Abstract
Objective
To compare the diagnostic role of Florine-18 2-fluoro-2-deoxy-D-glucose-Positron emission tomography/computed tomography (FDG-PET/CT) in the restaging of bladder cancer with other radiological methods and to determine its effect on the treatment management of patients with bladder cancer.
Material and methods
A total of 83 patients who showed suspicious lesions with radiologic methods and FDG-PET/CT images were enrolled in the study. Positive lesion sites were detected and compared in all imaging modalities. Positive lesions were confirmed by biopsy or serial radiological imaging. Furthermore, the rate of change of the management and treatment modalities of patients after FDG-PET/CT were noted.
Results
The most frequent metastasis was observed in lymphatic localizations in all imaging methods. Lymph node metastases was detected with FDG-PET/CT in 37/83 patients, with computed tomography in 28/80 patients, and with magnetic resonance imaging in 6/19 patients. Lymph node metastasis was detected most commonly in the pelvic region with all modalities. Following lymphatic localization in FDG-PET/CT and CT, metastases were found most frequently in the lung and bone regions. FDG-PET/CT also revealed 8 of the 12 local lesions that were detected by radiological methods and showed additional lesions in four patients that could not be demonstrated by radiological methods. FDG-PET/CT changed the treatment approaches in 34.9% (29/83) of patients. In 16 patients, it caused upstaging and commencement of advanced treatment methods. In 13 patients, malignancy was excluded in suspicious lesions and unnecessary advanced examination and treatment were avoided. FDG-PET/CT also caused three radical cystectomies, one partial cystectomy, and one urethrectomy in patients who had not undergone any operational procedure previously.
Conclusion
Despite physiological urinary tract uptake, FDG-PET/CT is superior to other imaging modalities not only in distant localizations but also in evaluating pelvic lesions and lymph nodes. In addition, the use of FDG-PET/CT during the restaging process contributes to the management of maximum number of patients.
Keywords: Bladder cancer, CT scan, metastasis, MR tomography, PET scan
Introduction
Bladder cancer, is the most frequently occuring out of the urinary tract carcinomas, 6th in all cancers, and approximately three times more common in men.[1] There are many risk factors for bladder cancer, including tobacco use, family history, occupational exposure, long-term urinary catheter use, and Schistosoma haematobium infection.[2] About 90% of bladder cancers present as pure urothelial carcinomas (transitional cell carcinoma), while the remaining 10% are squamous cell carcinomas, adenocarcinomas, and other subtypes of urothelial carcinoma.[3] Approximately 75% of the lesions are limited to the bladder at the time of diagnosis, while 25% are detected in the regional lymph nodes or in distant locations.[4]
Bladder carcinoma can be roughly divided into three groups; organ-limited type without muscle invasion, organ-limited type with muscle invasion, and metastatic disease. Intravesical bacillus Calmette-Guarin immunotherapy and/or intravesical chemotherapy following the transurethral resection of bladder tumor (TURBT) is recommended in the organ-limited type without muscle invasion.[5] Cisplatin-based neoadjuvant chemotherapy followed by radical cystectomy, bilateral pelvic lymph node dissection, and urinary diversion is recommended in patients with organ-limited type with muscle invasion depending upon the extension of disease. Cisplatin-based adjuvant chemotherapy can be considered in high-risk patients, especially if they have not been given neoadjuvant chemotherapy.[6] In metastatic disease, cisplatin-based chemotherapy is suggested. If the patient is not eligible for cisplatin, carboplatin-based protocols or a single drug protocol can be administered.[7]
In patients with bladder carcinoma the use of pelvic, abdominal, and thoracic computed tomography (CT) or magnetic resonance imaging (MRI) is recommended to evaluate local invasion and regional or distant lymph node and organ metastasis.[8] MRI has superiority over CT in local staging, especially in superficial and multiple lesions. In periviscreal fat tissue invasion and regional or distant lymph node metastases, these two imaging methods have a similar accuracy.[8–13]
Since only a morphological evaluation can be performed with imaging methods such as CT and MRI, issues arise especially in detecting metastatic lymph nodes that are smaller than 1 cm. They also can give false positive or negative results due to the lack of metabolical data. Therefore, Florine-18 2-fluoro-2-deoxy-D-glucose-Positron emission tomography/computed tomography (FDG-PET/CT) consisted of FDG-PET, which provides metabolic information and CT, which provides morphological information. It thus plays an important role in the staging and restaging of bladder carcinoma.[14–16] Urinary excretion of FDG makes bladder the ideal physiological uptake area for FDG-PET/CT and it limits the use of FDG-PET/CT in the evaluation of perivesical invasion and local staging of bladder carcinoma. On the other hand, the superiority of FDG-PET/CT over radiological methods has been reported in the detection of pelvic lymph nodes, distant lymph nodes, and metastases.[17–20] In addition, to overcome the disadvantage in the use of FDG-PET/CT evaluating bladder due to physiological FDG uptake, diuretic administiration or bladder catheterization methods have been tested and favorable results have been obtained.[21] Nevertheless, studies that investigate power of FDG-PET/CT in bladder carcinoma are not frequently reported. In this case series, we presented role of FDG-PET/CT in the restaging of bladder cancer and its effect on patient management.
Material and methods
Patient population
Between July 2011 and May 2018, the patients who were admitted to our clinic for FDG-PET/CT imaging and were pathologically diagnosed as bladder cancer were included in the study. A total of 83 patients were showed radiological images with suspicious lesions and were referred to the Nuclear Medicine Department for restaging. All these patients had sufficient medical data in the hospital database. Of these patients, 12 (14.5%) were female and 71 (85.5%) were male. The mean age was 66±10 years. The lesions reported as metastasis were confirmed by biopsy or serial radiological imaging.
All of the cases underwent TURBT or cystectomy according to the pathologic stage after the diagnosis of bladder cancer. Patients were followed up with CT or MRI after the first treatment. PET/CT images obtained for restaging were investigated retrospectively and the maximum time interval between radiological and FDG-PET/CT imaging was 1 month.
Before imaging protocol, the necessary information was given and all the potential risks were explained to the patients. Informed consent forms that included a statement that imaging data may be used for studies without revealing personal data, were read and signed by all the patients. The study was performed according to principles of the declaration of Helsinki.
PET/CT imaging
FDG-PET/CT imaging of all patients was performed with the Biograph™ PET/CT system (Siemens Molecular Imaging, Hoffman Estates, IL, USA) consisting of a PET unit and a 2-slice spiral CT in the Nuclear Medicine Department of Balcali Hospital, Cukurova University. It was ensured that the patients were fasting for at least 4 hours before the injection and the blood glucose level was between 70–200 mg/dL. Patients were given 2 liters of water until radiopharmaceutical injection. After 10–15 milliCurie FDG injection, the patients were rested in a comfortable and quiet place at an appropriate temperature for about 60 minutes. In this process, patients were given IV hydration and allowed to urinate immediately before image acquisition to prevent urinary tract artifacts. In addition, oral opaque material was given to patients for CT imaging from the 6th hour to the time of the FDG injection.
Whole body PET/CT images were obtained between the skull base and the mid-thigh region approximately 60 minutes after the FDG injection. The patients were placed in the supine position while maintaining a normal breathing speed during the image acquisition. All images were reconstructed with a 2.4 mm increment and a 5 mm slice thickness. The PET emission time was adapted to the patient weight; <60 kg: 2 minutes, 60–80 kg: 2.6 minutes, and <80 kg: 3 minutes of bed position. Applied activity, administration time, and patient weight were used for the calculation of maximum standardized uptake value (SUVmax).
Image interpretation
All images were evaluated by three nuclear physicians in three sections (axial, coronal, and sagittal) and in 3-D projection (maximum intensity projection or MIP). The SUVmax value of each focus that had a dense FDG uptake was calculated.
Statistical analysis
All analyses were performed using the IBM Statistical Package for the Social Sciences Statistics Version 20.0 statistical (IBM SPSS Corp.; Armonk, NY, USA) software package. Categorical variables were expressed as numbers and percentages, whereas continuous variables were summarized as mean and standard deviation and as median and minimum-maximum where appropriate. The Chi-square test was used to compare categorical variables between the groups. The statistical level of significance for all tests was set at a p-value of 0.05.
Results
Of the total patients, 90% of them were diagnosed with urothelial carcinoma, a rate that is similar to what has been previously recorded in the literature. In most of the cases that were diagnosed as urothelial carcinoma, the subtype was not observed, but the most common subtype is papillary urothelial carcinoma in patients whose subtypes were reported. Of the eight patients with non-urothelial carcinoma, two were diagnosed with mucinous adenocarcinoma, two had mixed carcinoma (urothelial + small cell carcinoma), one had urachal carcinoma, one had undifferentiated large cell neuroendocrine carcinoma, one had small cell carcinoma, and one was diagnosed with squamous cell carcinoma. At the time of diagnosis, submucosal invasion was detected in 73 (88%) patients and muscle invasion was observed in 51 (61.5%) patients. Before PET/CT, 27 patients (32.5%) underwent cystectomy and 56 patients (67.5%) underwent TURBT (Table 1).
Table 1.
Demographics and clinical features of patients
| Feature | Number of patients (%) |
|---|---|
| Gender | |
| Male | 71, 85.5% |
| Female | 12, 14.5% |
|
| |
| Age (years, mean) | 66±10 |
|
| |
| Pathologic type | |
| Urothelial carcinoma | 75, 90.4% |
| Papillar | 29, 38.7% |
| Squamous Differentiation | 6, 8.0% |
| Glandular Differentiation | 4, 5.3% |
| Micropapillar Variant | 2, 2.7% |
| Nested Variant | 1, 1.3% |
| Others | 8, 9.6% |
|
| |
| Submucosa invasion | |
| Negative | 10, 12% |
| Positive | 73, 88% |
|
| |
| Muscle invasion | |
| Negative | 32, 38.5% |
| Positive | 51, 61.5% |
|
| |
| Previous procedures | |
| Cystectomy | 27, 32.5% |
| Transurethral resection of bladder tumor (TURBT) | 56, 67.5% |
|
| |
| Excised metastatic lymph node | |
| Negative | 18, 66.6% |
| Positive | 9, 33.3% |
| ≤10 | 8, 88.8% |
| >10 | 1, 11.2% |
In FDG-PET/CT images, the most common pathological foci were lymph nodes (37/83; 44.5%). In the lymphatic system, pathological FDG accumulation was observed mostly in the pelvic lymph nodes (20/83; 24%). FDG-PET/CT has a higher pathological lesion detection rate than morphological diagnostic methods in the lymphatic system. The number of patients with suspected lymph node lesion was 28 (28/80; 35%) on CT and 6 (6/19; 31.5%) on MRI. Pelvic lymph nodes were also demonstrated most frequently with radiological methods. The percentage of suspected pelvic lymph nodes in the CT and MRI was 17.5% (14/80) and 15.7%, respectively (3/19). FDG-PET/CT detected bone lesions in 25.3% (21/83) of patients, while this rate was 8.7% (7/80) in CT and 15.7% (3/19) in MRI. Metastatic lesion detection rates of imaging methods according to regions are given in Table 2. In addition, FDG-PET/CT was positive in 8 of 12 patients with local spread shown with radiological methods. FDG-PET/CT demonstrated local invasion that was not shown with radiological methods in four additional patients (Figure 1–4).
Table 2.
Rates of determination of metastatic lesions by the imaging modalities based on the number of patients (number of patients: %)
| Regions | FDG-PET/CT | CT | MRI |
|---|---|---|---|
| Pelvic lymph node | 20/83, 24% | 14/80, 17.5% | 3/19, 15.7% |
| Abdominal lymph node | 14/83, 16.8% | 8/80, 10% | 1/19, 5.2% |
| Mediastinal lymph node | 6/83, 7.2% | 2/80, 2.5% | 1/2, 50%* |
| Lung | 23/83, 27.7% | 17/80, 21.2% | 2/2, 100%* |
| Bone | 20/83, 24% | 3/80, 3.75%○ | 1/19, 5.2%○ |
| Liver | 7/83, 8.4% | 4/80, 5% | 0/19, 0% |
| Sub-renal | 7/83, 8.4% | 6/80, 7.5% | 1/19, 5.2% |
Only two patients have thoracic MRI images.
In thoracic, abdominal, and pelvic image regions.
PET/CT: positron emission tomography/computed tomography; TURBT: transurethral resection of bladder tumor; MRI: magnetic resonance imaging
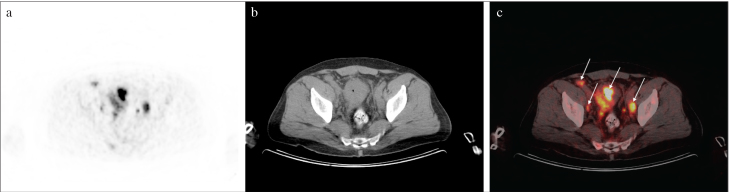
Figure 1. a–c.
Simultaneous PET (a), CT (b), and PET/CT (c) images of the patient who underwent TURBT and showed suspicious pelvic lymph nodes in the follow-up CT images. Metastatic lymph nodes were observed in the internal and external pelvic tracts. In addition, due to bladder catheterization, malignant metabolic activity is distinctly visualized in the bladder wall (arrows)
PET: positron emission tomography; CT: computed tomography; PET/CT: positron emission tomography/computed tomography
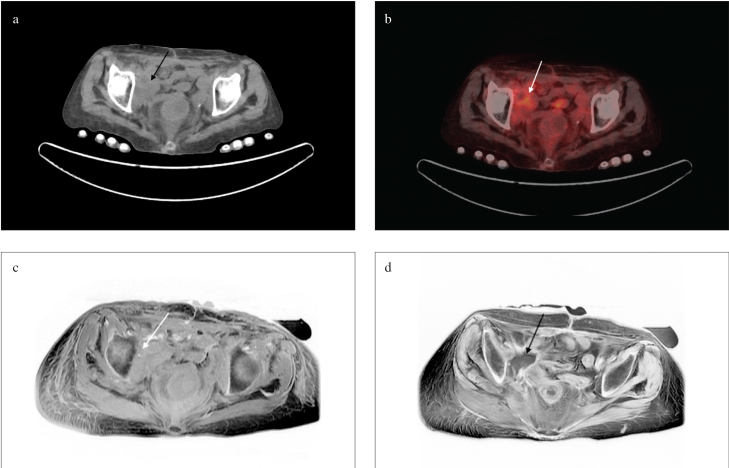
Figure 2. a–d.
Soft tissue mass (white and black arrows) adjacent to the iliac bone in the concurrent CT (a), PET/CT (b), T1 (c), and T2 (d) sequences of pelvic MRI images following cystectomy
CT: computed tomography; PET/CT: positron emission tomography/computed tomography; MRI: magnetic resonance imaging
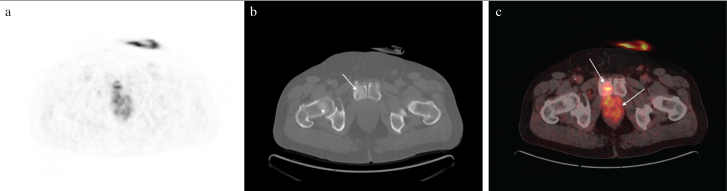
Figure 3. a–c.
PET (a), CT (b), and PET/CT (c) images of the patient who had recurrence in the bladder lodge and invasion of pubis (arrows) after cystectomy
PET: positron emission tomography; CT: computed tomography; PET/CT: positron emission tomography/computed tomography
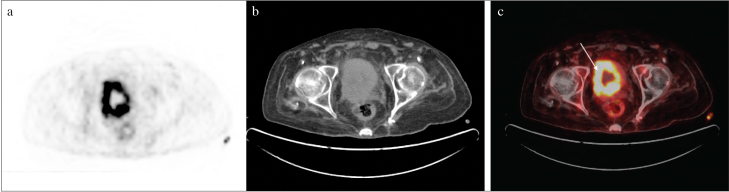
Figure 4. a–c.
After TURBT, recurrence and diffuse increased metabolic activity can be seen in the bladder wall (arrow) in the PET/CT scan with bladder catheterization
TURBT: transurethral resection of bladder tumor; PET/CT: positron emission tomography/computed tomography
The contribution of FDG-PET/CT to patient management was also investigated. The study group consisted of cases with suspected lesions during routine radiological follow-up with CT or MRI and who were referred to our clinic for restaging. FDG-PET/CT provided different or additional information in a total of 29 patients (34.9%) and caused a change in treatment planning according to radiological imaging methods. In 16 of these 29 patients, FDG-PET/CT positivity led to a progression in patient staging. In addition, nine patients (10.8%) showed malignant focus not only in suspected lesions but also in other foci. In the remaining 13 patients (15.6%), malignancy was excluded in the suspicious lesions and the stage was regressed, thus eliminating the possibility of distant metastasis or local spread, which saved these patients from a possible radiotherapy or chemotherapy regimen. The stage progression was detected with FDG-PET/CT and advanced treatment approaches differed according to the lesion site. In two patients, because metastasis was detected only in the pelvic region, radiotherapy was planned for the specific lesion sites. Systemic chemotherapy was initiated in 10 patients due to the presence of distant metastases. In 4 patients, a combination of radiotherapy and chemotherapy was applied because of local invasion or local lymph node spread and distant metastases. In the remaining 13 patients, possible malignant foci were excluded and unnecessary biopsy or other procedures were avoided. In 54 patients, FDG-PET/CT and radiological imaging reports were recorded simultaneously during patient management.
FDG-PET/CT also affected the operational decision of the patients. A total of 56 patients had undergone only TURBT and not cystectomy when PET/CT was performed. In 5 of these 56 patients (8.9%), the operational status changed according to the findings from FDG-PET/CT. Cystectomy was performed in three of these patients because no metastatic foci were detected in FDG-PET/CT. The pathological primer mass was compatible with the sample that was taken during TURBT, and none of extracted lymph nodes were metastatic. Among these 5 patients, 1 patient underwent partial cystectomy due to atypical localization of a nodular vesical mass that was unsuitable for biopsy. The mass was reported as a urachal carcinoma mixed-type (mucinous+neuroendocrine carcinoma) and three metastatic lymph nodes were detected. In the last one, a mass that had invaded to the urethra and penis was seen on the FDG-PET/CT, following which a urethrectomy was performed.
Discussion
Ultrasonography, CT, MRI, and FDG-PET/CT are used for radiological diagnosis and follow-up of bladder cancer. FDG-PET/CT is superior in the detection of distant organ and lymph node metastasis while contrast MRI is prominent in performing T-staging or evaluating the local spread. [12,13,16] CT has modarate accuracy in bladder carcinoma. The detection power of CT in local staging decreases with extravesical invasion of the tumor and microscopic metastases in regional and distant lymph nodes, which can cause a high rate of skipping.[10,22,23]
A study in which Tritschler et al.[22] investigated the value of CT in bladder cancer, the success of CT in local evaluation was found to be 49% and the success rate in lymph node evaluation was 54%. They also found that the success of CT went up to 24%, depending upon up or down staging. In the same study, the accuracy for lymph nodes was 54% and CT was shown to be more effective in showing negative lymph nodes. Tekes et al.[13] evaluated the strength of MRI in local staging and obtained a moderate level of accuracy on the basis of the patient and a high level of accuracy on the basis of the lesion in local staging. MRI can also correctly distinguish a superficial tumor from an invasive tumor and an organ-confined tumor from extensive disease at the rate of 85% and 82%, respectively.
The use of FDG-PET/CT in bladder cancer is still an area to be studied. The excretion of FDG via the urinary system limits the evaluation of local spread of bladder cancer and regional metastases. Therefore, there are few studies in the literature that investigated the role of FDG-PET/CT in bladder cancer. FDG-PET/CT is indispensable for cancer screening because of its ability to provide metabolic data and morphological data, which led to the emergence of some methods for the use of FDG-PET/CT for local assessment. One of the most commonly used methods for this purpose is intravenous furosemide injection, followed by forced diuresis and additional images from the pelvic region taken 1 hour later. Using this technique, Nayak et al.[20] found the sensitivity of FDG-PET/CT in 25 patients (78%) to be higher than contrast CT (44%) in both local assessment and in locoregional lymph nodes. In another similar study, Harkirat et al. detected 16 positive foci in the tumor region in the late diuretic images of 29 patients. Moreover, 7 of these 16 lesions could be obtained only with PET and could not be differentiated on CT images.[24] Anjos et al.[25] showed upstaging in late diuretic images by detecting positive new lesions in 6 of 16 patients in the bladder wall and in 2 of 16 patients in the paravesical lymph nodes.
A limited number of studies in the literature attempt to reveal the diagnostic value of FDG-PET/CT in bladder cancer. For this purpose, while Apolo et al.[26] compared the FDG-PET/CT findings with biopsy or consecutive CT and MRI images, the sensitivity and specificity on the lesion basis were found to be 87% and 87% and on the patient basis as 81% and 94%, respectively. In addition, in 40% of 57 patients, FDG-PET/CT revealed additional new lesions in comparison to the conventional morphological methods. Similar to our study, the metastases were most frequently observed in the lymph nodes, lungs, and skeletal system, respectively.
Kibel et al.[27] tested the diagnostic strength of FDG-PET/CT in 41 patients for both primary tumor and lymph node metastases. In patients without cystectomy, a positive lesion was seen in 28 of 34 patients with a mean SUVmax of 13.1 (±10.6) in the bladder. In the lymph nodes, the sensitivity and specificity of the proven metastases were reported as 70% and 94%, respectively; however, there was no discrimination between the different lymph node regions. With these results, the treatment management changed in 2 patients in total, which is quite low as compared to our results.
Although the use of PET in cancer imaging is a good metabolic detector of the lesion, it cannot provide anatomical information. Further, CT remains inadequate due to its inability to correctly distinguish between malignant and benign lesions in spite of correct anatomic positioning. Therefore, hybrid PET/CT is superior to these methods. Goodfellow et al.[28], according to their study in 207 high-risk patients the PET/CT outside of the pelvis was more sensitive than CT (sensitivity PET/CT: 54%, CT: 41%) but the specificity was approximately the same (specificity PET/CT: 97%, CT; 98%). In the same study, in the PET/CT combination of 93 patients who met the criteria when the pelvic region was evaluated, a significant increase was observed in the sensitivity to CT alone and the sensitivity increased from 45% to 69%. In our study, a similar increase was observed (pelvic region lymph node sensitivity: 17.5% in CT, 24% in FDG-PET/CT) and the sensitivity was lower for the pelvic region with both methods. In a multi-center retrospective study with 287 patients by Zattoni et al.[29], the FDG-PET/CT was found to be superior to contrast-enhanced CT and MRI in the lymph node, lung. and bone localizations. A higher proportion of patients also had more accurate results than other diagnostic methods.
The demonstration of distant metastases or new lesions by FDG-PET/CT is important in shaping the treatment management of the patient. While a local recurrence or a small number of regional lesions may require radiotherapy, widespread metastasis may require more aggressive systemic therapies or palliation. Therefore, determining the ability of FDG-PET/CT to change treatment management can provide important information about the follow-up of bladder cancer. A study in which the effect of radiotherapy management of FDG-PET/CT in invasive bladder carcinoma was investigated by Mertens et al.[30], FDG-PET/CT images and radiological imaging differed significantly in 21.9% of 96 patients, which occurs at a high rate (19.8%) and is seen as upstaging with additional lesions. As a result of these findings, the treatment was changed in 13 patients. This rate was relatively low as compared to 29 patients (34.9%) in our study. In seven of 13 patients, palliative treatments were recommended after PET/CT. Apolo et al.[26], as a result of the questionnaire that was admininstered among physicians, reported that physicians changed their approaches in 36 of 57 patients after reviewing PET/CT images, which resulted in avoidance of unnecessary biopsy and imaging. In our study, the most frequent distant metastases were detected with the help of FDG-PET/CT, and new systemic chemotherapy protocols were planned in 10 patients. Therefore, 13 patients were protected from unnecessary procedures.
Our study had some limitations. Firstly, it did not consist of a homogeneous group of patients. More precise results can be achieved in prospectively monitored patient groups that have the same pathological type, similar operational procedures, and treatment protocols of an equal duration. Secondly, our patient group consisted of relatively few patients. The accuracy of the results can be increased through meta-analysis or multi-center studies in which more patients are involved. Thirdly, long-term results of patients in our study group could not be obtained. The prognosis of the patients with new lesions, the patients whose treatment protocols changed, and even the patients that showed negative results with FDG-PET/CT remained unknown. The correlation of our data with long-term results may present new findings in terms of patient management in the future.
In conclusion, FDG-PET/CT is an effective imaging modality for not only distant organ or nodal metastasis, but also for the detection of regional nodal metastases, in spite of the physiological uptake of FDG in the urinary tract. Also, additional data provided by FDG-PET/CT in both the regional and distant foci have changed the treatment protocol in a significant part of the patients or protected patients from unnecessary procedures and treatments.
Footnotes
Ethics Committee Approval: Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects”, (amended in October 2013).
Informed Consent: Written informed consent was obtained from all patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - İ.B.G., K.A.K., V.İ., M.K.; Design - İ.B.G., K.A.K., V.İ., M.K.; Supervision - İ.B.G., K.A.K., V.İ., M.K.; Resources - İ.B.G., V.İ., M.K.; Materials - K.A.K.; Data Collection and/or Processing - İ.B.G., K.A.K., V.İ., M.K.; Analysis and/or Interpretation - İ.B.G., K.A.K.; Literature Search - K.A.K.; Writing Manuscript - İ.B.G., K.A.K.; Critical Review - İ.B.G., K.A.K., V.İ., M.K.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.PDQ® Adult Treatment Editorial Board. PDQ Bladder Cancer Treatment. Bethesda, MD: National Cancer Institute; [Accessed <07/27/2018>]. Updated <05/03/2018>. Available at: https://www.cancer.gov/types/bladder/patient/bladder-treatment-pdq. [Google Scholar]
- 3.Kantor AF, Hartge P, Hoover RN, Fraumeni JF. Epidemiological characteristics of squamous cell carcinoma and adenocarcinoma of the bladder. Cancer Res. 1988;48:3853–5. [PubMed] [Google Scholar]
- 4.Carroll PR. Urothelial carcinoma: cancers of the bladder, ureter, & renal pelvis. Smith and Tanagho’s General Urology. 2012;310 [Google Scholar]
- 5.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Compérat E, et al. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64:639–53. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Chang SS, Bochner BH, Chou R, Dreicer R, Kamat AM, Lerner SP, et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol. 2017;198:552–9. doi: 10.1016/j.juro.2017.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milowsky MI, Rumble RB, Lee CT. Guideline on muscle-invasive and metastatic bladder cancer (European Association of Urology guideline): American Society of Clinical Oncology clinical practice guideline endorsement summary. J Oncol Pract. 2016;12:588–90. doi: 10.1200/JOP.2016.012898. [DOI] [PubMed] [Google Scholar]
- 8.Witjes JA, Compérat E, Cowan NC, De Santis M, Gakis G, Lebret T, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65:778–92. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Kim JK, Park SY, Ahn HJ, Kim CS, Cho KS. Bladder cancer: analysis of multi–detector row helical CT enhancement pattern and accuracy in tumor detection and perivesical staging. Radiology. 2004;231:725–31. doi: 10.1148/radiol.2313021253. [DOI] [PubMed] [Google Scholar]
- 10.Baltaci S, Resorlu B, Yagcı C, Turkolmez K, Gogus C, Beduk Y. Computerized tomography for detecting perivesical infiltration and lymph node metastasis in invasive bladder carcinoma. Urol Int. 2008;81:399–402. doi: 10.1159/000167836. [DOI] [PubMed] [Google Scholar]
- 11.Oz II, Altinbas NK, Serifoglu I, Oz EB, Yagci C. The Role of Computerized Tomography in the Assessment of Perivesical Invasion in Bladder Cancer. Pol J Radiol. 2016;81:281–7. doi: 10.12659/PJR.896752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajesh A, Sokhi HK, Fung R, Mulcahy KA, Bankart MJG. Bladder cancer: evaluation of staging accuracy using dynamic MRI. Clin Radiol. 2011;66:1140–5. doi: 10.1016/j.crad.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Tekes A, Kamel I, Imam K, Szarf G, Schoenberg M, Nasir K, et al. Dynamic MRI of bladder cancer: evaluation of staging accuracy. AJR Am J Roentgenol. 2005;184:121–7. doi: 10.2214/ajr.184.1.01840121. [DOI] [PubMed] [Google Scholar]
- 14.Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. The J Nucl Med. 2003;44:1200–9. [PubMed] [Google Scholar]
- 15.Schöder H, Erdi YE, Larson SM, Yeung HW. PET/CT: a new imaging technology in nuclear medicine. Eur J Nucl Med Mol Imaging. 2003;30:1419–37. doi: 10.1007/s00259-003-1299-6. [DOI] [PubMed] [Google Scholar]
- 16.Lawrentschuk N, Lee ST, Scott AM. Current role of PET, CT, MR for invasive bladder cancer. Curr Urol Rep. 2013;14:84–9. doi: 10.1007/s11934-013-0308-y. [DOI] [PubMed] [Google Scholar]
- 17.Lu YY, Chen JH, Liang JA, Wang HY, Lin CC, Lin WY, et al. Clinical value of FDG PET or PET/CT in urinary bladder cancer: a systemic review and meta-analysis. Eur J Radiol. 2012;81:2411–6. doi: 10.1016/j.ejrad.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Jadvar H, Quan V, Henderson RW, Conti PS. [F-18]-Fluorodeoxyglucose PET and PET-CT in diagnostic imaging evaluation of locally recurrent and metastatic bladder transitional cell carcinoma. Int J Clin Oncol. 2008;13:42–7. doi: 10.1007/s10147-007-0720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soubra A, Hayward D, Dahm P, Goldfarb R, Froehlich J, Jha G, et al. The diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography and computed tomography in staging bladder cancer: a single-institution study and a systematic review with meta-analysis. World J Urol. 2016;34:1229–37. doi: 10.1007/s00345-016-1772-z. [DOI] [PubMed] [Google Scholar]
- 20.Nayak B, Dogra PN, Naswa N, Kumar R. Diuretic 18 F-FDG PET/CT imaging for detection and locoregional staging of urinary bladder cancer: prospective evaluation of a novel technique. Eur J Nucl Med Mol Imaging. 2013;40:386–93. doi: 10.1007/s00259-012-2294-6. [DOI] [PubMed] [Google Scholar]
- 21.Leisure GP, Vesselle HJ, Faulhaber PF, O’Donnell JK, Adler LP, Miraldi F. Technical improvements in fluorine-18-FDG PET imaging of the abdomen and pelvis. J Nucl Med Technol. 1997;25:115–9. [PubMed] [Google Scholar]
- 22.Tritschler S, Mosler C, Straub J, Buchner A, Karl A, Graser A, et al. Staging of muscle-invasive bladder cancer: can computerized tomography help us to decide on local treatment? World J Urol. 2012;30:827–31. doi: 10.1007/s00345-011-0817-6. [DOI] [PubMed] [Google Scholar]
- 23.Herr HW, Hilton S. Routine CT scan in cystectomy patients: does it chance management? Urology. 1996;47:324–5. doi: 10.1016/S0090-4295(99)80446-4. [DOI] [PubMed] [Google Scholar]
- 24.Harkirat S, Anand SS, Jacob MJ. Forced diuresis and dual-phase 18F-fluorodeoxyglucose-PET/CT scan for restaging of urinary bladder cancers. Indian J Radiol Imaging. 2010;20:13–9. doi: 10.4103/0971-3026.59746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anjos DA, Etchebehere ECSC, Ramos CD, Santos AO, Albertotti C, Camargo EE. 18F-FDG PET/CT delayed images after diuretic for restaging invasive bladder cancer. J Nucl Med. 2007;48:764–70. doi: 10.2967/jnumed.106.036350. [DOI] [PubMed] [Google Scholar]
- 26.Apolo AB, Riches J, Schöder H, Akin O, Trout A, Milowsky MI, et al. Clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in bladder cancer. J Clin Oncol. 2010;28:3973–8. doi: 10.1200/JCO.2010.28.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kibel AS, Dehdashti F, Katz MD, Klim AP, Grubb RL, Humphrey PA, et al. Prospective study of [18F] fluorodeoxyglucose positron emission tomography/computed tomography for staging of muscle-invasive bladder carcinoma. J Clin Oncol. 2009;27:4314–20. doi: 10.1200/JCO.2008.20.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodfellow H, Viney Z, Hughes P, Rankin S, Rottenberg G, Hughes S, et al. Role of fluorodeoxyglucose positron emission tomography (FDG PET)-computed tomography (CT) in the staging of bladder cancer. BJU Int. 2014;114:389–95. doi: 10.1111/bju.12608. [DOI] [PubMed] [Google Scholar]
- 29.Zattoni F, Incerti E, Colicchia M, Castellucci P, Panareo S, Picchio M, et al. Comparison between the diagnostic accuracies of 18F-fluorodeoxyglucose positron emission tomography/computed tomography and conventional imaging in recurrent urothelial carcinomas: a retrospective, multicenter study. Abdom Radiol (NY) 2018;43:2391–9. doi: 10.1007/s00261-017-1443-6. [DOI] [PubMed] [Google Scholar]
- 30.Mertens LS, Fioole-Bruining A, Vegt E, Vogel WV, van Rhijn BW, Horenblas S. Impact of 18 F-fluorodeoxyglucose (FDG)-positron-emission tomography/computed tomography (PET/CT) on management of patients with carcinoma invading bladder muscle. BJU Int. 2013;112:729–34. doi: 10.1111/bju.12109. [DOI] [PubMed] [Google Scholar]