Abstract
Background:
Transition across eating disorder diagnoses is common, reflecting instability of specific eating disorder presentations. Previous studies have examined temporal stability of diagnoses in adult treatment-seeking samples, but have not uniformly captured initial presentation for treatment. The current study examines transitions across eating disorder diagnostic categories in a large, treatment-seeking sample of individuals born in Sweden and compares transitions across two birth cohorts and from initial diagnosis.
Methods:
Data from Swedish eating disorders quality registers were extracted in 2013, including 9,622 individuals who were seen at least twice from 1999-2013. Patterns of remission were examined in the entire sample and subsequently compared across initial diagnoses. An older (born prior to 1990) and younger birth cohort were also identified, and analyses compared these cohorts on patterns of diagnostic transition.
Results:
Although diagnostic instability was common, transition between threshold eating disorder diagnoses was infrequent. For all diagnoses, transition to remission was likely to occur following a diagnosis state that matched initial diagnosis, or through a subthreshold diagnostic state. Individuals in the younger cohort were more likely to transition to a state of remission than those in the older cohort.
Conclusions:
Results indicate more temporal continuity in eating disorder presentations than suggested by previous research and highlight the importance of early detection and intervention in achieving remission.
Keywords: eating disorders, anorexia nervosa, bulimia nervosa, binge-eating disorder, crossover, diagnosis, classification
Introduction
Although eating disorders are classified into distinct diagnostic categories such as anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED), clinical presentation commonly fluctuates during the course of illness [e.g., restricting AN to binge-purge AN], reflecting temporal instability of core symptoms (Milos et al., 2005; Eddy et al., 2008; Castellini et al., 2011; Ekeroth et al., 2013). As research and treatment development often assume specific within-diagnosis risk and resilience processes, understanding the extent to which such categories represent discrete classes of illness is conceptually and empirically relevant. For example, some encourage a “transdiagnostic” approach, which posits that eating disorder psychopathology is maintained by a largely common set of mechanisms and highlights shared psychopathological features (Fairburn et al., 2003; American Psychiatric Association, 2013; Welch et al., 2016). Alternatively, response to some interventions differs across eating disorder presentations [e.g., fluoxetine (Herpertz et al., 2011; McElroy et al., 2015)]. Improving our understanding of patterns of diagnostic transitions will inform etiology, case conceptualization, and intervention for these poorly understood and often debilitating conditions (Treasure et al., 2015).
An additional reason to investigate diagnostic instability across eating disorders is that transition from one presentation to another may itself be a prognostic indicator (Eddy et al., 2007; Castellini et al., 2011). For example, women who present with a current diagnosis of BN and a history of AN are less likely to experience recovery than women who present with BN with no history of AN over a longitudinal follow-up period spanning several years (Eddy et al., 2007). Alternatively, transition through an eating disorder not otherwise specified (EDNOS) diagnosis, as defined in DSM-IV, may represent symptom change secondary to the process of recovery (Eddy et al., 2010) (which may change as DSM-5 enables the coding of partial and full remission as well as severity and offers the newly minted category of other specified feeding and eating disorders [OSFED]). In sum, identification of patterns of diagnostic instability or stability may be useful in flagging risk among patients in efforts to optimally tailor interventions.
Risk for transition between diagnostic categories varies by diagnosis (Eddy et al., 2008; Eddy et al., 2010; Castellini et al., 2011) and length of illness (Bulik et al., 1997; Milos et al., 2005; Tozzi et al., 2005). Retrospective reports implicate the first several years of illness as highest risk for transitions (Bulik et al., 1997; Tozzi et al., 2005), although studies examining adult patients many years into the course of illness report persistent diagnostic instability (Milos et al., 2005; Eddy et al., 2008). Few prospective studies have evaluated diagnostic transitions after first detection in adolescent and young adult samples, and those that have are limited in statistical power (Stice et al., 2009; Ackard et al., 2011; Stice et al., 2013); thus, prospective evaluation from first treatment presentation would provide useful information regarding diagnostic transition early in the course of illness. Finally, it is important to consider whether observed diagnostic transitions represent truly distinct presentations versus transitions through subthreshold presentations (i.e., EDNOS) as symptoms resolve (Agras et al., 2009; Eddy et al., 2010).
The aim of the current study was to evaluate the risk of diagnostic stability, crossover, and remission in a large sample of treatment-seeking individuals born in Sweden and to compare rates of transition and stability across eating disorder diagnoses at annual treatment visits as reported in quality registers. In accordance with other studies (Milos et al., 2005; Eddy et al., 2008; Castellini et al., 2011), we hypothesized that diagnostic crossover and remission would be common (e.g., fewer than half of participants would retain their prior diagnosis at a subsequent time point), and that an EDNOS diagnosis could reflect transition through a subthreshold presentation on the course to remission, and would therefore better predict transitions to remission than threshold diagnoses. We further evaluated differences between birth cohorts in diagnostic patterns and transition rates among these disorders, as diagnostic stability may differ between first and subsequent diagnoses. Again, we hypothesized that diagnostic crossover would be common and the probability of transitioning would differ depending on initial diagnosis (eating disorder diagnosis made at the first reported visit), with full threshold diagnoses being more likely to predict subsequent diagnostic stability than an EDNOS diagnosis.
Methods
Procedure
Data from the following Swedish population registers were extracted in 2013. 1) The eating disorders national quality registers, Riksät-National Quality Register for Eating Disorders Treatment (Swedish Association of Local Authorities and Regions, 2007) and Stepwise (Birgegård et al., 2010), include eating disorder diagnostic information. Specifically, Riksät began in 1999 and is an Internet-based register for individuals being treated for eating disorders. As long as the patient is in treatment, follow-up assessments are designed to occur annually. Stepwise contains detailed information on clinical presentation of eating disorders, course, outcome, and psychopathology since 2005. As of 2013, 90% of all specialized eating disorders centers were included in these registers. 2) The Migration Register contains information about immigration to and emigration from Sweden (Statistics Sweden, 2013). 3) The Cause of Death Register (National Board of Health and Welfare, 2010), which has data available since 1958, provides information of the date of death and diagnostic codes for the principal and contributing cause(s) of death. The registers can be linked using the unique personal identification number assigned to all Swedish residents. More detailed information about the registers is available elsewhere (D’Onofrio et al., 2013).
With the exception of Stepwise, the Swedish registers do not require informed consent. Riksät has an opt-out for inclusion in the register, but <.05% of patients choose this, and Stepwise has an opt-out for research use of data, which is selected by ~3% of individuals (Runfola et al., 2014). This study was approved by the Regional Ethical Review Board in Stockholm, Sweden and the University of North Carolina Biomedical Institutional Review Board.
Sample
To be included in either Riksät (Swedish Association of Local Authorities and Regions, 2007) or Stepwise (Birgegård et al., 2010), individuals had to present to a participating treatment unit and have a diagnosed eating disorder, either AN, BN, BED, or EDNOS (e.g. those who reported episodes of purging, but not binge eating; individuals who restricted intake significantly but who were not underweight) once intent to treat was established. Eating disorder diagnoses are determined by clinicians at each assessment (American Psychiatric Association, 2000) (follow-up data exist for ~69% of the patients registered in Riksät or Stepwise). In Riksät, structured diagnostic interviews are used to assess DSM-IV eating disorder criteria. In Stepwise, a semi-structured interview is completed to determine initial diagnosis. Prior to August 2008, the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID)–research H module (First et al., 2002) was used. The Structured Eating Disorder Interview (SEDI; [de Man Lapidoth and Birgegård, 2010] ) has been used since August 2008. Concurrent validity of the SEDI and Eating Disorder Examination is estimated at Kendall’s tau-b = .69 (de Man Lapidoth and Birgegård, 2010).
Since we are evaluating diagnostic transition, we included individuals who had at least two assessments coded in the registers. Thus, some individuals were excluded from analyses for reasons related to number or timing of assessments. Specifically, there were 15,486 individuals assessed in Riksät between October 26, 2000 and December 31, 2013. Of these individuals, 5,316 were only assessed once and were thus excluded. Four-hundred five individuals (1,465 events) had multiple events, but these events were recorded on the same date and were therefore excluded. Another 134 individuals (558 events) had successive observations recorded within the same week and thus were also excluded. Ten individuals were excluded who had follow-ups registered following their death. The full study sample comprises 9,622 individuals seen at least twice, with successive observations at least one week apart.
Our sample was also divided into birth cohorts. The older cohort included individuals born up until December 31, 1989 (n=6,454). The younger cohort included individuals born between January 1, 1990 and December 31, 2003 (n=3,168). These birth dates were chosen because register data began in 1999; thus, we can presume that, for the younger cohort, all presentation to treatment centers for eating disorders in Sweden were captured during their lifetime.
Data Analysis
This investigation was designed as a case only study with multiple case groups. Each individual was followed from first visit through subsequent clinical assessments as reported in Riksät or Stepwise until a censoring event such as death, migration, or end of follow-up period (December 31, 2013) occurred. Rates of censoring due to death, migration, or lack of follow-up are reported.
Longitudinal Multistate Models.
The transition probability is the probability of moving to state j from the current state i, pi,j = P (St+1 = j∣St = i), where St is the state at time t. To estimate the transition probabilities, we used longitudinal multistate models (MSMs). The states consisted of the diagnosis given at each assessment. In instances where no eating disorder diagnosis was coded in the register at an assessment visit, the state at this assessment was classified remission (RM): S = RM, AN, BN, BED, EDNOS. The transition probabilities form the transition probability matrix P which, since we estimated transition probabilities to and from any pair of states, is a five-by-five matrix (25 possible transitions). The diagonal entries in P are the probabilities of an individual retaining the same state between two assessments (diagnostic stability) and the off-diagonal entries are the probabilities of diagnostic crossover. The transition probabilities were estimated using the MSM-package in R. Due to variation in time between assessments, only the chronological order of states was considered in the modelling (t = 1, 2, …,T). This restricts the transition probabilities from being interpreted as probabilities on a continuous time scale. The limiting probabilities, in which the probability of transitioning between a pair of states is independent of the current state, were examined for each estimated transition matrix. These were calculated by multiplying P with itself n times until the difference between Pn and Pn+1 was negligible. The limiting probabilities reflect the prevalence of the different diagnostic states in the sample.
Transition probabilities were completed on the full sample, with total number of visits (2 to 6 or more) entered as a covariate. We calculated 95% confidence intervals by bootstrapping the models with replacement (2000 replicates). In order to evaluate patterns of diagnostic crossover and remission more specifically, transition probabilities were also examined within the subsample of individuals who presented with each specific diagnosis at their first visit (e.g., transition probabilities were evaluated for only those who initially presented with AN). In analyses of patterns of diagnostic crossover and remission across age cohort, we created ratios by dividing the transition probabilities evaluated as the effect of being in of the older cohort (born before January 1, 1990) with the effect of being in the younger cohort (born after January 1, 1990). Thus, a ratio between 0 and 1 indicates a higher likelihood of the younger cohort transitioning and a ratio greater than 1 indicates a higher likelihood of the older cohort. As a ratio can be skewed, bootstrapped confidence intervals were calculated using the log of the ratio and then transformed back.
Results
Sample Description
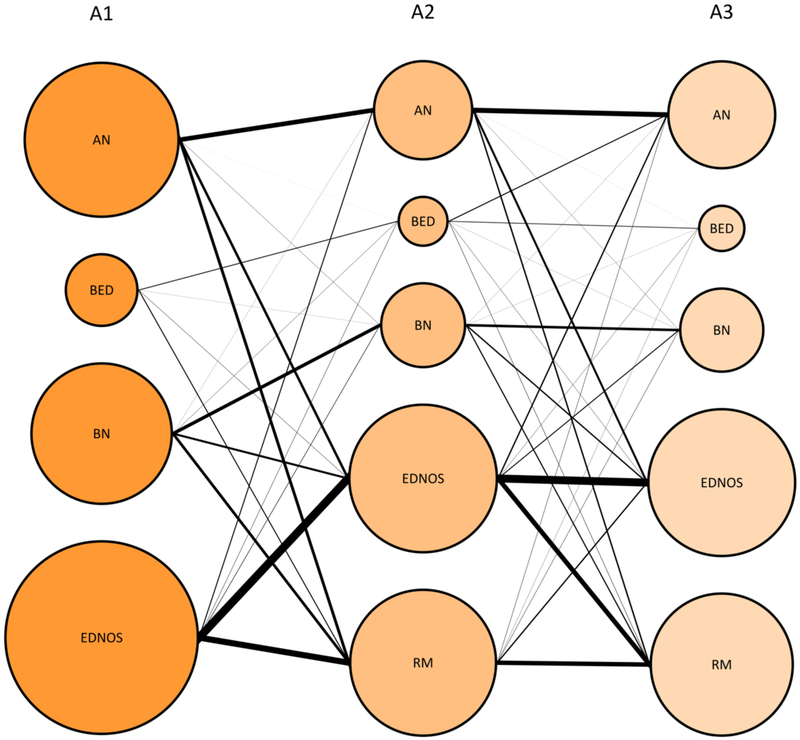
Descriptive statistics by initial diagnosis for the full sample are shown in Table 1a. These 9,622 individuals were assessed 25,641 times yielding an average of 2.67 events per individual. Average length of time between each assessment point was 1.1 years (range: 0.2-10.4 years; interquartile range: 0.8-1.1 years). Approximately one quarter had an AN diagnosis and one quarter had a BN diagnosis at first visit, whereas a smaller portion of the sample was diagnosed with BED at first visit (~6%) and larger portion of the sample (43%) was diagnosed with EDNOS at first visit. A bubble plot of the frequencies for each disease state in the first three assessment points is provided in Figure 1.
Table 1a.
Descriptive statistics by diagnosis at first visit for the full sample.
| All | AN | BN | BED | EDNOS | |
|---|---|---|---|---|---|
| N | 9,643 | 2,668 | 2,225 | 570 | 4,180 |
| % Diagnosis | --- | 27.67 | 23.07 | 5.91 | 43.35 |
| % Male | 3.44 | 4.54 | 1.84 | 4.39 | 3.47 |
| Mean Age | 23.14 | 20.28 | 25.95 | 30.66 | 22.44 |
| Mean BMI | 20.44 | 15.96 | 23.05 | 31.10 | 20.47 |
| N Visits | 2.67 | 2.97 | 2.64 | 2.41 | 2.52 |
| Mean Follow-up Time (years) | 5.86 | 6.01 | 6.05 | 5.59 | 5.29 |
Figure 1.
Plot showing transitions among the eating disorders across the first three assessment points (A1-3). Size of the bubbles indicate the proportion of individuals presenting with each diagnosis, within each assessment point. The width of a line between two diagnoses represents the share of the total flow between two assessment points. AN = anorexia nervosa, BED = binge-eating disorder, BN = bulimia nervosa, ENDOS = eating disorder not otherwise specified, A1-A2: n = 9622, A3: n = 3499.
Descriptive information for the older cohort is presented in Table 1b. This group was assessed 6,454 times, averaging 2.74 visits per individual during the study period. EDNOS was the most common diagnosis at first visit (39%), followed by BN (30%), and AN (23%).
Table 1b.
Descriptive statistics by diagnosis at first visit for the older cohort.
| All | AN | BN | BED | EDNOS | |
|---|---|---|---|---|---|
| N | 6454 | 1467 | 1936 | 528 | 2523 |
| % Diagnosis | --- | 22.73 | 30.00 | 8.18 | 39.09 |
| % Male | 2.67 | 3.55 | 1.81 | 4.74 | 2.38 |
| Mean Age | 26.34 | 23.77 | 27.06 | 31.48 | 26.20 |
| Mean BMI | 21.49 | 16.02 | 23.20 | 31.40 | 21.30 |
| N Visits | 2.74 | 3.18 | 2.69 | 2.44 | 2.60 |
| Mean Follow-up Time (years) | 6.47 | 7.23 | 6.43 | 5.75 | 6.21 |
In the younger cohort, all transitions beginning with first presentation for eating disorder treatment were captured. Descriptive information by diagnosis at first visit for these individuals is provided in Table 1c. This younger group was assessed 7,909 times, averaging 2.50 visits per individual during the study period. EDNOS was the most common diagnosis at first visit (52%), followed by AN (38%), and fewer than 10% were diagnosed with either BN or BED at first visit.
Table 1c.
Descriptive statistics by diagnosis at first visit for the younger cohort
| All | AN | BN | BED | EDNOS | |
|---|---|---|---|---|---|
| N | 3168 | 1198 | 282 | 40 | 1648 |
| % Diagnosis | --- | 37.82 | 8.90 | 1.26 | 52.02 |
| % Male | 5.02 | 5.76 | 2.13 | 0 | 5.10 |
| Mean Age | 16.58 | 16.01 | 18.35 | 18.73 | 16.64 |
| Mean BMI | 18.30 | 15.88 | 22.04 | 27.32 | 19.20 |
| N Visits | 2.50 | 2.71 | 2.28 | 2.08 | 2.39 |
| Mean Follow-up Time (years) | 4.07 | 4.50 | 3.47 | 3.28 | 3.88 |
Note. AN = anorexia nervosa; BN = bulimia nervosa; BED = binge eating disorder; EDNOS = eating disorder not otherwise specified.
Transition Probabilities
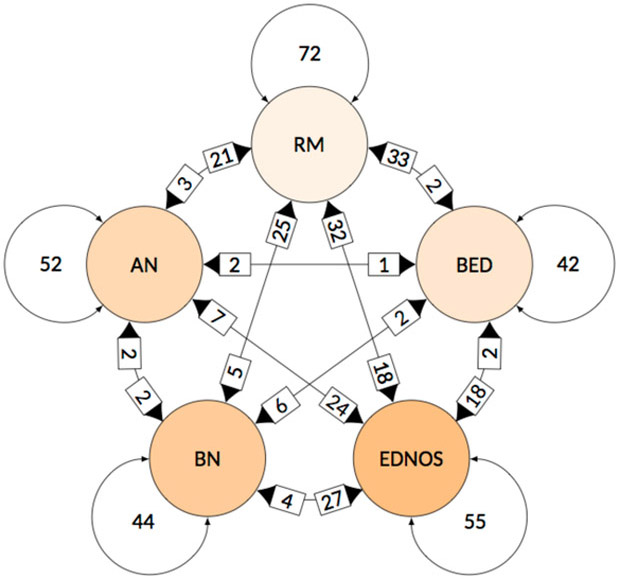
The fit of the model estimating transition probabilities for the full sample with age cohort included as a covariate was compared to fit of the model adjusting for number of visits: a likelihood ratio test indicated a better fit for the model adjusting for number of visits so those results are presented (see Figure 2). In the overall sample, the majority of individuals had only two assessment visits (63.6%). As such, unadjusted transition probabilities are reflective of a sample of individuals with relatively few eating disorder-related visits.
Figure 2.
Figure showing transition probabilities from diagnosis at Time X to diagnosis at Time Y for the full sample, controlling for number of visits. Arrows exogenous to each diagnosis indicate the likelihood of stability from Time X to Time Y (e.g. the likelihood of AN cases at Time X retaining an AN diagnosis at Time Y is .52). Bolded arrows pointing towards a diagnosis represent the likelihood of transition to that diagnosis at Time Y from the diagnosis where the arrow originates at Time X (e.g. the likelihood of AN cases at Time X transitioning to RM at Time Y is .21). AN = anorexia nervosa, BN = bulimia nervosa, BED = binge-eating disorder, EDNOS = eating disorder not otherwise specified; RM = remission.
In general, transitions across full-threshold disorders (AN, BN, and BED) were rare (pij < 0.03). Stability in the RM state, once this state was achieved, was common (pij > 0.5). For AN and BN diagnoses, diagnostic stability was the most likely outcome. The combined likelihood of transitioning to remission or EDNOS from AN was roughly equal to the probability of retaining AN. The combined likelihood of remission or transition to EDNOS from BN exceeded the probability of retaining BN.
Transition to remission or retaining BED were the most common pattern for this diagnosis. Stability and remission were also the most common transitions from EDNOS, with retention of EDNOS being the most likely outcome.
Transition Probabilities by Initial Diagnosis
Transition probabilities by initial diagnosis are provided in Table 2. This allows examination of patterns of remission and relapse based on transition to another illness state after initial presentation. In those initially diagnosed with AN, transition to remission was most likely to occur following a transition to EDNOS. The second most frequent pattern was direct transition to remission from AN. Transition to remission was less likely to occur after a transition to BN or BED. Relapse back to AN was most likely to follow from EDNOS, with similar probabilities of relapse from BN and remission states.
Table 2.
Estimated transition probabilities going from diagnosis at Time X to diagnosis at Time Y by diagnosis at first assessment for the full sample.
| Time Y | |||||||
|---|---|---|---|---|---|---|---|
| RM | AN | BN | BED | EDNOS | |||
| AN at First Assessment | Time X | RM | 0.68 (0.64; 0.73) | 0.11 (0.08; 0.14) | 0.02 (0.01; 0.03) | <0.01 (0.00; 0.01) | 0.18 (0.15; 0.22) |
| AN | 0.23 (0.22; 0.25) | 0.51 (0.50; 0.53) | 0.02 (0.01; 0.02) | <0.01 (0.00; 0.00) | 0.23 (0.22; 0.25) | ||
| BN | 0.14 (0.09; 0.23) | 0.10 (0.05; 0.18) | 0.46 (0.34; 0.58) | 0.03 (0.00; 0.07) | 0.26 (0.17; 0.36) | ||
| BED | 0.12 (0.03; 0.31) | 0.07 (0.02; 0.12) | 0.21 (0.01; 0.44) | 0.34 (0.04; 0.68) | 0.26 (0.08; 0.44) | ||
| EDNOS | 0.32 (0.28; 0.35) | 0.19 (0.16; 0.22) | 0.03 (0.02; 0.05) | 0.01 (0.00; 0.01) | 0.45 (0.42; 0.49) | ||
| BN at First Assessment | Time X | RM | 0.58 (0.52; 0.64) | 0.02 (0.01; 0.04) | 0.16 (0.11; 0.21) | 0.05 (0.02; 0.07) | 0.20 (0.15; 0.25) |
| AN | 0.12 (0.08; 0.20) | 0.46 (0.33; 0.60) | 0.21 (0.11; 0.31) | 0.02 (0.01; 0.06) | 0.19 (0.09; 0.29) | ||
| BN | 0.29 (0.27; 0.30) | 0.01 (0.01; 0.03) | 0.44 (0.42; 0.46) | 0.02 (0.02; 0.03) | 0.24 (0.22; 0.25 | ||
| BED | 0.31 (0.16; 0.46) | 0.01 (0.00; 0.02) | 0.17 (0.07; 0.30) | 0.31 (0.17; 0.47) | 0.19 (0.10; 0.34) | ||
| EDNOS | 0.29 (0.25; 0.34) | 0.03 (0.01; 0.04) | 0.19 (0.15; 0.23) | 0.02 (0.01; 0.03) | 0.47 (0.42; 0.51) | ||
| BED at First Assessment | Time X | RM | 0.62 (0.52; 0.76) | 0.01 (0.00; 0.01) | 0.08 (0.02; 0.12) | 0.14 (0.05; 0.23) | 0.15 (0.06; 0.25) |
| AN | 0.17 (0.02; 0.52) | 0.42 (0.03; 0.94) | 0.02 (0.01; 0.06) | 0.05 (0.01; 0.13) | 0.34 (0.02; 0.57) | ||
| BN | 0.25 (0.11; 0.45) | 0.01 (0.00; 0.02) | 0.40 (0.20; 0.62) | 0.15 (0.04; 0.30) | 0.19 (0.05; 0.37) | ||
| BED | 0.43 (0.39; 0.47) | 0.00 (0.00; 0.01) | 0.05 (0.03; 0.06) | 0.39 (0.36; 0.43) | 0.13 (0.10; 0.15) | ||
| EDNOS | 0.31 (0.18; 0.41) | 0.03 (0.00; 0.06) | 0.04 (0.01; 0.09) | 0.13 (0.05; 0.23) | 0.49 (0.37; 0.63) | ||
| EDNOS at First Assessment | Time X | RM | 0.66 (0.62; 0.71) | 0.04 (0.02; 0.06) | 0.05 (0.03; 0.07) | 0.01 (0.00; 0.02) | 0.24 (0.20; 0.29) |
| AN | 0.18 (0.13; 0.22) | 0.51 (0.44; 0.57) | 0.02 (0.01; 0.05) | 0.01 (0.00; 0.01) | 0.29 (0.23; 0.34) | ||
| BN | 0.33 (0.23; 0.42) | 0.03 (0.02; 0.03) | 0.24 (0.15; 0.32) | 0.03 (0.01; 0.07) | 0.38 (0.28; 0.47) | ||
| BED | 0.26 (0.15; 0.38) | 0.02 (0.02; 0.03) | 0.02 (0.01; 0.02) | 0.38 (0.26; 0.52) | 0.33 (0.21; 0.45) | ||
| EDNOS | 0.36 (0.35; 0.37) | 0.06 (0.05; 0.07) | 0.03 (0.03; 0.04) | 0.02 (0.01; 0.02) | 0.53 (0.52; 0.54) | ||
Note. RM = remission; AN = anorexia nervosa; BN = bulimia nervosa; BED = binge eating disorder; EDNOS = eating disorder not otherwise specified.
For those with an initial diagnosis of BN, the probability of transition to remission from BN, BED, or EDNOS was similar (pij: 0.29-0.31); whereas the probability of transition to remission from AN was lower. Likelihood of relapse back to BN was similar after passing through AN, BN, BED, or remission.
For those initially diagnosed with BED, transition to remission was more likely to occur directly from BED than through EDNOS. Relapse to BED was less common in individuals initially diagnosed with BED compared with relapse in groups with other initial diagnoses. When relapse to BED did occur, it was most likely to occur from remission, BN, or EDNOS (pij: 0.13-0.15). Of note, transitions from AN or BN in this subsample have wide confidence intervals due to the small number of individuals with a BED diagnosis who transitioned to AN or BN, indicating that these results should be interpreted with caution.
For those with an initial diagnosis of EDNOS, transition to remission was likely to occur after a diagnosis of BN, BED, or EDNOS (pij: 0.26-0.36), and the probability of transition to remission was lower through AN. Likelihood of relapse back to EDNOS was similar after AN, BN, BED, or remission.
Comparison between Older and Younger Cohorts
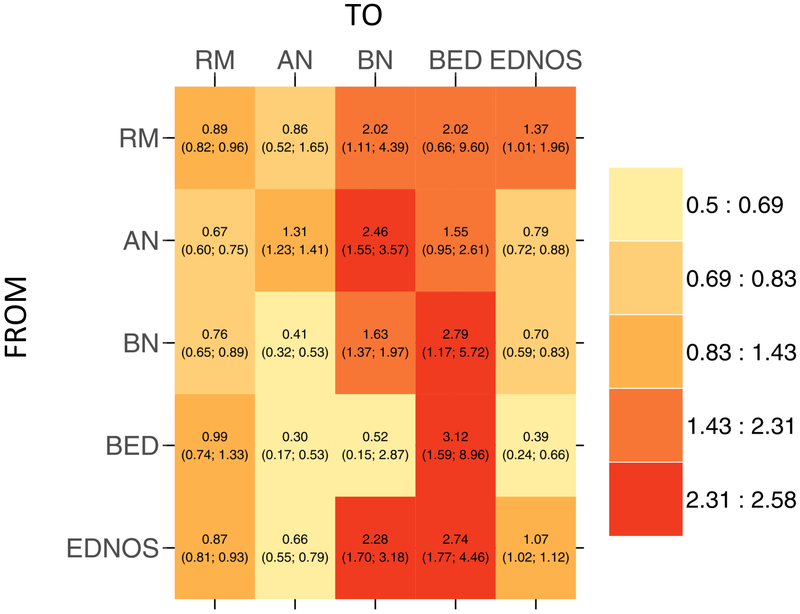
Direct comparison of transition probabilities by age are presented in Figure 3. Transition probability ratios greater than 1 indicate that individuals in the older cohort were more likely to make the transition; whereas, transition probabilities less than 1 indicate that the specified transition was more likely in the younger cohort. Overall, individuals in the older cohort were more likely to transition to BN or BED from all states, compared with those in the younger cohort—with the exception of transitioning from BED to BN. Individuals in the younger cohort were more likely to transition to remission from all illnesses except for BED, and were more likely than those in the older cohort to transition to AN and EDNOS from other illnesses. Although transitions to AN were less common for individuals in the older cohort, retaining AN was more likely for those in the older than in the younger cohort. Stability of BN and BED was also more likely for those in the older cohort, whereas individuals in the younger cohort were more likely to remain in remission.
Figure 3.
Heat map showing transitions among the eating disorders comparing younger and older cohorts. Ratios less than one indicate that transition is more likely in the younger cohort; ratios greater than one indicate that transition is more likely in the older cohort. AN = anorexia nervosa, BN = bulimia nervosa, BED = binge-eating disorder, ENDOS = eating disorder not otherwise specified; RM = remission.
Limiting Probabilities
The proportion of cases that at any point in time are expected to be in each state are presented in Table 3. In the full sample, about half of individuals were in remission at any given time point, whereas about a third were diagnosed with EDNOS. Threshold diagnoses were less common states, even among this sample presenting to eating disorder treatment. The older cohort was less likely to be in a remitted state than the younger cohort. When examining the likelihood of being in a remitted state by initial diagnosis, those with BN were less likely than those with other initial diagnoses to be in remission over time.
Table 3.
Steady state probabilities—proportion of cases that at any point in time are expected to be in each state—for the full sample, the older cohort, and the younger cohort, and by eating disorder diagnosis at first assessment in the full cohort.
| RM | AN | BN | BED | EDNOS | |
|---|---|---|---|---|---|
| Full sample | 0.49 (0.47; 0.51) | 0.10 (0.09; 0.11) | 0.07 (0.06; 0.08) | 0.03 (0.02; 0.03) | 0.31 (0.29; 0.33) |
| Full sample, adjusted for n visits | 0.51 (0.49; 0.54) | 0.08 (0.07; 0.09) | 0.07 (0.06; 0.09) | 0.03 (0.02; 0.04) | 0.30 (0.28; 0.33) |
| Older cohort | 0.44 (0.42; 0.46) | 0.10 (0.09; 0.12) | 0.10 (0.08; 0.11) | 0.03 (0.03; 0.04) | 0.33 (0.31; 0.35) |
| Younger cohort | 0.60 (0.56; 0.65) | 0.09 (0.06; 0.11) | 0.04 (0.02; 0.05) | 0.01 (0.01; 0.02) | 0.27 (0.23; 0.30) |
| AN at first assessment | 0.46 (0.42; 0.50) | 0.22 (0.19; 0.25) | 0.04 (0.03; 0.06) | 0.01 (0.00; 0.02) | 0.27 (0.24; 0.30) |
| BN at first assessment | 0.40 (0.36; 0.44) | 0.04 (0.02; 0.06) | 0.24 (0.20; 0.27) | 0.05 (0.03; 0.07) | 0.28 (0.25; 0.32) |
| BED at first assessment | 0.47 (0.36; 0.59) | 0.02 (0.00; 0.15) | 0.09 (0.03; 0.15) | 0.18 (0.10; 0.24) | 0.24 (0.14; 0.33) |
| EDNOS at first assessment | 0.49 (0.45; 0.53) | 0.08 (0.06; 0.10) | 0.05 (0.04; 0.06) | 0.02 (0.01; 0.03) | 0.36 (0.32; 0.39) |
Note. RM = remission; AN = anorexia nervosa; BN = bulimia nervosa; BED = binge eating disorder; EDNOS = eating disorder not otherwise specified
Discussion
The current study characterized patterns of diagnostic transition in a large national sample of individuals presenting for eating disorder treatment. Average time between visits was approximately one year; thus, the current findings reflect transition during this follow-up period.
Diagnostic stability was common. Encouragingly, stability was highest in the remitted state. Of note, diagnostic stability was similar for EDNOS compared with other eating disorders, indicating EDNOS was not merely a diagnosis that individuals passed through in a transient manner. In general, the older cohort was more likely to retain their original diagnosis. It is possible that the sample delineation led to a higher proportion of individuals in the older age cohort who had a more enduring eating disorder, as individuals in that age cohort who recovered prior to the assessment period are not captured in this study. This pattern of findings does support the notion that recovery is more likely at younger age (which may often coincide with a shorter illness duration) (Treasure and Russell, 2011). These findings align with literature noting that the initial years after illness onset may represent a critical period for maximally effective intervention (Treasure and Russell, 2011; Treasure et al., 2015).
Overall, the picture of remission in the current sample was promising, and the likelihood of stable remission was high. Remission was more likely to occur through the initial diagnostic state and/or through an EDNOS presentation, compared to another full threshold diagnosis for all initial presentations. Transition to BN reduced the likelihood of remission for those initially diagnosed with AN, and transition to AN reduced the likelihood of remission for those initially diagnosed with BN. Although the reliability of this finding is unclear because transitions between these diagnoses were relatively uncommon in this sample, this pattern confirms previous literature indicating that diagnostic crossover, particularly between these two conditions, may indicate poor prognosis (Eddy et al., 2007). This pattern of findings has several potential explanations. At the level of individual differences, transitioning between AN and BN may be related to personality features (Tozzi et al., 2005); transitioning may also be associated with greater fluctuation in body weight, and therefore might indicate that these individuals are metabolically unique from those who do not transition between these states (Tozzi et al., 2005; Monteleone et al., 2011). Alternatively, these transitions may indicate deviance within the disorder and recovery process. Individuals who transition from AN to BN may represent a subset of those for whom disordered eating thoughts and behaviors persist at high levels even as weight is restored; while those who transition from BN to AN may experience greater reinforcement from compensatory behaviors as a result of enhanced weight loss. Initial research has cross-sectionally examined how specific cognitive and behavioral symptoms of eating disorders cluster together in symptom networks (Forbush et al., 2016); future examination of how symptom networks progress over time may assist in discriminating mechanisms associated with diagnostic transitions.
Limitations
The registers include cross-sectional snap-shots of diagnoses at specific time points and do not capture states between assessment points. For example, an individual who transitioned from AN to BN may have had an intermediate period during which they met criteria for EDNOS that was not captured by the assessment. Similarly, it is possible that those with longer periods of time between assessments may have traversed a period of remission before returning for follow-up, which was also not captured in the current study. Further, this sample only included treatment seeking individuals in Sweden and may not generalize to a broader population of untreated individuals with eating disorders. Indeed, diagnostic comparison of individuals who were excluded from the current sample due to only having one assessment visit were more likely to be diagnosed with EDNOS (50.8%) compared with those with two or more visits (43.3%), and less likely to be diagnosed with AN (19.4%) compared with those with two or more visits (27.7%). In addition, these participants had a shorter follow-up period from first assessment to the end of the observation period (M = 3.87 years vs. 5.86 years), indicating that some individuals did not complete a second assessment simply due to having a shorter time frame in which to complete this follow-up. Overall, it is estimated that 15% of individuals with eating disorder symptoms in the general population and 30% of those with a full-threshold eating disorder receive psychiatric care in Sweden (Sandeberg et al., 2009). Related to this point, attrition from the study sample at follow-up time points may have occurred for a variety of reasons, such as transition to remission, relocation, or treatment discontinuation. As reasons for failure to follow-up do not exist in the eating disorders quality registers, we are unable to address how the patterns of diagnostic transition might be affected by attrition. Further, transitions across diagnoses may reflect a range of processes. It is entirely possible that transitions may have occurred in response to treatment. Of note, all individuals in the current study received some amount of clinical attention, which may have affected rates and patterns of diagnostic transition and remission experienced in this population. For others, this may reflect the natural course of their disorder. It cannot be ruled out that in some cases, transition may be due to diagnostic misclassification at one or more time points.
Conclusion
Findings from this large cohort of treatment-seeking individuals support the hypothesis that eating disorder remission is both probable and likely. Diagnostic transition to and from EDNOS was common, whereas transition across threshold diagnoses of AN, BN, and BED was less likely. Transition between full-threshold diagnostic categories may signal increased risk for prolonged illness duration. Overall, our findings suggest greater stability in the core diagnostic structure of eating disorders across time than previously reported and support the notion that early detection and treatment is a clinically worthwhile goal to increase the likelihood of remission.
Acknowledgments
Financial Support:
This work was made possible by grants from the Swedish Research Council (VR Dnr: 538-2013-8864; PI Bulik); the Swedish Initiative for Research on Microdata in the Social and Medical Sciences (SIMSAM) framework (grant #340-2013-5867); the National Institute of Mental Health (T32MH076694; PI Bulik); and the Anorexia Nervosa Genetics Initiative (ANGI), an initiative of the Klarman Family Foundation; and the Foundation of Hope: Research and Treatment of Mental Illness.
Footnotes
Conflict of Interest Disclosures:
Cynthia Bulik has received research grants from and served on scientific advisory boards for Shire.
Henrik Larsson has served as a speaker for Eli-Lilly and Shire, and has received research grants from Shire.
Claes Norring is a consultant on a research grant from Shire.
Laura Thornton has been an investigator on a research grant from Shire.
References
- Ackard DM, Fulkerson JA, Neumark-Sztainer D. (2011). Stability of eating disorder diagnostic classifications in adolescents: five-year longitudinal findings from a population-based study. Eating Disorders, 19, 308–322 [DOI] [PubMed] [Google Scholar]
- Agras WS, Crow S, Mitchell JE, Halmi KA, Bryson S. (2009). A 4‐year prospective study of eating disorder NOS compared with full eating disorder syndromes. International Journal of Eating Disorders, 42, 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders. 5th Edition, American Psychiatric Press, Arlington, VA. [Google Scholar]
- American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition Text Revision. American Psychiatric Press, Washington, DC. [Google Scholar]
- Birgegård A, Björck C, Clinton D. (2010). Quality assurance of specialised treatment of eating disorders using large-scale Internet-based collection systems: methods, results and lessons learned from designing the Stepwise database. European Eating Disorders Review, 18, 251–259. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Fear J, Pickering A. (1997). Predictors of the development of bulimia nervosa in women with anorexia nervosa. The Journal of Nervous and Mental Disease, 185, 704–707. [DOI] [PubMed] [Google Scholar]
- Castellini G, Lo Sauro C, Mannucci E, Ravaldi C, Rotella CM, Faravelli C, Ricca V. (2011). Diagnostic crossover and outcome predictors in eating disorders according to DSM-IV and DSM-V proposed criteria: a 6-year follow-up study. Psychosomatic Medicine, 73, 270–279. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Class QA, Rickert ME, Larsson H, Langstrom N, Lichtenstein P. (2013). Preterm birth and mortality and morbidity: A population-based quasi-experimental study. JAMA Psychiatry, 70, 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Man Lapidoth J, Birgegård A. (2010). A Validation of the Structured Eating Disorder Interview (SEDI) against the Eating Disorder Examination (EDE). Karolinska Institutet, Stockholm. [Google Scholar]
- Eddy KT, Dorer DJ, Franko DL, Tahilani K, Thompson-Brenner H, Herzog DB. (2008). Diagnostic crossover in anorexia nervosa and bulimia nervosa: implications for DSM-V. American Journal of Psychiatry, 165, 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy KT, Dorer DJ, Franko DL, Tahilani K, Thompson-Brenner H, Herzog DB. (2007). Should bulimia nervosa be subtyped by history of anorexia nervosa? A longitudinal validation. International Journal of Eating Disorders, 40, 567–571. [DOI] [PubMed] [Google Scholar]
- Eddy KT, Swanson SA, Crosby RD, Franko DL, Engel S, Herzog DB. (2010). How should DSM-V classify eating disorder not otherwise specified (EDNOS) presentations in women with lifetime anorexia or bulimia nervosa. Psychological Medicine, 40, 1735–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekeroth K, Clinton D, Norring C, & Birgegård A. (2013). Clinical characteristics and distinctiveness of DSM-5 eating disorder diagnoses: findings from a large naturalistic clinical database. Journal of Eating Disorders 1, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn C, Stice E, Cooper Z Doll H, Norman P, & O'Conner M. (2003). Understanding persistence in bulimia nervosa: A 5-year naturalistic study. Journal of Consulting and Clinical Psychology, 71, 103–109. [PubMed] [Google Scholar]
- First MB, Spitzer R, Gibbon M, & Williams JB. (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patent Edition (SCID-I/P). Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Forbush KT, Siew CS & Vitevitch MS. (2016). Application of network analysis to identify interactive systems of eating disorder psychopathology. Psychological Medicine, 46, 2667–2677. [DOI] [PubMed] [Google Scholar]
- Herpertz S, Hagenah U, Vocks S, con Wietersheim J, Cuntz U, & Zeeck A. (2011). The diagnosis and treatment of eating disorders. Deutsches Ärzteblatt International, 108, 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Guerdjikova AI, Mori N, & Keck PE Jr. (2015). Psychopharmacologic treatment of eating disorders: emerging findings. Current Psychiatry Reports, 17, 35. [DOI] [PubMed] [Google Scholar]
- Milos G Spindler A, Schnyder U, & Fairburn CG. (2005). Instability of eating disorder diagnoses: prospective study. The British Journal of Psychiatry, 187, 573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Di Genio M, Monteleone AM, Di Filippo C, Maj M. (2011). Investigation of factors associated to crossover from anorexia nervosa restricting type (ANR) and anorexia nervosa binge-purging type (ANBP) to bulimia nervosa and comparison of bulimia nervosa patients with or without previous ANR or ANBP. Comprehensive Psychiatry, 52, 56–62. [DOI] [PubMed] [Google Scholar]
- National Board of Health and Welfare (2010). Causes of Death 2008 Stockholm, Sweden, National Board of Health and Welfare. [Google Scholar]
- Runfola CD, Thornton LM, Pisetsky EM, Bulik CM, Birgegard A. (2014). Self-image and suicide in a Swedish national eating disorders clinical register. Comprehensive Psychiatry, 55, 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeberg A-M, & Birgegård A, ML, Nordlander Ström C, Norring C, Siverstrand M-L. (2009). Regionalt Vårdprogram Ätstörningar. Stockholms Läns Landsting, [Google Scholar]
- Statistics Sweden (2013). Multi-Generation Register 2004.
- Stice E, Marti CN, Rohde P. (2013). Prevalence, incidence, impairment, and course of the proposed DSM-5 eating disorder diagnoses in an 8-year prospective community study of young women. Journal of Abnormal Psychology, 122, 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Marti CN, Shaw H, Jaconis M. (2009). An 8-year longitudinal study of the natural history of threshold, subthreshold, and partial eating disorders from a community sample of adolescents. Journal of Abnormal Psychology, 118, 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedish Association of Local Authorities and Regions. (2007). National Healthcare Quality Registries in Sweden. KLF Grafisk Producation, Edita, Stockholm. [Google Scholar]
- Tozzi F, Thornton L, Klump K, Bulik C, Fichter M, Halmi K, Kaplan A, Strober M, Woodside D, Crow S, Mitchell J, Rotondo A, Mauri M, Cassano C, Keel P, Plotnicov K, Pollice C, Lilenfeld L, Berrettini W, Kaye W. (2005). Symptom fluctuation in eating disorders: correlates of diagnostic crossover. American Journal of Psychiatry, 162, 732–740. [DOI] [PubMed] [Google Scholar]
- Treasure J & Russell G (2011). The case for early intervention in anorexia nervosa: theoretical exploration of maintaining factors. British Journal of Psychiatry, 199, 5–7. [DOI] [PubMed] [Google Scholar]
- Treasure J, Stein D, Maguire S (2015). Has the time come for a staging model to map the course of eating disorders from high risk to severe enduring illness? An examination of the evidence. Early Intervention in Psychiatry, 9, 173–184. [DOI] [PubMed] [Google Scholar]
- Welch E, Jangmo A, Thornton LM, Norring C, von Hausswolff-Juhlin Y, Herman BK, Pawaskar M, Larsson H, Bulik CM. (2016). Treatment-seeking patients with binge-eating disorder in the Swedish national registers: clinical course and psychiatric comorbidity. BMC Psychiatry, 16, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]