Abstract
Dust is suspected to be an important factor in transmission of livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) between pigs and pig farmers and their families. The aim of this study was to determine the rate of decay for Staphylococcus aureus and LA-MRSA in dust from swine farms. Electrostatic dust fall collectors (EDCs) were used for passive sampling of settling airborne dust in 11 stable sections from six swine farms. Extraction, plating, identification, and enumeration of cultivable S. aureus and LA-MRSA from the EDCs were performed after storage for 0–30 days postsampling. The survival of S. aureus was measured in 196 dust samples from all farms, and data were used to estimate the decay constant λ according to a model for exponential decay: N(t) = N0 × e−λt. The number of S. aureus colonies was up to 600-fold higher than the number of LA-MRSA colonies on MRSA selective agar. The data showed a good fit to the model (λ = 0.13, r2 = 0.86) even with a large difference in initial concentrations of S. aureus between stables. The loads of S. aureus and LA-MRSA in the dust were significantly reduced by storage time, and the half-life was 5 days for both S. aureus and LA-MRSA. In dust samples with high initial concentrations, LA-MRSA and S. aureus could still be cultivated 30 days after sampling. On all farms MRSA isolates belonged to the clonal complex (CC) 398, and at one farm some isolates also belonged to CC30. A screening for other Staphylococcus species in the farm dust revealed 13 different species numerically dominated by Staphylococcus equorum. Based on the exponential decay model, S. equorum had a half-life of 4 days. In conclusion, the presence of MRSA in airborne dust from five of six farms indicates that dust might be an important vehicle for transmission of LA-MRSA. LA-MRSA and S. aureus was found to survive well in farm dust with half-lives of 5 days, and dependent on the initial concentration they could be found in farm dust for weeks. The 99.9% die-off rate was 66 days for LA-MRSA. Thus, farm dust can pose an exposure risk for humans in the farm environment, but also when transported to other environments. On the other hand, the risk will decrease by time. These results provide important knowledge to diminish spread from farm environments to other environments on, e.g., tools or clothing, and in relation to cleaning of emptied LA-MRSA-positive stables.
Keywords: bacterial half-life, CoNS, exposure, farmer, stable dust
Introduction
Transmission of livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) between pigs and pig farmers and their families was described in 2005 in the Netherlands (Voss et al., 2005). In Denmark, the prevalence of LA-MRSA infected swine farms has increased from 16% in 2010 to 88% in 2016 (Ministry of Environment and Food of Denmark 2017). Concurrently, an increase in the number of cases of LA-MRSA-positive humans has been found (Larsen et al., 2015).
Direct contact with live pigs is a known risk factor for LA-MRSA colonization and farm workers are expected to be particularly highly exposed, but also veterinarians have a significantly elevated risk of becoming LA-MRSA carriers (Garcia-Graells et al., 2012; Dahms et al., 2014; Van Cleef et al., 2015). However, transmission of LA-MRSA is not restricted to persons in direct contact with infected animals, as household members show a higher level of LA-MRSA carriage in comparison to the general community (Verkade et al., 2014). Furthermore, a significant geographical association between the density of swine farms and the incidences of human LA-MRSA carriage has been shown (van Loo et al., 2007; van Rijen et al., 2014; van Alen et al., 2017). An increasing number of people without direct contact to livestock is registered as LA-MRSA positive (van Rijen et al., 2014; Larsen et al., 2015; Deiters et al., 2015; Nielsen et al., 2016).
Recent surveys show that approximately 40% of all new human MRSA-cases in both Denmark, the Netherlands, and in some regions of Germany are attributed to LA-MRSA CC398—the most widespread clone of LA-MRSA in Europe (Köck et al., 2013; NETHMAP, 2013; DANMAP, 2016). In Denmark, LA-MRSA CC398 was responsible for 208 documented cases of human infections in 2015, corresponding to a 13% infection rate of the 1173 reported cases of LA-MRSA CC398 (DANMAP, 2016). The colonization of farm workers and the dissemination of LA-MRSA into the community are an occupational as well as a public health concern, which has large economic implications for the society. To counteract this development by targeted interventions, a better understanding of LA-MRSA transmission is needed.
LA-MRSA has been measured in the air and in dust settling on surfaces within swine farms (Schulz et al., 2012; Agersø et al., 2013; Bos et al., 2016; Dahms et al., 2014). Apart from direct contact with infected animals, exposure to bioaerosols has therefore been proposed as a determinant for nasal carriage of LA-MRSA in farm workers (Bos et al., 2016). In addition, bioaerosols and dust may also function as a source of transmission to humans outside the farm. This may occur by emission of bioaerosols from the farms or indirectly by contamination of tools, farming implements, clothing, etc., which are brought outside the farm buildings. The concentration of viable LA-MRSA in dust may therefore be an important parameter when assessing the risk of secondary exposure. To our knowledge, the survival of LA-MRSA in dust from swine farms has not previously been determined. The aim of this study was to determine the survival of LA-MRSA in settling dust sampled in different swine farms in Denmark. The survival of Staphylococcus equorum was also investigated as we found this species to be commonly present in stable dust, and as this species is also of health concern (Marshall et al., 1998).
Materials and methods
Sampling and storage
Electrostatic dust fall collectors (EDCs) were used for sampling of bacteria in settling airborne dust within six swine farm stables (Table 1) located in different regions (Zealand, Jutland, and Funen) of Denmark. EDCs consist of a polypropylene map with four sterilized electrostatic dust cloths (each 19 cm × 11 cm). For sampling, the EDCs were placed in one to four different stable sections in each of the six swine farms of the study (Table 1). The EDCs were left open for passive sampling in a height of 1.0–1.5 m above the floor in 1–7 days, and thereafter transported to the laboratory. One cloth from each of the EDCs was analyzed at the time of arrival to the laboratory (2 h to 6 days postsampling—depending on the transportation time of the EDCs), and the remaining cloths were analyzed with different time intervals after storage in the laboratory for up to 30 days postsampling (Table 1). The EDCs were stored at ambient conditions with a mean temperature of 23°C (mean daily min. 22°C and mean daily max. 24°C) and a mean relative humidity of 30% (mean daily min. 17%, mean daily max. 33%).
Table 1.
Overview of dust sampling and detection of MRSA and Staphylococcus aureus types in the sampled dust.
| Pig farm | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| Stable sections | Weaning and nursing | Weaning | 4 Weaning sections | Farrowing and weaning | Slaughter and weaning | Farrowing and 2 weaning sections |
| Number of samplesa | 24 (n = 3) | 48 (n = 1–3) | 16 (n = 1) | 24 (n = 3) | 24 (n = 3) | 60 (n = 5) |
| Survival period studied (days postsampling) | 0, 10, 15, 20 | 0–7 (4 days analyzed)b | 1, 9, 17, 30 | 6, 12, 18, 24 | 5, 10, 14, 17 | 0, 1, 4 |
| S. aureus detected | Yes | Yes | Yes | Yes | Yes | Yes |
| MRSA detected | Yes | Yes | Yes | Yes | Yes | No |
| CC group | CC398 and CC30 | CC398 | CC398 | CC398 | CC398 | CC398 |
| Spa-type | t034, t4652, and t1333 | t011 | t034 | t571 | t034 | t034 |
Total number of samples (n = number of replicate samples from the same sampling occasion and section).
Sampling was performed at 8 different occasions, and survival was analyzed at Day 0 and after storage for three different time periods until Day 7.
Extraction and cultivation
Dust and bacteria from the EDC cloths were extracted in 50 ml tubes by addition of 15 ml extraction buffer (0.85% NaCl + 0.05% Tween 80) and shaking at 500 rpm for 15 min. The extractions were then plated on chromogenic agar plates selective for MRSA [Brilliance MRSA-2 agar, Oxoid (MRSA 2)] and for Staphylococcus spp. [SaSelect, Bio-Rad (SA)], in volumes of 100, 200, and 500 µl. SA is designed to select broadly for Staphylococcus. The agar plates were incubated at 37°C for 18–24 h before enumeration of colony-forming units (CFU) of MRSA, total S.aureus (on SA), and S. equorum (on SA). After identification by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) concentrations of the studied species were calculated as CFU m−2 sampling cloth. Samples of EDC dust extractions from all samples were also prepared for freeze storage by mixing 1.0 ml dust extraction with 0.5 ml 85% glycerol, and the tubes were placed at −80°C for later PCR and subtyping analyses.
Species identification by MALDI-TOF MS
To verify the species identity of bacterial colonies with specific color morphology on the selective agar plates we used MALDI-TOF MS. From MRSA-2 plates, presumptive MRSA isolates were identified and counted. To assess the diversity of Staphylococcus species, identification of all colonies on a SA plate from the first dust extraction from each farm were performed. From subsequent samples, only presumptive S. aureus and S. equorum colonies were identified and counted. For identification the extended direct transfer method was used as described earlier (Madsen et al., 2016). In brief, a toothpick was used to transfer a small amount of the bacterial colony onto the target plate (MSP 96 target polished steel BC, Bruker Daltonics, Bremen, Germany). The sample was then overlaid with 70% formic acid and allowed to dry before addition of an HCCA matrix solution (α-cyano-4-hydroxycinnamic acid, Bruker Daltonics). The MALDI-TOF MS analysis was performed on a Microflex LT mass spectrometer (Bruker Daltonics) using the Bruker Biotyper 3.1 software with the BDAL standard library. A bacterial test standard (Bruker Daltonics) was used to calibrate the instrument.
PCR verification and subtyping of MRSA and S. aureus isolates
Colonies isolated from MRSA 2 plates and confirmed by MALDI-TOF MS to be S. aureus were further verified to be MRSA by a PCR assay detecting mecA, a gene specific for CC398 and spa typing (Islam et al., 2017). Seven to ten isolates from each of the Farms A, B, C, D, and E were randomly chosen for PCR and subtyping. From Farm F, PCR and subtyping of 10 S. aureus presumptive isolates were instead performed on colonies isolated from SA plates.
Treatment of data
Concentrations of bacteria are presented as CFU m−2 EDC with standard error. The data were log-normal distributed. The concentrations as a function of storage time were analyzed using PROC GLM (General Linear Models Procedure) from SAS version 9.4 (SAS institute, Cary, NC, USA). To estimate the decay rate (λ) for MRSA and for total S. aureus and S. equorum we used the model N(t) = N0 × exp−λt, which can be converted to ln N(t)i = ln N(t0)I − λt + farmi + Ɛit, where i represents measurements from each of the different farm stable sections; Ɛit represents the error term; N(t) represents the CFU number of bacteria at time t and N0 represents the CFU number of bacteria at time t = 0. To test the validity of the model, we first determined the interaction (farmi × t), using the formula ln N(t)i = ln N(t0)i – λt + farmi + λ(farmi × t) + Ɛit. We found this interaction to be insignificant (P > 0.05), thus suggesting that the origin of the dust did not affect the decay rate of the bacteria. An exception was dust samples from Farm D, which showed a significantly slower decay of MRSA than dust from the other farms. Two model calculations of λ were therefore performed, with and without inclusion of data from Farm D. The 90, 99, and 99.9% die-off rates were calculated. The effect of time from sampling to the first measure and of the number of sampling days on the estimated half-lives was studied in a MIXED procedure COVTEST.
Results
MALDI-TOF MS analysis confirmed that the specific color of the colonies on the selective agar plates corresponded to the expected species identity for all tested MRSA, S. aureus, and S. equorum isolates. The characterized S. aureus isolates belonged to five spa-types and two CC-groups (Table 1).
Concentrations of MRSA and total S. aureus in dust samples
A large variation in the initial concentration of MRSA was found in the EDC samples from the different swine farms (means between 6 × 103 and 7 × 105 CFU m−2), but also between stable sections within the same farm (Fig. 1). In all EDC samples from Farm F, and in samples from 4 of 8 sampling occasions from Farm B the concentration of MRSA was below the level of detection (<1.4 × 103 CFU m−2). The concentration of S. aureus was below the level of detection in samples from one time point of dust extractions from the weaning section in Farm A, one section in Farm F, and one sample from Farm B.
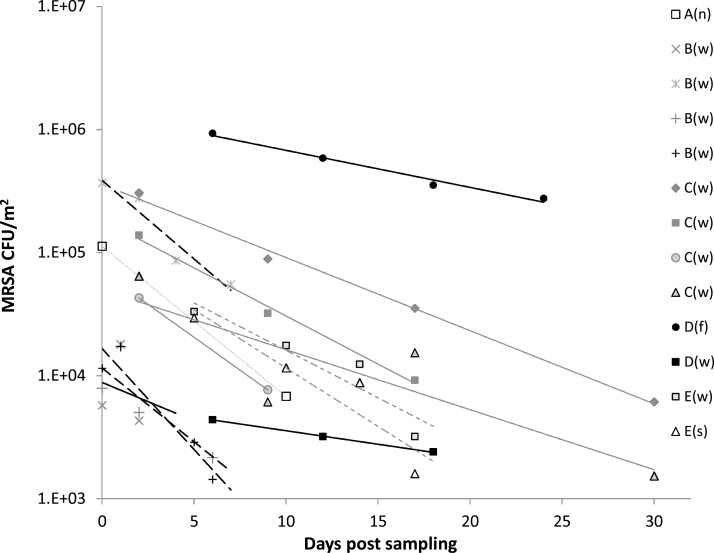
Figure 1.
Survival of MRSA in swine stable dust following storage as determined by CFU of MRSA on MRSA 2 agar plates. The symbols represent the mean of EDC samples (n = 1–3, Table 1) collected from each of the farm stable sections at different sampling occasions. Capital letters represent data from the five MRSA-positive farms, and letters in brackets designate the type of stable section: (n) nursing, (w) weaning, (f) farrowing, and (s) slaughter. For Farms A to E, the following symbols are used for the regression lines: A: ---; B: ----; C: — ; D: — ; E:-----.
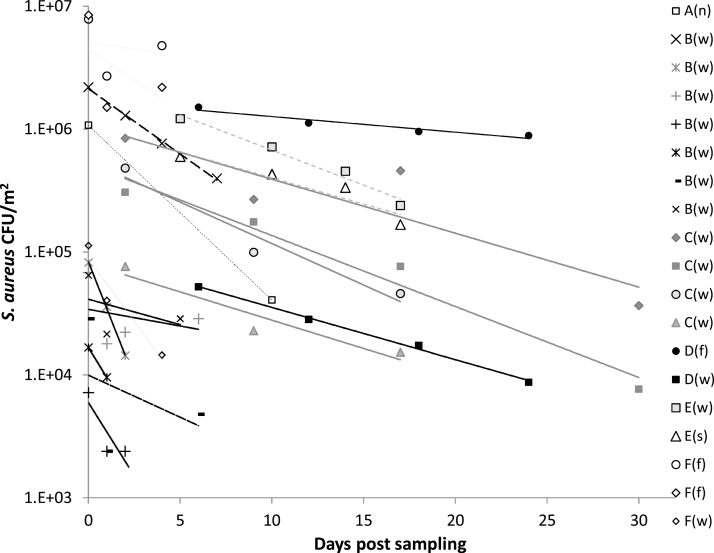
Comparative CFU measurements of total S. aureus (MRSA and nonresistant S. aureus colonies) on SA selective agar plates and MRSA on MRSA-2 agar showed that total S. aureus was between 2- and 600-fold higher than the number of MRSA (Figs 1 and 2). In the different stable sections from Farm B, C, and D, and in the nursing section from Farm A, the total number of S. aureus as measured on SA was 2- to 12-fold higher than the number of MRSA measured on MRSA-2 agar. In the slaughter and weaning section from Farm E the total number of S. aureus was 50-fold higher, and in the weaning section from Farm A, it was 600-fold higher than MRSA.
Figure 2.
Survival of total Staphylococcus aureus in swine stable dust following storage as determined by CFU of S. aureus on SA plates. The symbols represent the mean of individual sets of replicate EDC-samples (n = 1–5, Table 1) collected from each of the farm sections at different sampling occasions. Capital letters represent data from each of the farms A, B, C, D, E, and F, and letters in brackets designate type of stable section: (n) nursing, (w) weaning, (f) farrowing, and (s) slaughter. For Farms A to F, the following symbols are used for regression lines: A: ---; B: ---- ; C: — ; D: — ; E:-----; F: —.
Exponential decay models
For all measurements the concentrations of MRSA in stable dust were significantly (P < 0.001) reduced in response to storage time of the dust (Fig. 1). When all measurements of MRSA concentrations in dust were plotted into a model for exponential decay [N(t) = N0 × e−λt) storage time of the dust was found to be a significant factor (P < 0.001). The model parameters were calculated based on the assembled data, resulting in an estimate of the exponential decay constant λ = −0.140. This corresponds to a half-life of 5 days for MRSA in the dust (Table 2). The data showed a good model fit (r2 = 0.88), however, a significant effect of stable dust origin versus storage time was observed (P = 0.017). MRSA seemed to exhibit a better survival in dust from Farm D than in dust from the other farms, and when data from Farm D were excluded from the data analysis, no interaction was found between dust origin and survival (P = 0.35). The decay constants for EDC samples collected in Farm D were, λ = −0.069 and λ = −0.067 for the farrowing and the weaning section, respectively, both corresponding to a half-life of 10 days for MRSA in dust from Farm D. To determine the impact of Farm D on the model, we made a new calculation of model parameters with exclusion of Farm D. The estimated decay constant for this model was λ = −0.172, corresponding to a half-life of 4 days (Table 2).
Table 2.
Decay half-lives for MRSA and Staphylococcus aureus in a model including (Model 1) and excluding (Model 2) data from Farm D.
| MRSA Model 1 | MRSA Model 2 | S. aureus Model 1 | S. aureus Model 2 | |
|---|---|---|---|---|
| Farms | A, B, C, D, E | A, B, C, E | A, B, C, D, E, F | A, B, C, E, F |
| Measurements (n) | 92 | 71 | 140 | 118 |
| Correlation, r2 | 0.88 | 0.83 | 0.86 | 0.86 |
| Decay constant (λ) | −0.140 | −0.172 | −0.127 | −0.156 |
| SE (λ) | 0.014 | 0.017 | 0.016 | 0.020 |
| Bacterial concentration in CFU m−2 at t0 (N0) | 7.4 × 104 | 1.2 × 105 | 4.3 × 105 | 5.6 × 105 |
| Half-life (t½) in days | 5.0 (4.1–6.2) | 4.0 (3.4–5.0) | 5.5 (4.5–7.2) | 4.4 (3.6–5.9) |
| 90% die-off in days | 33 (28–41) | 28 (22–33) | 36 (29–48) | 30 (24–39) |
| 99% die-off in days | 49 (41–61) | 40 (34–50) | 54 (44–72) | 44 (35–59) |
| 99.9 % die-off in days | 66 (55–82) | 54 (45–66) | 72 (58–96) | 59 (47–79) |
CFU, colony-forming units. N0 represents the CFU number of bacteria at the first measure of the survival study (t0).
The estimated half-life for MRSA in Model 1 was not affected significantly by the number of sampling days (P = 0.24) and the time from sampling to the first measure of the concentration (P = 0.11). The same was seen for the Model 2 data (P > 0.05).
The concentration of S. aureus in stable dust was significantly (P < 0.001) reduced in response to storage time of the dust (Fig. 2). A significant negative correlation between the concentration of total S. aureus and storage time was shown when the measurements of S. aureus concentrations in dust from all farms were plotted into the model for exponential decay (P < 0.001). The data showed a good correlation to the model (r2 = 0.86), in which the estimated exponential decay constant was λ = −0.127. The decay rate for S. aureus was not significantly affected by the origin of the stable dust (P = 0.087). Yet, the survival of total S. aureus in dust from Farm D seemed to be higher than in dust from the other farms, as also found for MRSA. When data from Farm D were excluded from the data analysis, no interaction was found between dust origin and survival (P = 0.27). The decay constants for EDC samples from the farrowing section and the weaning section in Farm D were λ = −0.046 and λ = −0.099, respectively. This corresponds to a half-life of 15 days for S. aureus in dust from the farrowing section and a half-life of 7 days for the weaning section. When excluding data from Farm D from the model, the estimated decay rate was λ = −0.156, corresponding to a half-life of 4 days. Thus, the decay rates estimated for the models based on concentrations of total S. aureus and of MRSA showed to be consistent, both resulting in half-lives of 4–5 days (Table 2).
The estimated half-life for S. aureus in Model 1 was not affected significantly by the number of sampling days (P = 0.74) and the time from sampling to the first measure of concentration (P = 0.24). The same was seen for the Model 2 data (P > 0.05).
Presence and survival of other Staphylococcus species in dust samples
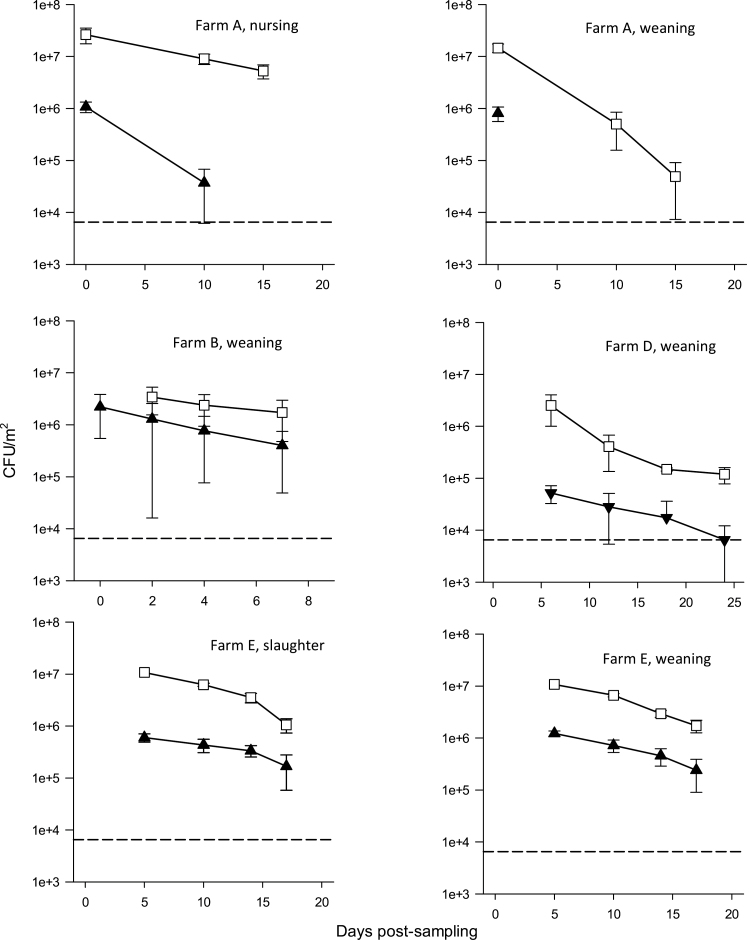
In addition to S. aureus, a variety of different Staphylococcus species were found in the dust samples from the swine farms. These included S. equorum, Staphylococcus haemolyticus, Staphylococcus simulans, Staphylococcus epidermidis, Staphylococcus xylosus, Staphylococcus chromogenes, Staphylococcus saprophyticus, Staphylococcus hyicus, Staphylococcus sciuri, Staphylococcus hominis, Staphylococcus pettenkoferi, Staphylococcus warneri, and Staphylococcus pasteuri. The concentration and survival of S. equorum was determined in samples from Farms A, B, D, and E. In all samples, the concentration of S. equorum was at least 10-fold higher than the concentration of S. aureus (Fig. 3). However, the survival of S. equorum in the stable dust seemed to be similar to the survival of S. aureus. Based on the exponential decay model, the decay rate for S. equorum was λ = −0.191, corresponding to a half-life of 3.6 days (3.0–4.6 days).
Figure 3.
Survival (mean and standard error of the mean) of Staphylococcus aureus (filled triangles) and Staphylococcus equorum (open squares) in stable dust during storage. The dotted line is the limit of detection.
Discussion
Stable dust is a complex aggregated compound including dried dung and urine, skin flakes, grain mites, spores, pollens, feed, and bedding particles (Pedersen et al., 2000) and therefore difficult to replace by artificial produced media. Previous studies of S. aureus survival in the environment has primarily been performed in relation to hospital hygiene control measures by transfer of pure bacterial cultures to different surfaces (Kramer et al., 2006). In this study, survival of staphylococci was measured in dust collected in the pig stables, which is important to get a better picture of LA-MRSA survival in the environment. Several factors may influence the survival of bacteria in aerosols and in settled dust, and hence affect the potential levels of human exposure. Thus, the composition and structure of dust particles and the clustering behavior of the bacteria as well as relative humidity and temperature affect the survival of bacteria (Madsen et al., 2009; Tang, 2009; Zhao et al., 2014). Yet, the bacterial response to environmental conditions is complicated and depends on the physiological state of the bacteria. In a study by Strasters and Winkler (1966), the effect of humidity on survival of cultured aerosolized hospital strains of staphylococci varied significantly in response to the bacterial culture age, the method of aerosolization, and the growth and suspension medium.
In our study, the community of LA-MRSA in the stable dust samples most likely represented different physiological stages of growth and stress conditions. The time preceding bacterial release from the host pig and thus favorable growth conditions for S. aureus, may have been variable—especially because the collected dust samples presumably contained both newly aerosolized particles and settled and re-aerosolized particles. The primary source of LA-MRSA in swine stables are the pigs, where MRSA proliferates as a normal part of the microbiota of skin and nostrils. During activity of the pigs, emission of LA-MRSA occurs from mucus or by abscess of skin particles, and thus bioaerosols are released to the stable air (Zhao et al., 2014). Depending on the size and the source of the bioaerosols, the LA-MRSA may have experienced different protection against environmental stress both as aerosols and in the settled dust.
We have found a half-life of LA-MRSA in settled stable dust of 5 days. To our knowledge, this is the first study to assess the rate of decay of LA-MRSA in stable dust. Studies on laboratory cultivated isolates typically have determined the total duration time until extinction of the bacteria, while we have obtained a measure of the rate of decay. This measure is particularly important in determining the risk of LA-MRSA exposure during, e.g., cleaning of stables emptied for animals, or by assessment of LA-MRSA survival on working clothes or working tools. Previous reports have suggested that an important route of LA-MRSA transmission from animals to humans is via bioaerosols (Bos et al., 2016). We recently found a significant correlation between the concentration of LA-MRSA in the stable air of a swine farm and the risk of nasal LA-MRSA carriage in voluntary people who were either in direct contact with animals or simply standing still in the corridor between the pig pens for an hour. The nasal LA-MRSA carriage in the volunteers was independent of the number of hand facial touches, signifying that bioaerosols were a primary source of contamination (Angen et al., 2017). Given a half-life of 5 days for LA-MRSA, the specific working task of cleaning following emptying of the stable for animals, seem to represent a risk of exposure due to the low rate of LA-MRSA decay and a re-aerosolisation of settled dust. The survival of LA-MRSA is also of relevance for assessment of exposure by carry-over of dust from the stable to the outside environment. In some countries, regulations demand that personal bath and change clothes before leaving an LA-MRSA infested stable. Considering the results of this study, these measures of control are sensible and apart from preventing transmission of LA-MRSA also counteract dissemination of other potential pathogenic microorganisms. Transmission of stable dust to the outside air via ventilation is another route, which has been discussed as a potential way of animal to human transmission (Gibbs et al., 2004). However, many factors like UV-radiation and humidity may be very different in the outside environment and the half-life of LA-MRSA determined for dust in this study cannot be extrapolated to the outdoor conditions.
The populations of LA-MRSA in the different stables seem to exhibit patterns of survival similar to those of the total populations of S. aureus. According to a review study, there are no obvious difference in survival of laboratory cultivated multiresistant and nonresistant S. aureus (Kramer et al., 2006). Staphylococcus aureus is generally recognized as a very robust species, which is highly resistant toward environmental stress, including desiccation (Clements and Foster, 1999), and a review paper based on studies where S. aureus has been cultivated in the laboratory and subsequently transferred to different surfaces, a persistence of 7 days to 7 months has been reported (Kramer et al., 2006). In this study, the survival of LA-MRSA was significantly higher in dust from Farm D compared to the other farms. This difference could be due to a different composition of the dust, but interestingly, Farm D was the only farm harboring LA-MRSA belonging to spa-type t571. It could therefore be speculated that the population of LA-MRSA with prevailing spa-type t571 exhibited better capabilities of survival compared to the populations of LA-MRSA strains from the other farms.
In all investigated stable sections from the six farms, S. aureus was found in the dust and MRSA was found in the dust in five of six farms. All presumptive MRSA colonies selected for verification belonged to previously described LA-MRSA lineages CC398 and CC30, but with different spa types dominated in the different farms, including t011, t034,and t571. In Farm A, t1333 (CC30) MRSA was found together with CC398 MRSA. The type CC398 is the most common in Danish pig farms while CC30 has been found (Seier-Petersen et al., 2015), but only rarely. LA-MRSA spa type t011 and t034 have also been found in other farm animals as, e.g., calves (Graveland et al., 2010).
A high prevalence of MRSA-positive air samples has also been found in swine farms in other studies (Friese et al., 2012; Agersø et al., 2013; Masclaux et al., 2013; Bos et al., 2016; Schmithausen et al., 2015). A large proportion of the S. aureus populations in pig farms has acquired resistance to antibiotics (Smith et al., 2013; Slifierz et al., 2015) The different fractions of MRSA (MRSA out of total S. aureus) observed in the different farms and farm sections might be caused by different levels of antibiotic usage (Smith et al., 2013), though this information is not available from the respective farms.
In addition to S. aureus, 13 other Staphylococcus species were found in the farm dust samples. These species included S. hyicus, which is coagulase-positive and a potential pathogen in pigs (Wegener et al., 1993). The 12 other species are coagulase-negative, and several of them have earlier been found on pig skin (Nagase et al., 2002). Some of these species may act as reservoirs for the SSCmec cassette and the mecA gene (Tulinski et al., 2012), and some can be oxacillin resistant (Marshall et al., 1998), or cause human clinical infections (Rogers et al., 2009). The following species, all found in the dust samples have caused human blood stream infections: S. aureus, S. equorum, S. haemolyticus, S. simulans, S. epidermidis, S. xylosus, S. saprophyticus, S. hyicus, S. hominis (Marshall et al., 1998), and S. pettenkoferi (Song et al., 2009). Therefore, the spread and survival of these species in and via farm dust are also of interest. Earlier studies have shown that bacteria are transported from one work environment to another environment (Normand et al., 2011; Madsen et al., 2016). In this study, we show that S. equorum was found in high concentrations in the farm dust and has a half-life of almost 4 days.
The number of sampling days was not identical throughout the study, since initially we expected a long sampling time to be necessary to collect sufficient dust levels. The different sampling times may hence be a limitation to this study. However, the number of sampling days does not seem to have a pronounced effect on the survival since the half-life of 4–7 days was found consistently. This may be because the airborne S. aureus, as mentioned earlier, in the stables is a mixture of S. aureus newly released from the pigs and S. aureus re-aerosolized from surfaces. In other studies, the EDC has also been used for sampling airborne bacteria on pig farms. The sampling time has typically been 14 days followed by extra time needed for shipping before extraction (Dorado-Garcia et al., 2013; Van Cleef et al., 2015). In light of a half-life of 5 days, a shorter sampling time should be considered if culturable MRSA are to be quantified.
In conclusion, LA-MRSA and S. aureus had half-lives of 5 days, and a 99.9% die-off rate of 66–72 days, thus it can be found in farm dust for weeks. Bacteria can be transported from one work environment to another environment. Consequently farm dust with LA-MRSA cannot only pose an exposure risk for weeks in the farm environment, but also if transported to other environments. These results indicate that dust might be an important vehicle for transmission of LA-MRSA, e.g., through air, on farm clothing, and during cleaning of stables.
Acknowledgements
We wish to thank Zeynab Nedaei, Amal Markouch, and Hanieh Kashefpour for valuable practical help with the extraction of EDC-samples, enumeration of CFU and performance of MALDI-TOF MS analysis. We also want to thank the Danish Working Environment Authority and Linda Sandberg Pedersen from SEGES for their involvement in the project and Prof. Dr Monika Raulf for EDC supply. Finally, we want to thank the six farms for their participation in the project. Funding for this project was provided by the Danish Working Environment Authority. The authors declare no conflict of interest relating to the material presented in this article. Its contents, including any opinions and/or conclusions expressed, are solely those of the authors.
References
- Agersø Y, Vigre H, Cavaco LM, et al. (2014)Comparison of air samples, nasal swabs, ear-skin swabs and environmental dust samples for detection of methicillin-resistant Staphylococcus aureus (MRSA) in pig herds. Epidemiol Infect; 142: 1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angen Ø, Feld L, Larsen J, et al. (2017)Transmission of MRSA to human volunteers visiting a swine farm. Appl Environ Microbiol; 83: e01489–17. 10.1128/AEM.01489-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos ME, Verstappen KM, van Cleef BA, et al. (2016)Transmission through air as a possible route of exposure for MRSA. J Expo Sci Environ Epidemiol; 26: 263–9. [DOI] [PubMed] [Google Scholar]
- Clements MO, Foster SJ (1999)Stress resistance in Staphylococcus aureus. Trends Microbiol; 7: 458–62. [DOI] [PubMed] [Google Scholar]
- Dahms C, Hübner NO, Cuny C, et al. (2014)Occurrence of methicillin-resistant Staphylococcus aureus in farm workers and the livestock environment in Mecklenburg-Western Pomerania, Germany. Acta Vet Scand; 56: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANMAP. (2016)DANMAP 2015 Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. Birgitte Borck Høg, Helle Korsgaard, and Ute Wolff Sönksen 1–144. [Google Scholar]
- Deiters C, Günnewig V, Friedrich AW, et al. (2015)Are cases of methicillin-resistant Staphylococcus aureus clonal complex (CC) 398 among humans still livestock-associated?Int J Med Microbiol; 305: 110–3. [DOI] [PubMed] [Google Scholar]
- Dorado-García A, Bos ME, Graveland H, et al. (2013)Risk factors for persistence of livestock-associated MRSA and environmental exposure in veal calf farmers and their family members: an observational longitudinal study. BMJ Open; 3: e003272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese A, Schulz J, Hoehle L, et al. (2012)Occurrence of MRSA in air and housing environment of pig barns. Vet Microbiol; 158: 129–35. [DOI] [PubMed] [Google Scholar]
- Garcia-Graells C, Antoine J, Larsen J, et al. (2012)Livestock veterinarians at high risk of acquiring methicillin-resistant Staphylococcus aureus ST398. Epidemiol Infect; 140: 383–9. [DOI] [PubMed] [Google Scholar]
- Gibbs SG, Green CF, Tarwater PM, et al. (2004)Airborne antibiotic resistant and nonresistant bacteria and fungi recovered from two swine herd confined animal feeding operations. J Occup Environ Hyg; 1: 699–706. [DOI] [PubMed] [Google Scholar]
- Graveland H, Wagenaar JA, Heesterbeek H, et al. (2010)Methicillin resistant Staphylococcus aureus ST398 in veal calf farming: human MRSA carriage related with animal antimicrobial usage and farm hygiene. PLoS One; 5: e10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MZ, Espinosa-Gongora C, Damborg P(2017)Horses in Denmark are a reservoir of diverse clones of methicillin-resistant and-susceptible Staphylococcus aureus. Front Microbiol; 8: 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köck R, Schaumburg F, Mellmann A, et al. (2013)Livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) as causes of human infection and colonization in Germany. PLoS One; 8: e55040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A, Schwebke I, Kampf G (2006)How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis; 6: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J, Petersen A, Sørum M, et al. (2015)Methicillin-resistant Staphylococcus aureus CC398 is an increasing cause of disease in people with no livestock contact in Denmark, 1999 to 2011. Euro Surveill; 20 doi: 10.2807/1560-7917.ES.2015.20.37.30021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen AM, Alwan T, Ørberg A, et al. (2016)Waste workers’ exposure to airborne fungal and bacterial species in the truck cab and during waste collection. Ann Occup Hyg; 60: 651–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen AM, Hansen VM, Nielsen SH, et al. (2009)Exposure to dust and endotoxin of employees in cucumber and tomato nurseries. Ann Occup Hyg; 53: 129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Wilke WW, Pfaller MA, et al. (1998)Staphylococcus aureus and coagulase-negative staphylococci from blood stream infections: frequency of occurrence, antimicrobial susceptibility, and molecular (mecA) characterization of oxacillin resistance in the SCOPE program. Diagn Microbiol Infect Dis; 30: 205–14. [DOI] [PubMed] [Google Scholar]
- Masclaux FG, Sakwinska O, Charrière N, et al. (2013)Concentration of airborne Staphylococcus aureus (MRSA and MSSA), total bacteria, and endotoxins in pig farms. Ann Occup Hyg; 57: 550–7. [DOI] [PubMed] [Google Scholar]
- Nagase N, Sasaki A, Yamashita K, et al. (2002)Isolation and species distribution of staphylococci from animal and human skin. J Vet Med Sci; 64: 245–50. [DOI] [PubMed] [Google Scholar]
- NETHMAP. (2013)Consumption of antimicrobial agents and antimicrobial resistance among medically important bacteria in the Netherlands. Nijmegen/Bilthoven: Dutch Working Party on Antibiotic Policy and Centre for Infectious Disease Control, National Institute for Public Health and the Environment of the Netherlands. [Google Scholar]
- Nielsen RT, Kemp M, Holm A, et al. (2016)Fatal septicemia linked to transmission of MRSA clonal complex 398 in hospital and nursing home, Denmark. Emerg Infect Dis; 22: 900–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normand AC, Sudre B, Vacheyrou M, et al. ; GABRIEL-A Study Group. (2011)Airborne cultivable microflora and microbial transfer in farm buildings and rural dwellings. Occup Environ Med; 68: 849–55. [DOI] [PubMed] [Google Scholar]
- Pedersen S, Nonnenmann M, Rautiainen R, et al. (2000)Dust in Pig Buildings. J Agric Saf Health; 6: 261–74. [DOI] [PubMed] [Google Scholar]
- Rogers KL, Fey PD, Rupp ME (2009)Coagulase-negative staphylococcal infections. Infect Dis Clin North Am; 23: 73–98. [DOI] [PubMed] [Google Scholar]
- Schmithausen RM, Schulze-Geisthoevel SV, Stemmer F, et al. (2015)Analysis of transmission of MRSA and ESBL-E among pigs and farm personnel. PLoS One; 10: e0138173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz J, Friese A, Klees S, et al. (2012)Longitudinal study of the contamination of air and of soil surfaces in the vicinity of pig barns by livestock-associated methicillin-resistant Staphylococcus aureus. Appl Environ Microbiol; 78: 5666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seier-Petersen MA, Nielsen LN, Ingmer H, et al. (2015)Biocide susceptibility of Staphylococcus aureus CC398 and CC30 isolates from pigs and identification of the biocide resistance genes, qacG and qacC. Microb Drug Resist; 21: 527–36. [DOI] [PubMed] [Google Scholar]
- Slifierz MJ, Friendship RM, Weese JS (2015)Methicillin-resistant Staphylococcus aureus in commercial swine herds is associated with disinfectant and zinc usage. Appl Environ Microbiol; 81: 2690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TC, Gebreyes WA, Abley MJ, et al. (2013)Methicillin-resistant Staphylococcus aureus in pigs and farm workers on conventional and antibiotic-free swine farms in the USA. PLoS One; 8: e63704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SH, Park JS, Kwon HR, et al. (2009)Human bloodstream infection caused by Staphylococcus pettenkoferi. J Med Microbiol; 58(Pt 2): 270–2. [DOI] [PubMed] [Google Scholar]
- Strasters KC, Winkler KC (1966)Viability of hospital staphylococci in air. Bacteriol Rev; 30: 674–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JW. (2009)The effect of environmental parameters on the survival of airborne infectious agents. J R Soc Interface; 6 (Suppl. 6): S737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulinski P, Fluit AC, Wagenaar JA, et al. (2012)Methicillin-resistant coagulase-negative staphylococci on pig farms as a reservoir of heterogeneous staphylococcal cassette chromosome mec elements. Appl Environ Microbiol; 78: 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alen S, Ballhausen B, Peters G, et al. (2017)In the centre of an epidemic: fifteen years of LA-MRSA CC398 at the University Hospital Münster. Vet Microbiol; 200: 19–24. [DOI] [PubMed] [Google Scholar]
- van Cleef BA, van Benthem BH, Verkade EJ, et al. . 2015. Livestock-associated MRSA in household members of pig farmers: transmission and dynamics of carriage, a prospective cohort study. PloS one; 10: e0127190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loo I, Huijsdens X, Tiemersma E, et al. (2007)Emergence of methicillin-resistant Staphylococcus aureus of animal origin in humans. Emerg Infect Dis; 13: 1834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijen MM, Bosch T, Verkade EJ, et al. ; CAM Study Group. (2014)Livestock-associated MRSA carriage in patients without direct contact with livestock. PLoS One; 9: e100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkade E, Kluytmans-van den Bergh M, van Benthem B, et al. (2014)Transmission of methicillin-resistant Staphylococcus aureus CC398 from livestock veterinarians to their household members. PLoS One; 9: e100823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss A, Loeffen F, Bakker J, et al. (2005)Methicillin-resistant Staphylococcus aureus in pig farming. Emerg Infect Dis; 11: 1965–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener HC, Andresen LO, Bille-Hansen V (1993)Staphylococcus hyicus virulence in relation to exudative epidermitis in pigs. Can J Vet Res; 57: 119–25. [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Aarnink AJ, De Jong MC, et al. . 2014. Airborne microorganisms from livestock production systems and their relation to dust. Crit Rev Environ Sci Technol; 44: 1071–128. [DOI] [PMC free article] [PubMed] [Google Scholar]