Abstract
The impact of human activity on the acoustic environment is overwhelming, with anthropogenic noise reaching even remote areas of the planet. The World Health Organization has identified noise pollution as one of the leading environmental health risks in humans, and it has been linked to a myriad of short- and long-term health effects in exposed individuals. However, less is known about the health effects of anthropogenic noise exposure on animals. We investigated long- and short-term effects of traffic noise on zebra finches breeding in small communal aviaries, using a repeated measures design. Birds bred in both noise and no-noise conditions, and we measured baseline plasma glucocorticoid levels before, during and after breeding. In addition, we assayed immune function, measured reproductive success and offspring growth and compared rates of extra-pair paternity of breeding adults. Breeding birds had significantly lower baseline plasma corticosterone levels when exposed to traffic noise than when they were not exposed to noise playback. In addition, the nestlings reared during noise exposure were lighter than nestlings of the same parents when breeding in control conditions. Our results suggest that traffic noise poses a more severe hurdle to birds at more vulnerable stages of their life history, such as during reproductive events and ontogeny. While chronic exposure to traffic noise in our birds did not, by itself, prove to be a sufficient stressor to cause acute effects on health or reproductive success in exposed individuals, it did result in disruptions to normal glucocorticoid profiles and delayed offspring growth. However, animals living in urban habitats are exposed to a multitude of anthropogenic disturbances, and it is likely that even species that appear to be thriving in noisy environments may suffer cumulative effects of these multiple disturbances that may together impact their fitness in urban environments.
Keywords: Anthropogenic noise, glucocorticoids, reproductive fitness, songbird, stress, urbanization
Introduction
Humans have been dramatically altering the environment for millennia, and as the global population swells, urbanization is ever increasing. The growth of urban areas is projected to reach exceptional levels in the coming decades, and this expansion of human developments will continue the fundamental alteration of ecosystems (United Nations, 2014). Some of the challenges that animals face in cities are habitat fragmentation, changes in micro-climate, limitation of resources, alteration of resource flow, changes in species interactions, as well as pollution (Grimm et al., 2008; Barber et al., 2009; Shanahan et al., 2014).
Pollution is one of the main consequences of human development and may take the form of chemical contamination as well as light and noise emissions. The past decade has seen a groundswell of interest in the effects of anthropogenic noise pollution on birds, primarily focused on changes in vocal behaviour. Dozens of studies have reported correlations between background noise levels and various song characteristics. For instance, in response to increases in the background noise levels, birds increase the amplitude of their songs, as well as the redundancy, duration, frequency and the timing of their vocalizations (reviewed in Brumm and Zollinger, 2013). These observations have led many to suggest that these modifications of vocal behaviour are an attempt of birds to ameliorate the negative effects of noise on acoustic communication (Gil and Brumm, 2013), although some tactics are likely to be more effective at mitigating the effects of noise than others (see Nemeth and Brumm, 2010). For signal receivers, noise can have negative fitness consequences by masking important acoustic signals and cues, such as alarm signals, sounds made by predators or prey or signals relevant for breeding behaviour and offspring care (Brumm, 2010; McIntyre et al., 2014; Read et al., 2014; Templeton et al., 2016). Birds may be particularly sensitive to noise disruption during their reproductive period, since many species rely on acoustic signals to attract mates and defend territories, to maintain pair bonds and coordinate offspring feeding and care (Catchpole and Slater, 2008). Thus, acoustic masking by noise is likely to have major fitness consequences (Brumm and Slabbekoorn, 2005).
There is some evidence from correlational studies comparing noisy and less noisy sites, that noise exposure can influence avian reproductive success in different ways. For example, great tits (Parus major) breeding in areas with high levels of traffic noise produced fewer fledglings and laid smaller clutches than their conspecifics in quieter areas (Halfwerk et al., 2011). Eastern bluebirds (Sialia sialis) nesting in noisy areas also had reduced reproductive success, with lower hatching rates and fledging success than their conspecifics at quieter sites (Kight et al., 2012). House sparrows (Passer domesticus) breeding at a noisy site hatched fewer young, of lower body mass, produced fewer recruits and had lower rates of offspring provisioning than sparrows in quiet sites (Schroeder et al., 2012). Noise can also mask parental alarm calls, reducing the response of nestlings towards predator threats (McIntyre et al., 2014). Detection of alarm calls by great tits is significantly impaired in the presence of traffic noise (Templeton et al., 2016), which is likely to increase the risk of predation in habitats with high levels of noise pollution. In line with the notion of increased predation risk in noise, some species have been shown to increase vigilance time in noise, resulting in a reduction of feeding rate (Fernández-Juricic and Tellería, 2000; Quinn et al., 2006), but see (Yorzinski and Hermann, 2016).
Noise can also have negative non-auditory effects, in that it may act as a stressor and could increase physiological stress responses such as elevated plasma glucocorticoids, which can lead to depressed immune function and increased oxidative stress in the brain and organs of the immune system (reviewed in Kight and Swaddle, 2011). Several species of birds have shown correlations between levels of the hormone corticosterone and chronic environmental noise, although the size or direction of these correlations seem to vary by species, life history stage and context (Wright et al., 2007; Crino et al., 2011, 2013; Blickley et al., 2012; Potvin and MacDougall-Shackleton, 2015a; Kleist et al., 2018). Elevated levels of corticosterone in mothers led to small body size and slow plumage development in offspring (Saino et al., 2005). Elevated corticosterone has also been found to reduce the time parents spend incubating eggs and to increase the instances of nest abandonment (Spée et al., 2011; Thierry et al., 2013). Parents with high levels of plasma corticosterone may also reduce feeding rates (Angelier et al., 2009). On the other hand, in some studies increased levels of corticosterone seemed to help parents adapt to new situations better (Escribano-Avila et al., 2013), for example by resulting in an increase of parental care and number of fledglings (Bonier et al., 2009, 2011).
To date, most studies on the effects of noise on birds have been conducted in the field. While field studies are important to identify potential relationships between environmental factors and changes in populations, they can present challenges for understanding causal relationships, as there are often many interacting factors that cannot be controlled for, such as chemical pollution (Isaksson, 2010), artificial light at night (Dominoni et al., 2016), avian community density and composition (McKinney, 2006), habitiat structure (Nemeth and Brumm, 2010), food type and availability (e.g. Biard et al., 2017) and others. To understand the causal effects that underlie observed correlations between anthropogenic noise and changes in behaviour or fitness, it is important to identify model systems that can be experimentally manipulated. Several recent studies have investigated the effects of experimental traffic noise exposure on glucocorticoids and reproductive success in breeding birds in controlled field experiments, and in a range of songbird species, with mixed results (e.g. Angelier et al., 2016; Halfwerk et al., 2016; Davies et al., 2017; Injaian et al., 2018). As far as we are aware, just one previous laboratory study has investigated the direct effect of noise on corticosterone levels and reproductive success on captive birds (Potvin and MacDougall-Shackleton, 2015b). This study exposed zebra finches to an artificial mix of noises from cars, trains, motorcycles and lawnmowers and found trends towards increased embryo mortality and reduced nestling growth, although their study had quite low statistical power, and neither of these effects reached significance. They further found no chronic effect of noise exposure on plasma corticosterone levels.
In the present study we experimentally exposed breeding zebra finches (Taeniopygia guttata) to realistic levels of recorded traffic noise and measured whether noise exposure alone was sufficient a stressor to affect baseline glucocorticoid levels, immune function, reproductive success or levels of extra-pair paternity. We included extra-pair paternity in our analysis as an earlier study found that very high levels of noise exposure reduced the preference of female zebra finches for their pair-bonded males (Swaddle and Page, 2007), but it is unclear whether more realistic levels of environmental noise pollution would have a similar effect. The experiment was conducted in a pair-wise design in which birds living in communal aviaries were allowed to reproduce twice, once exposed to traffic noise and once without noise playback. We hypothesized that traffic noise would act as chronic stressor. While there appears to be no one way in which different species respond to chronic stress, the majority of species in a recent meta-analysis were found to respond to chronic stress with an increase in baseline glucocorticoids (Dickens and Romero, 2013). Thus, we expected to find higher levels of baseline corticosterone, a weaker immune system and a lower performance in reproductive success, including a negative effect on the size and/or body condition of chicks.
Materials and methods
Study system
We allowed 88 adult zebra finches (1–2 years old) from the colonies at the Max Planck Institute for Ornithology in Seewiesen, Germany to breed in 6 aviaries. The birds used for breeding were the naïve offspring of a cross between populations 11 and 18 (Forstmeier et al., 2007a), which had been housed prior to our experiment in groups of 4–8 birds in single-sex cages (240 cm long, 40 cm deep and 45 cm high). Each of two experimental rooms contained three aviaries (1 × 2 × 2 m), separated by opaque dividers, housing 7–8 pairs of birds. Each aviary was provided with 12 wooden nest boxes and ad libitum nesting materials, seeds, commercial finch egg food and water. In addition, birds were provided with fresh vegetables and hard-boiled eggs twice weekly throughout the experimental period. Animal housing and care was in accordance with European and German laws governing the care and use of laboratory animals (Council of Europe Treaty ETS-123). All experimental procedures were approved by and done under license from the Government of Upper Bavaria (Regierung von Oberbayern), licence number 55.2-1-54-2532-51-2013.
Experimental treatment
To determine if typical city traffic noise affects baseline plasma corticosterone levels, reproductive success and immune function in adult birds, we used a crosseddesign experiment. This means that one half of the aviaries (in Room 1) were treated with traffic noise throughout the first round of breeding, egg laying and nestling care. The other half (in Room 2) was not treated with noise. After the youngest of the offspring of the first round of breeding reached 120 days post-hatch, the offspring were moved out of their natal aviaries into a separate building. After a 30-day pause, a second breeding round started, in which the noise treatment was switched between rooms, that is breeding pairs that were previously exposed to noise were allowed to breed without noise, and breeding pairs that were previously allowed to breed without noise were now exposed to noise.
Daytime noise playbacks consisted of 80, 5-min long recordings of street traffic noise, which were recorded at several busy intersections in and around Munich, Germany during April 2013. Recordings were made along busy four or six lane roads within the city and along six to eight lane motorways. The recordings varied in vehicle type and pass frequency, and all files included passenger cars, with some also including motorcycles, pedestrian and bicyclist noise including shouting, large trucks and emergency vehicles including sirens. During the daylight hours (06:30–20:30), the 80 recordings were played continuously, in randomized order, with playback levels fluctuating between 65 and 85 dB(A) re 20 μPa at the position of the nest boxes. Nighttime playback (20:30–06:30) consisted of randomized playbacks of an additional 40 noise recordings, which were less dense in the rate of passing traffic than the daytime recordings and were reduced in peak amplitudes, with playback level averages fluctuating between 45 and 75 dB(A). None of the nighttime noise files contained sirens or loud small engine revving that characterized some of the daytime files. Noise playback mimicked typical urban noise patterns, according to published noise maps (Bayerisches Landesamt, 2012). During the control condition no noise playback occurred, and the noise levels in the rooms (exclusive of bird sounds) were 40–48 dB(A), depending on the cycling of the ventilation system. We played noise from a laptop computer to an array of 12 pairs of amplified portable speakers (Hama Sonic Mobil 400 Alu PS1032), with 4 pairs arranged above each of the 3 aviaries in the room. Noise playback was run using a script written in MatLab (version 7.5.0; Natick, MA, USA; www.mathworks.com) to randomize playback during day and night. For the noise treatment, playback of noise began 4 weeks before the introduction of nesting materials and nest boxes and continued until the median juvenile in the room had fledged (the date when half of the offspring had fledged, with an average fledgling age of 17 days post-hatch).
Baseline corticosterone levels
To determine hormone profiles, we captured birds in the experimental rooms and collected blood samples within 3.5 min of entering the room. Baseline corticosterone levels collected in less than 3 min from the start of the disturbance are not thought to be greatly affected by capture and handling (Romero and Reed, 2005, but see Results). Each bird was sampled four times during the breeding cycle: (i) pre-treatment (before birds were moved into the group aviaries from the single-sex housing in which they were being held prior to our experiment); (ii) during the courtship period; (iii) during the nesting period (incubating eggs, but before hatching); and (iv) post-treatment (2 weeks after the grown offspring were removed from the group aviaries at 120 days post-hatch (10 days after the end of the experiment). Pairs were still together in the group aviaries, but nest boxes had been removed and no nesting materials were provided. Sampling period 4 (post-treatment) from round one, was used as sampling period 1 (pre-treatment) for the second breeding round; see timeline in Fig. 1. All blood samples were collected between 11:00 and 12:00 h. We did not take a blood sample during the nestling care period because during a pilot study we found that catching and bleeding of the parents during this period resulted in chicks that were delayed in somatic growth and increased levels of chick mortality.
Figure 1.
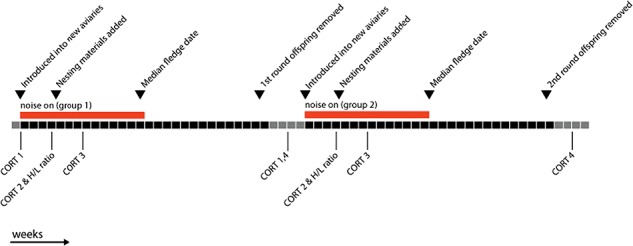
Timeline of the experimental procedure and blood sampling periods. Each block represents 1 week, black blocks are within the experimental reproductive rounds, while grey blocks are ‘rest periods’ during which birds were either housed in single-sex flight cages, or in mixed groups in home aviaries, but not provided with nesting materials.
Blood sampling techniques and hormone assays
Blood samples were collected by brachial venipuncture for each bird and were collected into heparinized capillary tubes (1.4 × 75 mm), transferred into Eppendorf tubes and centrifuged with 5000 rpm for 10 min to separate the plasma. Plasma was stored at −80°C. Corticosterone concentrations were determined by radioimmunoassay following Goymann et al. (2006). Corticosterone antibodies were obtained from Esoterix Endocrinology, Calabasas Hills, CA. Extraction efficiency (as calculated from tracer amounts of tritiated hormone added to each sample before extraction) was 83.2 ± 4.9% (N = 266) for 3H-corticosterone (Perkin Elmer, NET 399). The average limit of detection ranged from 2.94 to 3.39 pg corticosterone per tube. We ran a total of five corticosterone assays and the intra-assay variation for corticosterone standards ranged from 2.5% to 5.7% and from 1.7% to 17% for extracted chicken pool plasma. Inter-assay variation of unextracted standard corticosterone was 7.2% and for extracted chicken pool plasma 21.3%.
Immune function
To test the effect of traffic noise on immune function we measured leucocyte profiles of birds breeding in both treatments. We collected blood during the incubation period, at the same time as the samples for the corticosterone assays were collected, into separate capillaries and stored in separate Eppendorf tubes. Changes in leucocyte profiles, specifically, a higher heterophil/lymphocyte ratio (H/L ratio), have been found in several species of birds to be a good indicator of chronic stress (e.g. Vleck et al., 2000; Bedanova et al., 2010). We measured H/L ratios in birds twice, once when they were breeding in noise and once when they were breeding in the no-noise treatment and compared them within individuals.
We prepared blood smears using a standard two slide wedge procedure. The smears were air dried and then stained using the Differential Quik Stain Kit (Modified Giemsa). For each adult in the experiment we analysed two blood smears. In some preparations the smear was too thick and we were not able to see white blood cells, thus these samples were excluded, leaving a total sample size of 74 individuals. Each slide was inspected via a microscope with an oil immersion objective at ×100. We took a photo of every leucocyte that was present and counted the number of lymphocytes and granulocytes (eosinophils, basophils, heterophils) from the first 50 leucocytes we identified on each slide. We used the granulocyte to lymphocyte ratio as a proxy for heterophil to lymphocyte ratio (H/L ratio).
Reproductive success
After a 2-month period during which the birds were allowed to habituate to the aviaries and began courtship behaviours, each aviary was supplied with 12 nest boxes and nesting materials (coconut fibers and cotton strings). Every other day, nests were checked and the fate of each egg and each offspring was recorded. Individuals attending the nests (that is, the social parents) were identified by observation in person or by video. Each social pair was allowed to produce only a single clutch in each treatment (i.e. once the first brood fledged, eggs from further breeding attempts were removed). When eggs hatched, nestlings were weighed, and at Day 8 we collected a blood sample (~10 μL) for parentage analysis (described below). When nestlings died before Day 8, we stored a tissue sample in ethanol for parentage analysis. In addition, we opened all eggs that did not hatch and scored the eggs as either infertile (i.e. no visible embryonic development) or as containing embryos that died before hatching. Tissue from dead embryos was collected and used for parentage analysis. As fitness measures for each bird we measured the proportion of genetic offspring that reached adulthood (120 days old) and the proportion of embryos that died before hatch (embryo mortality). In addition, every offspring was weighed at Days 10, 21 and 120 post-hatch.
Paternity analysis
Since there is typically a considerable amount of extra-pair young in captive zebra finch colonies (Forstmeier et al., 2011), genetic paternity analysis is necessary to reliably assign parentage. To this end, all offspring were genotyped at 11 highly polymorphic microsatellite markers (chr2_109, chr2_47, chrZ_34, chr15_6, chr1a_39, chr22_3, chr3_58, chr11_8, chr27_1, chr5_34, chr6_16, see Table S2 in the online supplement for further information) following Forstmeier et al., (2007b) and Wang et al., (2017). Genetic parentage was determined by exclusion using the R package SOLOMON (Christie et al., 2013).
Statistics
All statistical analyses were performed in R 3.1.1 (R Core Team, 2013). We applied linear mixed-effects and generalized mixed-effects models to analyse our data for which we used the ‘lmer’ and ‘glmer’ function from the lme4 package (Bates et al., 2015). Additionally, we used the ‘sim’ function from the arm package (Gelman and Hill, 2007) to simulate the posterior distribution of the model parameters based on 2000 simulations. The statistical significance of fixed effects was assessed based on the 95% credible intervals (CrIs) around the mean estimate (and 95% CrI are provided as number ranges in brackets following estimates). We considered an effect to be ‘significant’ in the frequentist’s sense when the 95% CrI did not overlap zero (Nakagawa and Cuthill, 2007). For approximate orientation we also calculated P-values from t-values or z-values assuming infinite degrees of freedom.
For ‘corticosterone baseline levels’ we used the ‘lmer’ function, and the corticosterone levels were ln-transformed to approach normality as the dependent variable. Treatment (0 = control, 1 = noise), sampling period (0 = pre-treatment, 1 = during courtship, 2 = during nesting/incubation or 3 = post-treatment), sex (0 = females, 1 = males) and breeding round (1 = first breeding round, 2 = breeding round) were set as independent factors and sampling time as covariate. Pre- and post-treatment sampling periods were coded as control for treatment, since during that time birds were not exposed to traffic noise. Sampling time refers to the moment the sample was taken counting from the moment we entered the room [during the first, second, third or fourth minute (the maximum time from entering the room to finishing the blood sample was 3.5 min)]. Every individual was exposed to control and noise treatment and blood samples were taken at four sampling times, thus individual ID was included as a random effect. Aviary was included as a second random effect to account for effects of the common aviary. In addition, we allowed for different slopes of individuals across the two treatments [random slope effect, since individuals had repeated measures under both treatments, usually 2× noise and 6× control, since in the noise treatment, the noise playback occurred only during the middle 2 of the 4 sampling times, the first and fourth corticosterone samples were coded as control].
For ‘immune response’, we used the ‘lmer’ function, ‘H/L ratio’ was log transformed to approach normality and was set as the dependent variable. Treatment (0 = control, 1 = noise) and sex (0 = females, 1 = males) were set as independent factors. The individual ID and the aviary (to account for effects of the common aviary) were included as random effects.
For our model of ‘reproductive success’, we used the ‘glmer’ function with a binomial error distribution. We assigned to every egg an ‘egg fate’ code, depending on the developmental stage they reached, and each individual was coded as 0 or 1 for each category: offspring reaching adulthood and embryo died before hatch (embryo mortality). We ran a model for each of those categories as the dependent variables (offspring reaching adulthood and embryo mortality). Treatment and breeding round were independent factors. The order of treatment refers to whether the birds were exposed to the noise treatment during their first or second round of breeding. In the models for offspring reaching adulthood and embryo mortality, genetic mother ID, genetic father ID, genetic pair ID and aviary were included as random effects. In some cases, some pairs switched partners between breeding rounds, thus genetic pair ID was included.
The offspring body mass was measured three times per bird, when they were 10, 21 and 120 days old. We fitted three ‘lmer’ models, one model per age. As dependent variable we set mass, treatment and sex as independent factors. As random effects genetic mother ID, genetic father ID and aviary were included.
To measure extra-pair paternity each offspring was scored with 0 if its social father was the same as its genetic father and 1 otherwise. We fitted a ‘glmer’ model with a binomial error structure. As the dependent variable we set extra-pair paternity (yes/no) and as independent factors we set treatment, order of treatment (1 = control noise, 2 = noise control) and breeding round. Genetic mother ID, genetic clutch ID and aviary ID were included as random effects. We included the order of treatment since treatment could affect the establishment of couples, and therefore could affect extra-pair behaviour.
Results
Baseline corticosterone levels
Baseline corticosterone levels were lower during the courtship period when birds were exposed to noise (Fig. 2). The baseline corticosterone levels did not differ from those of birds in the control treatment during the pre-treatment, and post-treatment sampling periods, when neither treatment group was exposed to noise. In addition, sex, breeding round and sampling period did not have a significant effect on corticosterone levels (Table 1). The estimate of individual repeatability for corticosterone levels was 0.121 (variance in random intercepts) and there was also variation in how individuals responded to the two treatments (0.116 variance in random slopes). It is worth noting that sampling time (the number of minutes after entering the aviary room that the blood sample was taken) had a highly significant effect on corticosterone levels (Table 1). This is contrary to the common belief that acute stress does not significantly impact baseline corticosterone levels if collected within 3 min of the onset of the disturbance (Romero and Reed, 2005). Our study, with an N of 629 samples, shows that plasma corticosterone does increase, highly significantly, from the first to the second minute post-disturbance, and by a factor of 2.36 times between the first and last category: from 950 to 2250 pg/ml. In our study, time (seconds after entering the room) explains about 11.6% of the variance in baseline corticosterone measures.
Figure 2.
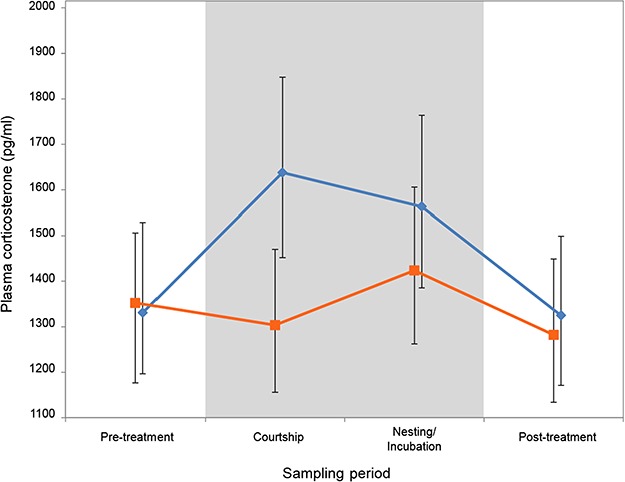
Mean baseline plasma corticosterone for noise exposed (orange squares) and control (no-noise playback, blue diamonds) breeding treatments at each sampling period. Error bars denote 95% CrIs.
Table 1.
Outcome of linear mixed-effects models testing the effects of noise on offspring mass at various ages, H/L ratio, plasma corticosterone levels, extra-pair paternity and reproductive success of adult zebra finches
| Mass Day 10 | Random effects | Variance | Levels/Obs | ||||
| MotherID | 0.70 | 39 | |||||
| FatherID | 0 | 33 | |||||
| AviaryID | 0.01 | 6 | |||||
| Residual | 1.05 | ||||||
| N | 201 | ||||||
| Fixed effects | Estimate | Standard error (SE) | t | P | 95% CI low | 95% CI high | |
| Intercept | 10.64 | 0.192 | 55.41 | 10.254 | 11.011 | ||
|
Treatment
(Noise vs contr) |
−0.39 | 0.159 | −2.48 | 0.013 | −0.708 | −0.097 | |
| Sex (male vs fem) | 0.22 | 0.154 | 1.42 | 0.156 | −0.084 | 0.518 | |
| Mass Day 21 | Random effects | Variance | Levels/Obs | ||||
| MotherID | 0.07 | 39 | |||||
| FatherID | 0.01 | 33 | |||||
| AviaryID | 0.12 | 6 | |||||
| Residual | 1.06 | ||||||
| N | 192 | ||||||
| Fixed effects | Estimate | SE | t | P | 95% CI low | 95% CI high | |
| Intercept | 12.22 | 0.199 | 61.31 | 11.830 | 12.624 | ||
| Treatment (noise vs contr) | −0.05 | 0.157 | −0.33 | 0.741 | −0.354 | 0.264 | |
| Sex (male vs fem) | 0.07 | 0.153 | 0.44 | 0.660 | −0.217 | 0.370 | |
| Mass Day 120 | Random effects | Variance | Levels/Obs | ||||
| MotherID | 0.05 | 39 | |||||
| FatherID | 0.26 | 33 | |||||
| AviaryID | 0.001 | 6 | |||||
| Residual | 1.12 | ||||||
| N | 194 | ||||||
| Fixed effects | Estimate | SE | t | P | 95% CI low | 95% CI high | |
| Intercept | 14.80 | 0.171 | 86.72 | 14.481 | 15.152 | ||
| Treatment (noise vs contr) | −0.23 | 0.171 | −1.36 | 0.174 | −0.566 | 0.091 | |
| Sex (male vs fem) | −0.15 | 0.162 | −0.94 | 0.347 | −0.483 | 0.170 | |
| H/L ratio (log transf.) | Random effects | Variance | Levels/Obs | ||||
| IndividualID | 0.07 | 74 | |||||
| AviaryID | 0 | 6 | |||||
| Residual | 0.69 | ||||||
| N | 144 | ||||||
| Fixed effects | Estimate | SE | t | P | 95% CI low | 95% CI high | |
| Intercept | −0.33 | 0.125 | −2.65 | −0.579 | −0.067 | ||
| Treatment (noise vs contr) | −0.22 | 0.139 | −1.58 | 0.114 | −0.492 | 0.055 | |
| Sex (male vs fem) | −0.31 | 0.153 | −2.04 | 0.041 | −0.602 | −0.018 | |
| Corticosterone (log transf.) | Random effects | Variance | Levels/Obs | ||||
| IndividualID | 0.06 | 87 | |||||
| Individual:treatment | 0.06 | ||||||
| AviaryID | 0.06 | 6 | |||||
| Residual | 0.34 | ||||||
| N | 629 | ||||||
| Fixed effects | Estimate | SE | t | P | 95% CI low | 95% CI high | |
| Intercept | 7.01 | 0.127 | 54.99 | 6.763 | 7.257 | ||
|
Treatment
(noise vs contr) |
−0.16 | 0.069 | −2.33 | 0.019 | −0.294 | −0.024 | |
|
Period
(courtship vs pre) |
0.16 | 0.074 | 2.24 | 0.025 | 0.017 | 0.313 | |
|
Period
(nesting vs pre) |
0.19 | 0.074 | 2.54 | 0.011 | 0.038 | 0.329 | |
| Period (post vs pre) |
−0.03 | 0.068 | −0.43 | 0.667 | −0.163 | 0.111 | |
| Sex (male vs fem) | −0.16 | 0.068 | −2.35 | 0.019 | −0.296 | −0.026 | |
|
Breeding round
(2nd vs 1st) |
−0.11 | 0.049 | −2.35 | 0.019 | −0.211 | −0.019 | |
|
Sampling time
(per min) |
0.26 | 0.030 | 8.91 | <0.0001 | 0.204 | 0.322 | |
| Extra-pair paternity (binomial) | Random effects | Variance | Levels/Obs | ||||
| GeneticClutchID | 11.79 | 61 | |||||
| MotherID | 1.84 | 38 | |||||
| AviaryID | 0 | 12 | |||||
| N | 232 | ||||||
| Fixed effects | Estimate | SE | z | P | 95% CI low | 95% CI high | |
| Intercept | 0.45 | 1.148 | 0.389 | 0.697 | −1.564 | 2.474 | |
| Treatment (noise vs contr) |
0.14 | 1.174 | 0.115 | 0.908 | −1.969 | 2.177 | |
| Order of treatment (noise first vs control first) | −2.47 | 1.378 | −1.789 | 0.074 | −4.706 | −0.178 | |
| Breeding round (2nd vs 1st) |
−0.12 | 1.169 | −0.102 | 0.919 | −2.168 | 1.950 | |
| Embryo mortality (binomial) | Random effects | Variance | Levels/Obs | ||||
| GeneticPairID | 0.99 | 66 | |||||
| GeneticMotherID | 1.03 | 40 | |||||
| GeneticFatherID | 0.001 | 35 | |||||
| AviaryID | 0.001 | 12 | |||||
| N | 297 | ||||||
| Fixed effects | Estimate | SE | z | P | 95% CI low | 95% CI high | |
| Intercept | −1.76 | 0.47 | −3.708 | >0.001 | −2.654 | −0.930 | |
| Treatment (noise vs contr) |
0.20 | 0.36 | 0.554 | 0.579 | −0.467 | 0.872 | |
| Order of treatment (noise first vs control first) | −0.33 | 0.56 | −0.579 | 0.563 | −1.373 | 0.740 | |
| Breeding round (2nd vs 1st) |
0.52 | 0.36 | 1.425 | 0.154 | −0.169161 | 1.2209318 | |
| Offspring survival (from early embryo to 120 days; binomial) | Random effects | Variance | Levels/Obs | ||||
| GeneticPairID | <0.001 | 66 | |||||
| GeneticMotherID | 0.78 | 40 | |||||
| GeneticFatherID | <0.001 | 35 | |||||
| AviaryID | <0.001 | 12 | |||||
| N | 297 | ||||||
| Fixed effects | Estimate | SE | z | P | 95%CI low | 95%CI high | |
| Intercept | 1.32 | 0.35 | 3.751 | <0.001 | 0.583 | 1.998 | |
| Treatment (noise vs contr) | −0.46 | 0.29 | −1.559 | 0.119 | −1.009 | 0.101 | |
| Order of treatment (noise first vs control first) | 0.59 | 0.41 | 1.441 | 0.149 | −0.204 | 1.409 | |
| Breeding round (2nd vs 1st) | −0.73 | 0.29 | −2.483 | 0.013 | −1.296 | −0.162 |
Immune system
Traffic noise did not have a significant effect on granulocyte/lymphocyte (H/L) ratio in adult zebra finches (Table 1). Birds exposed to noise showed a non-significant tendency towards lower H/L ratios (Fig. 3), opposite to the predicted direction—birds in noise exposure treatment had an estimated ratio of 0.68 (95% CrI 0.52–0.82) and control birds had a ratio of 0.87 (0.72–1.03) H/L (parameter estimates calculated on data prior to ln-transformation, hence different to the models in Table 1). Males had significantly lower H/L ratios than females [males 0.66 (0.50–0.81) H/L, females 0.89 (0.74–1.03) H/L].
Figure 3.
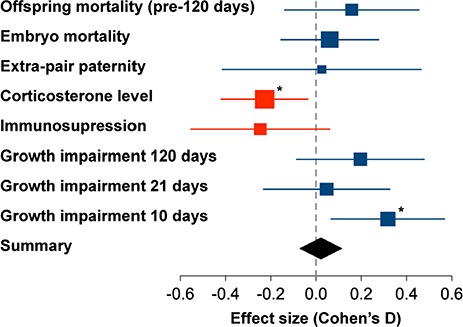
Effect size of each measured parameter for individuals in noise. Data points give the mean estimates of the models with 95% confidence intervals (1.96*SE). To make all effects comparable, variables were modelled as normally distributed, and all dependent variables were scaled to a standard deviation of 1 (scaled values can be found in Table S1, in the electronic supplemental materials). For illustration, the sign of the effect of noise was standardized such that we expected to obtain values >0 (e.g. the effect on offspring mass was multiplied by −1 in order to reflect growth impairment rather than growth). Data point size reflects the sample size. Red symbols indicate effects that were opposite to expectations. Asterisks indicate significance, without correction for multiple testing.
Reproductive success and extra-pair paternity
The traffic noise treatment did not have a significant effect on the proportion of fertile eggs that reached adulthood (Table 1), although there was a trend towards higher offspring mortality (Fig. 3). Birds had a significantly lower proportion of offspring surviving to adulthood in the second breeding round (Table 1), regardless of noise treatment.
Overall embryo mortality was 24% (N = 296), but we did not find significant differences in embryo mortality levels between noise and control treatments (Table 1).
Likewise, we found an overall level of extra-pair paternity of 42%, but the proportion of extra-pair paternity was not affected by traffic noise, breeding round or order of treatment (Table 1).
The offspring of parents in the noise exposure treatment were significantly lighter at 10 days post-shatch than those born to the same parents in control treatments (Table 1, effect estimate 0.39 g), but did not differ in body mass at later stages, although there were trends towards smaller mass for chicks born to noise-exposed parents at all three age categories (Fig. 3). The estimate of body mass of the offspring at Day 10 was 10.36 g (10.01–10.72) in the noise treatment and 10.75 g (10.41–11.08) in the control treatment; at 21 days 12.21 g (11.85–12.59) in noise and 12.26 g (11.90–12.61) in controls and at 120 days 14.49 g (14.17–14.80) in noise 14.72 g (14.43–15.02) in controls. No significant difference in body mass between female and male offspring could be detected (Table 1).
Discussion
We found that traffic noise had a small but significant effect on baseline corticosterone levels, which were lower in noise-exposed birds in the courtship, nest building, egg laying or incubation period of the reproductive cycle. These differences did not continue after the noise treatment ended, when their offspring reached adulthood (120 days post-hatch). We also found that offspring raised in noise conditions were smaller at 10 days post-hatch than offspring of the same parents raised in no-noise conditions. In our experiment, chronic traffic noise exposure did not have a direct, significant impact on any of the other variables we measured, although birds in noise treatments tended to have lower H/L ratios (a trend opposite to our prediction) and higher levels of offspring mortality than birds in a no-noise treatment.
Baseline corticosterone levels
We found that baseline corticosterone levels increased during the courtship and breeding periods in control birds (Fig. 2). However, when the same birds were chronically exposed to traffic noise during the same part of the reproductive cycle, their baseline corticosterone levels remained low. To date, previous studies investigating links between noise exposure and glucocorticoid stress responses have produced very mixed evidence. Some studies reported positive correlations between noise exposure and baseline corticosterone levels in adult songbirds (Davies et al., 2017), while others found no change in corticosterone baselines (Potvin and MacDougall-Shackleton, 2015a; Injaian et al., 2018), and still others found lower baseline corticosterone in noise-exposed adults (Kleist et al., 2018). These differences could result from methodological differences, as the noise exposure in these studies varied in both their duration (3 min to chronic throughout the lifetime of the bird) and in their composition (broad spectrum and constant amplitude to varying in both frequency content and amplitude over time), or they could be specific to a certain species (Kleist et al., 2018), their ontogenetic background or experience (Davies et al., 2017) or to geographic location (Partecke, 2013). Note that in our study, the observed effect is of small magnitude (see Fig. 2), reaching statistical significance based on >600 measures of corticosterone. Such effects would not be detectable with the usual sample sizes, which are often more than an order of magnitude smaller.
In animals exposed to a chronic stressor, it has been found in some cases that baseline levels of corticosterone are higher than in non-stressed individuals, but in other cases, they may have lower baselines corticosterone than controls. This is because animals might deal with long-term disturbances by suppressing corticosteroid responses (Rich and Romero, 2005). Birds may physiologically reduce activity of the hypothalamic–pituitary–adrenal (HPA) axis during periods of chronic stress to elude pathological effects linked with chronically elevated glucocorticoid concentrations, such as weight loss, impaired immune function and hyperglycemia (Cyr and Romero, 2007, 2009). Our birds were found to have lower levels of baseline corticosterone when breeding in traffic noise than they did during the no noise treatment, particularly at the second date, which occurred when the birds were in the pair formation and courtship period of the reproductive cycle. This difference does not represent a decrease from the pre-treatment baseline, but rather results from a lack of an increase in baseline corticosterone that was observed in the control condition. One hypothesis for this apparent suppression of the elevated corticosterone baseline during reproduction could be that chronic noise reduces corticosterone responsiveness in order to minimize the negative impacts of chronically elevated glucocorticoids as found by Cyr and Romero (2007, 2009). While we did not find differences in immune function in our noise exposed birds, without additional tests we cannot confirm that our birds in noise had lower corticosterone during breeding than controls because of a suppression of the normal glucocorticoid response or for some other reason.
While we did not find differences between treatments in H/L ratios, a measure of immune function, nor in our measures of reproductive success, we cannot yet conclude that our birds were habituated to the traffic noise. To test whether birds in chronic noise are down-regulating the activity of the HPA axis rather than simply becoming habituated to the noise, it would be necessary to follow up with further experimental testing, such as measuring acute glucocorticoid responses to a second, novel stressor (Wingfield and Romero, 2015).
Another possible interpretation of our results could be that traffic noise is initially a potent stressor, but that the birds quickly habituate to the noise (i.e. the birds learn that the stimulus is not harmful or aversive). To rule out habituation, one must look beyond baseline glucocorticoid levels at other measures, such as immune function, behavioural changes, oxidative damage on the cellular level or secondary responses to additional stressors (Wingfield and Romero, 2015). For instance, European starlings exposed to chronic stress had lower corticosterone levels than controls, but also had reduced reproductive success (Cyr and Romero, 2007), suffered decreases in body weight and altered blood chemistry (Awerman and Romero, 2010) and showed depressed corticosterone responses to novel stressors (Rich and Romero, 2005), all of which are diagnostic indications that habituation had not occurred (Cyr and Romero, 2009). In this study we investigated not only plasma corticosterone but also several measures of reproductive success, extra-pair paternity and immune function, in order to have a wider perspective on potential physiological consequences of chronic noise exposure in zebra finches.
Immune system
Zebra finches exposed to chronic noise did not have significantly higher H/L ratios, as we had predicted. Previous studies have found that an increase in H/L ratio was a reliable indicator of chronic stress in birds, including songbirds (Fourie and Hattingh, 1983; Gross and Siegel, 1986; Maxwell, 1993; Campbell, 1995; Ots and Horak, 1996; Müller et al., 2011). One study in chickens even found a noise-related increase in H/L ratio when chronically exposing them to a very loud unfamiliar sound (Bedanova et al., 2010). However, our study showed an opposite tendency to what we expected, namely a non-significant reduction in the H/L ratio for individuals exposed to noise. It is possible that we did not observe differences in H/L ratios, not because of a lack of increase in heterophils, but rather because there was also an increase in lymphocytes. An increase of lymphocytes could be related with the mating, nesting, hatching and chick rearing periods if birds need to be in the better condition to resist this demanding process. As we only measured H/L ratio at one point in the reproductive cycle, we may have missed an effect if one occurred earlier or later in the treatment. Also, it is important to take into account that other environmental conditions were stable in our study, such as ad lib food availability. Thus, birds might have, for instance, limited negative effects of other stressors on their immune system by changing food intake (Klasing, 2007). Finally, our data did show a differential response in females and males, in that males had lower H/L ratios. This observation suggests that breeding behaviour and/or being in reproductive condition may have different physiological consequences for males than for females.
Reproductive success and extra-pair paternity
As measures of reproductive success, we measured the proportion of offspring that reached adulthood and embryo mortality (embryos that died before hatching). We did not find any significant correlations between noise and either of these variables. However, offspring mortality tended to be higher in birds exposed to noise. In addition to these measures of reproductive success, we also measured body mass in chicks. We measured the body mass of offspring at 10, 21 and 120 days of age and we found that the offspring in the noise treatment were slightly, but significantly lighter at 10 days of age than those in the control treatment. However, the offspring from the two treatments were not different in mass by age 21 days, and so it is unclear whether the small difference in mass during the nestling phase would have much impact on the condition or fitness of these offspring later in life.
Several field studies reported correlations between anthropogenic noise and reproductive success (Kuitunen et al., 2003; Halfwerk et al., 2011; Kight et al., 2012; Injaian et al., 2018; Kleist et al., 2018). Western bluebirds (Sialia mexicana) near noisy oil and gas extraction sites had lower hatching success than conspecifics living near similar, but silent, oil and gas well pads (Kleist et al., 2018). European pied flycatchers (Ficedula hypoleuca) had a decreased number of fledglings per breeding attempt when they lived close to roads (Kuitunen et al., 2003). Both great tits and eastern bluebirds nesting in areas with higher levels of anthropogenic noise fledged fewer offspring than conspecifics in quieter sites (Halfwerk et al., 2011; Kight et al., 2012). Additionally, the offspring of tree swallows (Tachycineta bicolor) exposed to noise during breeding were smaller, fledged later and experienced higher levels of oxidative stress and had a higher rate of telomere attrition than those in quieter sites (Injaian et al., 2018, 2019). While we did not find a striking effect of experimental chronic traffic noise exposure on reproductive success in our laboratory experiment, we did find a tendency towards higher offspring mortality and a small but significant effect of lower body mass in chicks from parents exposed to noise. Our results may be accounted for by direct noise effects on the offspring embryos and hatchlings or by indirect effects via modulation of parental behaviours such as feeding rate. Supporting the latter idea, feeding rates in other songbird species have been shown to be lower in birds raising their young in noisy environments, possibly as a result of noise masking the begging calls of young in the nest (Leonard and Horn, 2008; Schroeder et al., 2012; Leonard et al., 2015). Begging calls are essential in parent–offspring communication since chicks produce begging calls that elicit feeding behaviour in adults (Burford et al., 1998; Budden and Wright, 2001). However, it will require experiments specifically targeting this issue in the future to work out exactly if and how noise exposure may influence parental feeding behaviour and how that affects reproductive success in zebra finches.
In line with our observation that offspring of birds exposed to noise were lighter at the nestling stage (10 days post-hatch) than the offspring from birds in quiet aviaries, previous studies have found that noise during ontogeny can retard growth, particularly in young chicks that are still in the nest (Potvin and MacDougall-Shackleton, 2015a). For example, noise exposure during egg development resulted in chicks with lower body and brain mass in domestic chickens Gallus gallus (Kesar, 2014). Chronic noise exposure impairs normal brain development in the auditory cortex in rats (Chang, 2003) and in areas of the song control system in zebra finches (Potvin et al., 2016). Further, in a related study, we found that juvenile zebra finches exposed to the same noise treatments as we used in this study had higher rates of telomere loss than juveniles from control groups or juveniles whose parents were exposed to noise (Dorado-Correa et al., 2018). Together, this evidence indicates that anthropogenic noise may indeed be an important source of stress-related health effects and is particularly potent during early life.
While our study did not find statistically significant effects of noise playback during breeding on the immune system and reproductive success of the parents, it may be premature to conclude that chronic noise has no negative impact on urban birds. Our experimental birds were housed in climate controlled, parasite- and predator-free aviaries, with unlimited access to food and water. They were not exposed to chemical pollutants, nor to light pollution at night. It may be that noise in itself is indeed a stressor, but not potent enough to cause serious effects on the breeding success or health of adult birds. In humans, noise effects on health may be augmented by, or in turn, may increase the impact of other stressors (reviewed in Stansfeld and Matheson, 2003). Similar results to those we report here have been found in response to other types of stressors in zebra finches breeding in captivity. For example, when breeding pairs were required to spend more time foraging for food, their offspring were lighter at fledging than offspring from parents with easy access to food (Spencer et al., 2003; Brumm et al., 2009; Honarmand et al., 2017). However, it is feasible to imagine that traffic noise pushes wild birds, which already experience a range of environmental challenges and stressors, closer to a threshold above which the combined effects of these stressors would result in more severe physiological effects and fitness consequences. To truly understand the effects of urban environments on bird fitness, it will thus be necessary to conduct experiments designed to investigate the combined effects of multiple aspects of urbanization.
Conclusion
This study is one of the first comprehensive experimental tests of effects of traffic noise exposure on captive songbirds, investigating reproductive success, immune function, glucocorticoid levels and extra-pair paternity in a repeated measures design. We found that noise depressed corticosterone levels in breeding birds and reduced the growth of their nestlings, but that noise did not, by itself, induce acute negative effects on immune status, reproductive success or extra-pair paternity in exposed breeding adults. Further studies into whether noise exposure may have more long-term fitness-related consequences, and if noise interacting with additional stressors leads to more serious acute effects, are needed before ruling out noise as a potent threat to the health and fitness of exposed wildlife. The results of our study correspond with the findings of a recent noise study (Kleist et al., 2018), but not with others (Potvin and MacDougall-Shackleton, 2015a; Davies et al., 2017; Injaian et al., 2018) in the details of when and in which way noise affects reproductive success and physiology. These differences make clear the importance of taking life history stage, species traits and noise profiles into account when designing future experiments, as one-off or short-term studies could easily underestimate impacts of noise pollution. Our study further adds to existing evidence that noise has a stronger negative impact on adult birds during more vulnerable life history stages, such as reproduction, nestling care, as well as during early ontogeny. In the face of increasing urbanization globally, it is critical to consider how to create urban environments that are as ‘livable’ for as many species as possible. Given that global transportation infrastructure spending and car ownership is predicted to double in the next 10 years (Oxford Economics, 2017), traffic noise exposure will potentially affect more wildlife than ever before. Noise mitigation strategies such as sinking roads below ground level or constructing walls to insulate surrounding areas from noise (Nemeth and Zollinger, 2014), creating quieter road surfaces (e.g. Kleizienė et al., 2019) and quieter vehicles can protect sensitive species from some of the negative impacts of road noise year round. However, our study suggests that when permanent noise-reducing structures are not possible, noise impacts could be lessened by developing more transient noise-mitigating strategies, for example, which are focused on reducing road noise during seasonal peaks in avian reproductive behaviour.
Supplementary Material
Acknowledgements
We are very grateful to Antje Bakker, Alexander Girg, Sylvia Kuhn, Melanie Schneider and Monika Trappschuh for help with the processing and analysis of samples, to Sándor Zsebők for writing the MatLab script used for noise playback and to our many colleagues that helped with collecting blood samples: Robin Abbey-Lee, Nicolas Adreani, Robert de Brujin, Javier Bueno-Enciso, Martin Bulla, Leticia Copera, Stefan Greif, Lisa Gill, Antje Girndt, Timothy Grieves, Michaela Hau, Moritz Hertel, Theresa Hügel, Malika Ihle, Sabine Jörg, Meng-Ching Ko, Klemen Koselj, Albertine Leitão, Stefan Leitner, Anja Lohrentz, Katrin Martin, Jaqueline van der Meij, Vincent van Meir, Lucia Mentesana, Maria Moiron, Amanda Monte, Sara Ocasio, Martina Oltrogge, Liviu Parau, Rene Quispe, Sukanya Ramani, Alfredo Sanchez-Tojar, Julia Schäfer, Lotte Schlicht, Julia Schroeder, Susanne Seltmann, Emily Stewart, Erica Stuber, Kim Teltscher, Rachele Trevisi, Lisa Trost, Camila Villavicencio and Andrea Wittenzellner. We also thank Bart Kempenaers for support and comments on the manuscript. Thanks as well to Alexander Lees and two anonymous referees for their helpful and constructive comments.
Funding
This work was supported by the German Research Foundation (award numbers BR 2309/7-1, BR 2309/8-1 and BR 2309/8-2) and the Max Planck Society.
References
- Angelier F, Clément-Chastel C, Welcker J, Gabrielsen GW, Chastel O (2009) How does corticosterone affect parental behaviour and reproductive success? A study of prolactin in black-legged kittiwakes. Funct Ecol 23: 784–793. [Google Scholar]
- Angelier F, Meillère A, Grace JK, Trouvé C, Brischoux F (2016) No evidence for an effect of traffic noise on the development of the corticosterone stress response in an urban exploiter. Gen Comp Endocrinol 232: 43–50. [DOI] [PubMed] [Google Scholar]
- Awerman JL, Romero LM (2010) Chronic psychological stress alters body weight and blood chemistry in European starlings (Sturnus vulgaris). Comp Biochem Physiol A Mol Integr Physiol 156: 136–142. [DOI] [PubMed] [Google Scholar]
- Barber JR, Crooks KR, Fristrup KM (2009) The costs of chronic noise exposure for terrestrial organisms. Trends Ecol Evol 25: 180–189. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48. [Google Scholar]
- Bayerisches Landesamt (2012) Lärmkartierung Vol 2014 https://www.muenchen.de/rathaus/Stadtverwaltung/Referat-fuer-Gesundheit-und-Umwelt/Laerm/Laermminderungsplanung/Laermkartierung.html, Landeshauptstadt Muenchen [Google Scholar]
- Bedanova I, Chloupek J, Chloupek P, Knotkova Z, Voslarova E, Pistekova V, Vecerek V (2010) Responses of peripheral blood leukocytes to chronic intermittent noise exposure in broilers. Berl Munch Tierarztl Wochenschr 123: 186–191. [PubMed] [Google Scholar]
- Biard C, Brischoux F, Meillère A, Michaud B, Nivière M, Ruault S, Vaugoyeau M, Angelier F (2017) Growing in cities: an urban penalty for wild birds? A study of phenotypic differences between urban and rural great tit chicks (Parus major). Front Ecol Evol 5: 79. [Google Scholar]
- Blickley JL, Blackwood D, Patricelli GL (2012) Experimental evidence for the effects of chronic anthropogenic noise on abundance of greater sage-grouse at leks. Conserv Biol 26: 461–471. [DOI] [PubMed] [Google Scholar]
- Bonier F, Moore IT, Martin PR, Robertson RJ (2009) The relationship between fitness and baseline glucocorticoids in a passerine bird. Gen Comp Endocrinol 163: 208–213. [DOI] [PubMed] [Google Scholar]
- Bonier F, Moore IT, Robertson RJ (2011) The stress of parenthood? Increased glucocorticoids in birds with experimentally enlarged broods. Biol Lett 7: 944–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumm H. (2010) Anthropogenic noise: implications for conservation. In Breed MD, Moore J, eds, Encyclopedia of Animal Behavior, Academic Press, Oxford, pp 89–93. [Google Scholar]
- Brumm H, Slabbekoorn H (2005) Acoustic communication in noise. Adv Study Behav 35: 151–209. [Google Scholar]
- Brumm H, Zollinger SA (2013) Avian vocal production in noise. In Brumm H, ed, Animal Communication and Noise. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 187–227. [Google Scholar]
- Brumm H, Zollinger SA, Slater PJB (2009) Developmental stress affects song learning but not song complexity and vocal amplitude in zebra finches. Behav Ecol Sociobiol 63: 1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budden AE, Wright J (2001) Begging in nestling birds. In Nolan V, Thompson CF, eds, Current Ornithology, Vol 16 Springer US, Boston, pp 83–118. [Google Scholar]
- Burford JE, Friedrich TJ, Yasukawa K (1998) Response to playback of nestling begging in the red-winged blackbird, Agelaius phoeniceus. Anim Behav 56: 555–561. [DOI] [PubMed] [Google Scholar]
- Campbell TW. (1995) Avian Hematology and Cytology. Iowa State University Press, Ames [Google Scholar]
- Catchpole C, Slater PJB (2008) Bird Song: Biological Themes and Variations, Ed 2 Cambridge University Press, Cambridge. [Google Scholar]
- Chang EF. (2003) Environmental noise retards auditory cortical development. Science 300: 498–502. [DOI] [PubMed] [Google Scholar]
- Christie MR, Tennessen JA, Blouin MS (2013) Bayesian parentage analysis with systematic accountability of genotyping error, missing data, and false matching. Bioinformatics 29: 725–732. [DOI] [PubMed] [Google Scholar]
- Crino OL, Johnson EE, Blickley JL, Patricelli GL, Breuner CW (2013) Effects of experimentally elevated traffic noise on nestling white-crowned sparrow stress physiology, immune function and life history. J Exp Biol 216: 2055–2062. [DOI] [PubMed] [Google Scholar]
- Crino OL, van Oorschot BK, Johnson EE, Malisch JL, Breuner CW (2011) Proximity to a high traffic road: glucocorticoid and life history consequences for nestling white-crowned sparrows. Gen Comp Endocrinol 173: 323–332. [DOI] [PubMed] [Google Scholar]
- Cyr NE, Romero LM (2007) Chronic stress in free-living European starlings reduces corticosterone concentrations and reproductive success. Gen Comp Endocrinol 151: 82–89. [DOI] [PubMed] [Google Scholar]
- Cyr NE, Romero LM (2009) Identifying hormonal habituation in field studies of stress. Gen Comp Endocrinol 161: 295–303. [DOI] [PubMed] [Google Scholar]
- Davies S, Haddad N, Ouyang JQ (2017) Stressful city sounds: glucocorticoid responses to experimental traffic noise are environmentally dependent. Biol Lett 13: 20170276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens MJ, Romero LM (2013) A consensus endocrine profile for chronically stressed wild animals does not exist. Gen Comp Endocrinol 191: 177–189. [DOI] [PubMed] [Google Scholar]
- Dominoni DM, Borniger JC, Nelson RJ (2016) Light at night, clocks and health: from humans to wild organisms. Biol Lett 12: 20160015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorado-Correa AM, Zollinger SA, Heidinger B, Brumm H (2018) Timing matters: traffic noise accelerates telomere loss rate differently across developmental stages. Front Zool 15: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano-Avila G, Pettorelli N, Virgós E, Lara-Romero C, Lozano J, Barja I, Cuadra FS, Puerta M (2013) Testing Cort-Fitness and Cort-Adaptation hypotheses in a habitat suitability gradient for roe deer. Acta Oecologica 53: 38–48. [Google Scholar]
- Fernández-Juricic E, Tellería JL (2000) Effects of human disturbance on spatial and temporal feeding patterns of Blackbird Turdus merula in urban parks in Madrid, Spain. Bird Study 47: 13–21. [Google Scholar]
- Forstmeier W, Martin K, Bolund E, Schielzeth H, Kempenaers B (2011) Female extrapair mating behavior can evolvevia indirect selection on males. Proc Natl Acad Sci USA 108: 10608–10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmeier W, Schielzeth H, Schneider M, Kempenaers B (2007a) Development of polymorphic microsatellite markers for the zebra finch (Taeniopygia guttata). Mol Ecol Notes 7: 1026–1028. [Google Scholar]
- Forstmeier W, Segelbacher G, Mueller JC, Kempenaers B (2007b) Genetic variation and differentiation in captive and wild zebra finches (Taeniopygia guttata). Mol Ecol 16: 4039–4050. [DOI] [PubMed] [Google Scholar]
- Fourie F, Hattingh J (1983) Comparative haematology of some South African birds. Comp Biochem Physiol A Physiol 74: 443–448. [Google Scholar]
- Gelman A, Hill J (2007) Data Analysis Using Regression and Hierarchical/Multilevel Models. Cambridge University Press, Cambridge [Google Scholar]
- Gil D, Brumm H (2014) Avian Urban Ecology: Behavioural and Physiological Adaptations. Oxford University Press, Oxford [Google Scholar]
- Goymann W, Trappschuh M, Jensen W, Schwabl I (2006) Low ambient temperature increases food intake and dropping production, leading to incorrect estimates of hormone metabolite concentrations in European stonechats. Horm Behav 49: 644–653. [DOI] [PubMed] [Google Scholar]
- Grimm NB, Foster D, Groffman P, Grove JM, Hopkinson CS, Nadelhoffer KJ, Pataki DE, Peters DPC (2008) The changing landscape: ecosystem responses to urbanization and pollution across climatic and societal gradients. Front Ecol Environ 6: 264–272. [Google Scholar]
- Gross WB, Siegel PB (1986) Effects of initial and second periods of fasting on heterophil/lymphocyte ratios and body weight. Avian Dis 30: 345. [PubMed] [Google Scholar]
- Halfwerk W, Holleman LJ, Lessells CK, Slabbekoorn H (2011) Negative impact of traffic noise on avian reproductive success. J Appl Ecol 48: 210–219. [Google Scholar]
- Halfwerk W, Both C, Slabbekoorn H (2016) Noise affects nest box choice of 2 competing songbird species, but not their reproduction. Behav Ecol 27: 1592–600. [Google Scholar]
- Honarmand M, Krause ET, Naguib M (2017) Implications of nutritional stress as nestling or fledgling on subsequent attractiveness and fecundity in zebra finches (Taeniopygia guttata). PeerJ 5: e3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Injaian AS, Taff CC, Patricelli GL (2018) Experimental anthropogenic noise impacts avian parental behaviour, nestling growth and nestling oxidative stress. Anim Behav 136: 31–39. [Google Scholar]
- Injaian AS, Gonzalez-Gomez PL, Taff CC, Bird AK, Ziur AD, Patricelli GL, Haussmann MF, Wingfield JC (2019) Traffic noise exposure alters nestling physiology and telomere attrition through direct, but not maternal, effects in a free-living bird. Gen Comp Endocrinol 276: 14–21. [DOI] [PubMed] [Google Scholar]
- Isaksson C. (2010) Pollution and its impact on wild animals: a meta-analysis on oxidative stress physiology. Ecohealth 7: 342–350. [DOI] [PubMed] [Google Scholar]
- Kesar A. (2014) Effect of prenatal chronic noise exposure on the growth and development of body and brain of chick embryo. Int J Appl Basic Med Res 4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kight R, Saha MS, Swaddle JP (2012) Anthropogenic noise is associated with reductions in the productivity of breeding Eastern Bluebirds (Sialia sialis). Ecol Appl 22: 1989–1996. [DOI] [PubMed] [Google Scholar]
- Kight CR, Swaddle JP (2011) How and why environmental noise impacts animals: an integrative, mechanistic review: environmental noise and animals. Ecol Lett 14: 1052–1061. [DOI] [PubMed] [Google Scholar]
- Klasing KC. (2007) Nutrition and the immune system. Br Poultry Sci 48: 525–537. [DOI] [PubMed] [Google Scholar]
- Kleist NJ, Guralnick RP, Cruz A, Lowry CA, Francis CD (2018) Chronic anthropogenic noise disrupts glucocorticoid signaling and has multiple effects on fitness in an avian community. Proc Natl Acad Sci USA 115: E648–E657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleizienė R, Šernas O, Vaitkus A, Simanavičienė R (2019) Asphalt pavement acoustic performance model. Sustainability 11: 2938. [Google Scholar]
- Kuitunen MT, Viljanen J, Rossi E, Stenroos A (2003) Impact of busy roads on breeding success in pied flycatchers Ficedula hypoleuca. Environ Manag 31: 79–85. [DOI] [PubMed] [Google Scholar]
- Leonard ML, Horn AG (2008) Does ambient noise affect growth and begging call structure in nestling birds? Behav Ecol 19: 502–507. [Google Scholar]
- Leonard ML, Horn AG, Oswald KN, McIntyre E (2015) Effect of ambient noise on parent–offspring interactions in tree swallows. Anim Behav 109: 1–7. [Google Scholar]
- Maxwell MH. (1993) Avian blood leucocyte responses to stress. Worlds Poult Sci J 49: 34–43. [Google Scholar]
- McKinney ML. (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127: 247–260. [Google Scholar]
- McIntyre E, Leonard ML, Horn AG (2014) Ambient noise and parental communication of predation risk in tree swallows, Tachycineta bicolor. Anim Behav 87: 85–89. [Google Scholar]
- Müller C, Jenni-Eiermann S, Jenni L (2011) Heterophils/lymphocytes-ratio and circulating corticosterone do not indicate the same stress imposed on Eurasian kestrel nestlings: H/L-ratio and corticosterone in kestrel nestlings. Funct Ecol 25: 566–576. [Google Scholar]
- Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82: 591–605. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Brumm H (2010) Birds and anthropogenic noise: are urban songs adaptive? Am Nat 176: 465–475. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Zollinger SA (2014) The application of signal transmission modelling in conservation biology: on the possible impact of a projected motorway on avian communication. In Gil D, Brumm H, , Avian Urban Ecology. Oxford University Press, Oxford, pp 192–200. [Google Scholar]
- Ots I, Horak P (1996) Great tits Parus major trade health for reproduction. Proc Biol Sci 263: 1443–1447. [DOI] [PubMed] [Google Scholar]
- Oxford Economics (2017) Global Infrastructure Outlook Report. Global Infrastructure Hub, a G20 Initiative, Oxford, UK: https://www.oxfordeconomics.com/publication/open/283970. Accessed 10 June 2019. [Google Scholar]
- Partecke J. (2013) Mechanisms of phenotypic responses following colonization of urban areas: from plastic to genetic adaptation. In Gil D, Brumm H, , Avian Urban Ecology. Oxford University Press, Oxford, pp 131–142. [Google Scholar]
- Potvin DA, Curcio MT, Swaddle JP, MacDougall-Shackleton SA (2016) Experimental exposure to urban and pink noise affects brain development and song learning in zebra finches (Taenopygia guttata). PeerJ 4: e2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin DA, MacDougall-Shackleton SA (2015a) Experimental chronic noise exposure affects adult song in zebra finches. Anim Behav 107: 201–207. [Google Scholar]
- Potvin DA, MacDougall-Shackleton SA (2015b) Traffic noise affects embryo mortality and nestling growth rates in captive zebra finches. J Exp Zool A Ecol Genet Physiol 323: 722–730. [DOI] [PubMed] [Google Scholar]
- Quinn JL, Whittingham MJ, Butler SJ, Cresswell W (2006) Noise, predation risk compensation and vigilance in the chaffinch Fringilla coelebs. J Avian Biol 37: 601–608. [Google Scholar]
- R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. [Google Scholar]
- Read J, Jones G, Radford AN (2014) Fitness costs as well as benefits are important when considering responses to anthropogenic noise. Behav Ecol 25: 4–7. [Google Scholar]
- Rich EL, Romero LM (2005) Exposure to chronic stress downregulates corticosterone responses to acute stressors. Am J Physiol Regul Integr Comp Physiol 288: R1628–R1636. [DOI] [PubMed] [Google Scholar]
- Romero LM, Reed JM (2005) Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp Biochem Physiol A Mol Integr Physiol 140: 73–79. [DOI] [PubMed] [Google Scholar]
- Saino N, Romano M, Ferrari RP, Martinelli R, Møller AP (2005) Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. J Exp Zool A Ecol Genet Physiol 303: 998–1006. [DOI] [PubMed] [Google Scholar]
- Schroeder J, Nakagawa S, Cleasby IR, Burke T (2012) Passerine birds breeding under chronic noise experience reduced fitness. PLoS One 7: e39200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan DF, Strohbach MW, Warren PS, Fuller RA (2014) The challenges of urban living. In Gil D, Brumm H, eds, Avian Urban Ecology: Behavioural and Physiological Adaptations. Oxford University Press, Oxford, pp 3–20. [Google Scholar]
- Spée M, Marchal L, Lazin D, Le Maho Y, Chastel O, Beaulieu M, Raclot T (2011) Exogenous corticosterone and nest abandonment: a study in a long-lived bird, the Adélie penguin. Horm Behav 60: 362–370. [DOI] [PubMed] [Google Scholar]
- Spencer KA, Buchanan KL, Goldsmith AR, Catchpole CK (2003) Song as an honest signal of developmental stress in the zebra finch (Taeniopygia guttata). Horm Behav 44: 132–139. [DOI] [PubMed] [Google Scholar]
- Stansfeld SA, Matheson MP (2003) Noise-pollution: non-auditory effects on health. Br Med Bull 68: 243–257. [DOI] [PubMed] [Google Scholar]
- Swaddle JP, Page LC (2007) High levels of environmental noise erode pair preferences in zebra finches: implications for noise pollution. Anim Behav 74: 363–368. [Google Scholar]
- Templeton CN, Zollinger SA, Brumm H (2016) Traffic noise drowns out great tit alarm calls. Curr Biol 26: R1173–R1174. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Massemin S, Handrich Y, Raclot T (2013) Elevated corticosterone levels and severe weather conditions decrease parental investment of incubating Adélie penguins. Horm Behav 63: 475–483. [DOI] [PubMed] [Google Scholar]
- United Nations (2014) World Urbanization Prospects: The 2014 Revision, Highlights. Department of Economic and Social Affairs. Population Division, United Nations; New York. [Google Scholar]
- Vleck CM, Vertalino N, Vleck D, Bucher TL (2000) Stress, corticosterone, and heterophil to lymphocyte ratios in free-living Adélie penguins. Condor 102: 392. [Google Scholar]
- Wang D, Kempenaers N, Kempenaers B, Forstmeier W (2017) Male zebra finches have limited ability to identify high-fecundity females. Behav Ecol 28: 784–792. [Google Scholar]
- Wingfield JC, Romero LM (2015) Tempests, Poxes, Predators, and People: Stress in Wild Animals and How They Cope. Oxford University Press, New York. [Google Scholar]
- Wright AJ, Soto NA, Baldwin AL, Bateson M, Beale CM, Clark C, Deak T, Edwards EF, Fernández A, Godinho A (2007) Anthropogenic noise as a stressor in animals: a multidisciplinary perspective. Int J Comp Psych 20: 250–273. [Google Scholar]
- Yorzinski JL, Hermann FS (2016) Noise pollution has limited effects on nocturnal vigilance in peahens. PeerJ 4: e2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


