Abstract
Intravenous iron is commonly prescribed for treatment of iron deficiency, with modern formulations demonstrating an acceptable safety profile in the majority of patients. We report the case of a patient who was hospitalised with muscle pain, deteriorating mobility and multiple fractures following repeated ferric carboxymaltose infusions. Investigations revealed severe hypophosphatemia with serum phosphate of 0.27 mmol/L, 25‐hydroxyvitamin D (25OHD) level of 32 nmol/L and insufficiency fractures of the sacrum and L5 transverse process. The patient's hypophosphatemia was corrected with several infusions of intravenous phosphate, as well as oral phosphate and calcitriol, with subsequent resolution of her muscle aches, back pain and immobility.
The risk of persistent hypophosphatemia and osteomalacia may be higher with iron carboxymaltose than other iron formulations and a transient increase in intact fibroblast growth factor‐23 with reduced renal tubular phosphate absorption has been postulated as the key mechanism. This risk appears increased by repeated iron infusions, underlying malnutrition, hypophosphatemia at baseline, vitamin D deficiency, hyperparathyroidism or anti‐resorptive medication use. The true risk and incidence of hypophosphatemia need to be clarified so that appropriate monitoring, prevention and treatment strategies can be developed.
Keywords: adverse effects, ferric carboxymaltose, gastrointestinal bleeding, hypophosphatemia, intravenous iron, iron deficiency
Introduction
Intravenous iron infusions are commonly prescribed to rapidly replace iron stores in the setting of blood loss or malabsorption of iron. Ferric carboxymaltose has been demonstrated to have significant advantages due to a short infusion time and reported low rate of adverse effects. Hypophosphatemia, most often asymptomatic and transient, has been described in numerous clinical trials. Recently, persistent hypophosphataemia with osteomalacia has been reported following repeated iron infusions, most commonly following ferric carboxymaltose. We report the case of a patient with severe hypophosphatemia and sacral and lumbar insufficiency fractures following multiple ferric carboxymaltose infusions.
Case report
A 73‐year‐old woman was hospitalized with symptomatic, severe hypophosphatemia and fractures following multiple ferric carboxymaltose (FCM) infusions for persistent gastrointestinal blood loss. She reported a 10‐month history of proximal upper and lower limb muscle pain, weakness, back pain, and deteriorating mobility without falls. Examination demonstrated an antalgic gait and proximal muscle tenderness.
Blood tests showed severe hypophosphatemia (serum phosphate 0.27 [normal range 0.75–1.50] mmol/L) and hypocalcemia (corrected calcium of 2.04 [2.10–2.60] mmol/L). The patient was also found to have a 25‐hydroxyvitamin D (25OHD) level of 32 nmol/L (>75 nmol/L), a serum parathyroid hormone (PTH) level of 29.8 pmol/L (1.6–6.9 pmol/L), and alkaline phosphatase (ALP) of 229 IU/L (30–110 IU/L), with otherwise normal liver biochemistry; 24 hour urinary phosphate excretion was 92 (13–42) mmol/day. A bone scan demonstrated bilateral insufficiency fractures of the sacral wings and degenerative changes in the right hip (Fig. 1a). A computed tomography (CT) pelvis showed a further acute left L5 transverse process fracture and a fracture of the lateral mass of sacrum (Fig. 1b).
Figure 1.
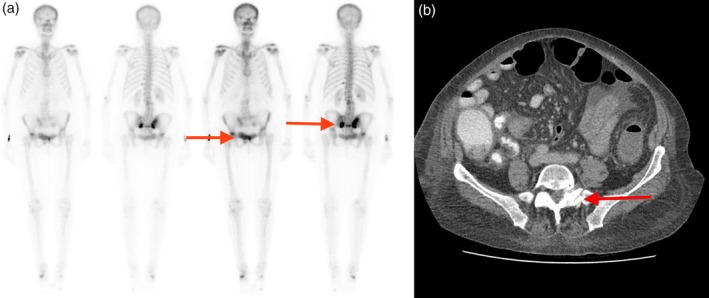
Single Photon Emission Computed Tomography (SPECT) imaging demonstrating increased uptake in the bilateral sacral wings and right hip joint. (b) Computed Tomography (CT) of the pelvis demonstrating left‐sided sacral fracture.
The patient had a past history of hepatitis B cirrhosis, portal hypertensive gastropathy with gastric antral vascular ectasia (GAVE), and subsequent recurrent iron deficiency anemia despite several treatments with gastroscopy and argon plasma coagulation (APC). In the 2 years before presentation, she had received 11 g of intravenous FCM (in 1 g infusions), the last of which was 2 months prior to her admission. Her regular medications comprised spironolactone, 50 mg daily, and entecavir, 0.5 mg daily. Having found to be osteopenic (bone mineral density T score − 2.0 in lumbar spine, T score −2.5 in femur), she also received a single infusion of denosumab 1 month before presentation.
The patient's hypophosphatemia was corrected with multiple infusions of intravenous phosphate over the course of 3 weeks (totaling 150 mmol phosphorous), in addition to oral phosphate and calcitriol. The patient's GAVE was managed with gastroscopy and APC during her admission, and her hemoglobin stabilized. Subsequently, her iron deficiency was managed with oral iron supplementation only. At follow‐up 6 months later, her serum phosphate and other biochemical markers of bone health were normal, with complete resolution of her proximal muscle aches, back pain, and immobility.
Discussion
Hypophosphatemia, defined as a serum phosphate level <0.75 mmol/L, is a common finding in hospitalized patients. Causes are diverse and include decreased oral intake (severe prolonged illness, starvation, alcohol abuse), redistribution of body phosphate (acute respiratory alkalosis, refeeding syndrome, treatment of diabetic ketoacidosis), decreased intestinal absorption (drugs, chronic diarrhea, vitamin D deficiency), and increased renal loss (renal tubular disorders, drugs, hyperparathyroidism, presence of phosphatonin). Severe hypophosphatemia (arbitrarily defined as serum phosphate <0.35 mmol/L) is prevalent and is a marker of morbidity and mortality in patients with critical illness. Symptoms of hypophosphatemia depend on both the severity and chronicity but may include irritability, fatigue, paraesthesia, proximal myopathy, dysphagia, ileus, and prolonged ventilator dependency in critically ill patients. Prolonged hypophosphatemia and disordered calcium–vitamin D metabolism may also result in osteomalacia.
Intravenous iron is recommended as the first‐line modality for total dose iron replacement in cases of severe iron deficiency, where a rapid increase in hemoglobin is required. Although several formulations of intravenous iron are available, FCM is commonly prescribed due to its 15‐min administration time and low rate of immediate adverse reactions.1
Transient, asymptomatic hypophosphatemia is described in several trials using FCM. In a large randomized controlled trial (RCT) of 485 patients with iron deficiency anemia secondary to inflammatory bowel disease (IBD),1 serum phosphate fell from 1.12 ± 0.22 mmol/L to 0.69 ± 0.24 mmol/L at Week 2 and returned to normal between Weeks 4 and 12 following FCM administration. No symptoms were reported among these patients. In a further RCT of postpartum women with iron deficiency anemia, a transient, asymptomatic lowering of serum phosphate to 1.3 mg/dL (0.42 mmol/L) occurred, reaching nadir approximately 2 weeks after initiating therapy and spontaneously resolving thereafter.2
However, there have also been isolated case reports of patients with severe, symptomatic hypophosphatemia after FCM infusions,3 with patients presenting with fatigue, generalized muscle weakness, and myalgia. Severe hypophosphatemia and osteomalacia have been also been described after the administration of other intravenous iron compounds, including iron polymaltose4 and the older saccharated ferric oxide.
A transient increase in intact fibroblast growth factor‐23 (iFGF‐23), a consistent finding after FCM infusions,5 has been postulated as the mechanism through which intravenous iron reduces serum phosphate. Produced by osteocytes, its physiological actions appear diverse, but in conjunction with membrane‐bound α‐Klotho on the proximal renal tubules, it inhibits the NaPi2a and 2c cotransporters, thereby increasing phosphate excretion. It also decreases active 1,25‐hydroxyvitamin D by causing preferential hydroxylation to the inactive 24,25‐hydroxyvitamin D.6 The resultant reduction in active hormone indirectly reduces intestinal phosphate absorption through the inhibition of the gut NaPi2b cotransporter.
The degree of risk of hypophosphatemia does appear to relate to the specific intravenous iron formulation. In a study of women with a history of heavy uterine bleeding, serum phosphate fell below 0.65 mmol/L in 10 of 25 patients who received FCM compared to none of the 30 women who received iron dextran.5 In a retrospective study, hypophosphatemia (<0.8 mmol/L) was noted more commonly in the 55 patients who received FCM (45.5%) than in the 26 who received iron isomaltoside (4%).7 In a prospective RCT, hypophosphatemia (<0.6 mmol/L) also occurred in 0.4% of 997 patients administered ferumoxytol and 38.7% of 1000 who received FCM.8 The physiological mechanisms involved in certain iron formulations being more likely to cause hypophosphatemia likely relate to the carbohydrate side chain associated with the specific formulation, which in turn variably affects the intracellular proteolysis of iFGF‐23. In the presence of other clinical conditions, this may result in prolonged hypophosphatemia and osteomalacia.4, 9
Additional risk factors have been described in patients experiencing severe and/or sustained hypophosphatemia following the administration of intravenous iron, most of which are themselves independently associated with a reduced serum phosphate. Such conditions include coexistent disorders in phosphate homeostasis, such as hyperparathyroidism; vitamin D deficiency; malnutrition;10 and use of drugs that predispose to hypocalcemia, such as receptor activator of nuclear factor kappa‐B ligand (RANKL) inhibitors, and bisphosphonates. The effect of multiple intravenous iron infusions, often required in patients with ongoing blood loss, might also increase the risk of developing sustained hypophosphatemia, with an associated risk of clinically significant disease.4
Symptoms of hypophosphatemia, if present, are often nonspecific and may be confused with those of underlying iron deficiency. However, for patients receiving recurrent iron infusions, particularly FCM, and especially in the presence of other relevant pathology such as hyperparathyroidism, malnutrition, vitamin D deficiency, and relevant concurrent medications such as denosumab, the risk of significant clinical sequelae is increased. Our patient developed severe hypophosphatemia and osteomalacia‐related fractures, and the additive effect of denosumab may well have been a potentiating factor. It would thus seem prudent to monitor the serum phosphate in such patients and manage both iron and phosphate supplementation accordingly. Thus, there is a need to clarify the relative risk and severity of hypophosphatemia in association with different iron formulations in order to implement more effective interventions and to adopt appropriate preventative strategies.
Declaration of conflict of interest: Lawrence P McMahon has served on the advisory boards and received speaker fees and research or travel grants from Pfizer, Pharmacosmos, Amgen, Roche, and Vifor Pharma. Stephen Bloom has served on the advisory boards of Merck Sharp and Dohme and has received speaker fees or travel grants from Abbvie, Merck Sharp and Dohme, and Bristol‐Myers Squibb. Mayur Garg has served on the advisory board of Pfizer and Pharmacosmos and has received speaker fees and research or travel grants from Abbvie, Janssen, Pfizer, Pharmacosmos, Shire, Vifor, and Takeda.
References
- 1. Evstatiev R, Marteau P, Iqbal T et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011; 141: 846.e2–53.e2. [DOI] [PubMed] [Google Scholar]
- 2. Seid MH, Derman RJ, Baker JB, Banach W, Goldberg C, Rogers R. Ferric carboxymaltose injection in the treatment of postpartum iron deficiency anemia: a randomized controlled clinical trial. Am. J. Obstet. Gynecol. 2008; 199: 435.e1–7. [DOI] [PubMed] [Google Scholar]
- 3. Zoller H, Schaefer B, Glodny B. Iron‐induced hypophosphatemia: an emerging complication. Curr. Opin. Nephrol. Hypertens. 2017; 26: 266–75. [DOI] [PubMed] [Google Scholar]
- 4. Schouten BJ, Doogue MP, Soule SG, Hunt PJ. Iron polymaltose‐induced FGF23 elevation complicated by hypophosphataemic osteomalacia. Ann. Clin. Biochem. 2009; 46: 167–9. [DOI] [PubMed] [Google Scholar]
- 5. Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J. Bone Miner. Res. 2013; 28: 1793–803. [DOI] [PubMed] [Google Scholar]
- 6. Chanakul A, Zhang MY, Louw A et al. FGF‐23 regulates CYP27B1 transcription in the kidney and in extra‐renal tissues. PLoS One. 2013; 8: e72816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schaefer B, Würtinger P, Finkenstedt A et al. Choice of high‐dose intravenous iron preparation determines hypophosphatemia risk. PLoS One. 2016; 11: e0167146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adkinson NF, Strauss WE, Macdougall IC et al. Comparative safety of intravenous ferumoxytol versus ferric carboxymaltose in iron deficiency anemia: a randomized trial. Am. J. Hematol. 2018; 93: 683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hardy S, Vandemergel X. Intravenous iron administration and hypophosphatemia in clinical practice. Int. J. Rheumatol. 2015; 2015: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fierz Y, Kenmeni R, Gonthier A, Lier F, Pralong F, Bertrand PC. Severe and prolonged hypophosphatemia after intravenous iron administration in a malnourished patient. Eur. J. Clin. Nutr. 2014; 68: 531–3. [DOI] [PubMed] [Google Scholar]


