussion and consensus, and by consulting an additional experienced senior reviewer if needed (M.H.F).
Types of publications
The review included all human and animal studies published in English. Letters, editorials, PhD theses, and abstracts were excluded.
Types of studies
Present review included all retrospective and prospective follow-up studies, case-control studies, case report series, cohort studies, experiments, comparative analyses and observational studies without time cut.
Types of participants/population
The subjects of the included studies were humans or animals submitted to tooth extraction procedures, with or without any further intervention.
Inclusion and exclusion criteria
The inclusion criteria were as follows:
In vitro, in vivo and clinical studies published in the English language without time cut;
No systemic disorders associated;
Clinical, histological, morphological or cellular/molecular outcome parameters and/or imagiologic alveolar bone dimensions outcome parameters.
The exclusion criteria were:
Studies that involved the application of any additional therapy, other than graft materials and autologous platelet concentrates, that could have affected socket healing outcomes;
Studies involving the same population and reporting the same outcome variables, as other included studies.
Sequential search strategy
Following the initial literature search, all article titles were screened to eliminate irrelevant publications. Next, within the abstract reading stage, inclusion and exclusion criteria were applied to the information given in abstracts; if any information was missing, the full-text reading was performed. At the final stage, all the included articles were carefully screened and only relevant articles were included for further analysis.
Data extraction
Two independent reviewers extracted the relevant data using an Excel spreadsheet (Microsoft, Redmond, WA). The extracted data were study design, study setting, follow-up duration, wound healing process phases, classification of the cytokines, chemokines and growth factors and regenerative biomaterials types. Additional data were extracted and descriptive from relevant aspects involved in the research.
Statistical analysis
No meta-analyses could be performed due to the heterogeneity between the studies (different study designs, control groups, and observation periods).
RESULTS AND DISCUSSION
Socket healing process
Haemostasis and coagulation
Immediately after tooth extraction, the socket is filled with blood resulting from the haemorrhagic process, followed by the formation of a stable blood clot embedded in a network of fibrin [6,10]. The clot initially fills the socket volume up to the margins of the soft tissue, being in direct contact with the injured periodontal ligament [11]. Clot formation aims twofold - it prevents blood loss, and provides a structured scaffold for the adhesion of recruited cells that will orchestrate healing in the subsequent phases of the process [12]. Haemostasis, in the alveolar socket, results from the dynamic interplay of platelets and endothelial cells, as well as the equilibrium between coagulation and fibrinolysis, giving rise to the formation of a stable clot. The blood clot seems to be entirely remodelled throughout the first week post extraction, as demonstrated in human studies, with only scattered erythrocytes being identified at subsequent time points [13,14].
Mechanistically, teeth extraction leads to microvascular damage and blood extravasation, processes that are rapidly controlled by the reflex vasoconstriction, responsible by the retrenchment of vascular smooth muscle cells - able to control bleeding in arterioles up to 0.5 cm in diameter [15]. Notwithstanding, the vascular tone control is only temporarily effective until acidosis and hypoxia develop within the microenvironment, leading to passive relaxation, and resuming bleeding. If it were not for the establishment of an insoluble fibrin network - resulting from the activation of the coagulation cascade through different pathways for the aggregation of platelets and clot formation - haemostatic mechanisms would become ineffective [16].
Platelets release clotting factors upon activation, which occurs following the contact with extracellular matrix molecules. Blood clot and associated platelets, besides haemostatic functions, further play a fundamental role for proper tissue healing due to the presence of many cytokines (as those from chemokine and interleukin families) and growth factors (e.g., tumour necrosis factor alfa family, transforming growth factor beta family, fibroblast growth factor family, epidermal growth factor family, and individual factors such as platelet-derived growth factor, vascular endothelial growth factor, granulocyte macrophage colony stimulating factor and connective tissue growth factor, among others), that are able to modulate subsequent cellular processes - cell migration, proliferation and differentiation - fundamental to promote angiogenesis and bone regeneration [17-19]. In fact, clot organization, maintenance and retraction are determining factors of the subsequent phases involving connective tissue formation, as demonstrated experimentally in a socket healing study in which the blood clot was removed 6 - 8 minutes postoperatively, greatly compromising the subsequent healing outcome [20].
Platelets also contain and release vasoactive amines and arachidonic acid-derived metabolism products that play a fundamental role in the initiation and modulation of the subsequent inflammatory phase. In this context, adhesion molecules - as vitronectin and distinct integrins - were found to be positively regulated during the initial events, assisting on the subsequent cell recruitment, adhesion and activation [21].
Inflammatory phase
A transitory and moderate inflammatory process is essential for adequate bone healing/regeneration, as embraced by the concept of constructive inflammation, with the activation of both humoral and cellular inflammatory response [22].
Upon blood clot establishment, the recruitment and migration of inflammatory cells are verified, throughout the first days following tooth extraction [7]. The complement system is activated and neutrophils early colonize the clot tissue, migrating according to an established chemokine concentration gradient - majorly governed by complement molecules C3a and C5a, TGF- β and bacteria released products. Neutrophils enrol an active phagocytosis, removing the clot structure, bacteria and eventual foreign material, through the release of free radical species and proteolytic enzymes [23]. Experimental studies in rat healing socket revealed an early neutrophilic infiltration, separating viable tissues from tissue debris and bacteria [24]. The more apically located neutrophils were characterized by a dense granular structure and occasional phagosomes, whether the more coronal located possessed fewer granules, but a high distribution of phagosomes engulfing bacteria - supporting the fundamental neutrophils’ role in bacteria clearance and infection control [24].
Macrophages are following recruited, from circulating monocytes that experience phenotypic maturation, being responsible for the continuation of phagocytosis and further providing the release of effective growth factors - transforming growth factor-alfa, transforming growth factor-beta, fibroblast growth factor, and epidermal growth factor - that activate subsequently recruited fibroblasts and osteoblasts [25]. Lymphocytes are lastly recruited, broadly in response to interleukin-1 and breakdown products of local molecules [26].
Proliferative phase
The proliferative phase is initiated by fibroplasia. At this time, there is an intense fibroblast migration and proliferation, as well as an increase in collagen synthesis and other extracellular matrix proteins. The newly formed abundant extracellular matrix further supports cell migration, allowing for enhanced cell adhesion and anchorage thorough filopodia and pseudopodia extensions that attach fibronectin and collagen proteins of the matrix [2,14].
Histological data reveals that, during the first week of healing, extraction socket is suffused with loosely organized cell-rich granulation tissue with an intense infiltration of inflammatory cells, vascular sprouts and fibroblasts - replacing the initial blood clot that undergoes coagulative necrosis, in a centrifugal process [11,27].
Early activation of TGF-β1 and FGF-2 seem to modulate activation and proliferation of fibroblastic populations, greatly determining the synthesis and maturation of the extracellular matrix and organization of the granulation tissue [28]. At the same time, major components of the extracellular matrix, such as collagen type I - coded by COL1A1 and COL1A2 genes - further acknowledged as early bone markers, also report an abundant expression at the early healing phases [29], in parallel with the high expression of enzymes responsible for extracellular matrix remodelling - as matrix metalloproteinases (MMPs), particularly MMP-2 and MMP-9 - known to further modulate the movement of inflammatory cell populations, as well as the angiogenic process [30].
In fact, modelling and blood circulation re-establishment is essential and elapses from the fine balance of pro- and anti-angiogenic factors that populate the microenvironment, modulating endothelial cells’ functionality [1,31]. Hypoxic conditions further promote endothelial proliferation and differentiation, assisting angiogenesis in a timely ordered process characterized by the enzyme-mediated basal lamina degradation allowing original vessel crawling; cell chemotaxis; cell proliferation and differentiation; and finally, maturation and remodelling of the newly formed structures [32]. Accordingly, vessel sprouts from the surrounding edges invade the tissue and a competent network of capillaries is formed in a few days.
Progressively, the granulation tissue is replaced by a provisional matrix formed by immature connective tissue, rich in collagen fibres and recruited cells. Experimental animal studies have revealed that residual periodontal ligament fibres - identified perpendicularly to the socket wall and inserted in the bundle bone - are embedded within the newly formed matrix in the direction to the socket centre. This matrix progressively replaces the remnants of periodontal ligament, as well as those of the blood clot and granulation tissue [11]. Quantitative histomorphometric data revealed differences in tissue composition and volume, in the three zones of the socket, 7 days following tooth extraction: in the most coronal region, clot corresponded to 79 (SD 6.4)% of the tissues, 13 (SD 3.8)% corresponded to granulation tissue and 8 (SD 11.1)% to provisional matrix; the middle zone encompassed 64 (SD 3.4)% of clot and 36% of provisional matrix, and the most apical region comprised 100 (SD 4.9)% of the newly formed provisional matrix [6]. In human studies, broadly in the first week post extraction, the clot is almost completely remodelled with granulation tissue [11], being progressively substituted by the provisional matrix during the subsequent weeks postoperatively [14]. Throughout this period, distributed erythrocytes can still be identified but no remnants of the organized clot are present, and granulation tissue and provisional matrix dominate socket volume, constituting around 30% and 50% of the total, respectively [14].
At this phase of healing, the formation of woven bone begins through the penetration of undifferentiated mesenchymal cells that differentiate into the osteogenic lineage. Fingerlike projections of mineralized tissue are broadly identified, which progressively extend from the walls of the socket to the centre of the wound, in a connective tissue matrix laid with collagen fibres, without a structured organization [2,14]. These fingerlike projections surround blood vessels, giving rise to the formation of the Haversian system (primary osteons) - sporadically strengthened by parallel-fibered bone [2,6,14]. In distinct locations the active resorption of the bundle bone originates a direct communication between the newly organized woven mineralized structure and the marrow of the adjacent interdental septa. Trabecular organization thus extends from the old bone at the socket wall, centripetally into the centre of the defect, closely associated with the formation of new blood vessels. The formation of the mineralized tissue gradually confines the provisional matrix structure to the very most central part of the socket [11]. Throughout this period, the maximum content of mineralized tissue is generally achieved, despite the verified decrease of the mineral apposition rate from the lingual to the buccal regions.
Throughout the bone formation phase and osteogenesis activation, several GFs, such as platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), transforming growth factor β (TGF- β), bone morphogenetic proteins (BMPs), vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF), have been evidenced in the early and intermediate stages, with some differences in peak expressions [4,33-36].
BMPs and TGF-β are classically known to regulate a variety of cellular processes, such as cell-cycle progression, differentiation, motility and adhesion, as well as tissue specific functions such as neuronal growth, bone morphogenesis and wound healing [35,36]. Within the socket healing process, and despite the fact that the molecular expression pattern is not entirely clear, it is known that BMPs play a key role in tissue formation and maturation, with peaks of the osteogenic BMPs being evidenced at the initial steps of the healing process [4,37]. In fact, increased expression of BMP2, BMP4, and BMP7 is essential in the differentiation of osteoblasts during repair osteogenesis, being evidenced that BMP-2 activity is required for the initiation of bone healing through experimental mice models carrying a conditional mutation of the BMP-2 gene, showing failure in the bone healing process. Furthermore, it has been demonstrated that the absence of BMP-2 signalling during the intermediate stages of intramembranous repair is a critical event to prevent the formation of cartilage [4,37,38]. As well, BMP-7 is highly expressed during the early and intermediary stages of bone healing [39], as further confirmed in a human socket healing study, which showed the increase in BMP-7 positive cells between the early and intermediate healing phases of alveolar socket healing [14].
Interestingly, BMPs may also stimulate the synthesis and secretion of VEGF, PDGF, FGF, and IGF which regulate angiogenesis [35]. In the rat experimental socket healing model, VEGF expression peak was verified at 7 days [34]. It was observed that a partial blockade of VEGF signalling resulted in the blockade of intramembranous ossification and this growth factor, particularly the VEGF-A isoform, was described as expressed in maturing osteoblasts within the primary mineralization [40]. VEGFR2 is the main receptor for VEGF-A, which is expressed by VEGF-producing cells of the osteoblastic lineage and can regulate survival, migration, and differentiation through autocrine signalling processes [41,42]. Overall, major GFs showed a peak expression between the third and seventh day of bone healing, followed by a gradual reduction in mRNA levels [4].
In addition to the progressive formation of woven bone, this phase is also characterized by the absence of the periodontal ligament in experimental animal models, such as the rat [43] or the dog [6], whereas a residual periodontal ligament structure was found to be still present, structured towards the centre of the alveolus, and contained the epithelial remains of Malassez and cementicles, in a human extraction socket study [44]. Likewise, in an experimental monkey socket healing, Sharpey’s fibres were still identified up to 30 days following extraction [7].
Throughout this period, histomorphometric data from the dog revealed that provisional matrix reduced from 75 (SD 14.4)% to 22 (SD 7.4)% within the most coronal region of the socket, from 44 (SD 5.7)% to 10 (SD 2.2)% at mid region, and from 28 (SD 10.4)% to 5 (SD 2.4)% at the most apical portion. Contrariwise, mineralized bone tissue increased significantly within this period, from 15 (SD 11.1)% to 78 (SD 7.1)% at the marginal region, from 56 (SD 7.3)% to 90 (SD 2.1)% at mid-defect and from 72 (SD 11.2)% to 95 (SD 2.7)% at the apical region, sustaining the high metabolic activity of this phase with a high proliferation rate [6]. In the human, throughout the 6 to 8 week period of healing, granulation tissue is broadly replaced by the provisional matrix and woven bone, corresponding broadly to 60% and 35% of the tissue volume of the socket [14]. Nevertheless, the formed woven bone has limited biomechanical properties, being temporary and sustaining the need to be replaced by a mature bone type [6,45,46].
Bone tissue modelling and remodelling
The final phase of the socket healing process embraces changes in the structure of the bone tissue, which can occur with modification of the architecture and shape (modelling), or without the modification of these parameters (remodelling). Organizationally, the substitution of woven bone with lamellar bone tissue/bone marrow is regarded as remodelling, whereas the resulting dimensional alterations of the alveolar ridge, at the socket wall, comprise bone tissue modelling [2,14,46]. The modelling/remodelling process results from the active interplay between osteoblasts and osteoclasts, being highly modulated by the presence of factors such as macrophage colony stimulating factor (M-CSF), receptor activator of nuclear factor kappa B (RANK), receptor activator of nuclear factor kappa B ligand (RANKL) and osteoprotegerin (OPG) [14,47,48]. The process is normally initiated by the recruitment of osteoclasts, identified lining on the native socket wall and through the marginal trabecular structure of the woven bone. Modelling is similarly distributed between the buccal and lingual surfaces, but since the lingual plate is normally wider, an increased vertical bone loss is attained at the thinner buccal plate. Of additional relevance, modelling occurs earlier than remodelling, as around two-thirds of the process occur up to 3 months postoperatively [49].
Of additional relevance, it is possible to verify that the mesio-distal distance of the extraction socket becomes progressively reduced, due to the mature bone remodelling in the coronal region. This corticalization process - corresponding to the formation of the hard tissue bridge covering the socket entrance, separating the marginal mucosa from the extraction socket [50], takes a considerably long timeframe until complete in humans, sustaining a complete occurrence between 9 and 12 months postoperatively. Concomitantly, the largest part of woven bone apical of the bridge is substituted by lamellar bone and bone marrow, with collagen fibres becoming anchored in the newly formed cortical structure, further fostering the formation of a periosteum-like assembly [11].
90 days after tooth extraction, it becomes very difficult to identify the socket limits in the different experimental models and human clinical studies. Within monkeys’ experimental socket healing model, mature bone was identified within all the assessed regions (i.e., coronal, middle and apical), with emphasis of the formation of mature cortical bone within the coronal region, leading to the socket corticalization, with the middle and apical locations found to be filled by newly formed trabecular bone and bone marrow [7]. After 120 and 180 days of socket healing, a similar trend was observed in the dog model, in which the marginal hard tissue bridge covering the socket entrance had become reinforced by layers of lamellar bone. Simultaneously, a periosteum-like structure had been established through the insertion of collagen fibres into the newly formed cortical bone. After 180 days, 85% of the extraction socket was formed of bone marrow [6]. The identified bone marrow developed into a maturation process, occupying the middle and apical zones with a limited amount of trabecular bone [6,7].
In humans, 12 - 24 weeks after tooth extraction, lamellar bone and bone marrow were broadly identified, populated with vessels, adipocytes, mesenchymal stem cells and inflammatory cells [14]. Other study reported that, at 16 weeks after tooth extraction, 60 - 65% of tissue volume was composed of lamellar bone and bone marrow [51].
In summary, the modelling and remodelling processes promote qualitative and quantitative alterations in the socket, which results in a reduction of the bone crest dimension. These dimensional changes occur most significantly during the first 8 weeks after extraction, due to the high osteoclastic activity present in this period [2,52]. It is known that during the first year after tooth extraction, major morphological and dimensional changes occur, especially in the first three months, in which the most pronounced bone loss is attained [49]. However, post extraction dimensional alterations seem to be related to several additional factors including local site and patients’ characteristics, as well as surgery-related variables [2].
Socket healing with graft materials
Ridge preservation, or socket preservation involves placement of graft material within the socket, which can be further combined with a membrane, or rotated flap. The rationale for socket preservation is sustained for the fact that, once positioned in the fresh socket, graft materials act as solid scaffolds which assist on coagulum stabilization during the early phases of healing, by impeding the interference of destabilizing factors on the clot maturation process [3]. The application of ridge preservation techniques at fresh extractions sites is performed in order to improve the quality and maximize the quantity of bone for the placement and osseointegration of a dental implant, and to avoid post extraction alterations of the ridge profile [11]. A number of biomaterials for socket grafting have been reported, including autogenous, allogeneic, xenogeneic, and alloplastic bone grafts, as well as combinations of the above [53]. Bone grafts differ in terms of their properties of osteoconduction, osteoinduction, osteogenesis, and structural support. These materials may be broadly categorized into slow and fast resorbing grafts. In the slow resorbing category, graft materials maintain their presence and integrity over the long term, and graft particles essentially osseointegrate and have direct contact with newly formed bone. Fast-resorbing materials, on the other hand, degrade through osteoclastic-mediated resorption or dissolution with the degradation products ideally fuelling and enhancing the osteogenic process [54]. Furthermore, some graft materials may have a direct modulatory effect on the cellular behaviour, leading to an increased production of extracellular matrix and its subsequent maturation.
Ideally, the graft material should sustain the entire healing process of an extraction socket, being progressively resorbed and replaced by vital, mature bone [11]. The clinical advantages of bone fillers’ implantation in the extraction socket, aiming alveolar ridge volume preservation and prevention of additional bone grafting procedure prior or during implant placement, are largely supported by the available literature and recent meta-analytical studies [55-57].
Evidence from the experimental data from the dog model reveal that, after tooth extraction and socket grafting procedure, a fibrin network entraps the graft particles. Inflammatory cells and osteoclasts migrate to the surface of the particles, originating a slow and minimal removal of material from the outer surface of the graft particles [58,59]. This response is typical of a foreign body reaction, which is non-immunogenic in nature, non-toxic and chemically inert, but induces a delayed healing response during the earliest stages of socket healing [58]. After 1 - 2 weeks, the osteoclasts are replaced by osteoblasts, that initiate the apposition of osteoid in the collagen bundles of the provisional matrix, leading to a progressive integration of the graft particles [59]. After 2 weeks, immature, newly formed trabecular bone may be observed, particularly in the lateral and apical compartments of the socket, while the central and marginal compartments are broadly occupied by connective tissue that entraps graft particles and inflammatory cells. At this stage of healing, grafted sockets reveal a lower amount of newly formed bone, in the apical and lateral compartments, as compared to non-grafted sockets. In the intermediate and latest phases of healing, the presence of the graft seems not to exert a detrimental effect on bone formation, neither within modelling and remodelling of the socket walls, or regarding the amount of formed mineralized tissue, at 3 months of healing [60]. It thus can be extrapolated that residual particles occupy part of the volume that would have been occupied by bone marrow, in the absence of grafting [61]. Furthermore, when considering the composition of the mineralized tissue present in grafted and non-grafted sockets, similar proportions of woven bone and lamellar bone were observed [62].
From the histological point of view, the implantation of distinct grafts promoted new bone formation, possibly by osteoconduction at the apical and the middle part of the socket, while the coronal and central part of the socket have been found to be mainly occupied by graft particles surrounded by dense connective tissue, even some months following the alveolar ridge preservation surgery [63]. Areas of mineral apposition within the connective tissue fibres were also identified [64]. In accordance, connective tissue showed an inverse tendency, being more present in the coronal compartments (52.4%) compared to the apical compartments (9.5%) of the socket [65]. The greater proportion of the newly formed bone in the coronal area was found to be of the woven-type, while lamellar structures were observed mainly in the apical region. More specifically, the lamellar/woven bone ratio varied from 1 : 12.9 in the coronal area to 1 : 3.8 in the mid-section area, and 1 : 1.7 in the apical area [65].
The composition of vital bone formation recorded in the literature was highly variable, with an amount ranging from 19.3% [66] to 61% [67]. Attained differences between studies could be majorly due to different follow-up times and the degradation profile of the grafting materials [68]. In fact, bioceramics or xenogenic mineralized grafting materials seem to interfere with the earliest stages of socket healing, with the degradation and progressive substitution by mature bone tissue requiring several years, or being nonresorbable even in the long term [69]. Accordingly, residual and/or encapsulated graft particles were found to range from 0% - within fast resorbing materials (e.g., polylactide sponge) [70], to 45,8% - within corticocancellous xenogenic grafts [71]. The majority of the graft particles were in direct contact with mineralized tissue and presented small areas of decalcification on their outer surface [65]. Long term reports addressing residual grafting of dense hydroxyapatite revealed a remaining volume of around 38%, 20 years following the procedure, with direct bone contact and absence of gaps or fibrous tissues at the bone-biomaterial interface [73]. Similarly, the assessment of DBBM resorption with histomorphometric analyses at 8 months (29.8% newly formed bone and 70.2% DBBM), 2 years (69.7% newly formed bone), and 10 years (86.7% newly formed bone), further underlined the slow biodegradation of this graft but a high integration with new bone, providing a dense scaffold for further bone deposition and a good support for dental implants’ placement [74].
Overall, and despite the verified hindrances on the initial phases of the healing process, socket grafting seems to be effective on allowing for the formation of mature bone, further promoting ridge preservation by limiting the physiologic reduction, as compared to ungrafted healing, particularly on the preservation of the mid-buccal and mid-lingual height [55-57].
Socket healing with autologous platelet concentrates
In addition to the use of autogenous, allogeneic, xenogeneic, and alloplastic biomaterials for socket preservation, autologous platelet concentrates (APCs) are widely reported in the literature to enhance extraction socket healing [73-76]. APCs refer to a group of products that are prepared from autologous blood and are intended to enhance tissue regeneration by triggering the natural healing process with a supplement of highly concentrated bioactive factors [77]. APCs are classified into 4 main families depending on their leukocyte and fibrin content: pure platelet-rich plasma (P-PRP), leukocyte- and platelet-rich plasma (L-PRP), pure platelet-rich fibrin (P-PRF), and leukocyte- and platelet-rich fibrin (L-PRF) [78]. Each family of platelet preparations has different preparation protocol, composition, biological content, and potential application [79]. Therefore, although APCs are widely described as surgical adjuvants to promote tissue healing [73,74,80], the clinical impact of their use in socket healing, either regarding soft or hard tissue, is inconsistent [75,76], as the use of different compositions and preparation methodologies alters the clinical outcome.
The beneficial effect of APCs in extraction socket healing to promote osteoid formation and extraction socket epithelization is mainly attributed to the release of cytokines and growth factors, immersed in the fibrin mesh, platelets, and leukocytes [81].
According to the fibrin structure, APCs can be present as liquid solutions or in an activated gel form [78]. L-PRP and P-PRP form loose fibrin meshwork after platelet activation and most of the growth factors are released within the first 24 hours of preparation [79]. In contrast, fibrin polymerizes under centrifugation in P-PRF and L-PRF, resulting in strong and dense fibrin network that entraps cells and their growth factors for sustained release, up to 28 days upon application [79,82]. Fibrin matrix is described as a favourable scaffold for mesenchymal stem cells proliferation, differentiation [83], vascular ingrowth [84] and is safe to be left exposed in oral cavity to guide epithelial cell migration to its surface, leading to natural wound reepithelization by secondary intention healing [85,86]. Multiple studies have found accelerated soft tissue healing over APCs’ filled extraction sockets, as compared to regular blood clot controls [87-89].
The idea of using platelet supplementation to enhance extraction wound healing is primarily based on the ability of platelets to trigger healing response upon release of various growth factors. Platelets contain alpha granules, which degranulate upon platelet activation following the release of growth factors and stimulate cell migration and enhance cellular-level events to expedite wound healing. Experimental and clinical studies have found that platelet growth factors, such as the FGF and TGFβ-1, stimulate bone formation during osseous healing [90,91]. PDGF regulates the migration and proliferation of mesenchymal stem cells in the extraction site and stimulates endothelial, fibroblastic and osteoblastic proliferation to facilitate socket healing [92-94]. Additionally, VEGF, released from platelets, stimulates the proliferation and differentiation of numerous cell types essential for vascular formation during angiogenesis and vasculogenesis, helping to transport nutrients and oxygen mandatory for the extraction wound healing process [95].
The leukocytes within the leukocyte-containing APCs mainly consist of lymphocytes, followed by neutrophils, monocytes, eosinophils, and basophils [96,97]. Although there are some controversies in the literature regarding the potential positive effect of leukocytes on wound healing [98,99], overpopulation of lymphocytes within the L-PRF and L-PRP, as compared to regular blood samples, seems to display an improved immunomodulatory role within the healing processes, as lymphocytes are determinant effectors of the cellular component of the innate immune response [100,101]. Lymphocytes were also shown to release insulin-like growth factor 1 (IGF-1) [102], which plays a central role in cellular growth, differentiation, survival, and cell cycle progression [103].
Neutrophils are known to participate in the antimicrobial host defence, both as the first line of the cell-based innate immune defence, as well as within the initiation of the adaptive immunity [104]. This contributes toward the prevention of bacterial contamination within the surgical site in addition to platelet antimicrobial peptides (platelet factor 4, RANTES, connective tissue activating peptide 3, platelet basic protein, thymosin β-4, fibrinopeptide B, and fibrinopeptide A), which were shown to be potent against Escherichia coli and Staphylococcus aureus, and other pathogenic bacteria [105]. Leukocyte containing APCs were found to significantly reduce infection-related complications and the incidence of alveolar osteitis after mandibular third molar surgical extractions [89,106,107].
Monocytes, within L-PRP and L-PRF delivered to the alveolar socket, differentiate into tissue macrophages. Macrophages are key mediators of the wound healing process, playing a pivotal role in the transition between wound inflammatory and repair phase, with particular emphasis on osteogenesis [108,109]. Functions of macrophages also involve phagocytosis and the release of enzymes, such as collagenase, elastase, and plasminogen activator, facilitating tissue debridement and wound repair [109]. Macrophages release TGF to stimulate keratinocytes, IL-1, FGF, and TNFα that stimulate collagen production by the fibroblasts and improve angiogenesis. PDGF, which was formerly considered to be specific to platelets, was also found to be produced by macrophages [110].
Apart from the fundamental contribute of leukocytes to the immune-inflammatory activation, major cell populations also release various bioactive factors, when at the extraction wound site. Leukocytes present anti-nociceptive effects through the release of different chemokines, anti-inflammatory interleukins (IL-4, IL-10, and IL-13), and opioid peptides (b-endorphin, metenkephalin, and dynorphin-A), and can, therefore, promote a clinically relevant inhibition of postoperative pain. During the inflammatory phase of wound healing, these cytokines counteract the effects of the pro-inflammatory mediators generated naturally in the early stages of inflammation [111], thus significantly diminishing the postoperative pain sensation, as it was shown in multiple clinical studies [89,107,112].
Socket preservation procedure demands slow resorption and adequate space maintaining biomaterials to stabilize the coagulum and counteract post extraction ridge resorption process, as suggested by Araújo et al. in 2008 [61]. APCs are considered as a weak osteoconductive scaffold [114], and are not expected to serve as an osteoconductive biomaterial for ridge preservation alone, in contrast to slow resorption xenografts, allografts, or alloplasts, used specifically within socket preservation therapeutic approaches. Recent studies by Mendoza-Azpur et al. [115] and De Angelis et al. [116] found significantly greater horizontal and vertical bone resorption within sole APCs filled extraction sockets compared to sockets augmented with osteoconductive grafting biomaterials. Notwithstanding, APCs, being a source of growth factors, containing living cells, as well as matrix proteins (thrombospondin-1, fibronectin, and vitronectin), glycosaminoglycans (heparin, hyaluronic acid), and a complex set of regulatory cytokines, when delivered to the extraction socket seem to improve the capacity of tissues to regenerate by enhancing cellular chemoattraction, angiogenesis, proliferation and differentiation [86]. Despite the fact that the study by Zhang et al. [117] did not detected significant differences in post extraction alveolar bone remodelling among L-PRF treated and regular blood clot sites, L-PRF significantly improved gingival healing and exhibited a significantly greater new bone production. APCs were shown to improve new bone formation either alone [118,119] or in combination with osteoconductive materials [120,121] in healing extraction sockets. To et al. [118] found significantly higher bone formation ratio and higher expressions of osteocalcin and osteopontin in the extraction sockets filled with platelet-rich fibrin derivative than in the control blood clot group in the beagle dog model. Similarly, Hauser et al. [119] found significantly improved trabecular microarchitecture and significantly higher bone quality in the L-PRF group compared to blood clot controls at 8 weeks microcomputed tomographic analysis, in human post extraction sites. This is in accordance with another clinical study in which histologic evaluation of the human bone specimen retrieved at 6 weeks post extraction, with the use of L-PRF alone, revealed accelerated bone formation, with bone trabeculae occupying approximately 30% of the total tissue area [122]. Similarly, histological evaluation of 8 weeks biopsies of FDBA/β-TCP/PRP grafted sockets showed 37% newly formed bone and 28% remaining graft particles compared to 28% new bone formation and 35% remaining graft particles in FDBA/β-TCP group, with no addition of PRP [120]. Another study investigated addition of L-PRF to xenograft particulate graft, which resulted in significantly less postoperative pain, better wound healing and significantly less vertical and horizontal post extraction bone resorption compared to xenograft alone group [116].
CONCLUSIONS
In this manuscript, the alveolar socket healing process following tooth extraction was critically described, from a molecular and cellular point of view, further addressing histological and histomorphometric data from experimental animal models and human clinical studies. It was verified that each tissue component participating in the healing process (i.e. the blood clot, granulation tissue, provisional matrix, woven bone and lamellar bone) revealed a time- and space-dependent interplay, with discrete differences between models and a high inter-individual variability, but converging to mature bone formation and regeneration of the lost tissues. The biological aspects of the healing process following socket grafting were also detailed, sustaining the capability of particulate grafts and autologous platelets concentrates to modulate the molecular and cellular events throughout the healing process.
Overall, the detailed knowledge of the biological events associated with the socket healing process, as well as the temporal sequence of the healing events, is of foundational importance to improve clinical treatment outcomes and assist on the development of innovative biologically-based regenerative approaches for enhanced tissue healing.
Of additional relevance, and aiming to establish more robust and reliable comparisons and conclusions between assayed interventions for enhanced socket healing, future studies shall focus on unbiased control reports and experimental data homogeneity, assisting on effective determination of efficacy, effectiveness and patient satisfaction of the assayed therapeutic options. Also, experimental outcomes should be reformed to include clinical-meaningful and patient-centred outcomes, improving evidence-based decision making. Lastly, and since the current body of evidence derives majorly from single socket healing experiments, this poses potential limitations on the translational application to multiple extraction sites, being imperative to increase the evidence base within this path.
Acknowledgments
ACKNOWLEDGMENTS AND DISCLOSURE STATEMENTS
The work was partially supported by the project UID/QUI/5006/2019 with funding from FCT/MCTES through national funds. The authors report no conflicts of interest related to the present review.
REFERENCES
- 1.Araújo MG, Silva CO, Misawa M, Sukekava F. Alveolar socket healing: what can we learn? Periodontol 2000. 2015 Jun;68(1):122-34. [DOI] [PubMed]
- 2.Pagni G, Pellegrini G, Giannobile WV, Rasperini G. Postextraction alveolar ridge preservation: biological basis and treatments. Int J Dent. 2012;2012:151030. [DOI] [PMC free article] [PubMed]
- 3.Vieira AE, Repeke CE, Ferreira Junior Sde B, Colavite PM, Biguetti CC, Oliveira RC, Assis GF, Taga R, Trombone AP, Garlet GP. Intramembranous bone healing process subsequent to tooth extraction in mice: micro-computed tomography, histomorphometric and molecular characterization. PLoS One. 2015 May 29;10(5):e0128021. [DOI] [PMC free article] [PubMed]
- 4.Smith N. A comparative histological and radiographic study of extraction socket healing in the rat. Aust Dent J. 1974 Aug;19(4):250-4. [DOI] [PubMed]
- 5.Cardaropoli G, Araújo M, Lindhe J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J Clin Periodontol. 2003 Sep;30(9):809-18. [DOI] [PubMed]
- 6.Scala A, Lang NP, Schweikert MT, de Oliveira JA, Rangel-Garcia I Jr, Botticelli D. Sequential healing of open extraction sockets. An experimental study in monkeys. Clin Oral Implants Res. 2014 Mar;25(3):288-295. [DOI] [PubMed]
- 7.Richardson M. Acute wounds: an overview of the physiological healing process. Nurs Times. 2004 Jan 27-Feb 2;100(4): 50-3. [PubMed]
- 8.Aukhil I. Biology of wound healing. Periodontol 2000. 2000 Feb;22:44-50. [DOI] [PubMed]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097. [DOI] [PMC free article] [PubMed]
- 10.Amler MH. The time sequence of tissue regeneration in human extraction wounds. Oral Surg Oral Med Oral Pathol. 1969 Mar;27(3):309-18. [DOI] [PubMed]
- 11.Farina R, Trombelli L. Wound healing of extraction sockets. Endodon Top. 2011 Sep;25(1):16-43. [DOI]
- 12.Davies JE, Hosseini MM. Histodynamics of Endosseous Wound Healing. In: Davies JE, editors. Bone engineering. Toronto: em squared inc.; 2000. p. 1-14.
- 13.Amler MH, Johnson PL, Salman I. Histological and histochemical investigation of human alveolar socket healing in undisturbed extraction wounds. J Am Dent Assoc. 1960 Jul;61:32-44. [DOI] [PubMed]
- 14.Trombelli L, Farina R, Marzola A, Bozzi L, Liljenberg B, Lindhe J. Modeling and remodeling of human extraction sockets. Plast Reconstr Surg. 2006 Jun;117(7 Suppl):12S-34S. [DOI] [PubMed]
- 15.Broughton G 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006 Jun; 117(7 Suppl):12S-34S. [DOI] [PubMed]
- 16.Monroe DM, Hoffman M. The clotting system - a major player in wound healing. Haemophilia. 2012 Jul;18 Suppl 5:11-6. [DOI] [PubMed]
- 17.Schenk RK, Buser D, Hardwick WR, Dahlin C. Healing pattern of bone regeneration in membrane-protected defects: a histologic study in the canine mandible. Int J Oral Maxillofac Implants. 1994 Jan-Feb;9(1):13-29. [PubMed]
- 18.Chappuis V, Araújo MG, Buser D. Clinical relevance of dimensional bone and soft tissue alterations post-extraction in esthetic sites. Periodontol 2000. 2017 Feb;73(1):73-83. [DOI] [PubMed]
- 19.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008 Sep-Oct;16(5):585-601. [DOI] [PubMed]
- 20.Okamoto T, Okamoto R, Alves Rezende MC, Gabrielli MF. Interference of the blood clot on granulation tissue formation after tooth extraction. Histomorphological study in rats. Braz Dent J. 1994;5(2):85-92. [PubMed]
- 21.Garlet GP, Horwat R, Ray HL Jr, Garlet TP, Silveira EM, Campanelli AP, Trombone AP, Letra A, Silva RM. Expression analysis of wound healing genes in human periapical granulomas of progressive and stable nature. J Endod. 2012 Feb;38(2):185-90. [DOI] [PubMed]
- 22.Eming SA, Hammerschmidt M, Krieg T, Roers A. Interrelation of immunity and tissue repair or regeneration. Semin Cell Dev Biol. 2009 Jul;20(5):517-27. [DOI] [PubMed]
- 23.Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B, Brückner UB, Nilsson B, Gebhard F, Lambris JD, Huber-Lang M. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010 Nov 1;185(9):5628-36. [DOI] [PMC free article] [PubMed]
- 24.McMillan MD. Neutrophils in the molar tooth extraction wound in the rat: a transmission electron microscope (TEM) study. J Oral Pathol Med. 1999 Aug;28(7):297-302. [DOI] [PubMed]
- 25.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011 Jul 11;13:e23. [DOI] [PMC free article] [PubMed]
- 26.De Coster P, Browaeys H, De Bruyn H. Healing of extraction sockets filled with BoneCeramic® prior to implant placement: preliminary histological findings. Clin Implant Dent Relat Res. 2011 Mar;13(1):34-45. [DOI] [PubMed]
- 27.Pang P, Shimo T, Takada H, Matsumoto K, Yoshioka N, Ibaragi S, Sasaki A. Expression pattern of sonic hedgehog signaling and calcitonin gene-related peptide in the socket healing process after tooth extraction. Biochem Biophys Res Commun. 2015 Nov 6;467(1):21-6. [DOI] [PubMed]
- 28.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002 Jun;84(6):1032-44. [DOI] [PubMed]
- 29.Berendsen AD, Pinnow EL, Maeda A, Brown AC, McCartney-Francis N, Kram V, Owens RT, Robey PG, Holmbeck K, de Castro LF, Kilts TM, Young MF. Biglycan modulates angiogenesis and bone formation during fracture healing. Matrix Biol. 2014 Apr;35:223-31. [DOI] [PMC free article] [PubMed]
- 30.Kanyama M, Kuboki T, Akiyama K, Nawachi K, Miyauchi FM, Yatani H, Kubota S, Nakanishi T, Takigawa M. Connective tissue growth factor expressed in rat alveolar bone regeneration sites after tooth extraction. Arch Oral Biol. 2003 Oct;48(10):723-30. [DOI] [PubMed]
- 31.Schmidt-Bleek K, Schell H, Lienau J, Schulz N, Hoff P, Pfaff M, Schmidt G, Martin C, Perka C, Buttgereit F, Volk HD, Duda G. Initial immune reaction and angiogenesis in bone healing. J Tissue Eng Regen Med. 2014 Feb;8(2):120-30. [DOI] [PubMed]
- 32.Carano RA, Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today. 2003 Nov 1;8(21):980-9. [DOI] [PubMed]
- 33.Lin Z, Rios HF, Volk SL, Sugai JV, Jin Q, Giannobile WV. Gene expression dynamics during bone healing and osseointegration. J Periodontol. 2011 Jul;82(7):1007-17. [DOI] [PMC free article] [PubMed]
- 34.Abreu FA, Ferreira CL, Silva GA, Paulo Cde O, Miziara MN, Silveira FF, Alves JB. Effect of PDGF-BB, IGF-I growth factors and their combination carried by liposomes in tooth socket healing. Braz Dent J. 2013;24(4):299-307. [DOI] [PubMed]
- 35.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005 Dec;36(12):1392-404. [DOI] [PubMed]
- 36.Majidinia M, Sadeghpour A, Yousefi B. The roles of signaling pathways in bone repair and regeneration. J Cell Physiol. 2018 Apr;233(4):2937-2948. [DOI] [PubMed]
- 37.Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006 Dec;2(12):e216. [DOI] [PMC free article] [PubMed]
- 38.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006 Dec;38(12): 1424-9. [DOI] [PubMed]
- 39.Spector JA, Luchs JS, Mehrara BJ, Greenwald JA, Smith LP, Longaker MT. Expression of bone morphogenetic proteins during membranous bone healing. Plast Reconstr Surg. 2001 Jan;107(1):124-34. [DOI] [PubMed]
- 40.Runyan CM, Gabrick KS. Biology of Bone Formation, Fracture Healing, and Distraction Osteogenesis. J Craniofac Surg. 2017 Jul;28(5):1380-1389. [DOI] [PubMed]
- 41.Watson EC, Adams RH. Biology of Bone: The Vasculature of the Skeletal System. Cold Spring Harb Perspect Med. 2018 Jul 2;8(7). pii: a031559. [DOI] [PMC free article] [PubMed]
- 42.Hu K, Olsen BR. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest. 2016 Feb;126(2):509-26. [DOI] [PMC free article] [PubMed]
- 43.Hsieh YD, Devlin H, Roberts C. Early alveolar ridge osteogenesis following tooth extraction in the rat. Arch Oral Biol. 1994 May;39(5):425-8. [DOI] [PubMed]
- 44.Devlin H, Sloan P. Early bone healing events in the human extraction socket. Int J Oral Maxillofac Surg. 2002 Dec;31(6):641-5. [DOI] [PubMed]
- 45.Araújo MG, Berglundh T, Albrekstsson T, Lindhe J. Bone formation in furcation defects. An experimental study in the dog. J Clin Periodontol. 1999 Oct;26(10):643-52. [DOI] [PubMed]
- 46.Van der Weijden F, Dell'Acqua F, Slot DE. Alveolar bone dimensional changes of post-extraction sockets in humans: a systematic review. J Clin Periodontol. 2009 Dec;36(12):1048-58. [DOI] [PubMed]
- 47.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000 Jan;15(1):2-12. [DOI] [PubMed]
- 48.Karst M, Gorny G, Galvin RJ, Oursler MJ. Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-beta regulation of osteoclast differentiation. J Cell Physiol. 2004 Jul;200(1):99-106. [DOI] [PMC free article] [PubMed]
- 49.Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003 Aug;23(4):313-23. [PubMed]
- 50.Bertl K, Kukla EB, Albugami R, Beck F, Gahleitner A, Stavropoulos A. Timeframe of socket cortication after tooth extraction: A retrospective radiographic study. Clin Oral Implants Res. 2018 Jan;29(1):130-138. [DOI] [PubMed]
- 51.Lindhe J, Cecchinato D, Bressan EA, Toia M, Araújo MG, Liljenberg B. The alveolar process of the edentulous maxilla in periodontitis and non-periodontitis subjects. Clin Oral Implants Res. 2012 Jan;23(1):5-11. [DOI] [PubMed]
- 52.Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005 Feb;32(2):212-8. [DOI] [PubMed]
- 53.Stumbras A, Kuliesius P, Januzis G, Juodzbalys G. Alveolar Ridge Preservation after Tooth Extraction Using Different Bone Graft Materials and Autologous Platelet Concentrates: a Systematic Review. J Oral Maxillofac Res. 2019 Mar 31;10(1):e2. [DOI] [PMC free article] [PubMed]
- 54.Kassim B, Ivanovski S, Mattheos N. Current perspectives on the role of ridge (socket) preservation procedures in dental implant treatment in the aesthetic zone. Aust Dent J. 2014 Mar;59(1):48-56. [DOI] [PubMed]
- 55.Willenbacher M, Al-Nawas B, Berres M, Kämmerer PW, Schiegnitz E. The Effects of Alveolar Ridge Preservation: A Meta-Analysis. Clin Implant Dent Relat Res. 2016 Dec;18(6):1248-1268. [DOI] [PubMed]
- 56.Iocca O, Farcomeni A, Pardiñas Lopez S, Talib HS. Alveolar ridge preservation after tooth extraction: a Bayesian Network meta-analysis of grafting materials efficacy on prevention of bone height and width reduction. J Clin Periodontol. 2017 Jan;44(1):104-114. [DOI] [PubMed]
- 57.Avila-Ortiz G, Elangovan S, Kramer KW, Blanchette D, Dawson DV. Effect of alveolar ridge preservation after tooth extraction: a systematic review and meta-analysis. J Dent Res. 2014 Oct;93(10):950-8. [DOI] [PMC free article] [PubMed]
- 58.Luttikhuizen DT, Harmsen MC, Van Luyn MJ. Cellular and molecular dynamics in the foreign body reaction. Tissue Eng. 2006 Jul;12(7):1955-70. [DOI] [PubMed]
- 59.Araújo MG, Liljenberg B, Lindhe J. Dynamics of Bio-Oss Collagen incorporation in fresh extraction wounds: an experimental study in the dog. Clin Oral Implants Res. 2010 Jan;21(1):55-64. [DOI] [PubMed]
- 60.Araújo MG, Lindhe J. Ridge preservation with the use of Bio-Oss collagen: A 6-month study in the dog. Clin Oral Implants Res. 2009 May;20(5):433-40. [DOI] [PubMed]
- 61.Araújo M, Linder E, Wennström J, Lindhe J. The influence of Bio-Oss Collagen on healing of an extraction socket: an experimental study in the dog. Int J Periodontics Restorative Dent. 2008 Apr;28(2):123-35. [PubMed]
- 62.Cardaropoli G, Araújo M, Hayacibara R, Sukekava F, Lindhe J. Healing of extraction sockets and surgically produced - augmented and non-augmented - defects in the alveolar ridge. An experimental study in the dog. J Clin Periodontol. 2005 May;32(5):435-40. [DOI] [PubMed]
- 63.Molly L, Vandromme H, Quirynen M, Schepers E, Adams JL, van Steenberghe D. Bone formation following implantation of bone biomaterials into extraction sites. J Periodontol. 2008 Jun;79(6):1108-15. [DOI] [PubMed]
- 64.Mardas N, Chadha V, Donos N. Alveolar ridge preservation with guided bone regeneration and a synthetic bone substitute or a bovine-derived xenograft: a randomized, controlled clinical trial. Clin Oral Implants Res. 2010 Jul;21(7):688-98. [DOI] [PubMed]
- 65.Artzi Z, Tal H, Dayan D. Porous bovine bone mineral in healing of human extraction sockets. Part 1: histomorphometric evaluations at 9 months. J Periodontol. 2000 Jun;71(6):1015-23. [DOI] [PubMed]
- 66.Beck TM, Mealey BL. Histologic analysis of healing after tooth extraction with ridge preservation using mineralized human bone allograft. J Periodontol. 2010 Dec;81(12):1765-72. [DOI] [PubMed]
- 67.Vance GS, Greenwell H, Miller RL, Hill M, Johnston H, Scheetz JP. Comparison of an allograft in an experimental putty carrier and a bovine-derived xenograft used in ridge preservation: a clinical and histologic study in humans. Int J Oral Maxillofac Implants. 2004 Jul-Aug;19(4):491-7. [PubMed]
- 68.Sadeghi R, Babaei M, Miremadi SA, Abbas FM. A randomized controlled evaluation of alveolar ridge preservation following tooth extraction using deproteinized bovine bone mineral and demineralized freeze-dried bone allograft. Dent Res J (Isfahan). 2016 Mar-Apr;13(2):151-9. [DOI] [PMC free article] [PubMed]
- 69.Mordenfeld A, Hallman M, Johansson CB, Albrektsson T. Histological and histomorphometrical analyses of biopsies harvested 11 years after maxillary sinus floor augmentation with deproteinized bovine and autogenous bone. Clin Oral Implants Res. 2010 Sep;21(9):961-70. [DOI] [PubMed]
- 70.Serino G, Biancu S, Iezzi G, Piattelli A. Ridge preservation following tooth extraction using a polylactide and polyglycolide sponge as space filler: a clinical and histological study in humans. Clin Oral Implants Res. 2003 Oct;14(5):651-8. [DOI] [PubMed]
- 71.Serrano CA, Castellanos P, Botticelli D. Use of Combination of Allografts and Xenografts for Alveolar Ridge Preservation Procedures: A Clinical and Histological Case Series. Implant Dent. 2018 Aug;27(4):467-473. [DOI] [PubMed]
- 72.Mangano C, Piattelli A, Perrotti V, Iezzi G. Dense hydroxyapatite inserted into postextraction sockets: a histologic and histomorphometric 20-year case report. J Periodontol. 2008 May;79(5):929-33. [DOI] [PubMed]
- 73.Sartori S, Silvestri M, Forni F, Icaro Cornaglia A, Tesei P, Cattaneo V. Ten-year follow-up in a maxillary sinus augmentation using anorganic bovine bone (Bio-Oss). A case report with histomorphometric evaluation. Clin Oral Implants Res. 2003 Jun;14(3):369-72. [DOI] [PubMed]
- 74.Temmerman A, Vandessel J, Castro A, Jacobs R, Teughels W, Pinto N, Quirynen M. The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: a split-mouth, randomized, controlled clinical trial. J Clin Periodontol. 2016 Nov;43(11):990-999. [DOI] [PubMed]
- 75.Alissa R, Esposito M, Horner K, Oliver R. The influence of platelet-rich plasma on the healing of extraction sockets: an explorative randomised clinical trial. Eur J Oral Implantol. 2010 Summer;3(2):121-34. [PubMed]
- 76.Del Fabbro M, Bortolin M, Taschieri S. Is autologous platelet concentrate beneficial for post-extraction socket healing? A systematic review. Int J Oral Maxillofac Surg. 2011 Sep;40(9):891-900. [DOI] [PubMed]
- 77.Donos N, Dereka X, Calciolari E. The use of bioactive factors to enhance bone regeneration: A narrative review. J Clin Periodontol. 2019 Jun;46 Suppl 21:124-161. [DOI] [PubMed]
- 78.Yung YL, Fu SC, Cheuk YC, Qin L, Ong MT, Chan KM, Yung PS. Optimisation of platelet concentrates therapy: Composition, localisation, and duration of action. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2017 Jan 30;7:27-36. [DOI] [PMC free article] [PubMed]
- 79.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009 Mar;27(3):158-67. [DOI] [PubMed]
- 80.Dohan Ehrenfest DM, Bielecki T, Jimbo R, Barbé G, Del Corso M, Inchingolo F, Sammartino G. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF). Curr Pharm Biotechnol. 2012 Jun;13(7):1145-52. [DOI] [PubMed]
- 81.Bielecki T, Dohan Ehrenfest DM. Leukocyte- and platelet-rich Plasma (L-PRP)/fibrin (L-PRF) in medicine - past, present, future. Curr Pharm Biotechnol. 2012 Jun;13(7):i-ii. [DOI] [PubMed]
- 82.Jeyaraj PE, Chakranarayan A. Soft Tissue Healing and Bony Regeneration of Impacted Mandibular Third Molar Extraction Sockets, Following Postoperative Incorporation of Platelet-rich Fibrin. Ann Maxillofac Surg. 2018 Jan-Jun;8(1):10-18. [DOI] [PMC free article] [PubMed]
- 83.He L, Lin Y, Hu X, Zhang Y, Wu H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009 Nov;108(5):707-13. [DOI] [PubMed]
- 84.Dohan Ehrenfest DM, Doglioli P, de Peppo GM, Del Corso M, Charrier JB. Choukroun's platelet-rich fibrin (PRF) stimulates in vitro proliferation and differentiation of human oral bone mesenchymal stem cell in a dose-dependent way. Arch Oral Biol. 2010 Mar;55(3):185-94. [DOI] [PubMed]
- 85.Ratajczak J, Vangansewinkel T, Gervois P, Merckx G, Hilkens P, Quirynen M, Lambrichts I, Bronckaers A. Angiogenic Properties of 'Leukocyte- and Platelet-Rich Fibrin'. Sci Rep. 2018 Oct 2;8(1):14632. [DOI] [PMC free article] [PubMed]
- 86.Jain V, Triveni MG, Kumar AB, Mehta DS. Role of platelet-rich-fibrin in enhancing palatal wound healing after free graft. Contemp Clin Dent. 2012 Sep;3(Suppl 2):S240-3. [DOI] [PMC free article] [PubMed]
- 87.Thakkar DJ, Deshpande NC, Dave DH, Narayankar SD. A comparative evaluation of extraction socket preservation with demineralized freeze-dried bone allograft alone and along with platelet-rich fibrin: A clinical and radiographic study. Contemp Clin Dent. 2016 Jul-Sep;7(3):371-6. [DOI] [PMC free article] [PubMed]
- 88.Marenzi G, Riccitiello F, Tia M, di Lauro A, Sammartino G. Influence of Leukocyte- and Platelet-Rich Fibrin (L-PRF) in the Healing of Simple Postextraction Sockets: A Split-Mouth Study. Biomed Res Int. 2015;2015:369273. [DOI] [PMC free article] [PubMed]
- 89.Dutta SR, Passi D, Singh P, Sharma S, Singh M, Srivastava D. A randomized comparative prospective study of platelet-rich plasma, platelet-rich fibrin, and hydroxyapatite as a graft material for mandibular third molar extraction socket healing. Natl J Maxillofac Surg. 2016 Jan-Jun;7(1):45-51. [DOI] [PMC free article] [PubMed]
- 90.Daugela P, Grimuta V, Sakavicius D, Jonaitis J, Juodzbalys G. Influence of leukocyte- and platelet-rich fibrin (L-PRF) on the outcomes of impacted mandibular third molar removal surgery: A split-mouth randomized clinical trial. Quintessence Int. 2018;49(5):377-388. [DOI] [PubMed]
- 91.He B, Ou Y, Chen S, Zhao W, Zhou A, Zhao J, Li H, Jiang D, Zhu Y. Designer bFGF-incorporated d-form self-assembly peptide nanofiber scaffolds to promote bone repair. Mater Sci Eng C Mater Biol Appl. 2017 May 1;74:451-458. [DOI] [PubMed]
- 92.Poniatowski ŁA, Wojdasiewicz P, Gasik R, Szukiewicz D. Transforming growth factor Beta family: insight into the role of growth factors in regulation of fracture healing biology and potential clinical applications. Mediators Inflamm. 2015;2015:137823. [DOI] [PMC free article] [PubMed]
- 93.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006 Mar;101(3):e45-50. [DOI] [PubMed]
- 94.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006 Mar;101(3):e56-60. [DOI] [PubMed]
- 95.Fiedler J, Etzel N, Brenner RE. To go or not to go: Migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. J Cell Biochem. 2004 Nov 15;93(5):990-8. [DOI] [PubMed]
- 96.Boëck-Neto RJ, Artese L, Piattelli A, Shibli JA, Perrotti V, Piccirilli M, Marcantonio E Jr. VEGF and MVD expression in sinus augmentation with autologous bone and several graft materials. Oral Dis. 2009 Mar;15(2):148-54. [DOI] [PubMed]
- 97.Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB. Three-dimensional architecture and cell composition of a Choukroun's platelet-rich fibrin clot and membrane. J Periodontol. 2010 Apr;81(4):546-55. [DOI] [PubMed]
- 98.Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, Landes C, Sader R, Kirkpatrick C, Choukroun J. Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol. 2014 Dec;40(6):679-89. [DOI] [PubMed]
- 99.Anitua E, Zalduendo MM, Prado R, Alkhraisat MH, Orive G. Morphogen and proinflammatory cytokine release kinetics from PRGF-Endoret fibrin scaffolds: evaluation of the effect of leukocyte inclusion. J Biomed Mater Res A. 2015 Mar;103(3):1011-20. [DOI] [PubMed]
- 100.Dohan Ehrenfest DM, Zhang CQ, Pinto NR, Bielecki T. Merchants shall be expelled from the Temple: the PRGF(®) (Plasma-Preparation Rich in Growth Factors)-Endoret(®) case. Muscles Ligaments Tendons J. 2015 Feb 5;4(4):473-7. [DOI] [PMC free article] [PubMed]
- 101.Turner ML. Cell adhesion molecules: a unifying approach to topographic biology. Biol Rev Camb Philos Soc. 1992 Aug;67(3):359-77. [DOI] [PubMed]
- 102.Cieslik-Bielecka A, Dohan Ehrenfest DM, Lubkowska A, Bielecki T. Microbicidal properties of Leukocyte- and Platelet-Rich Plasma/Fibrin (L-PRP/L-PRF): new perspectives. J Biol Regul Homeost Agents. 2012 Apr-Jun; 26(2 Suppl 1):43S-52S. [PubMed]
- 103.Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, Rudolph R, Jameson J, Havran WL. A role for human skin-resident T cells in wound healing. J Exp Med. 2009 Apr 13;206(4):743-50. [DOI] [PMC free article] [PubMed]
- 104.Yakar S, Courtland HW, Clemmons D. IGF-1 and bone: New discoveries from mouse models. J Bone Miner Res. 2010 Dec;25(12):2543-52. [DOI] [PMC free article] [PubMed]
- 105.Mócsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013 Jul 1;210(7):1283-99. [DOI] [PMC free article] [PubMed]
- 106.Tang YQ, Yeaman MR, Selsted ME. Antimicrobial peptides from human platelets. Infect Immun. 2002 Dec;70(12): 6524-33. [DOI] [PMC free article] [PubMed]
- 107.Eshghpour M, Dastmalchi P, Nekooei AH, Nejat A. Effect of platelet-rich fibrin on frequency of alveolar osteitis following mandibular third molar surgery: a double-blinded randomized clinical trial. J Oral Maxillofac Surg. 2014 Aug;72(8): 1463-7. [DOI] [PubMed]
- 108.Al-Hamed FS, Tawfik MA-M, Abdelfadil E. Clinical effects of platelet-rich fibrin (PRF) following surgical extraction of lower third molar. Saudi J Dent Res. 2017 Jan-Jul;8(1-2):19-25. [DOI]
- 109.Sinder BP, Pettit AR, McCauley LK. Macrophages: Their Emerging Roles in Bone. J Bone Miner Res. 2015 Dec;30(12):2140-9. [DOI] [PMC free article] [PubMed]
- 110.Clark RA. Fibrin and wound healing. Ann N Y Acad Sci. 2001;936:355-67. [DOI] [PubMed]
- 111.Shimokado K, Raines EW, Madtes DK, Barrett TB, Benditt EP, Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277-86. [DOI] [PubMed]
- 112.Dohan Ehrenfest DM, Andia I, Zumstein MA, Zhang CQ, Pinto NR, Bielecki T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014 May 8;4(1):3-9. [DOI] [PMC free article] [PubMed]
- 113.Bilginaylar K, Uyanik LO. Evaluation of the effects of platelet-rich fibrin and piezosurgery on outcomes after removal of ımpacted mandibular third molars. Br J Oral Maxillofac Surg. 2016 Jul;54(6):629-33. [DOI] [PubMed]
- 114.Kobayashi M, Kawase T, Horimizu M, Okuda K, Wolff LF, Yoshie H. A proposed protocol for the standardized preparation of PRF membranes for clinical use. Biologicals. 2012 Sep;40(5):323-9. [DOI] [PubMed]
- 115.Mendoza-Azpur G, Olaechea A, Padial-Molina M, Gutiérrez-Garrido L, O'Valle F, Mesa F, Galindo-Moreno P. Composite Alloplastic Biomaterial vs. Autologous Platelet-Rich Fibrin in Ridge Preservation. J Clin Med. 2019 Feb 9;8(2). pii: E223. [DOI] [PMC free article] [PubMed]
- 116.De Angelis P, De Angelis S, Passarelli PC, Liguori MG, Manicone PF, D'Addona A. Hard and Soft Tissue Evaluation of Different Socket Preservation Procedures Using Leukocyte and Platelet-Rich Fibrin: A Retrospective Clinical and Volumetric Analysis. J Oral Maxillofac Surg. 2019 Sep;77(9):1807-1815. [DOI] [PubMed]
- 117.Zhang Y, Ruan Z, Shen M, Tan L, Huang W, Wang L, Huang Y. Clinical effect of platelet-rich fibrin on the preservation of the alveolar ridge following tooth extraction. Exp Ther Med. 2018 Mar;15(3):2277-2286. [DOI] [PMC free article] [PubMed]
- 118.To M, Su CY, Hidaka K, Okudera T, Matsuo M. Effect of advanced platelet-rich fibrin on accelerating alveolar bone formation in dogs: a histological and immunofluorescence evaluation. Anat Sci Int. 2019 Jun;94(3):238-244. [DOI] [PubMed]
- 119.Hauser F, Gaydarov N, Badoud I, Vazquez L, Bernard JP, Ammann P. Clinical and histological evaluation of postextraction platelet-rich fibrin socket filling: a prospective randomized controlled study. Implant Dent. 2013 Jun;22(3):295-303. [DOI] [PubMed]
- 120.Kotsakis GA, Boufidou F, Hinrichs JE, Prasad HS, Rohrer M, Tosios KI. Extraction Socket Management Utilizing Platelet Rich Fibrin: A Proof-of-Principle Study of the "Accelerated-Early Implant Placement" Concept. J Oral Implantol. 2016 Apr;42(2):164-8. [DOI] [PubMed]
- 121.Geurs N, Ntounis A, Vassilopoulos P, Van der Velden U, Loos BG, Reddy M. Using growth factors in human extraction sockets: a histologic and histomorphometric evaluation of short-term healing. Int J Oral Maxillofac Implants. 2014 Mar-Apr;29(2):485-96. [DOI] [PubMed]
- 122.Camarini ET, Zanoni JN, Leite PC, Boos FB. Use of biomaterials with or without platelet-rich plasma in postextraction sites: a microscopic study in dogs. Int J Oral Maxillofac Implants. 2009 May-Jun;24(3):432-8. [PubMed]